Ultra-Low Friction on Tetrahedral Amorphous Diamond-Like Carbon (ta-C) Lubricated with Ethylene Glycol
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ta-C Coatings
2.2. Tribological Characterization
2.3. Surface Characterization
3. Results and Discussion
3.1. Tribological Experiments
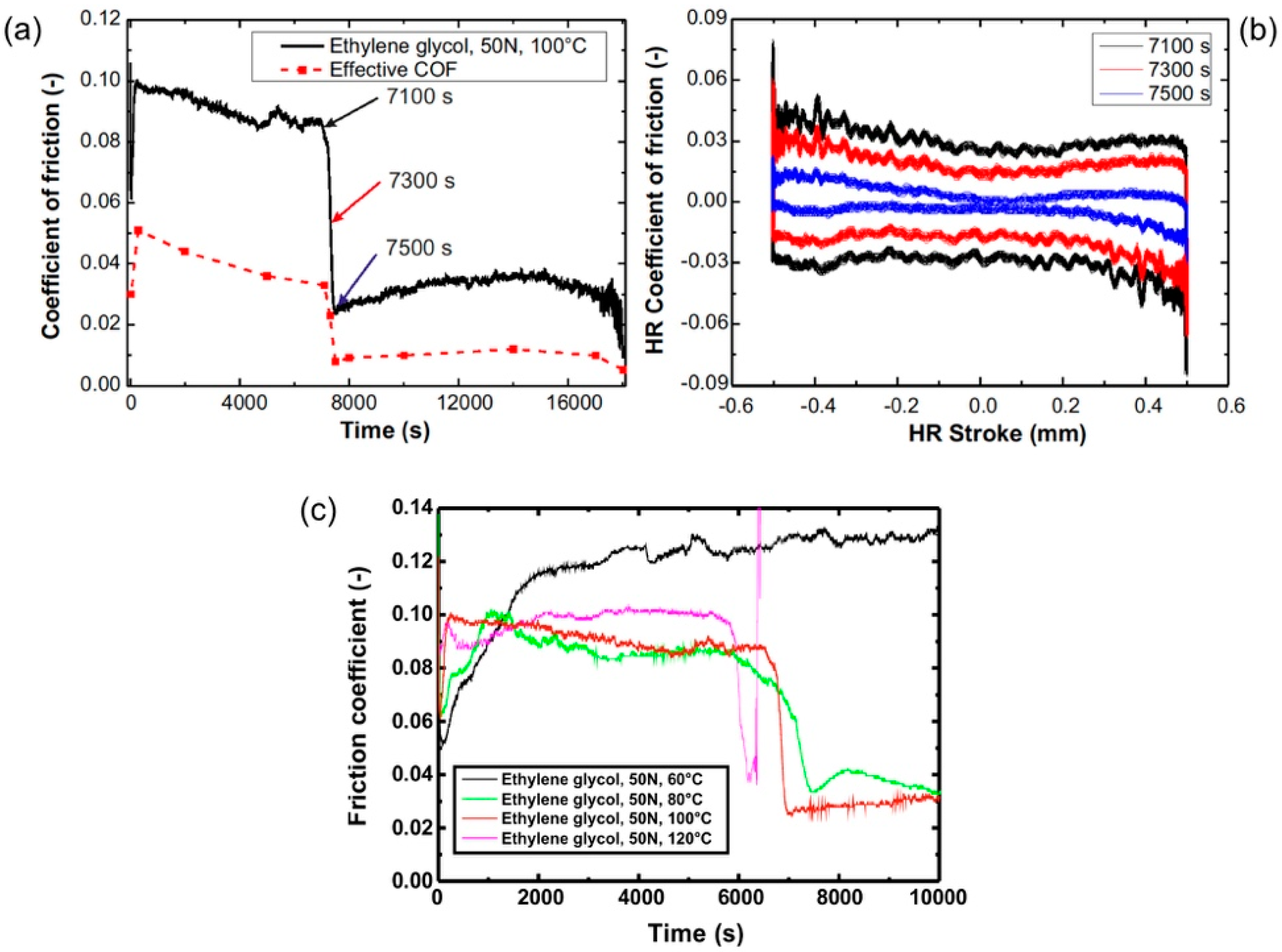
3.1.1. ta-C Lubricated by Ethylene Glycol
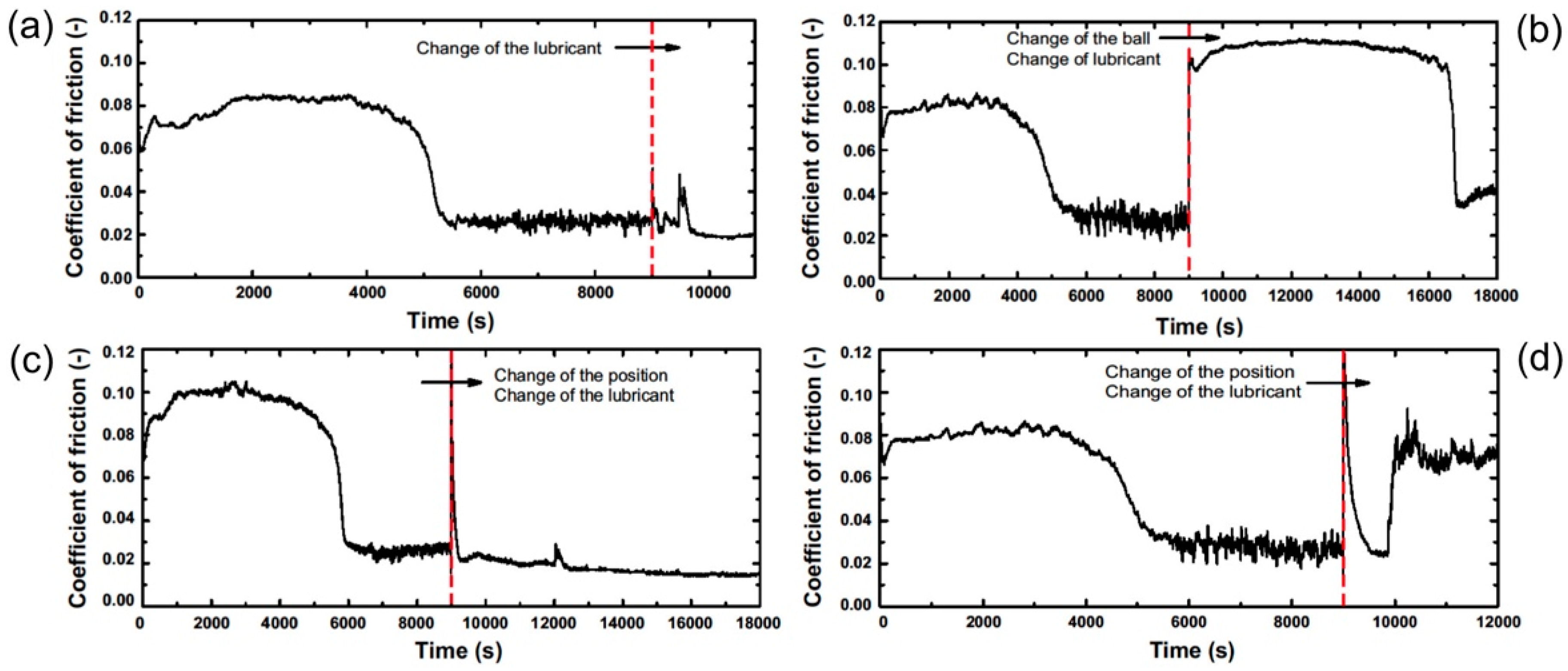
3.1.2. Variation of the Tribological System
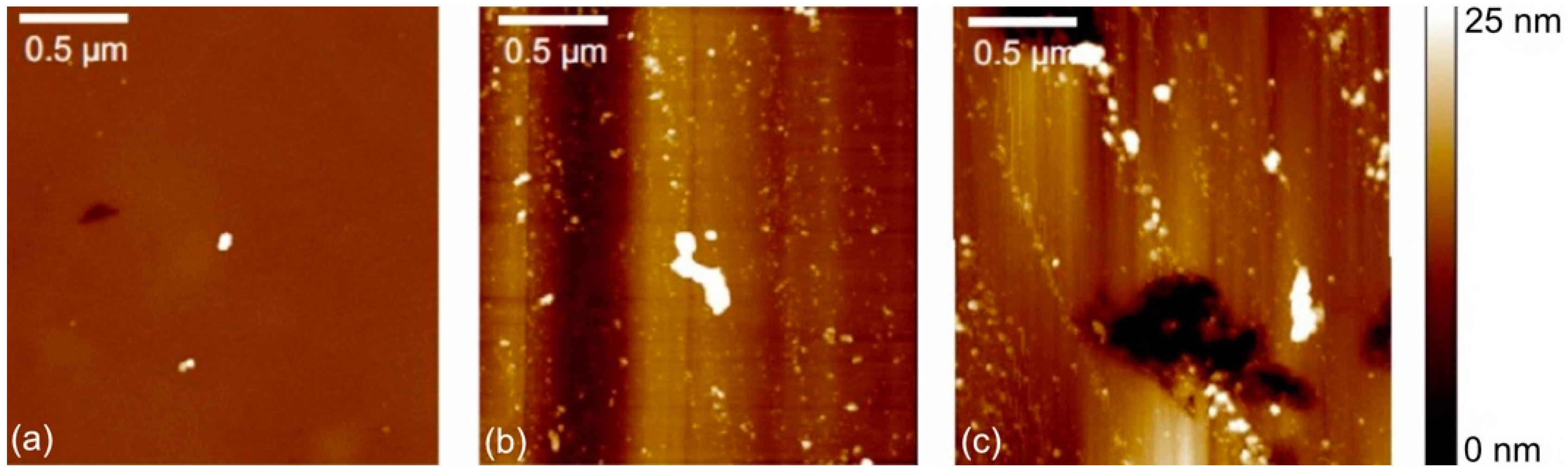
3.2. Surface Characterization
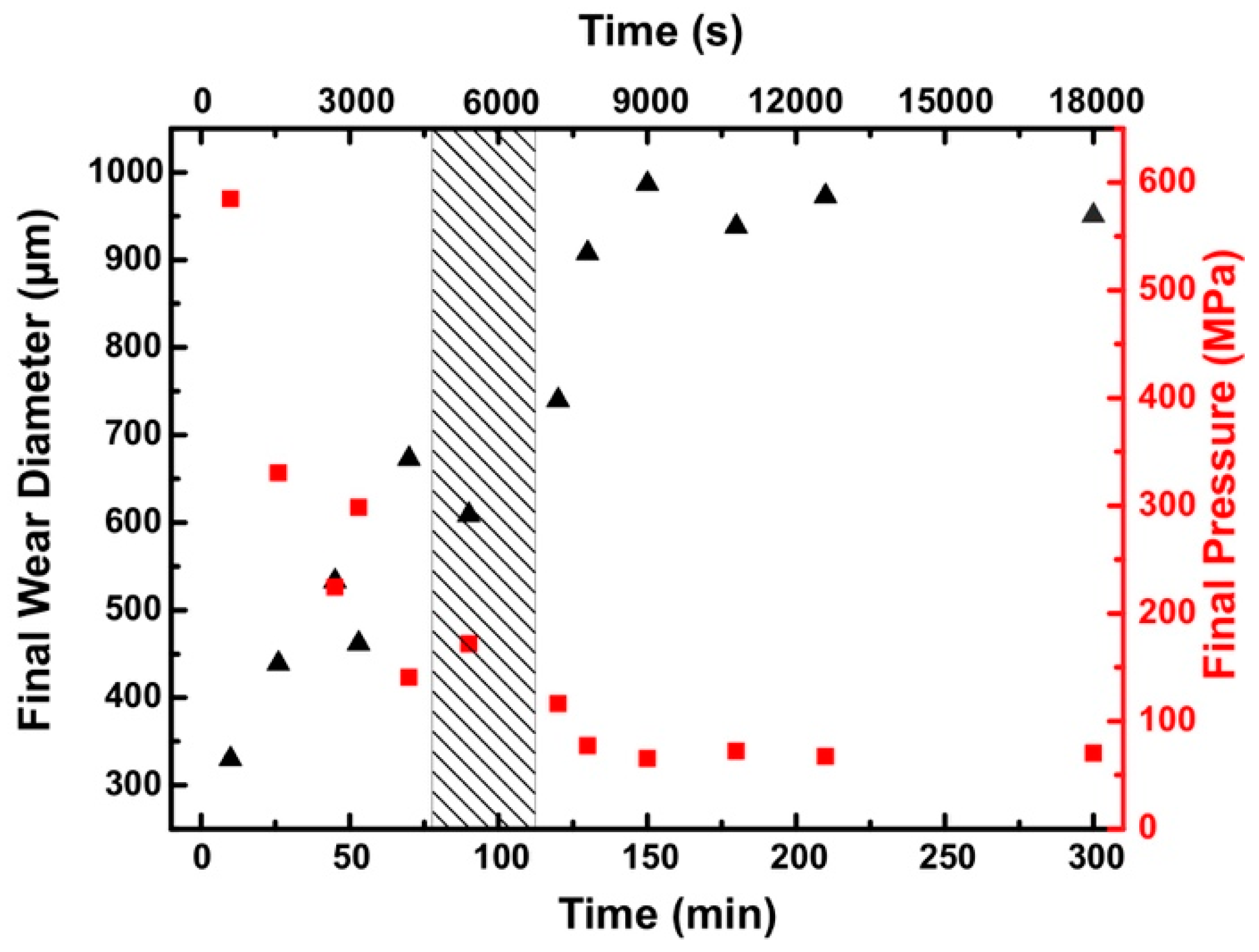
3.2.1. Wear of the Steel Counter-Body
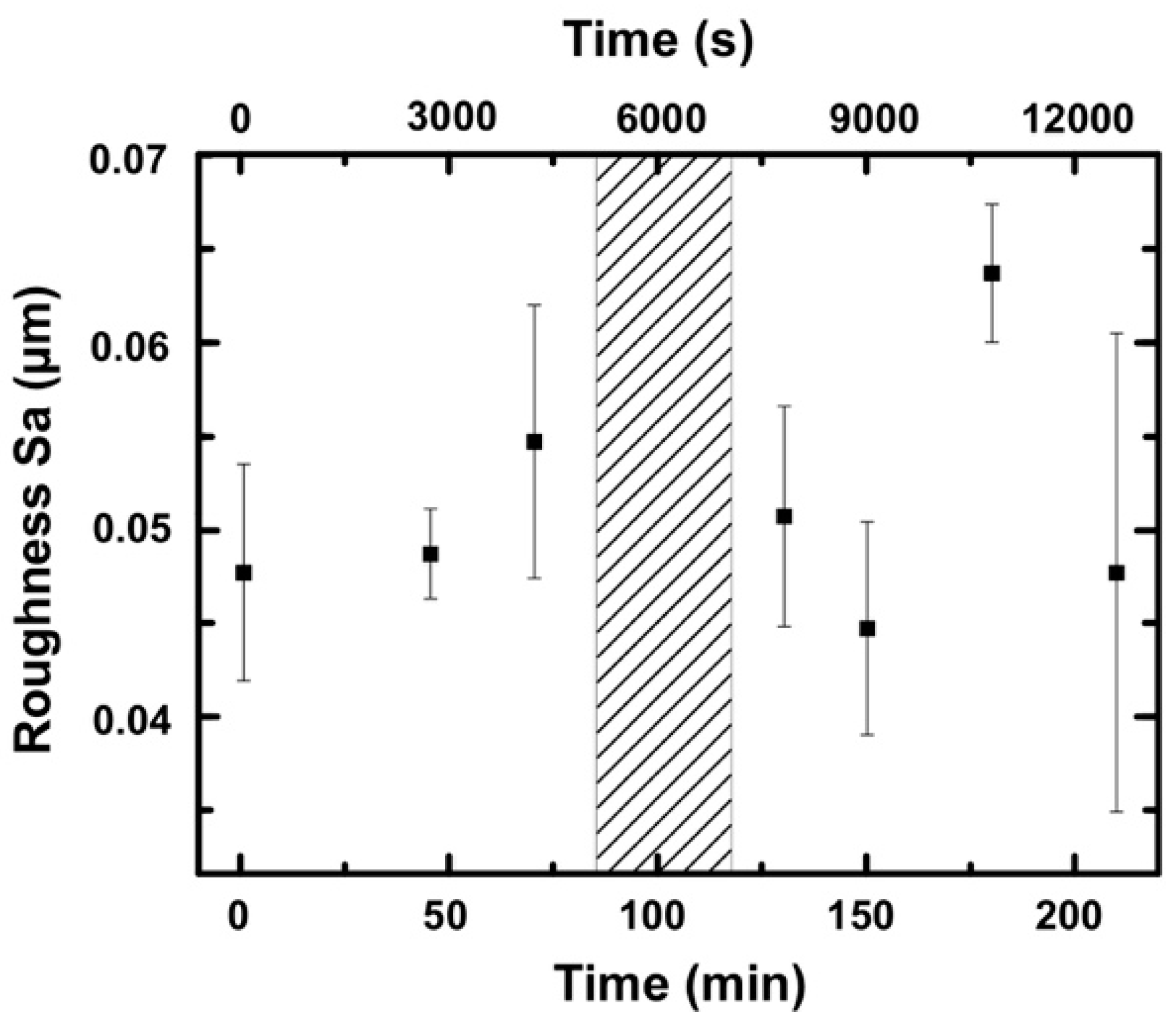
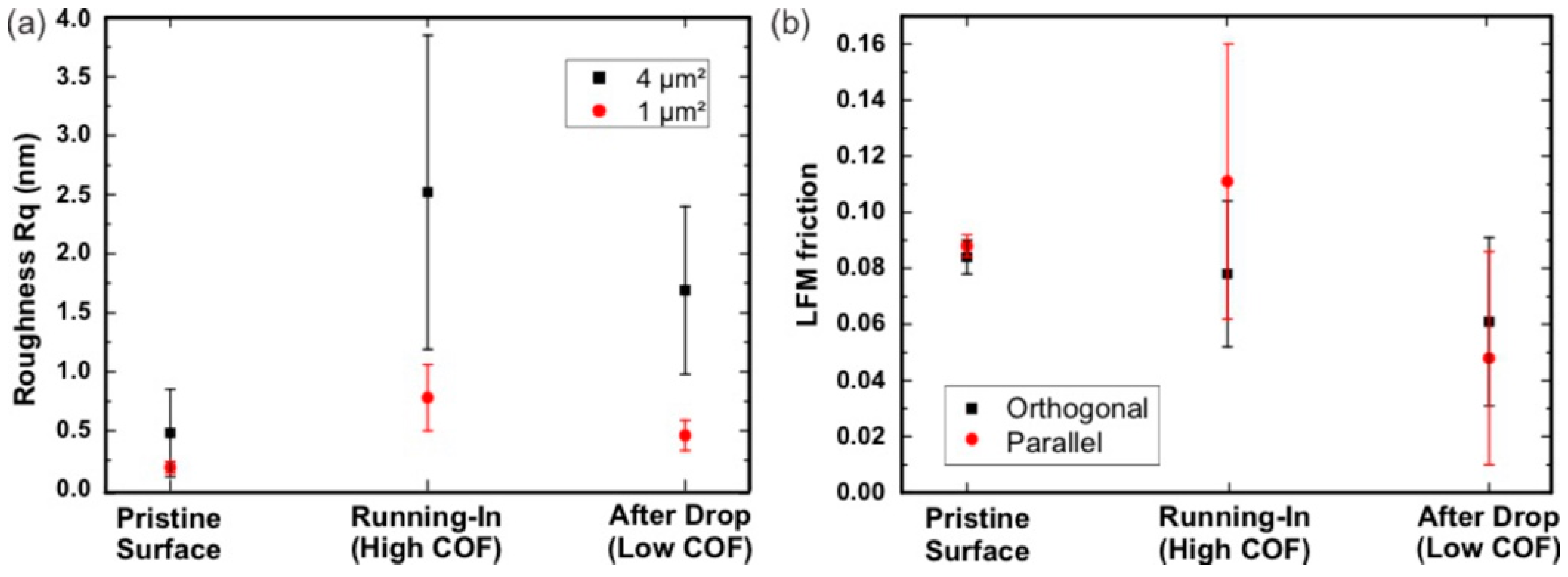
3.2.2. Roughness and Friction
3.2.3. Nano-Hardness
3.2.4. ToF-SIMS Study of the ta-C Coating
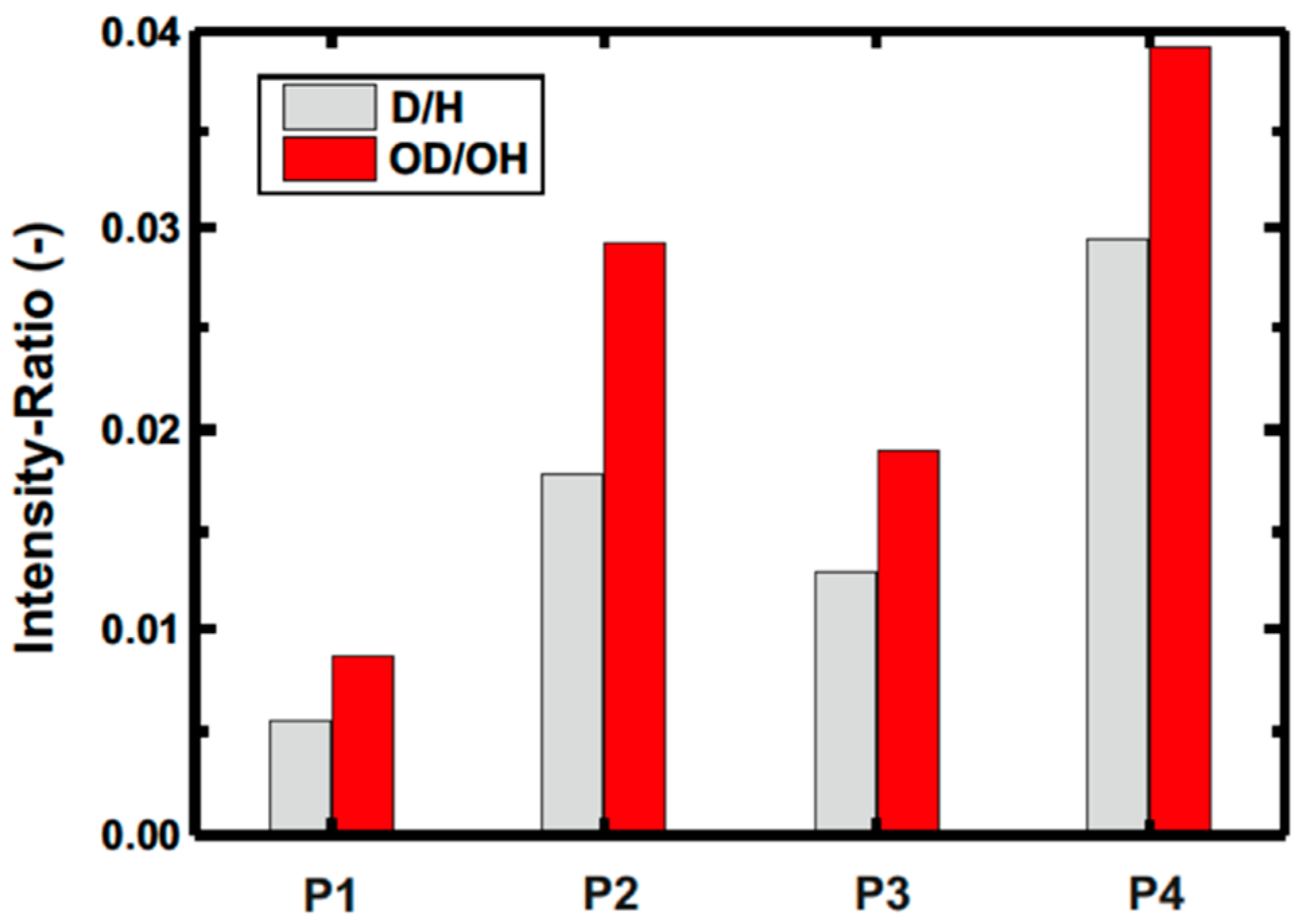
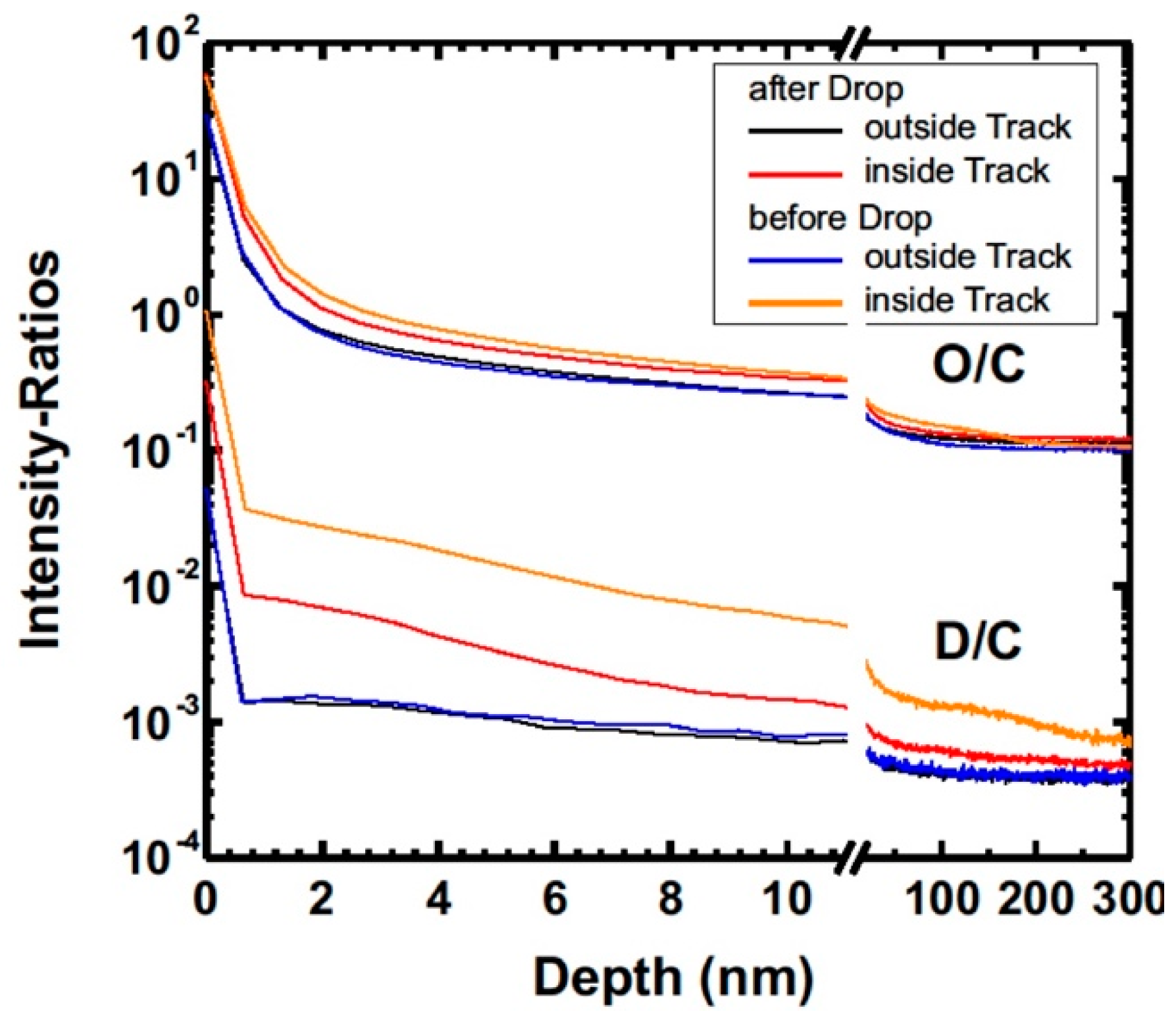
3.2.5. SIMS Study of the ta-C Coating
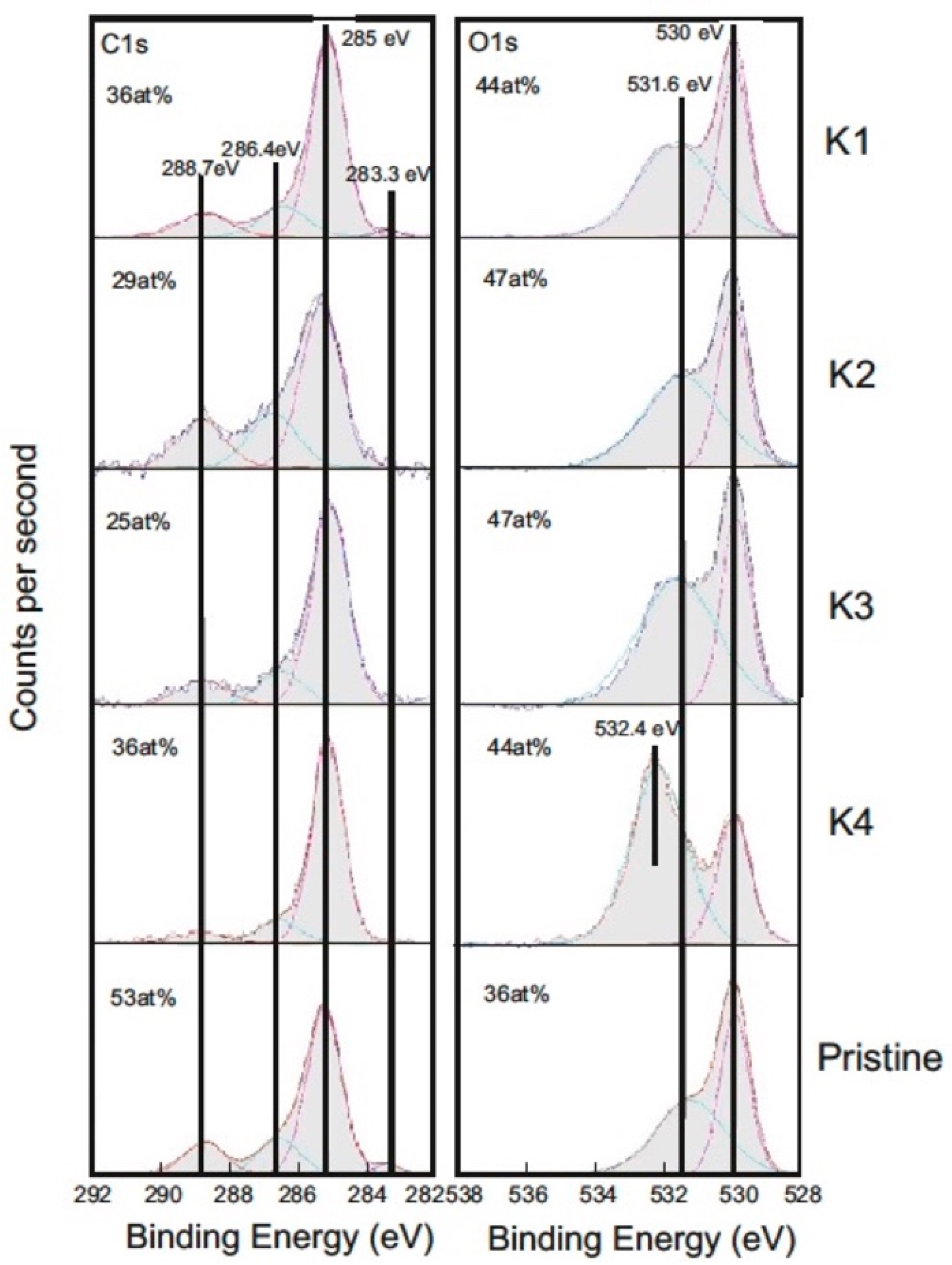
3.2.6. XPS Study of the Steel Counter-Body
