Perfluorotetradecanoic Acid as an Additive for Friction Reduction in Full-Film EHD Contacts: The Role of Functional Group, Base Oil Polarity, Additive Concentration and Contact Pressure
Abstract
1. Introduction
2. Materials and Methods
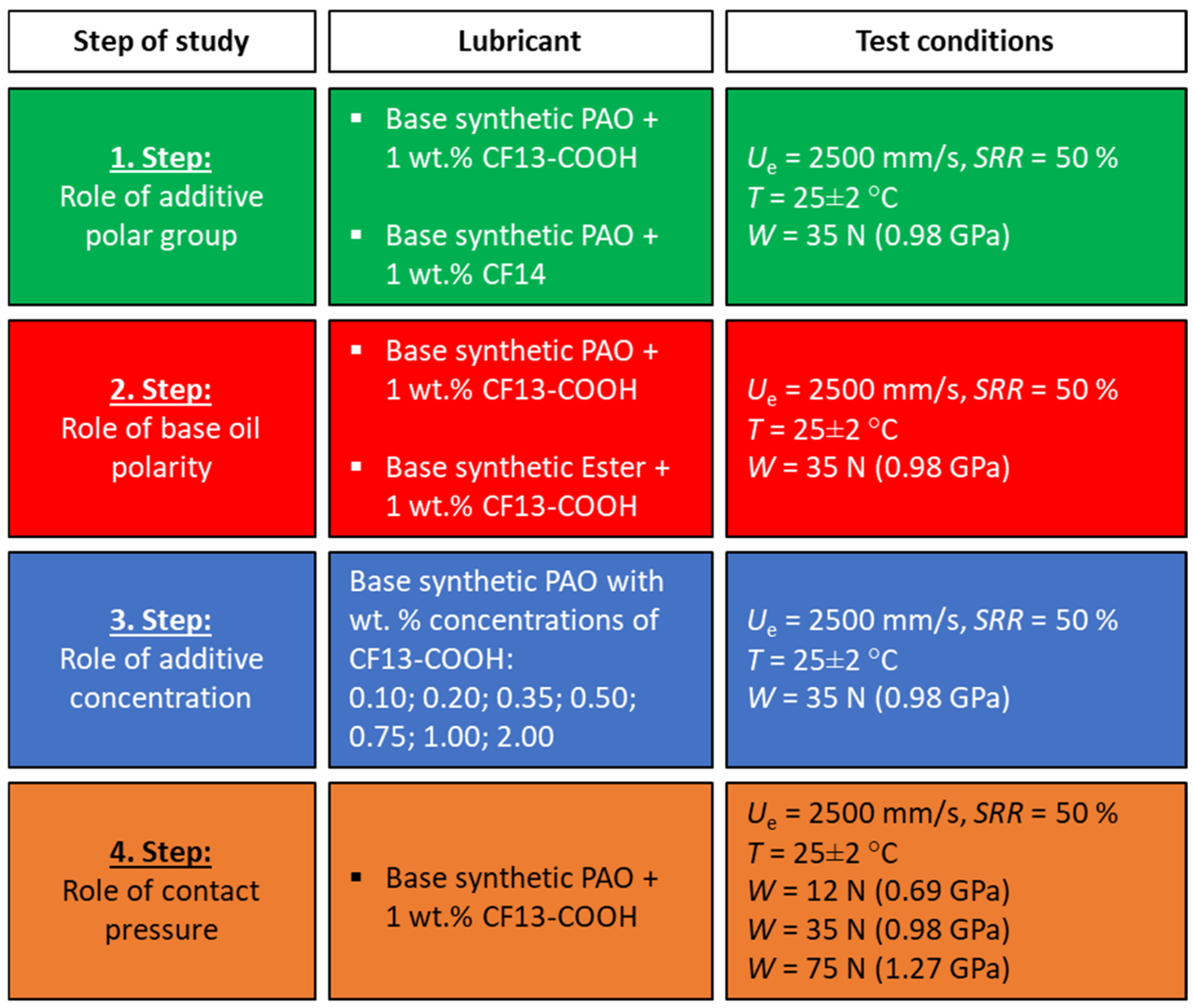
2.1. Specimens and Lubricants
2.2. Tribological Tests
- 12 N, corresponding to a Hertzian contact pressure of 0.69 GPa;
- 35 N, corresponding to 0.98 GPa;
- And 75 N, corresponding to 1.27 GPa.
