Molecular Mechanisms Underlying Lubrication by Ionic Liquids: Activated Slip and Flow
Abstract
1. Introduction
2. Results
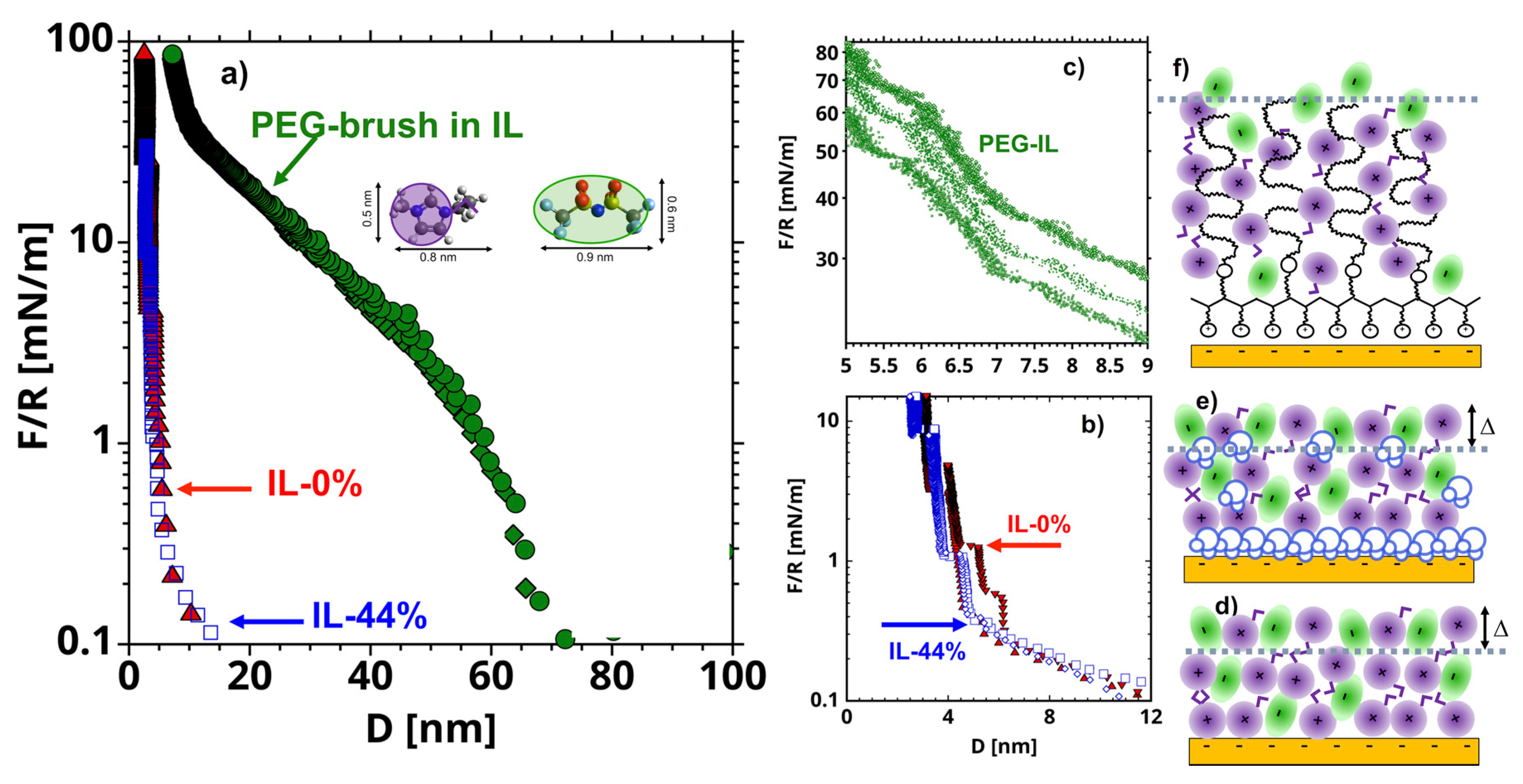
2.1. Interfacial Structure