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hauert, R. An overview on the tribological behavior of diamond-like carbon in technical and medical applications. Tribol. Int. 2004, 37, 991–1003. [Google Scholar] [CrossRef]
- Lawes, S.D.A.; Fitzpatrick, M.E.; Hainsworth, S.V. Evaluation of the tribological properties of DLC for engine applications. J. Phys. Appl. Phys. 2007, 40, 5427–5437. [Google Scholar] [CrossRef]
- Zeng, A.; Neto, V.F.; Gracio, J.J.; Fan, Q.H. Diamond-like carbon (DLC) films as electrochemical electrodes. Diam. Relat. Mater. 2014, 43, 12–22. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 1999, 8, 428–434. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Logothetidis, S.; Kassavetis, S.; Charitidis, C.; Panayiotatos, Y.; Laskarakis, A. Nanoindentation studies of multilayer amorphous carbon films. Carbon 2004, 42, 1133–1136. [Google Scholar] [CrossRef]
- Verein Deutscher Ingenieure. VDI 2840: Kohlenstoffschichten: Grundlagen, Schichttypen und Eigenschaften; Verein Deutscher Ingenieure: Düsseldorf, Germany, 2005. [Google Scholar]
- Makowski, S.; Weihnacht, V.; Leson, A. Diesel-lubricated ta-C coatings. Lubr. Sci. 2013, 269–274. [Google Scholar] [CrossRef]