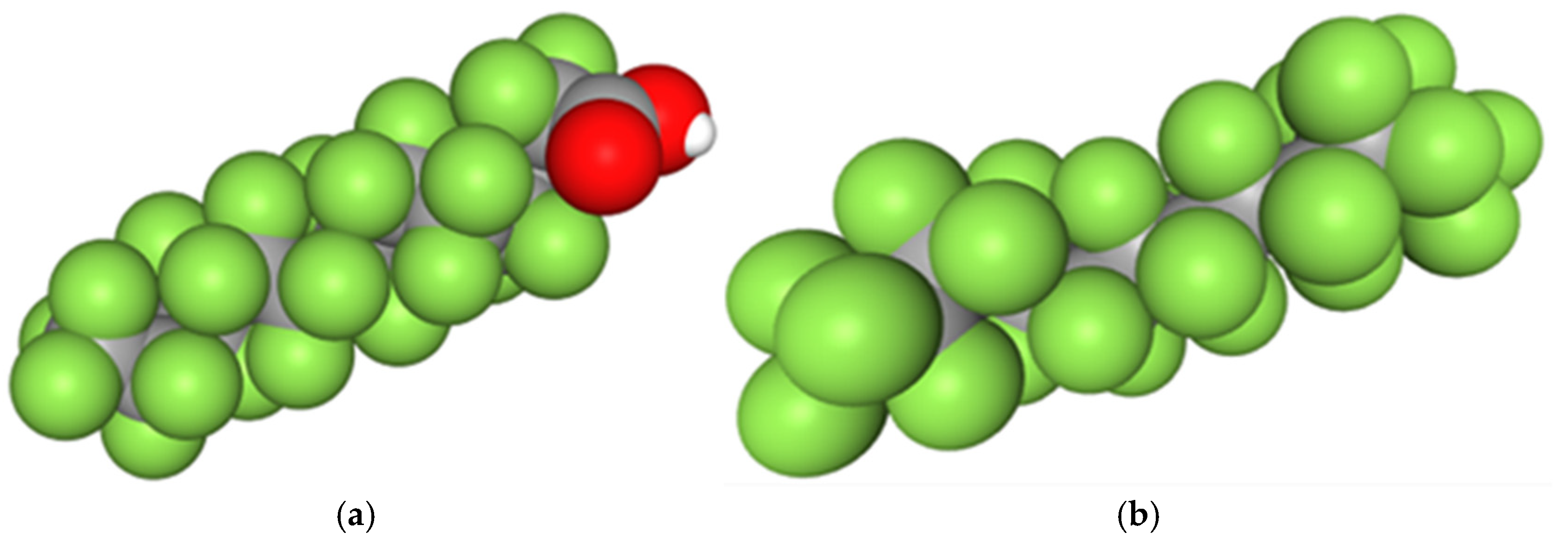
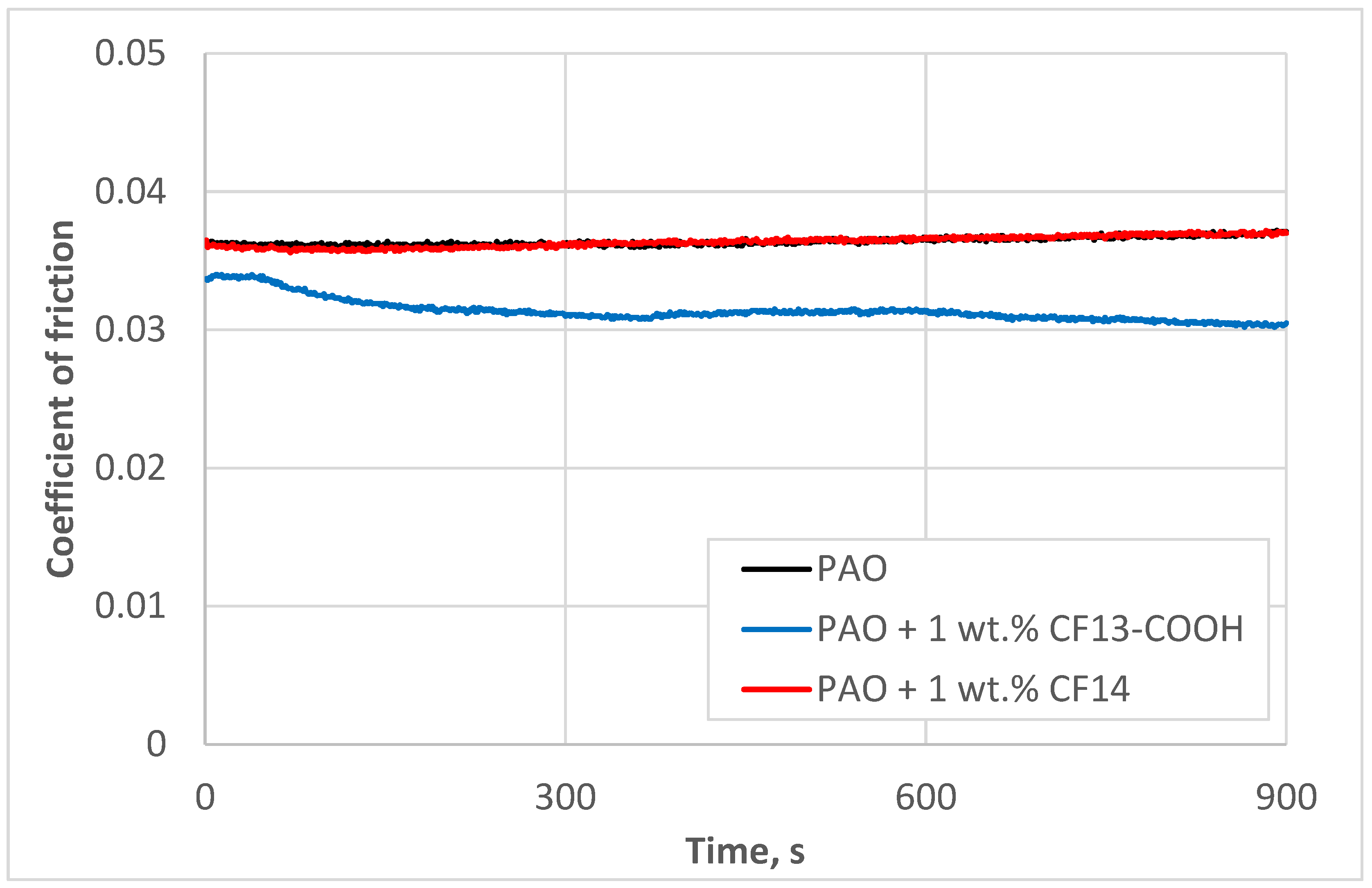
- Step 1: We investigated the effect of the carboxylic acid functional group on friction reduction. In this phase, both additives—CF13-COOH (with the acid group) and CF14 (without the acid group)—were tested under identical conditions, with a constant additive concentration and testing parameters.
- Step 2: We examined the influence of the base oil polarity by testing two different base oils, both containing 1 wt.% of the CF13-COOH additive. All other testing conditions and the additive concentration were kept constant.
- Step 3: We studied the effect of additive concentration by varying the amount of CF13-COOH in the PAO base oil. The testing parameters remained unchanged throughout this step.
