Abstract
Tribology is the branch of science and engineering that focuses on understanding friction, wear, and lubrication, which is essential for saving energy, improving performance, reducing vibration, and creating eco-friendly lubricants and wear resistance. Over the past decade, nanomaterials have captured the immense interest of tribology science. This review aimed to analyze how graphene and its derivatives can be incorporated into lubricants to enhance their properties, particularly in mitigating friction and wear. This is due to graphene’s excellent specific properties, such as a low friction coefficient, mechanical strength, high thermal and electrical conductivity, biocompatibility, high load-carrying capacity, wear resistance, and chemical stability. This study briefly introduces graphite, graphene, and graphene oxide, as well as presents graphene as a material for tribological applications. Among other things, the environmentally friendly possibilities of chemical reduction of reduced graphene oxide are analyzed here, as well as the macro-, micro-, and nano-tribological examination of graphene and its derivatives. Despite what is already known about graphene in tribology, further research is needed to gain a deeper understanding of development regarding integration with different materials, long-term performance, eco-friendly synthesis using green reducing agents, and comprehending how these approaches may affect systems at various scales.
1. Introduction
Wear and friction at the interface of moving elements are primary sources of energy loss and mechanical system failure [1]. Friction-related losses, which account for approximately 5–7% of the gross domestic product in many countries, lead to an increased demand for lubricants. These oils are essential for minimizing friction, wear, and tear, controlling temperature, preventing oxidation, improving viscosity, and providing anti-corrosive benefits—ultimately ensuring the efficient performance and extended lifespan of machinery [2,3]. Lubrication plays a crucial role in minimizing wear and controlling friction by forming a film layer that reduces friction between moving surfaces in contact [4].
Tribology, a multidisciplinary science that explores the complex interactions between surfaces under both dry and lubricated conditions, is essential in various industries such as automotive, food, and space. This field encompasses wear, friction, adhesion, abrasion, and tribochemistry, and its applications span from space exploration to biomedicine [5,6]. Addressing challenges such as frictional and wear losses, CO2 emissions, and energy inefficiencies is central to tribology, contributing to enhanced productivity and sustainability across diverse sectors [5,7,8]. In this context, the performance of lubrication systems is vital in reducing friction and wear, ultimately improving overall system efficiency [9]. Lubrication remains one of the most efficient methods to minimize friction, wear, corrosion, and environmental contamination between moving surfaces [10].
Previous studies have highlighted the significant advantages of environmentally friendly oils over conventional alternatives. Furthermore, prior research has demonstrated that additives play a significant role in enhancing lubricity, improving thermal stability, preventing corrosion, and extending the lubricant’s service life and the component’s overall lifespan. For instance, zinc dialkyl dithiophosphate, boron-based additives, and molybdenum disulfide have been extensively studied as anti-wear agents to decrease metal-to-metal contact under extreme conditions, including high temperatures and pressures [11]. A study showed that graphene coatings protect against oxidation by blocking oxygen atoms with a high energy barrier, preventing them from reaching reactive areas below [5]. Another study demonstrated that nanofluids with graphene nano-platelets (GNP) and MWCNT showed higher thermal and electrical conductivity as the weight concentration of the carbon-based additives increased [5]. In other words, a pivotal component in this context is the incorporation of carbon-based lubricant additives, with graphene emerging as a subject of considerable scientific interest owing to its unparalleled and exceptional properties.
Graphene, a single layer of carbon atoms arranged in a honeycomb lattice, has garnered significant attention in the scientific world due to its electronic, electron transport, electrical, optical, mechanical, chemical, and thermal properties [12,13,14]. Graphene holds immense potential across many industries, including medicine, biomaterials, electronics, optics, defense, energy, and many other fields [15,16]. Graphene and its derivatives possess remarkable attributes, including low shear resistance, exceptional stiffness and thermal conductivity, and self-lubrication, making their tribological properties highly attractive due to their low friction, superior stiffness, and unparalleled mechanical stability [9]. This review delves into exploring the tribological properties of graphene and its derivatives, shedding light on their potential applications and prospects.