- Vengudusamy, B.; Green, J.H.; Lamb, G.D.; Spikes, H.A. Tribology International Behaviour of MoDTC in DLC/DLC and DLC/steel contacts. Tribol. Int. 2012, 54, 68–76. [Google Scholar] [CrossRef]
- De Barros Bouchet, I.M.; Martin, J.M.; Avila, J.; Kano, M.; Yoshida, K.; Bai, S.; Higuchi, Y.; Ozawa, N.; Kubo, M.; Asensio, M.C. Diamond-like carbon coating under oleic acid lubrication: Evidence for graphene oxide formation in superlow friction. Sci. Rep. 2017, 7, 46394. [Google Scholar] [CrossRef] [PubMed]
- Minami, I.; Kubo, T.; Nanao, H.; Mori, S.; Sagawa, T.; Okuda, S. Investigation of Tribo-Chemistry by Means of Stable Isotopic Tracers, Part 2: Lubrication Mechanism of Friction Modifiers on Diamond-Like Carbon Investigation of Tribo-Chemistry by Means of Stable Isotopic Tracers, Part 2: Lubrication Mechanism of Fri. Tribol. Trans. 2007, 37–41. [Google Scholar] [CrossRef]
- Wu, X.; Ohana, T.; Tanaka, A.; Kubo, T. Tribochemical investigation of DLC coating in water using stable isotopic tracers. Appl. Surf. Sci. 2008, 254, 3397–3402. [Google Scholar] [CrossRef]