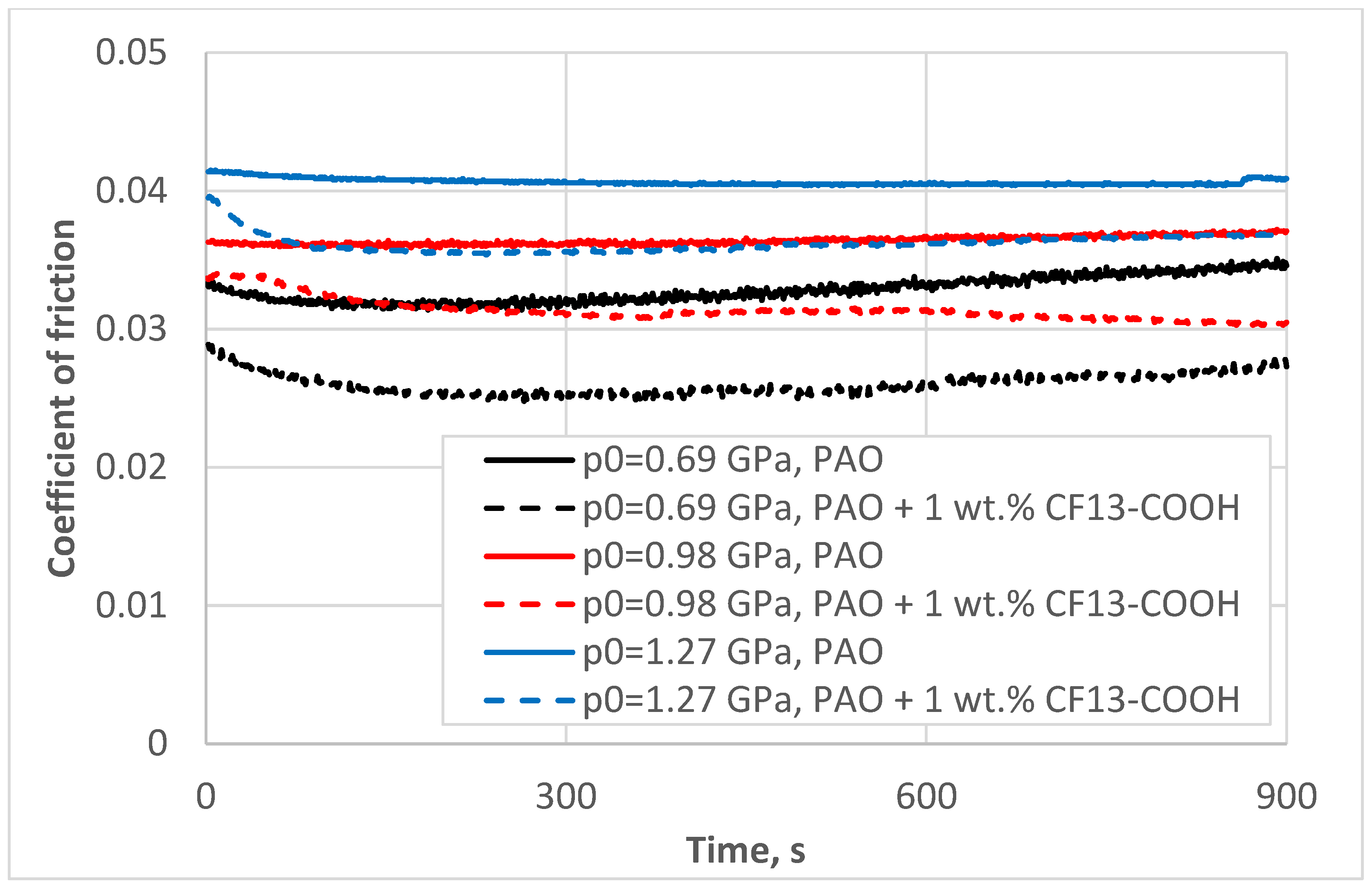
- Step 4: We explored the influence of contact pressure by varying the normal load applied, thereby generating different Hertzian contact pressures, while keeping all other test conditions constant. The CF13-COOH additive mixed in 1 wt.% in PAO oil was used in this step.