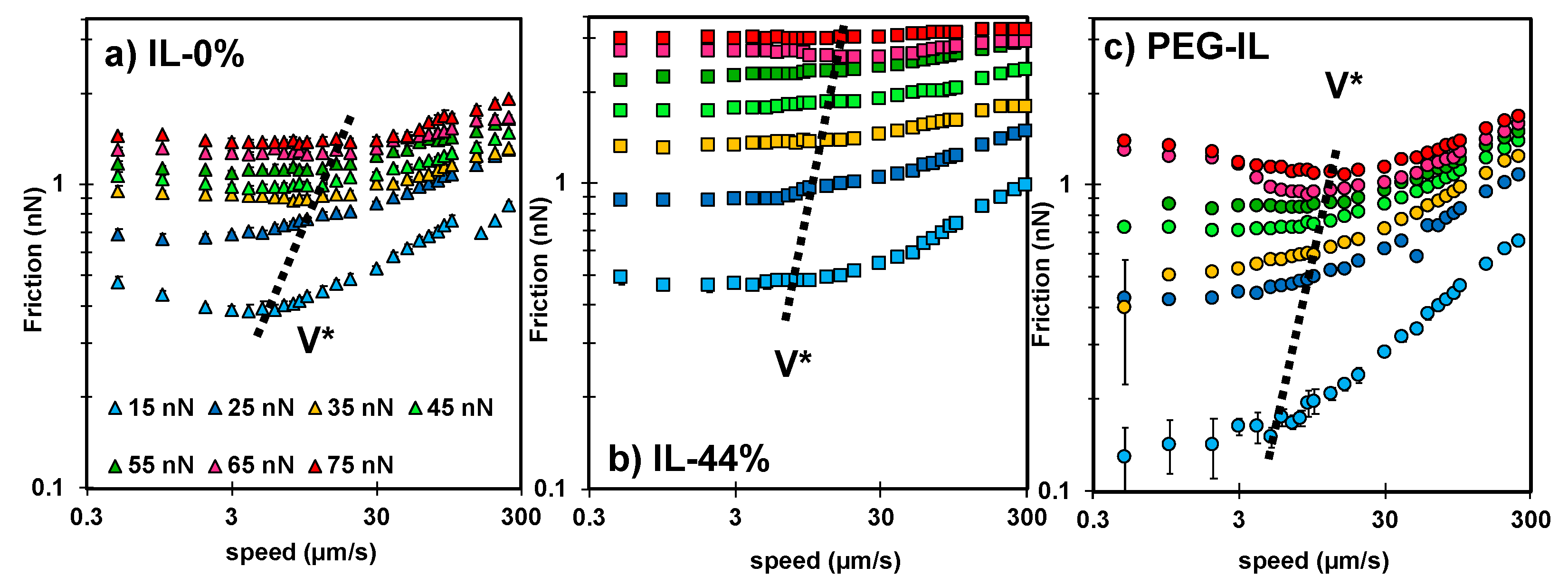
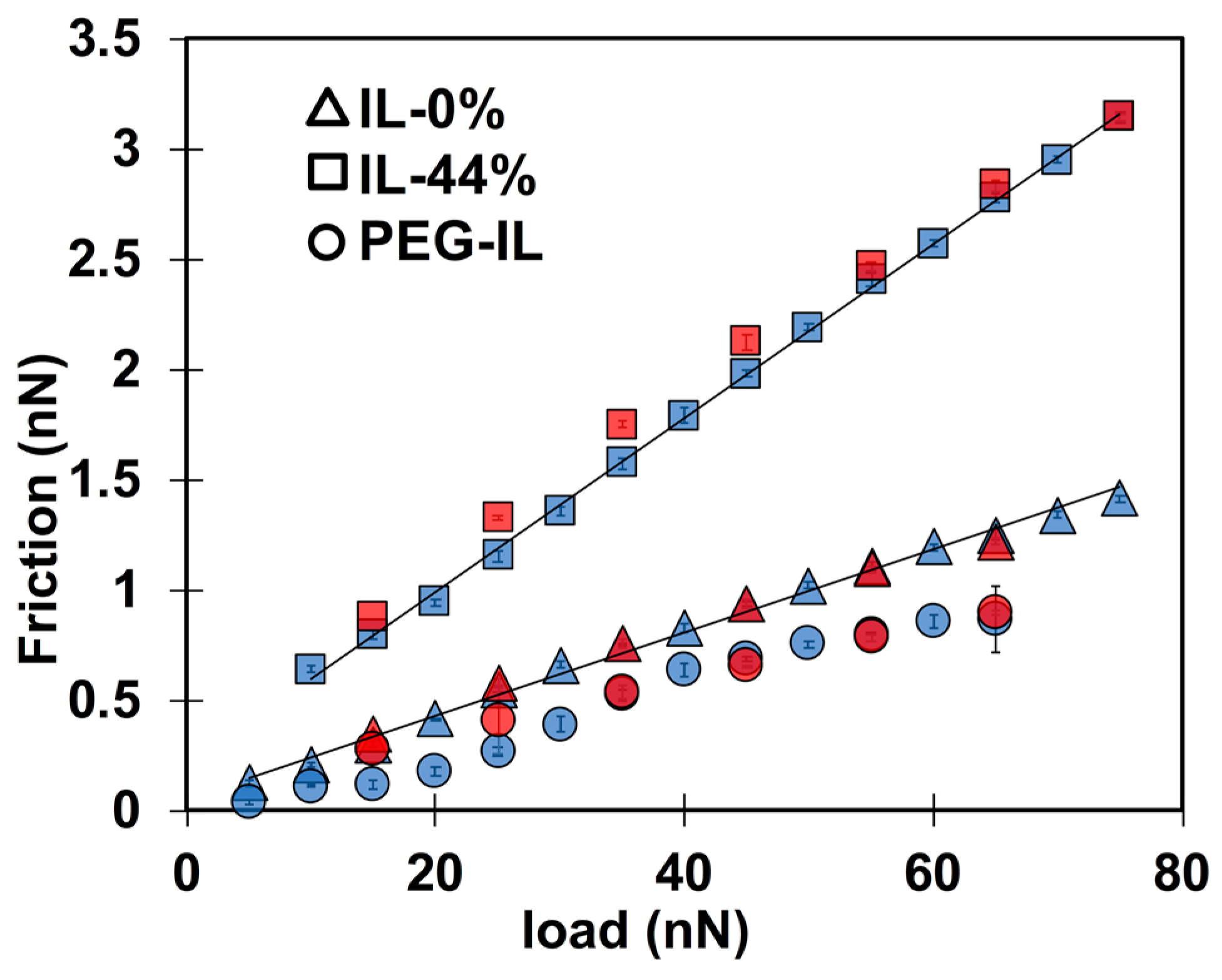
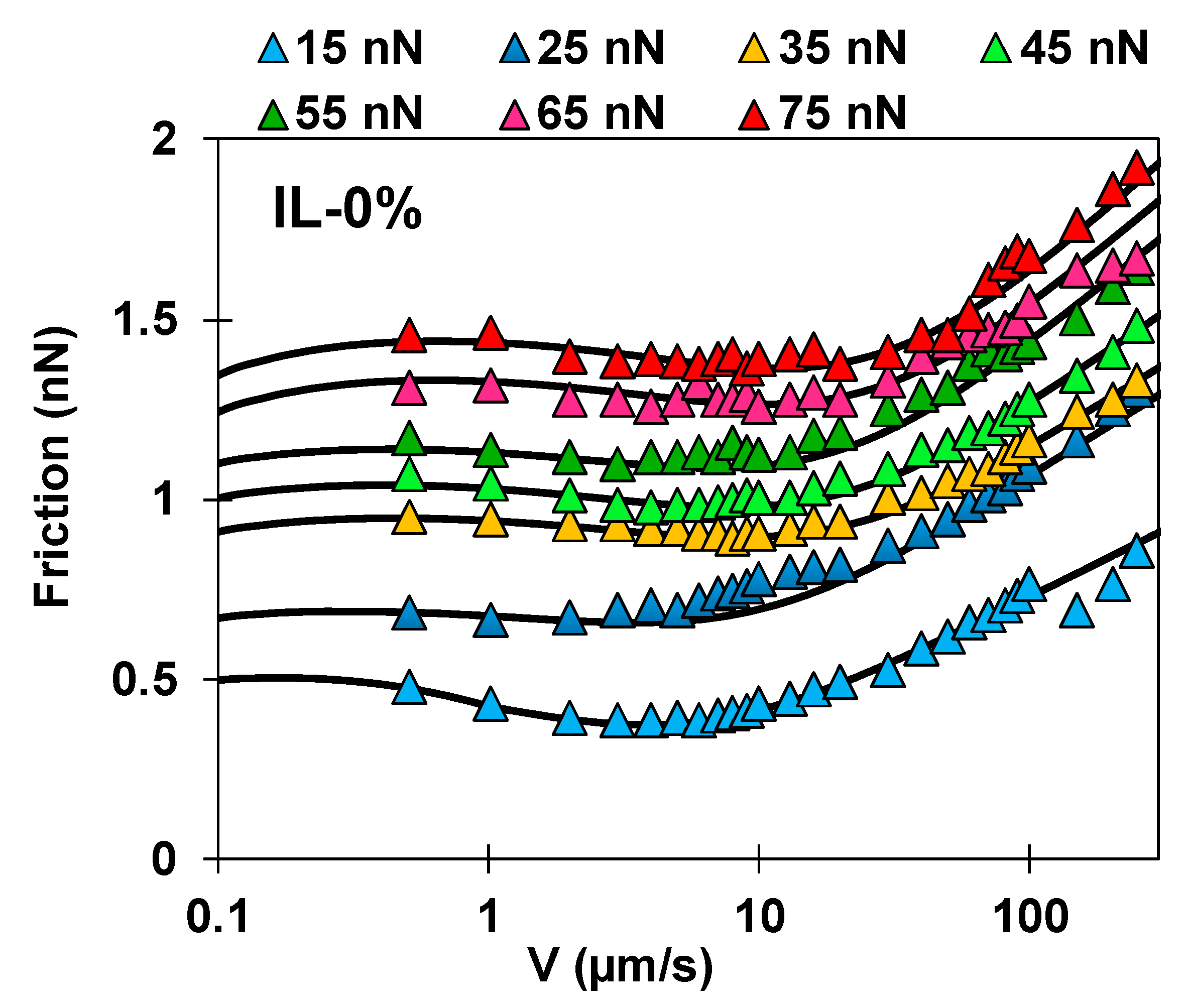
2.2. Friction-Force Measurements
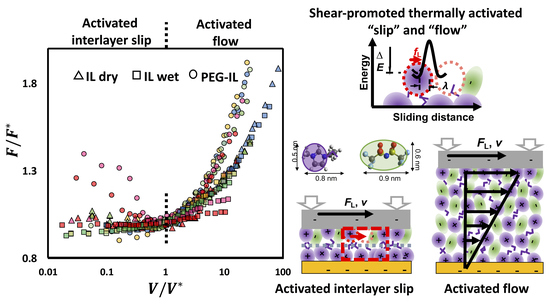