- Ichiro, M.; Furesawa, T.; Kubo, T.; Nanao, H.; Mori, S. Investigation of tribo-chemistry by means of stable isotopic tracers: Mechanism for durability of monomolecular boundary film. Tribol. Int. 2008, 41, 1056–1062. [Google Scholar] [CrossRef]
- Matta, C.; De Barros Bouchet, M.I.; Vachet, B.; Martin, J.M. Tribochemistry of tetrahedral hydrogen-free amorphous carbon coatings in the presence of OH-containing lubricants. Lubr. Sci. 2008, 20, 137–149. [Google Scholar] [CrossRef]
- Matta, C.; Joly-Pottuz, L.; De Barros Bouchet, M.I.; Martin, J.; Kano, M.; Zhang, Q.; Goddard, W. Superlubricity and tribochemistry of polyhydric alcohols. Phys. Rev. B 2008, 78, 085436. [Google Scholar] [CrossRef]
- De Barros Bouchet, M.I.; Matta, C.; Le-Mogne, T.; Martin, J.M.; Zhang, Q.; Goddard, W.; Kano, M.; Mabuchi, Y.; Ye, J. Superlubricity mechanism of diamond-like carbon with glycerol. Coupling of experimental and simulation studies. J. Phys. Conf. Ser. 2007, 89, 012003. [Google Scholar] [CrossRef]