2. An Overview of Graphene and Its Derivatives
2.1. Carbon
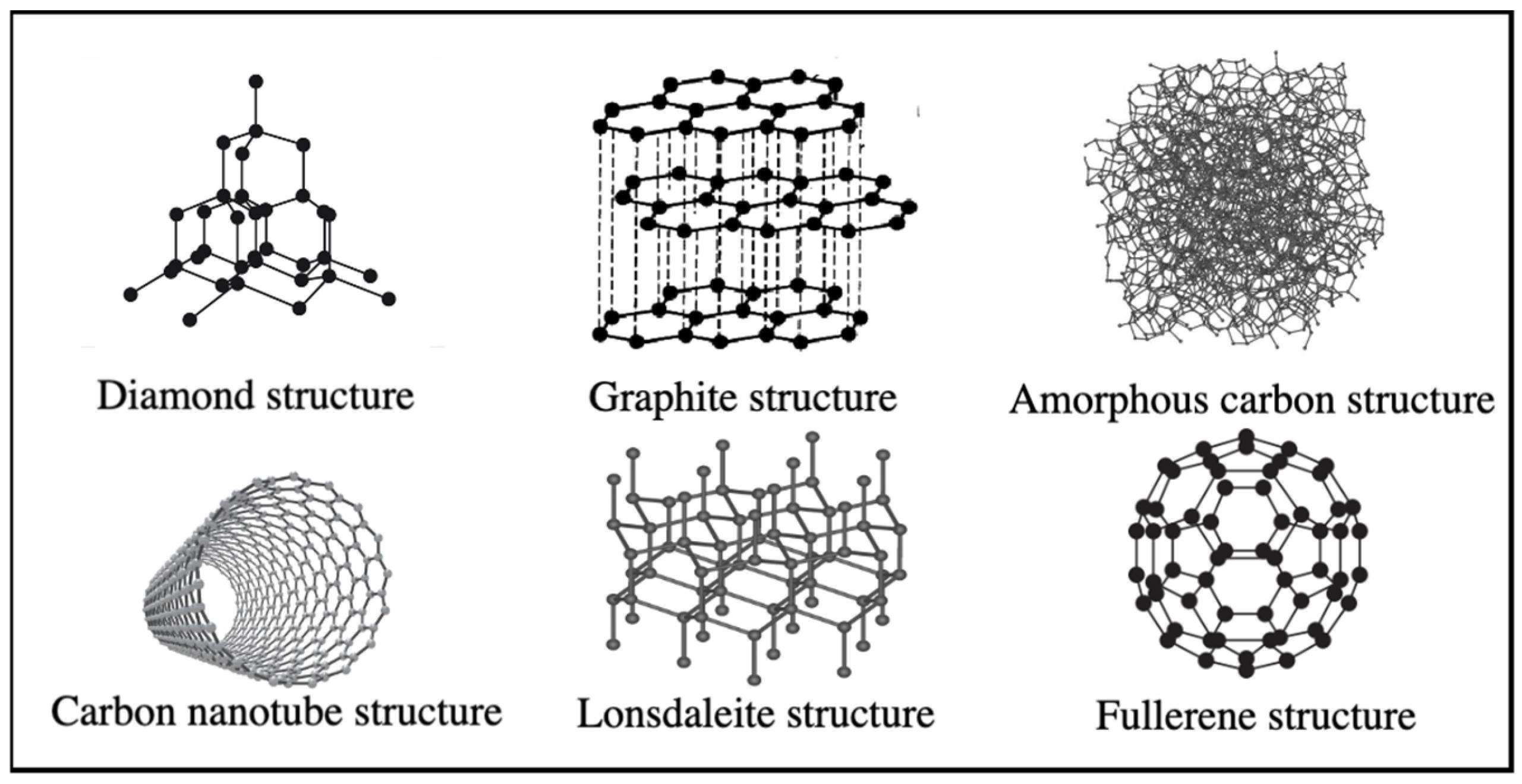
Carbon, a nonmetal with a grayish-black, opaque appearance, derives its name from the Latin word “carbo”, meaning coal. It forms the foundation of organic chemistry and life by bonding in various ways and is naturally abundant in nature, positioned in group 4A of the periodic table. One of carbon’s most crucial properties is its ability to form three distinct hybridizations (sp, sp2, and sp3), which, in turn, leads to the formation of various allotropes (Figure 1) [17,18,19,20,21].
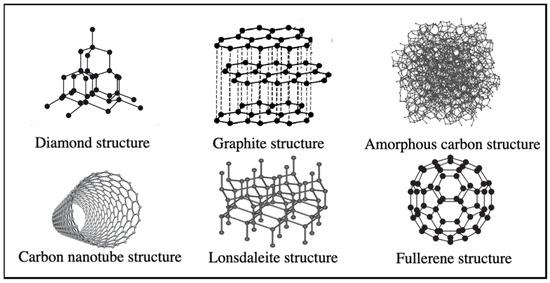
Figure 1.
Allotropes of carbon atoms.