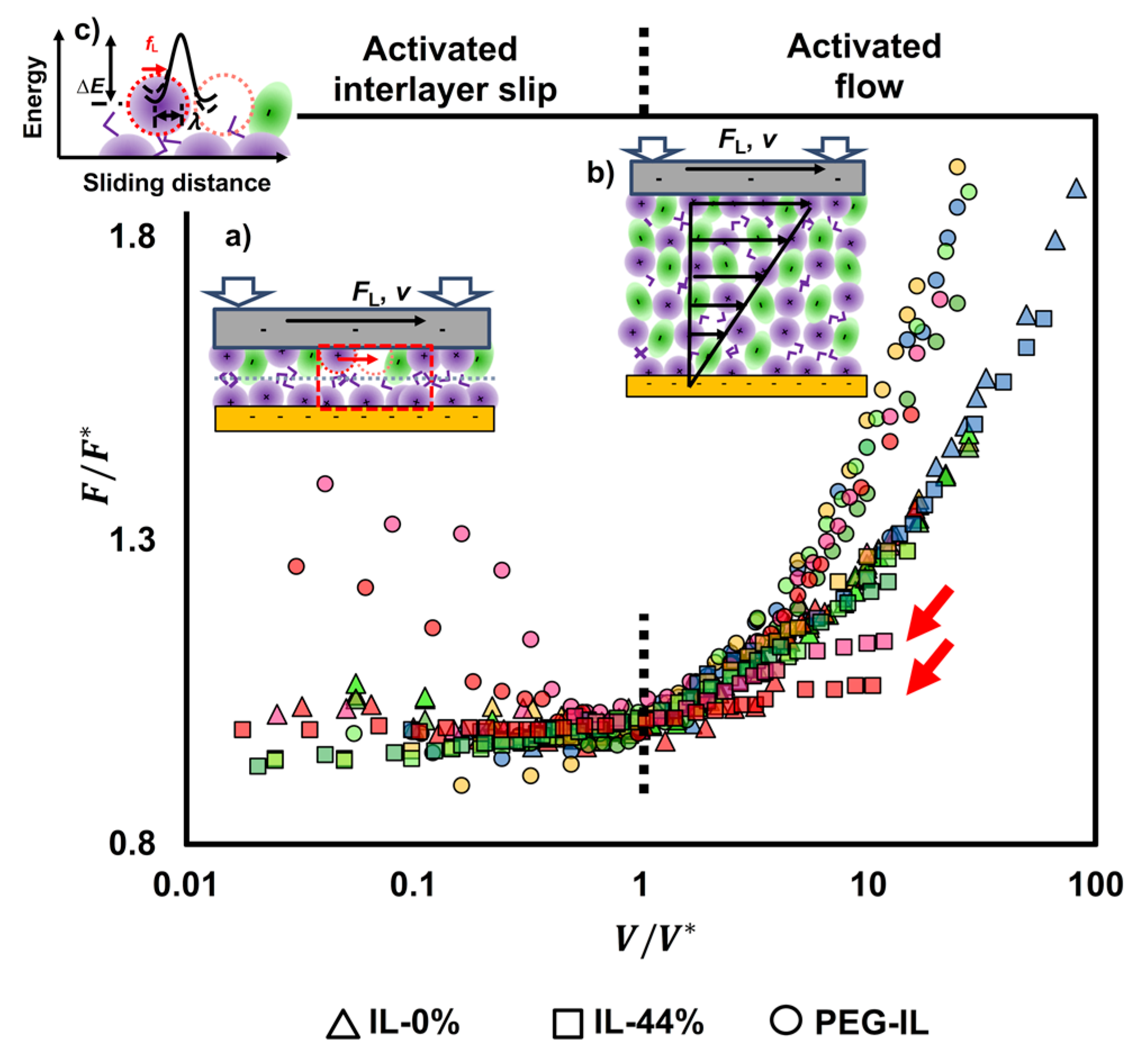
2.3. Identifying Molecular Mechanisms of Lubrication: Stress Activated Slip and Viscous Flow
2.4. Molecular Model of Friction
- The plateau of the friction force at the slowest sliding velocities is exclusively dictated by , and hence, it can be fit without knowledge of / and . At the fastest sliding velocities, can be determined independently of other parameters.