- Kano, M.; Martin, J.M.; Yoshida, K.; De Barros Bouchet, M.I. Super-low friction of ta-C coating in presence of oleic acid. Friction 2014, 2, 156–163. [Google Scholar] [CrossRef]
- Simic, R.; Kalin, M.; Kovac, J.; Jaksa, G. Adsorption of alcohols and fatty acids onto hydrogenated (a-C:H) DLC coatings. Appl. Surf. Sci. 2016, 363, 466–476. [Google Scholar] [CrossRef]
- Erdemir, A.; Bindal, C.; Fenske, G.R.; Zuiker, C.; Wilbur, P. Characterization of Transfer Layers forming on Surfaces sliding against Diamond-Like Carbon. Surf. Coat. Technol. 1996, 86, 692–697. [Google Scholar] [CrossRef]
- Scholz, C. Dirk Spaltmann, Matias Woydt, Friction Reduction on Powertrains by Polyglycols, Alternative Steels and/or Thin Films; STLE: Park Ridge, IL, USA, 2013; p. 1556885. [Google Scholar]
- Scheibe, H.-J.; Leonhardt, M.; Leson, A.; Meyer, C.-F.; Stucky, T.; Weihnacht, V. Abscheidung superharter Kohlenstoffschichten mittels Laser-Arco auf dem Weg vom Labor in die industrielle Serienfertigung. Vak. Forsch. Prax. 2008, 20, 26–31. [Google Scholar] [CrossRef]