The allotropes of carbon atoms, formed by bonding carbon atoms in various configurations, exhibit distinct physical and chemical properties. These allotropes also reflect the bonding geometry. In sp hybridization, carbon atoms form a linear structure with two bonds. Furthermore, in sp2 hybridization, carbon atoms form a triangular structure with three bonds per atom, while in sp3 hybridization, they arrange in a tetrahedral geometry, with four bonds per atom. Each distinct geometric arrangement of carbon atoms results in a different material, making carbon the unique element capable of exhibiting all three bonding geometries [20,21,22]. Materials composed of carbon atoms exhibit vastly different properties depending on the bonding geometry between the atoms.
Graphite is an allotrope of carbon with remarkable catenation ability, enabling it to bond with carbon and other chemical elements in various ways, forming the foundation of organic chemistry and life [20]. Graphite, the most widely recognized allotropic form of carbon since ancient times, consists of layers of sp2 hybridized carbon atoms stacked on top of one another, and held together by weak van der Waals bonds [19,20]. The C-C bond length in the graphite layer, which exhibits anisotropic behavior concerning thermal and electrical conductivity, is 0.134 nm, while the graphene layers in graphite are separated by 0.335 nm [19,20,21]. Table 1 provides information on the properties of various carbon allotropes.

Table 1.
Properties of some allotropes of carbon [23,24,25].
In graphite, elemental carbon adopts its lowest energy state under ambient temperature and pressure conditions. The graphite crystal lattice consists of stacked, parallel two-dimensional graphene sheets, where sp2-hybridized carbon atoms are tightly bonded in hexagonal rings. Graphene sheets possess the lowest energy state because the 2pz orbitals of carbon atoms overlap most effectively when aligned parallel. As a result, graphite exhibits anisotropy given the in-plane and out-of-plane bonding of carbon atoms [12,14,15].
2.2. Graphene
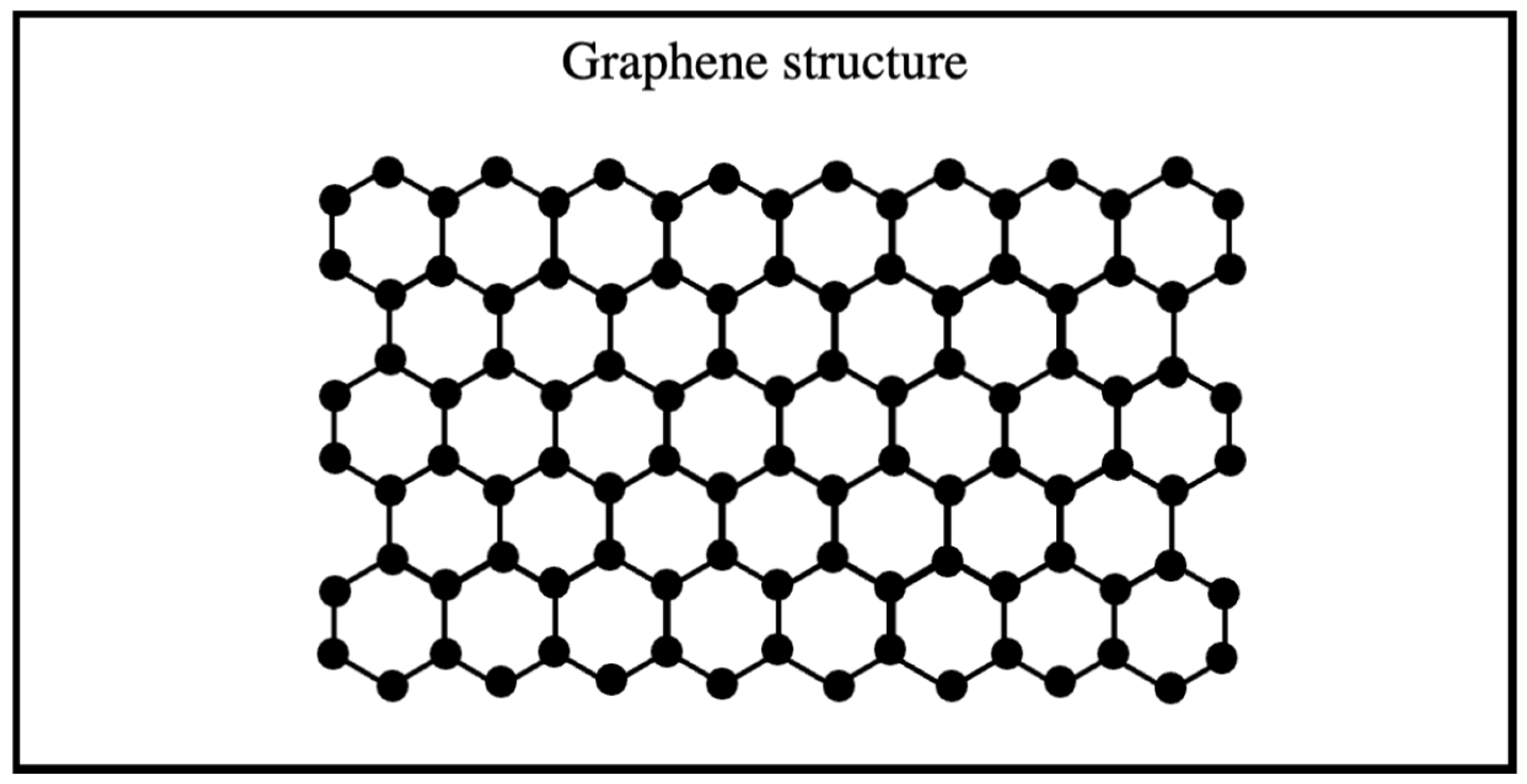
Graphene is a two-dimensional structure composed of a monolayer of sp2-hybridized carbon atoms arranged in hexagonal layers stacked on each other (Figure 2), serving as the fundamental building block of all other graphitic materials in various sizes [21,26]. On the other hand, graphene, despite being theoretically considered thermodynamically unstable as a two-dimensional crystal at finite temperature, and thus unlikely to exist, has gained significant attention resulting from its extraordinary properties (exceptional mechanical strength, high flexibility, superior thermal and electrical conductivity, optical transparency, and atomic scale thinness) [17,27]. Graphene is widely used in various applications, including electrochemiluminescence (ECL) sensors, transistors, water filtration, energy storage, biosensors, and solar cells [28].