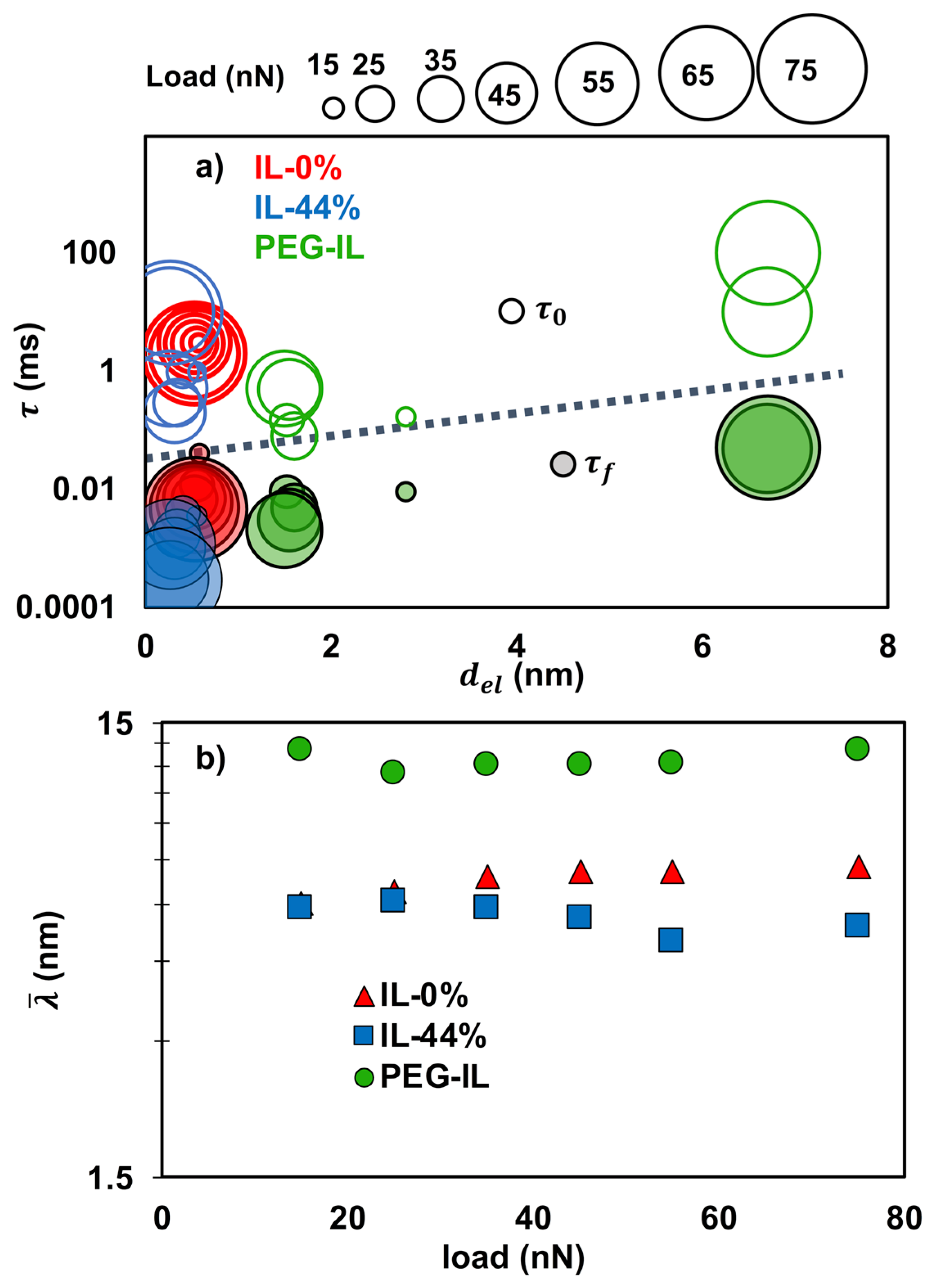
- The described model assumes the junction length to be close to the ion size, which is nm in IL-0% and IL-44% (the initial guess), so that can be roughly estimated at the plateau. We expect and to remain approximately constant with a change in load, however, as discussed later, needs to be decreased in IL-44% to describe the behavior at the highest loads. The stretching length must be smaller than . Good fits to the experimental data for IL-0% and IL-44% are achieved with ~ 0.12 nm and ~ 3.2 MPa at all loads.
- For the brush-bearing mica surface, a transitional response of the elastic friction was observed with an increase in load. Considering the compression of the brush with load (Figure 1), we assume that, at the smallest loads (<65 nN), the slip plane is located within the liquid film that separates the brush and the countersurface—as shown by MD simulations [45]. Here, , and are expected to be close to the values for IL-0%. At the highest loads (65 and 75 nN), the IL is squeezed out so that the polymer directly intervenes in the sliding process. Here, the slip plane is assumed to be located between the polymer and the colloid. The polymer junctions and the characteristic stretching length are expected to be greater than in IL-0%. Our initial guess is ~ 7 nm based on the force-distance curves in Figure 1a: the -values at the loads of 65 and 75 nN are 26 and 30 mN/m, respectively, which leads to ~ 14 nm for the brush-brush system, and thus, for the brush-colloid system in the friction-force measurements. Good fits to the experimental results are achieved with equal to 0.73 MPa and 0.9 nm for the polymer junctions.
- An increase in elastic friction with velocity requires very small characteristic times for bond formation (e.g., 0.1 µs), and it is strongly dependent on according to .
- The decrease in elastic friction with velocity is well described by and it is strongly influenced by , while only slightly affects friction at the slowest sliding velocity in our experiments.