3. Results
3.1. Role of Additive Polar Group
3.2. Role of Base Oil Polarity
3.3. Role of Additive Concentration
3.4. Role of Contact Pressure
4. Discussion
4.1. Role of Additive Polar Group
4.2. Role of Base Oil Polarity
4.3. Role of Additive Concentration
4.4. Role of Contact Pressure
5. Conclusions
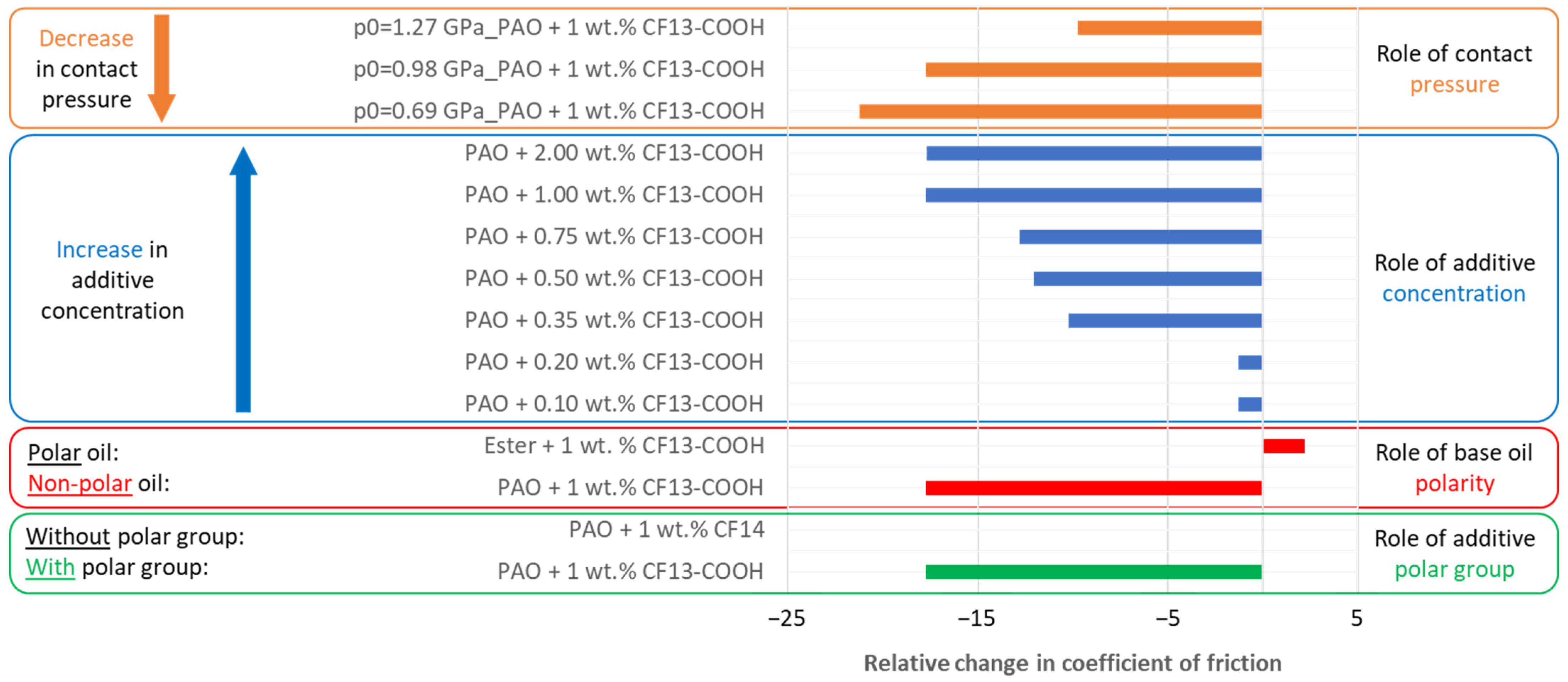
- A polar carboxyl functional group is indispensable in the additive to reduce the coefficient of friction relative to the base oil.