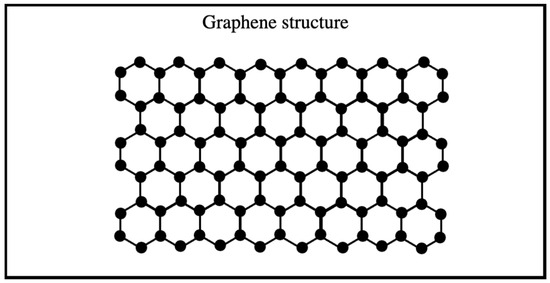
Figure 2.
Graphene structure.
The term “graphene” was first introduced in 1986 by Boehm et al. [29]. In 2004, monolayer graphene was successfully obtained by Andre Geim and Konstantin Novoselov [30], who used adhesive tape to peel graphite, which ultimately led them to receive the Nobel Prize in Physics in 2010 [31].
Thermal conductivity is the ability of a material to conduct heat and is usually calculated by Fourier’s law. Acoustic phonons typically contribute to the thermal conductivity of solids. While the thermal conductivity of metals is provided by high concentrations of free electrons (Ke), the thermal conductivity of carbon materials is supplied by phonons (Kp). The thermal conductivity of amorphous carbon is ~0.01 Wm−1 K−1, for diamond it is ~2000 Wm−1K−1, and for graphene it is 5000 Wm−1K−1 [32,33,34].
Graphene is a highly thermally stable material with an exceptionally high specific surface area (theoretically 2630 m2/g), superior electronic and optical properties, and remarkable electron transport capabilities, making it an excellent conductor. Graphene also has a high electron mobility (250,000 cm2/V·s) [21,34,35]. Moreover, graphene, with an electrical conductivity (200,000 cm2/V·s), has each carbon atom bond with three neighboring carbon atoms, whereas the 2pz orbitals from the 2p orbitals link the graphene layers in the layered graphite structure through weak van der Waals bonds. Single-layer graphene exhibits a non-stacked, wavy structure, while multilayer graphene can adopt stacking patterns such as Bernal or rhombohedral [32,35,36]. The thickness of a graphene layer is 0.34 nm. The distance between adjacent carbon atoms on the graphene layer is 1.2 Å [21]. The carbon–carbon bond length in the graphene structure is 0.142 nm, and its high Young’s modulus has been reported to be 1100 GPa [31]. Graphene, a two-dimensional hexagonal carbon allotrope, exhibits a three-fold rotational symmetry of 120 degrees and an optical transmittance of 97.7% [18,34].
Due to its unique energy band structure, graphene exhibits superior electrical properties not typically found in other materials. These include the ambipolar field effect, defined as the variation in self-conductivity through carrier density modulation via gate voltage, along with minimal conductivity and magnetoresistance [37]. The valence and conduction bands of graphene exhibit a conical structure that intersects at six distinct points, referred to as Dirac points, situated in the Brillouin zone around the K points. At these Dirac points, the energy dispersion is linear, and the charge carriers behave as massless Dirac fermions, resulting in graphene’s exceptional electrical conductivity at room temperature [38].