- Varenberg, M.; Etsion, I.; Halperin, G. An improved wedge calibration method for lateral force in atomic force microscopy. Rev. Sci. Instrum. 2003, 74, 3362–3367. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Luo, J. Investigation of the difference in liquid superlubricity between water- and oil-based lubricants. RSC Adv. 2015, 5, 63827–63833. [Google Scholar] [CrossRef]
- Dai, L.; Sorkin, V.; Zhang, Y.-W. Effect of Surface Chemistry on the Mechanisms and Governing Laws of Friction and Wear. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Bobji, M.S.; Biswas, S.K.; Pethica, J.B. Effect of roughness on the measurement of nanohardness-a computer simulation study. Appl. Phys. Lett. 1997, 71, 22–25. [Google Scholar] [CrossRef]
- Bobji, M.S.; Biswas, S.K. Deconvolution of hardness from data obtained from nanoindentation of rough surfaces. J. Mater. Res. 1999, 14, 2259–2268. [Google Scholar] [CrossRef]
- Liu, Y.; Erdemir, A.; Meletis, E.I. A study of the wear mechanism of diamond-like carbon films. Surf. Coat. Technol. 1996, 82, 48–56. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Friction behaviors of hydrogenated diamond-like carbon film in different environment sliding against steel ball. Appl. Surf. Sci. 2005, 249, 257–265. [Google Scholar] [CrossRef]