3. Discussion
4. Conclusions and Implications
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Li, J. Ionic Liquids in Surface Electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Wakeham, D.; Atkin, R. Interfaces of Ionic Liquids (2). In Ionic Liquids Uncoiled; John Wiley & Sons, Inc.: New York, NY, USA, 2012; pp. 51–85. [Google Scholar]
- Espinosa-Marzal, R.M.; Han, M.; Arcifa, A.; Spencer, N.D.; Rossi, A. Ionic Liquids at Interfaces and Their Tribological Behavior. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2017; pp. 172–194. [Google Scholar]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Electrotunable Lubricity with Ionic Liquid Nanoscale Films. Sci. Rep. 2015, 5, 7698. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Hausen, F.; Hayes, R.; Webber, G.B.; Endres, F.; Rutland, M.W.; Bennewitz, R.; Atkin, R. Control of Nanoscale Friction on Gold in an Ionic Liquid by a Potential-Dependent Ionic Lubricant Layer. Phys. Rev. Lett. 2012, 109, 155502. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-Temperature Ionic Liquids: A Novel Versatile Lubricant. Chem. Commun. 2001, 2244–2245. [Google Scholar] [CrossRef]
- Perkin, S.; Albrecht, T.; Klein, J. Layering and Shear Properties of an Ionic Liquid, 1-Ethyl-3-Methylimidazolium Ethylsulfate, Confined to Nano-Films between Mica Surfaces. Phys. Chem. Chem. Phys. 2010, 12, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Werzer, O.; Cranston, E.D.; Warr, G.G.; Atkin, R.; Rutland, M.W. Ionic Liquid Nanotribology: Mica-Silica Interactions in Ethylammonium Nitrate. Phys. Chem. Chem. Phys. 2012, 14, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rutland, M.W.; Atkin, R. Ionic Liquid Lubrication: Influence of Ion Structure, Surface Potential and Sliding Velocity. Phys. Chem. Chem. Phys. 2013, 15, 14616–14623. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Lovelock, K.R.; Gosvami, N.N.; Welton, T.; Perkin, S. Quantized Friction across Ionic Liquid Thin Films. Phys. Chem. Chem. Phys. 2013, 15, 15317–15320. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Marzal, R.M.; Arcifa, A.; Rossi, A.; Spencer, N.D. Microslips to “Avalanches” in Confined, Molecular Layers of Ionic Liquids. J. Phys. Chem. Lett. 2014, 5, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Marzal, R.M.; Arcifa, A.; Rossi, A.; Spencer, N.D. Ionic Liquids Confined in Hydrophilic Nanocontacts: Structure and Lubricity in the Presence of Water. J. Phys. Chem. C 2014, 118, 6491–6503. [Google Scholar] [CrossRef]
- Sweeney, J.; Webber, G.B.; Rutland, M.W.; Atkin, R. Effect of Ion Structure on Nanoscale Friction in Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 16651–16658. [Google Scholar] [CrossRef] [PubMed]
- Arcifa, A.; Rossi, A.; Espinosa-Marzal, R.M.; Spencer, N.D. Influence of Environmental Humidity on the Wear and Friction of a Silica/Silicon Tribopair Lubricated with a Hydrophilic Ionic Liquid. Acs. Appl. Mater. Interfaces 2016, 8, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An Ionic Liquid Lubricant Enables Superlubricity to Be “Switched on” in Situ Using an Electrical Potential. Chem. Commun. 2014, 50, 4368–4370. [Google Scholar] [CrossRef] [PubMed]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Eyring, H. Viscosity, Plasticity, and Diffusion as Examples of Absolute Reaction Rates. J. Chem. Phys. 1936, 4, 283–291. [Google Scholar] [CrossRef]
- Spikes, H.; Tysoe, W. On the Commonality between Theoretical Models for Fluid and Solid Friction, Wear and Tribochemistry. Tribol. Lett. 2015, 59, 21. [Google Scholar] [CrossRef]
- Briscoe, B.; Evans, D. The Shear Properties of Langmuir-Blodgett Layers; The Royal Society: Cambridge, UK, 1982; pp. 389–407. [Google Scholar]
- Schallamach, A. A Theory of Dynamic Rubber Friction. Wear 1963, 6, 375–382. [Google Scholar] [CrossRef]
- Drummond, C.; Israelachvili, J.; Richetti, P. Friction between Two Weakly Adhering Boundary Lubricated Surfaces in Water. Phys. Rev. E 2003, 67, 066110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.W.; Stock, P.; Moeremans, B.; Baimpos, T.; Banquy, X.; Renner, F.U.; Valtiner, M. Characterizing the Influence of Water on Charging and Layering at Electrified Ionic-Liquid/Solid Interfaces. Adv. Mater. Interfaces 2015, 2, 150159. [Google Scholar] [CrossRef]
- Arcifa, A.; Rossi, A.; Ramakrishna, S.N.; Espinosa-Marzal, R.; Sheehan, A.; Spencer, N.D. Lubrication of Si-Based Tribopairs with a Hydrophobic Ionic Liquid: The Multiscale Influence of Water. J. Phys. Chem. C 2018, 122, 7331–7343. [Google Scholar] [CrossRef]