2.3. Graphene Oxide
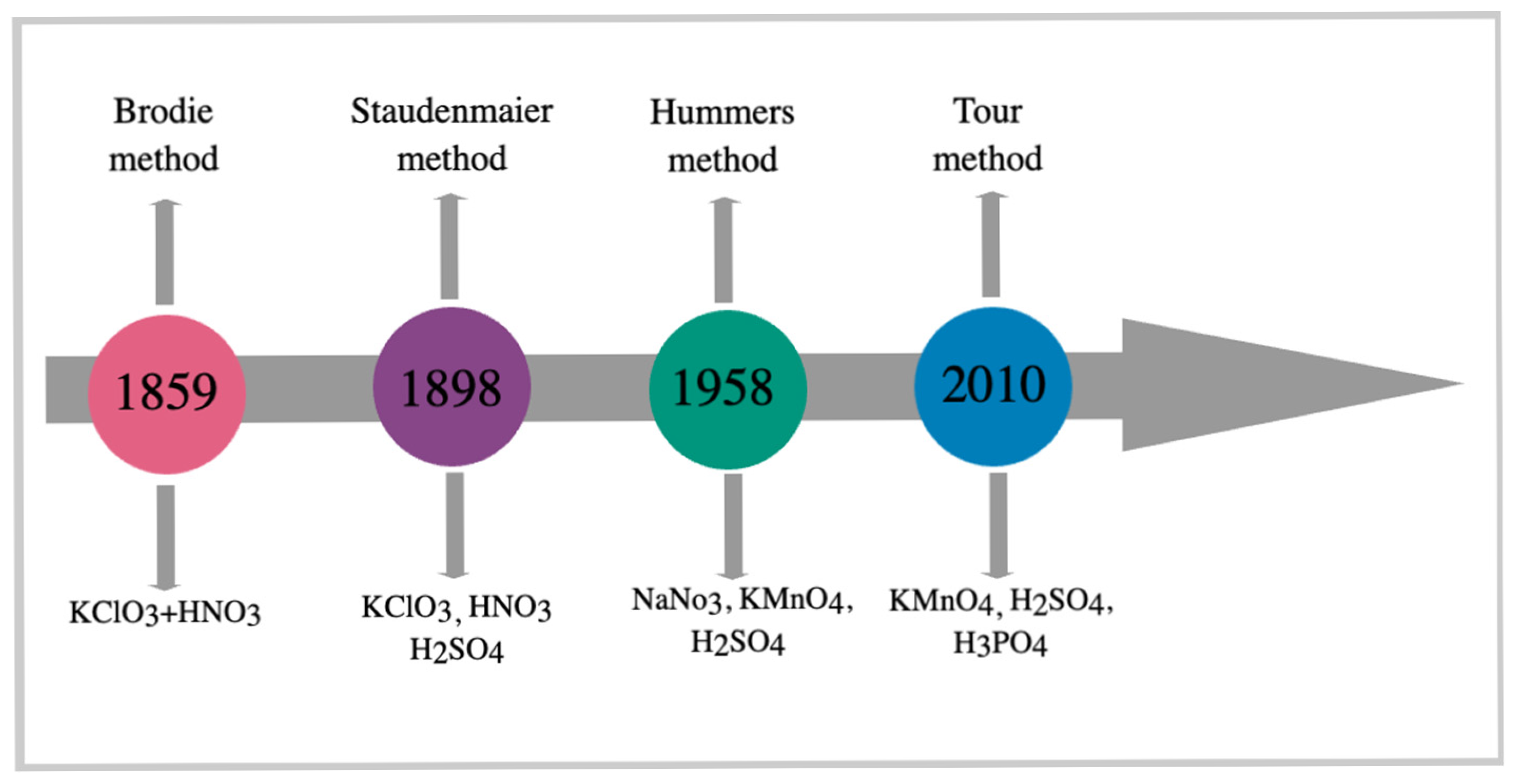
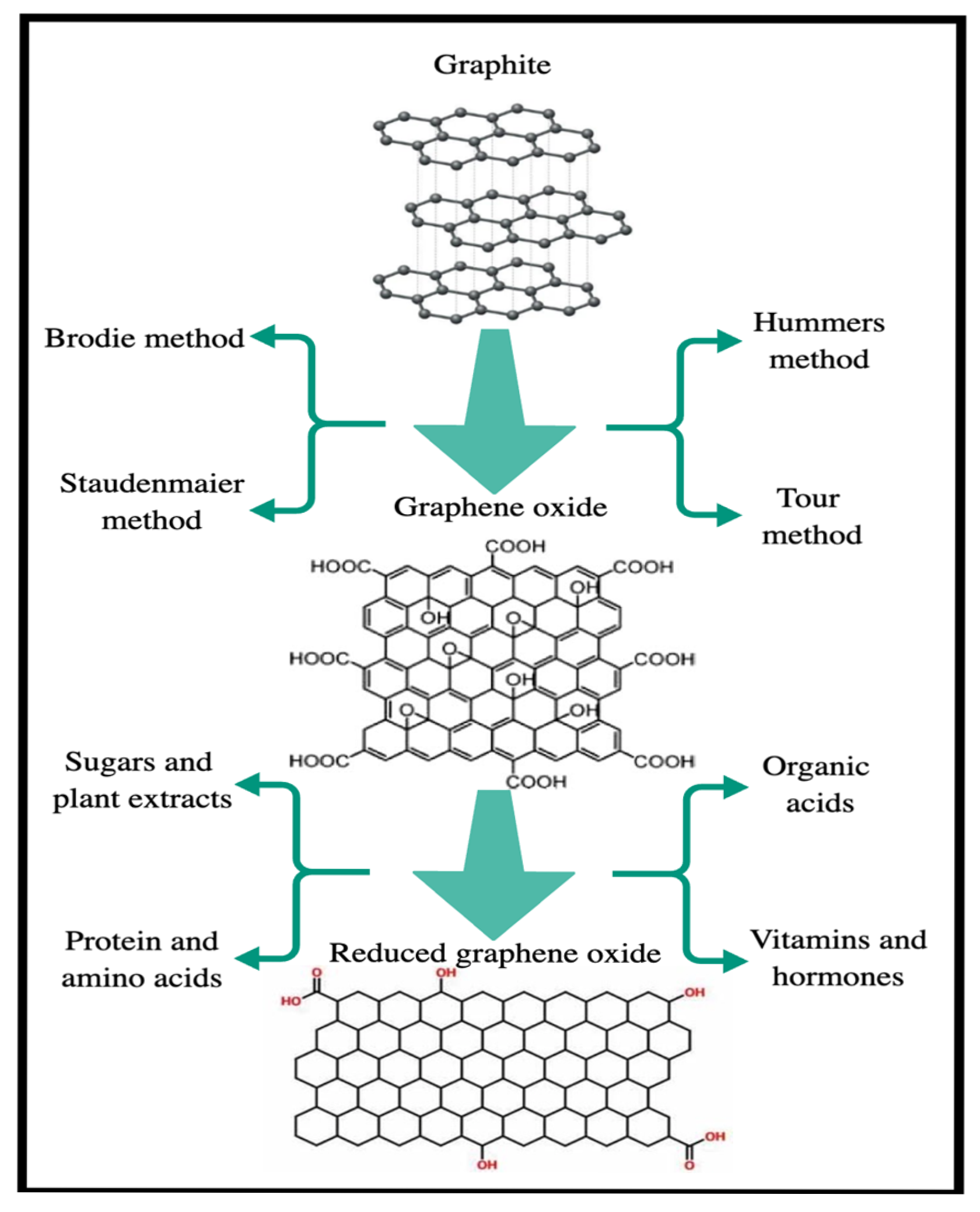
Graphene oxide (GO) was first studied in 1859 by the British chemist B.C. Brodie, who investigated the reactivity of flake graphite [39]. GO is a layered, hydrophilic material with various functional groups on its basal planes and edges [40]. Graphene oxide can be synthesized through various methods, including the Brodie, Staudenmaier, Hummers, and Tour methods (Figure 3) [13,41,42]. Studies on the synthesis of graphene oxide by the Brodie method, which mixed equal weights of graphite with three equivalent weights of potassium chlorate (KClO3) and reacted the mixture with nitric acid (HNO3) at 60 °C for 4 days, have laid the foundation for further research in this area [27,43,44]. In 1898, Staudenmaier developed an alternative method for synthesizing graphene oxide [43], where he replaced approximately two-thirds of HNO3 with concentrated sulfuric acid (H2SO4) and added more than one part of KClO3 [27,44].
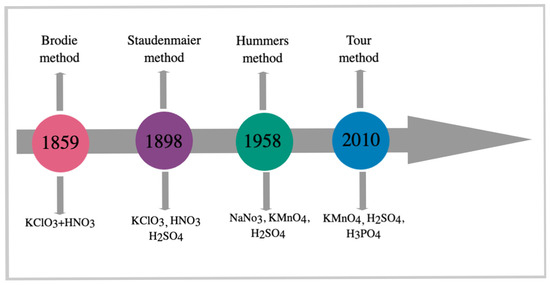
Figure 3.
Graphene oxide synthesis methods.