- Jao, J.Y.; Han, S.; Chang, L.S.; Chen, Y.C.; Chang, C.L.; Shih, H.C. Formation and characterization of DLC:Cr:Cu multi-layers coating using cathodic arc evaporation. Diam. Relat. Mater. 2009, 18, 368–373. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
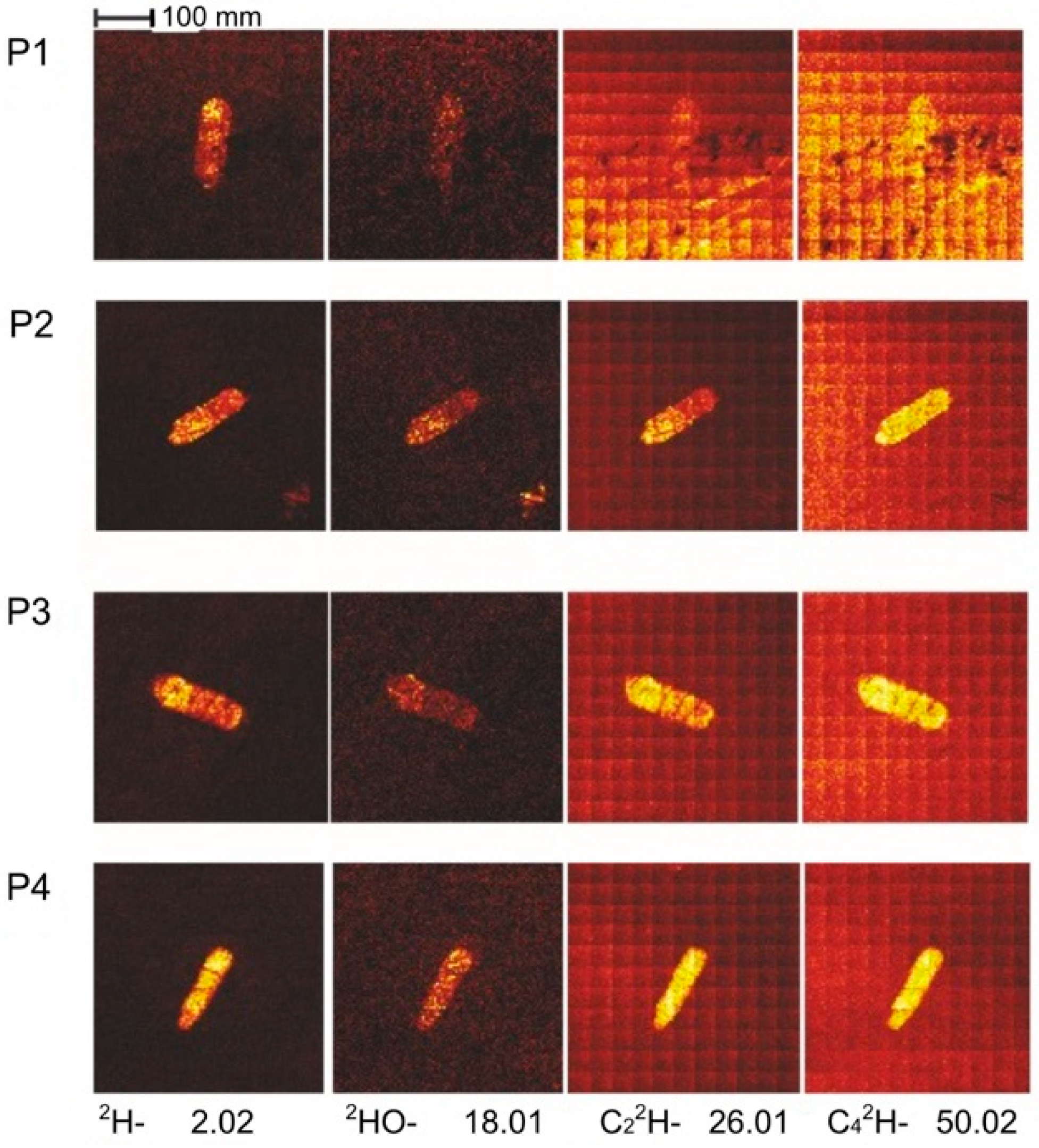
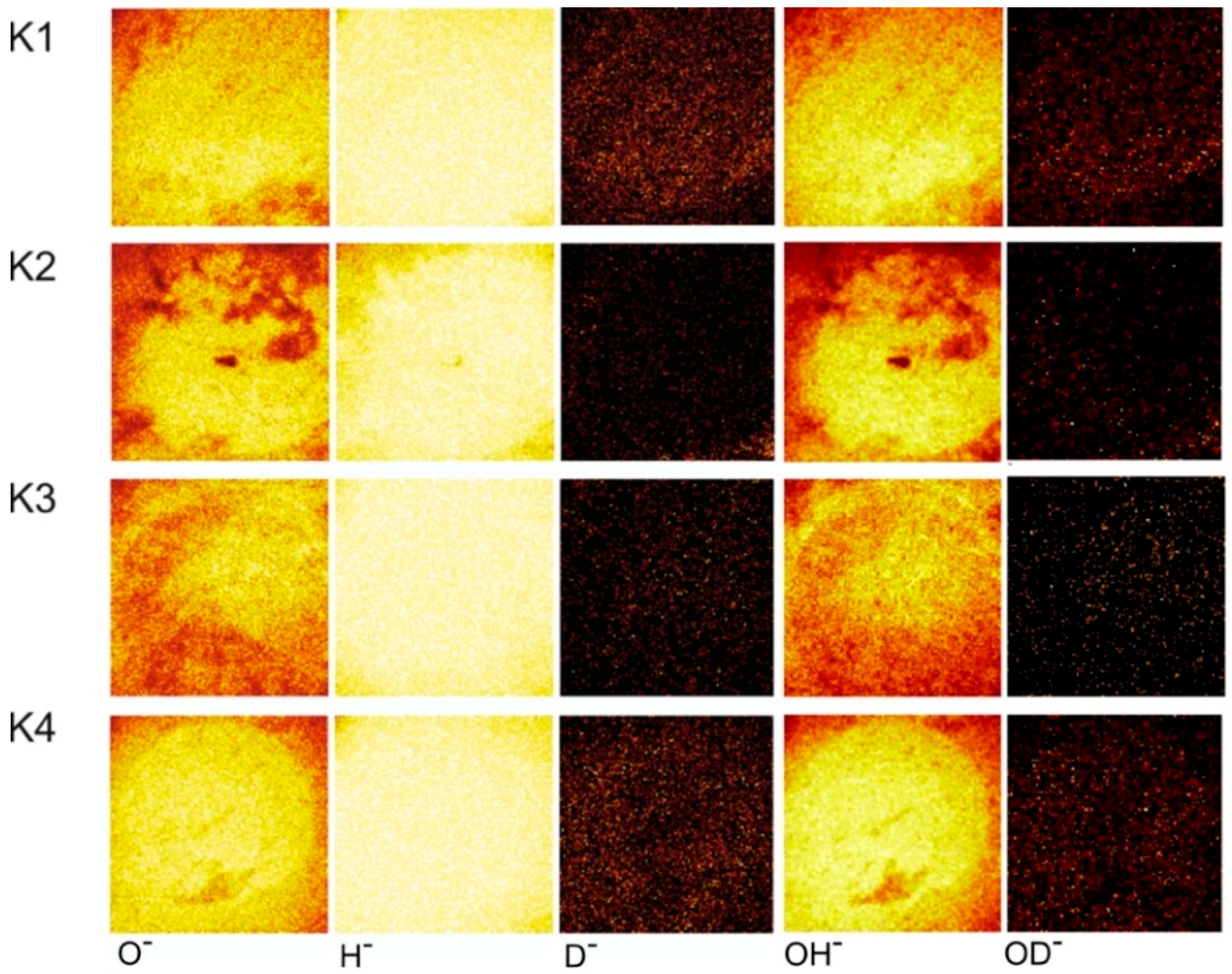

| Wear Track/Ball | Running Time | Final COF | Deuterated Version |
|---|---|---|---|
| P1/K1 | 12,000 s | 0.021 | d2 |
| P2/K2 | 3180 s | 0.090 | d2 |
| P3/K3 | 5400 s | 0.021 | d4 |
| P4/K4 | 1560 s | 0.076 | d4 |
| Core Level | Energy (eV) | Corresponding Binding Structure |
|---|---|---|
| C1s | 283.3 | C-Cr |
| C1s | 285 | Carbon inside aliphatic hydrocarbons (sp3) and DLC (sp3) |
| C1s | 286.5 | Carbon with one oxygen as binding partner (alcohol, ether) (sp3) |
| C1s | 288.7 | Carbon in carboxyl respectively carbonyl groups (sp3) |
| O1s | 530 | Oxygen in metal oxides |
| O1s | 531.6 | Oxygen in organics |