- Han, M.; Espinosa-Marzal, R.M. Strong Stretching of Poly(Ethylene Glycol) Brushes Mediated by Ionic Liquid Solvation. J. Phys. Chem. Lett. 2017, 8, 3954–3960. [Google Scholar] [CrossRef] [PubMed]
- Gebbie, M.A.; Valtiner, M.; Banquy, X.; Fox, E.T.; Henderson, W.A.; Israelachvili, J.N. Ionic Liquids Behave as Dilute Electrolyte Solutions. Proc. Natl. Acad. Sci. USA 2013, 110, 9674–9679. [Google Scholar] [CrossRef] [PubMed]
- Gebbie, M.A.; Smith, A.M.; Dobbs, H.A.; Lee, A.A.; Warr, G.G.; Banquy, X.; Valtiner, M.; Rutland, M.W.; Israelachvili, J.N.; Perkin, S.; et al. Long Range Electrostatic Forces in Ionic Liquids. Chem. Commun. 2017, 53, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.G.; Evans, D.F.; Ninham, B.W. Double-Layer and Solvation Forces Measured in a Molten-Salt and Its Mixtures with Water. J. Phys. Chem. 1988, 92, 3531–3537. [Google Scholar] [CrossRef]
- Atkin, R.; Warr, G.G. Structure in Confined Room-Temperature Ionic Liquids. J. Phys. Chem. C 2007, 111, 5162–5168. [Google Scholar] [CrossRef]
- Lhermerout, R.; Perkin, S. Nanoconfined Ionic Liquids: Disentangling Electrostatic and Viscous Forces. Phys. Rev. Fluids 2018, 3, 014201. [Google Scholar] [CrossRef]
- Zhou, H.; Rouha, M.; Feng, G.; Lee, S.S.; Docherty, H.; Fenter, P.; Cummings, P.T.; Fulvio, P.F.; Dai, S.; McDonough, J.; et al. Nanoscale Perturbations of Room Temperature Ionic Liquid Structure at Charged and Uncharged Interfaces. ACS Nano 2012, 6, 9818–9827. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Water in Ionic Liquid Lubricants: Friend and Foe. ACS Nano 2017, 11, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Fujii, K.; Nishi, K.; Sakai, T.; Ohara, K.; Umebayashi, Y.; Shibayama, M. Solvation Structure of Poly(Ethylene Glycol) in Ionic Liquids Studied by High-Energy X-Ray Diffraction and Molecular Dynamics Simulations. Macromolecules 2013, 46, 2369–2375. [Google Scholar] [CrossRef]
- Costa, L.T.; Ribeiro, M.C. Molecular Dynamics Simulation of Polymer Electrolytes Based on Poly(Ethylene Oxide) and Ionic Liquids. Ii. Dynamical Properties. J. Chem. Phys. 2007, 127, 164901. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier Science: Amsterdam, The Netherland, 2011. [Google Scholar]
- Shoaib, T.; Heintz, J.; Lopez-Berganza, J.A.; Muro-Barrios, R.; Egner, S.A.; Espinosa-Marzal, R.M. Stick-Slip Friction Reveals Hydrogel Lubrication Mechanisms. Langmuir 2018, 34, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K.J.; Goren, T.; Crockett, R.; Spencer, N.D. Load-Induced Transitions in the Lubricity of Adsorbed Poly(l-Lysine)-G-Dextran as a Function of Polysaccharide Chain Density. ACS Appl. Mater. Interfaces 2011, 3, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Iler, R.K. The Chemistry of Silica; Wiley: New York, NY, USA, 1979. [Google Scholar]
- He, Z.; Alexandridis, P. Nanoparticles in Ionic Liquids: Interactions and Organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef] [PubMed]
- Esfahanian, M.; Hamrock, B.J. Fluid-Film Lubrication Regimes Revisited. Tribol. Trans. 1991, 34, 628–632. [Google Scholar] [CrossRef]
- Johnson, K.L.; Tevaarwerk, J.L. Shear Behavior of Elastohydrodynamic Oil Films. Proc. R. Soc. Lond. Ser. A 1977, 356, 215–236. [Google Scholar] [CrossRef]
- Bair, S.; Martinie, L.; Vergne, P. Classical Ehl Versus Quantitative Ehl: A Perspective Part Ii-Super-Arrhenius Piezoviscosity, an Essential Component of Elastohydrodynamic Friction Missing from Classical Ehl. Tribol. Lett. 2016, 63, 37. [Google Scholar] [CrossRef]
- O’neill, M.; Stewartson, K. On the Slow Motion of a Sphere Parallel to a Nearby Plane Wall. J. Fluid Mech. 1967, 27, 705–724. [Google Scholar] [CrossRef]
- Grest, G.S. Interfacial Sliding of Polymer Brushes: A Molecular Dynamics Simulation. Phys. Rev. Lett. 1996, 76, 4979–4982. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Carson, G.A.; Granick, S. Relaxation Time of Confined Liquids under Shear. Phys. Rev. Lett. 1991, 66, 2758–2761. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Colby, R.H. Polymer Physics; OUP: Oxford, UK, 2003. [Google Scholar]
- Granick, S. Motions and Relaxations of Confined Liquids. Science 1991, 253, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jiang, X.; Qiao, R.; Kornyshev, A.A. Water in Ionic Liquids at Electrified Interfaces: The Anatomy of Electrosorption. ACS Nano 2014, 8, 11685–11694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fang, C.; Qiao, R. Effects of Water on Mica-Ionic Liquid Interfaces. J. Phys. Chem. C 2018, 122, 9035–9045. [Google Scholar] [CrossRef]