In 1958, Hummer developed a method for synthesizing graphene oxide that offers numerous advantages, including producing simple, easy-to-use, and cost-effective products. In this method, graphene oxide is obtained by oxidizing graphite with equal weights of graphite powder in a concentrated H2SO4 solution containing three equivalent weights of potassium permanganate (KMnO4) and an equal weight of sodium nitrate (NaNO3) [27,43,44,45]. The method developed by Hummer has successfully synthesized graphene oxide due to its high efficiency and reliability [19]. However, the drawbacks of this method include the release of toxic gases, such as nitrous oxide (NO) and nitrogen tetroxide (N2O4), as well as environmental damage [27].
In 2010, the Tour method was developed as a modified version of the Hummers method in which the amount of KMnO4 is doubled. In the Tour method, chemical agents with toxic properties are not used [46]. The advantages of the Tour method include high production yield, low cost, short synthesis time, no explosions, and no formation of toxic gases [47].
GO is an oxidized form of graphene with a hydrophilic structure, readily dispersible in water, forming stable colloidal suspensions. Graphene oxide does not exfoliate directly in non-aqueous solvents, likely due to the strong interlayer hydrogen bonds between the functional groups of adjacent graphene oxide layers in GO [48].
2.4. Reduced Graphene Oxide
The methods used to reduce graphene oxide can be classified into three main types: thermal, chemical, and multi-step reduction. The reduction of graphene oxide by the thermal method can be achieved by applying high heat; however, it is not a preferred method due to low production yield and potential degradation of graphene layers [48]. The microwave reduction method for rGO synthesis, developed as an alternative to the thermal method, has been shown to enhance the yield by transferring energy to the reaction system, causing a sudden internal temperature increase. This method is considered a preferred approach [15,49,50]. Table 2 presents some of the properties of graphene, graphene oxide, and reduced graphene oxide as reported in the literature.

Table 2.
Properties of graphene, graphene oxide, and reduced graphene oxide [23,24,25].
Typically, the chemical reduction of GO is performed using reducing agents, such as hydrazine and its derivatives, which are known for their toxic and explosive nature. To address these concerns, it is essential to employ environmentally friendly, cost-effective, and efficient alternatives, commonly referred to as green reducing agents, that offer high reducing power [19,51]. In the reduction of graphene oxide, multi-step reduction methods are more efficient than single-step reduction approaches. Studies have shown that factors such as the choice of reducing agents and reduction temperature significantly influence the synthesis process [31]. Reduced graphene oxide obtained through different reduction methods exhibits varying properties and affects the distribution of functional groups in distinct ways [52].
3. Tribological Properties of Graphene
Lubricants can be categorized into several types, including solid, liquid, and semi-solid lubricants [53]. Lubricants should be non-volatile, non-corrosive, durable over time, and chemically and thermally stable to prevent adverse effects on the system. Therefore, lubricants should provide desirable properties, such as reducing maintenance costs and minimizing surface wear and tear [5]. Improved stability and dispersibility of additives enable formulators to incorporate higher concentrations into the lubricant without causing sedimentation or aggregation, which is essential for shelf life and, consequently, for practical applications. Moreover, 2D materials can effectively create boundary films due to their superior properties and mechanisms [54]. Graphene and its derivatives can be used as lubricant additives to enhance efficiency by forming tribofilms that prevent direct contact between surfaces [9]. Graphene offers exceptional mechanical and tribological properties, including a hardness of 950 (SL), a Young’s modulus of 890–1000 GPa, a coefficient of friction of 0.15, 101 bonds broken/nm, a wear rate of mm3/Nm, as well as an ultra-high specific surface area and an extremely low interlayer van der Waals barrier.
Graphene exhibits superlubricity behavior. At the nanotribology scale, superlubricity has been observed in graphene nanoflakes on graphene, with a more pronounced effect at lower temperatures [1,55]. On the other hand, factors such as atomic vacancies or replacements, the number of layers, interlayer spacing, structural defects, and surface and edge functional groups can influence its lubrication performance [1].
Graphene can also be a coating to enhance wear resistance and lower friction. Regarding the latest studies, Baiocco et al. (2024) investigated improving anti-wear and low-friction graphene nanoplatelets (GNPs) coating on copper substrates through cathodic electrophoretic deposition in a water-based solution. In this study, deposition times (1, 2, and 3 times) and voltages (15, 30, and 45 V) on the morphology, adhesion, and tribological performances of the coatings were evaluated. The findings indicate that the application of GNP coatings facilitated the development of a tribofilm, which significantly reduced the coefficient of friction to around 0.12 and enhanced wear resistance by more than 88% [56]. Mingione et al. (2025) evaluated the improvement of thermal, electrical, and tribological performances of GnP composites produced by laser sintering. The results showed that electrical conductivity improved from 10−11 S/cm of the unfilled PA-12 matrix up to 10−4 S/cm for the 10 wt% GnP samples. In addition, thermal performance improved up to 33.6% with GnP reinforcement. It is detected that 10 wt% GnPs reduced friction and wear (25% and 81%, respectively) [57].