- Perfluorinated tetradecanoic acid reduces friction in non-polar synthetic PAO oil, while in a more polar synthetic ester, it is unable to reduce friction compared to the base oil.
- Increasing the concentration of perfluorinated tetradecanoic acid also increases the reduction in friction. The minimum concentration required, at which a perceptible reduction in friction is obtained (by about 10%) is 0.35 wt.%; however, the highest reduction in friction (by about 18%) is obtained at both largest concentrations of 1.00 and 2.00 wt.%.
- Perfluorinated tetradecanoic acid is best at reducing friction at lower pressures, with a reduction by about 22% at the lowest pressure tested (0.69 GPa) and just below 10% at the highest pressure (1.27 GPa).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EHD | Elasto-hydrodynamic |
| PAO | Poly-alpha-olefin |
| PTFE | Polytetrafluoroethylene |
References
- Kalin, M.; Kus, M. New Strategy for Reducing the EHL Friction in Steel Contacts Using Additive-Formed Oleophobic Boundary Films. Friction 2021, 9, 1346–1360. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ando, J.; Tsuda, T.; Takahashi, N.; Tohyama, M.; Murase, A.; Ohmori, T.; Hokkirigawa, K. Sliding Velocity Dependency of the Friction Coefficient of Si-Containing Diamond-like Carbon Film under Oil Lubricated Condition. Tribol. Int. 2011, 44, 1296–1303. [Google Scholar] [CrossRef]
- Ponjavic, A.; Wong, J.S. The Effect of Boundary Slip on Elastohydrodynamic Lubrication. RSC Adv. 2014, 4, 20821–20829. [Google Scholar] [CrossRef]
- Guo, F.; Yang, S.Y.; Ma, C.; Wong, P.L. Experimental Study on Lubrication Film Thickness under Different Interface Wettabilities. Tribol. Lett. 2014, 54, 81–88. [Google Scholar] [CrossRef]
- Fu, Z.; Wong, P.L.; Guo, F. Effect of Interfacial Properties on EHL under Pure Sliding Conditions. Tribol. Lett. 2013, 49, 31–38. [Google Scholar] [CrossRef]
- Jing, Z.; Guo, F.; Jin, W.; Kalin, M.; Polajnar, M. Study on the Influence of Interfacial Slip on the Lubrication Performance of a Step Slider Bearing. Tribol. Int. 2022, 176, 107822. [Google Scholar] [CrossRef]
- Fuks, G.I.; Berlin, L.I. Perfluorinated Aliphatic Acids as Additives Regulating the Wetting of Metals by Oils. Chem. Technol. Fuels Oils 1971, 7, 305–308. [Google Scholar] [CrossRef]
- Guo, L.; Wong, P.L.; Guo, F. Boundary Yield Stress and Interfacial Potential Energy Barrier in Thin Film Hydrodynamic Lubrication. Tribol. Lett. 2016, 62, 7. [Google Scholar] [CrossRef]
- Guo, L.; Wong, P.L.; Guo, F. Effects of Viscosity and Sliding Speed on Boundary Slippage in Thin Film Hydrodynamic Lubrication. Tribol. Int. 2017, 107, 85–93. [Google Scholar] [CrossRef]
- Hu, M.; Jing, L.; An, Q.; Ming, W.; Bian, C.; Chen, M. Tribological Properties and Milling Performance of HSS-Co-E Tools with Fluorinated Surfactants-Based Coatings against Ti–6Al–4V. Wear 2017, 376, 134–142. [Google Scholar] [CrossRef]
- Guo, F.; Wong, P.L.; Fu, Z.; Ma, C. Interferometry Measurement of Lubricating Films in Slider-on-Disc Contacts. Tribol. Lett. 2010, 39, 71–79. [Google Scholar] [CrossRef]
- Polajnar, M.; Požar, T.; Kalin, M. Perfluorocarboxylic Acids as Additives for Friction Reduction in Full-Film Ehd Contacts: Influence of Additive Chain Length; Elsevier BV: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Tallian, T.E. On Competing Failure Modes in Rolling Contact. ASLE Trans. 1967, 10, 418–439. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Dowson, D. Isothermal Elastohydrodynamic Lubrication of Point Contacts: Part III—Fully Flooded Results. J. Lubr. Tech. Apr. 1977, 99, 264–275. [Google Scholar] [CrossRef]