| 532.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, S.; Schulze, M.; Krell, L.; Merz, R.; Wahl, M.; Stark, R.W. Ultra-Low Friction on Tetrahedral Amorphous Diamond-Like Carbon (ta-C) Lubricated with Ethylene Glycol. Lubricants 2018, 6, 59. https://doi.org/10.3390/lubricants6030059
Bachmann S, Schulze M, Krell L, Merz R, Wahl M, Stark RW. Ultra-Low Friction on Tetrahedral Amorphous Diamond-Like Carbon (ta-C) Lubricated with Ethylene Glycol. Lubricants. 2018; 6(3):59. https://doi.org/10.3390/lubricants6030059
Chicago/Turabian StyleBachmann, Svenja, Marcus Schulze, Lisa Krell, Rolf Merz, Michael Wahl, and Robert W. Stark. 2018. "Ultra-Low Friction on Tetrahedral Amorphous Diamond-Like Carbon (ta-C) Lubricated with Ethylene Glycol" Lubricants 6, no. 3: 59. https://doi.org/10.3390/lubricants6030059
APA StyleBachmann, S., Schulze, M., Krell, L., Merz, R., Wahl, M., & Stark, R. W. (2018). Ultra-Low Friction on Tetrahedral Amorphous Diamond-Like Carbon (ta-C) Lubricated with Ethylene Glycol. Lubricants, 6(3), 59. https://doi.org/10.3390/lubricants6030059