- Lemaitre, A. Rearrangements and Dilatancy for Sheared Dense Materials. Phys. Rev. Lett. 2002, 89, 195503. [Google Scholar] [CrossRef] [PubMed]
- Grest, G.S. Computer Simulations of Shear and Friction between Polymer Brushes. Curr. Opin. Colloid Interfaces Sci. 1997, 2, 271–277. [Google Scholar] [CrossRef]
- Irfachsyad, D.; Tildesley, D.; Malfreyt, P. Dissipative Particle Dynamics Simulation of Grafted Polymer Brushes under Shear. Phys. Chem. Chem. Phys. 2002, 4, 3008–3015. [Google Scholar] [CrossRef]
- Nalam, P.C.; Ramakrishna, S.N.; Espinosa-Marzal, R.M.; Spencer, N.D. Exploring Lubrication Regimes at the Nanoscale: Nanotribological Characterization of Silica and Polymer Brushes in Viscous Solvents. Langmuir 2013, 29, 10149–10158. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Perahia, D.; Warburg, S. Forces between Polymer-Bearing Surfaces Undergoing Shear. Nature 1991, 352, 143–145. [Google Scholar] [CrossRef]
- Klein, J.; Kumacheva, E.; Mahalu, D.; Perahia, D.; Fetters, L.J. Reduction of Frictional Forces between Solid-Surfaces Bearing Polymer Brushes. Nature 1994, 370, 634–636. [Google Scholar] [CrossRef]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.F.; Jerome, R.; Klein, J. Lubrication by Charged Polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.E.; Bermudez, M.D. Imidazolium Ionic Liquids as Additives of the Synthetic Ester Propylene Glycol Dioleate in Aluminium-Steel Lubrication. Wear 2008, 265, 787–798. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Bermudez, M.D.; Carrion, F.J.; Martinez-Nicolas, G. Room Temperature Ionic Liquids as Lubricant Additives in Steel-Aluminium Contacts: Influence of Sliding Velocity, Normal Load and Temperature. Wear 2006, 261, 347–359. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.Q.; Luo, H.M.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-Miscible and Non-Corrosive Phosphonium-Based Ionic Liquids as Candidate Lubricant Additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Blass, J.; Albrecht, M.; Wenz, G.; Guerra, R.; Urbakh, M.; Bennewitz, R. Multivalent Adhesion and Friction Dynamics Depend on Attachment Flexibility. J. Phys. Chem. C 2017, 121, 15888–15896. [Google Scholar] [CrossRef]
- Cannara, R.J.; Eglin, M.; Carpick, R.W. Lateral Force Calibration in Atomic Force Microscopy: A New Lateral Force Calibration Method and General Guidelines for Optimization. Rev. Sci. Instrum. 2006, 77, 053701. [Google Scholar] [CrossRef]
- Heuberger, M.; Vanicek, J.; Zach, M. The Extended Surface Forces Apparatus. Ii. Precision Temperature Control. Rev. Sci. Instrum. 2001, 72, 3556–3560. [Google Scholar] [CrossRef]
- Heuberger, M. The Extended Surface Forces Apparatus. Part I. Fast Spectral Correlation Interferometry. Rev. Sci. Instrum. 2001, 72, 1700–1707. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Thin Film Studies Using Multiple-Beam Interferometry. J. Colloid Interfaces Sci. 1973, 44, 259–272. [Google Scholar] [CrossRef]
| IL-0% | IL-44% | PEG-IL | |||||
|---|---|---|---|---|---|---|---|
| 15 nN | 75 nN | 15 nN | 75 nN | 15 nN | 75 nN | ||
| (ms) | 3.00 | 2.00 | 1.00 | 10.00 | 0.17 | 100.00 | |
| (ms) | 0.040 | 0.005 | 0.0035 | 0.0003 | 0.01 | 0.05 | |
| (nm) | 0.14 | 0.11 | 0.12 | 0.11 | 0.11 | 0.92 | |
| G | MPa | 3.18 | 3.18 | 3.20 | 3.18 | 1 | 0.7 |
| nm | 0.58 | 0.53 | 0.55 | 0.26 | 1.5 | 6.70 | |
| kPa | 37.20 | 21.32 | 40.69 | 52.91 | 3.63 | 3.63 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Espinosa-Marzal, R.M. Molecular Mechanisms Underlying Lubrication by Ionic Liquids: Activated Slip and Flow. Lubricants 2018, 6, 64. https://doi.org/10.3390/lubricants6030064
Han M, Espinosa-Marzal RM. Molecular Mechanisms Underlying Lubrication by Ionic Liquids: Activated Slip and Flow. Lubricants. 2018; 6(3):64. https://doi.org/10.3390/lubricants6030064
Chicago/Turabian StyleHan, Mengwei, and Rosa M. Espinosa-Marzal. 2018. "Molecular Mechanisms Underlying Lubrication by Ionic Liquids: Activated Slip and Flow" Lubricants 6, no. 3: 64. https://doi.org/10.3390/lubricants6030064
APA StyleHan, M., & Espinosa-Marzal, R. M. (2018). Molecular Mechanisms Underlying Lubrication by Ionic Liquids: Activated Slip and Flow. Lubricants, 6(3), 64. https://doi.org/10.3390/lubricants6030064