- Tsubouchi, T.; Hata, H.; Yoshida, Y. Optimisation of Molecular Structure for Traction Fluids. Lubr. Sci. 2004, 16, 393–403. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, A.; Spikes, H. Effect of Base Oil Structure on Elastohydrodynamic Friction. Tribol. Lett. 2017, 65, 13. [Google Scholar] [CrossRef]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef]
- Yu, H.; Qin, L.; Zhou, J. Effect of Oil Polarity on the Protein Adsorption at Oil–Water Interfaces. Langmuir 2023, 39, 10701–10710. [Google Scholar] [CrossRef]
- Lee, B.; Yu, Y.; Cho, D.-S.; Cho, Y. Role of Additive Concentration in Slow-Speed Sliding Contact under Boundary Lubrication Conditions. J. Mech. Sci. Technol. 2019, 33, 5361–5368. [Google Scholar] [CrossRef]
- Choo, J.H.; Spikes, H.A.; Ratoi, M.; Glovnea, R.; Forrest, A. Friction Reduction in Low-Load Hydrodynamic Lubrication with a Hydrophobic Surface. Tribol. Int. 2007, 40, 154–159. [Google Scholar] [CrossRef]
- Leong, J.Y.; Reddyhoff, T.; Sinha, S.K.; Holmes, A.S.; Spikes, H.A. Hydrodynamic Friction Reduction in a MAC–Hexadecane Lubricated MEMS Contact. Tribol. Lett. 2013, 49, 217–225. [Google Scholar] [CrossRef]
- Jahanmir, S.; Hunsberger, A.Z.; Heshmat, H. Load Capacity and Durability of H-DLC Coated Hydrodynamic Thrust Bearings. J. Tribol. 2011, 133, 031301. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, P.L. Influences of Boundary Slip Coating on Elastohydrodynamically Lubricated Contacts. Tribol. Lett. 2022, 70, 54. [Google Scholar] [CrossRef]
- Fu, Z.; Wong, P.L.; Guo, F. Localized Boundary Effect on Elastohydrodynamic Lubricated Film Shape. Tribol. Lett. 2014, 56, 471–479. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, F.; Wong, P.L. Friction–Speed Characteristics of Elastohydrodynamically Lubricated Contacts with Anomalous Film Shapes. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2012, 226, 81–86. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polajnar, M.; Požar, T.; Kalin, M. Perfluorotetradecanoic Acid as an Additive for Friction Reduction in Full-Film EHD Contacts: The Role of Functional Group, Base Oil Polarity, Additive Concentration and Contact Pressure. Lubricants 2025, 13, 263. https://doi.org/10.3390/lubricants13060263
Polajnar M, Požar T, Kalin M. Perfluorotetradecanoic Acid as an Additive for Friction Reduction in Full-Film EHD Contacts: The Role of Functional Group, Base Oil Polarity, Additive Concentration and Contact Pressure. Lubricants. 2025; 13(6):263. https://doi.org/10.3390/lubricants13060263
Chicago/Turabian StylePolajnar, Marko, Tomaž Požar, and Mitjan Kalin. 2025. "Perfluorotetradecanoic Acid as an Additive for Friction Reduction in Full-Film EHD Contacts: The Role of Functional Group, Base Oil Polarity, Additive Concentration and Contact Pressure" Lubricants 13, no. 6: 263. https://doi.org/10.3390/lubricants13060263
APA StylePolajnar, M., Požar, T., & Kalin, M. (2025). Perfluorotetradecanoic Acid as an Additive for Friction Reduction in Full-Film EHD Contacts: The Role of Functional Group, Base Oil Polarity, Additive Concentration and Contact Pressure. Lubricants, 13(6), 263. https://doi.org/10.3390/lubricants13060263