Graphene advantages [9,58,59,60,61]:
- Mechanical strength and thermal stability;
- Low coefficient of friction and surface energy, and well-established lubricity;
- High thermal and electrical conductivity;
- Chemical inertness and shield against abrasion and corrosion;
- Super-hydrophobic coatings;
- Thermal shielding;
- Compatibility with different fluids.
Graphene challenges [5,62]:
- High temperatures can cause wear debris and degrade graphene-based additives, underscoring the need for more research and their use in various liquid lubricants;
- The high cost and poor dispersion stability of graphene-based additives remain major challenges for their industrial application in liquid lubricants;
- There are no standard guidelines for producing additive compounds, and key parameters like particle size, layer number, type, and functional group concentration for specific applications are still unclear;
- Sulfur compounds present in commonly used organic and inorganic components, such as sodium dodecyl sulfate and molybdenum disulfide, in graphene-based nanomaterials may contribute to the release of pollutants. Additionally, optimizing graphene production methods is essential for cost reduction and enabling large-scale production of additives for future applications.
The lubricating properties of graphene and its derivatives offer extremely low friction, wear rates, strength, and flexibility, facilitating the reduction in direct contact [63]. Graphene oxide (GO) and reduced graphene oxide (rGO) are graphene derivatives. GO and rGO contain functional groups such as hydroxyl and epoxy groups on their surfaces, carboxyl groups at their edges, aromatic rings, and π−π electrons [64]. Table 3 summarizes literature studies related to graphene, GO, and rGO.

Table 3.
Literature studies on the wear rate and coefficient of friction for graphene, graphene oxide, and reduced graphene oxide.
Graphene and its derivatives can affect the tribological properties of lubricants and contact surfaces. Past studies have found that several parameters influence these properties, including the form of graphene, production methods, additive concentration, and environmental conditions.
3.1. Graphene and Its Derivatives as an Additive
Lubricants are among the most essential substances for mitigating friction and wear, including various lubricating oils such as mineral, synthetic, semi-synthetic, and bio-based [71]. Environmentally sustainable lubricants have become an alternative to conventional petroleum-based lubricants [72]. Therefore, eco-friendly lubricants have become a significant issue for controlling rising pollution levels. Figure 4 illustrates the solution for sustainable and eco-friendly lubrication [73].
Figure 4.
An outline of the 2D lubrication theory.
Two-dimensional materials, including graphene, exhibit outstanding properties such as self-lubrication, anti-friction, anti-wear, thermal stability, and a large specific surface area, making them excellent lubricant additives due to their unique bonding characteristics [1,74]. Graphene-based materials can be added to various oils to evaluate their tribological properties. Researchers have studied the tribological properties of graphene and its derivatives as additives in different types of oils.
Zhao et al. (2021) investigated the application of graphene as an additive in PAO4 oil to study the frictional interaction between graphene and the ZDDP composite. They found that the reduction in wear rate was attributed to graphene’s exceptional properties, including the tribofilm and the protective polyphosphate film [75].
Wen et al. (2020) evaluated two-dimensional layered nanomaterials (GO, rGO) as lubricant additives in PAO 10 oil to assess the tribological behavior of these additives. The tribological evaluation showed that GCF can form a protective film on contact surfaces by interacting with metal ions through p-d feedback coordination, offering high stability against tribo-stress and heat. Its covalent bonds strengthen the film, ensuring durability, while its ultra-thin layered structure reduces shear stress. This combination allows GCF (at just 0.008 wt%) to significantly reduce friction (53.5%) and wear (95.4%) and improve load capacity (150 N). In contrast, GO and rGO additives, with poorer lipophilicity and oxygen group decomposition under stress, fail to provide adequate lubrication [76].
Jafari et al. (2024) published a review on the tribological properties of synthetic and biosourced lubricants containing graphene and its derivatives as additives. According to their review, biolubricants exhibit high biodegradability, renewability, and lower toxicity than traditional lubricants. Due to its excellent properties, such as low shear resistance, suitable size for reaching contact regions, and effective heat distribution, graphene oxide is considered more ideal than graphene for tribological applications [8].
Fuadi et al. (2022) investigated the tribological properties of a bacterial cellulose/polyolester oil bio-lubricant. They discovered that oxygen plays a crucial role in the friction and wear performance of polyolester oil with graphene additives. Additionally, the combination of graphene and bacterial cellulose (BC) in the oil enhanced friction and wear performance, despite the presence of oxygen on the contact interface [77].
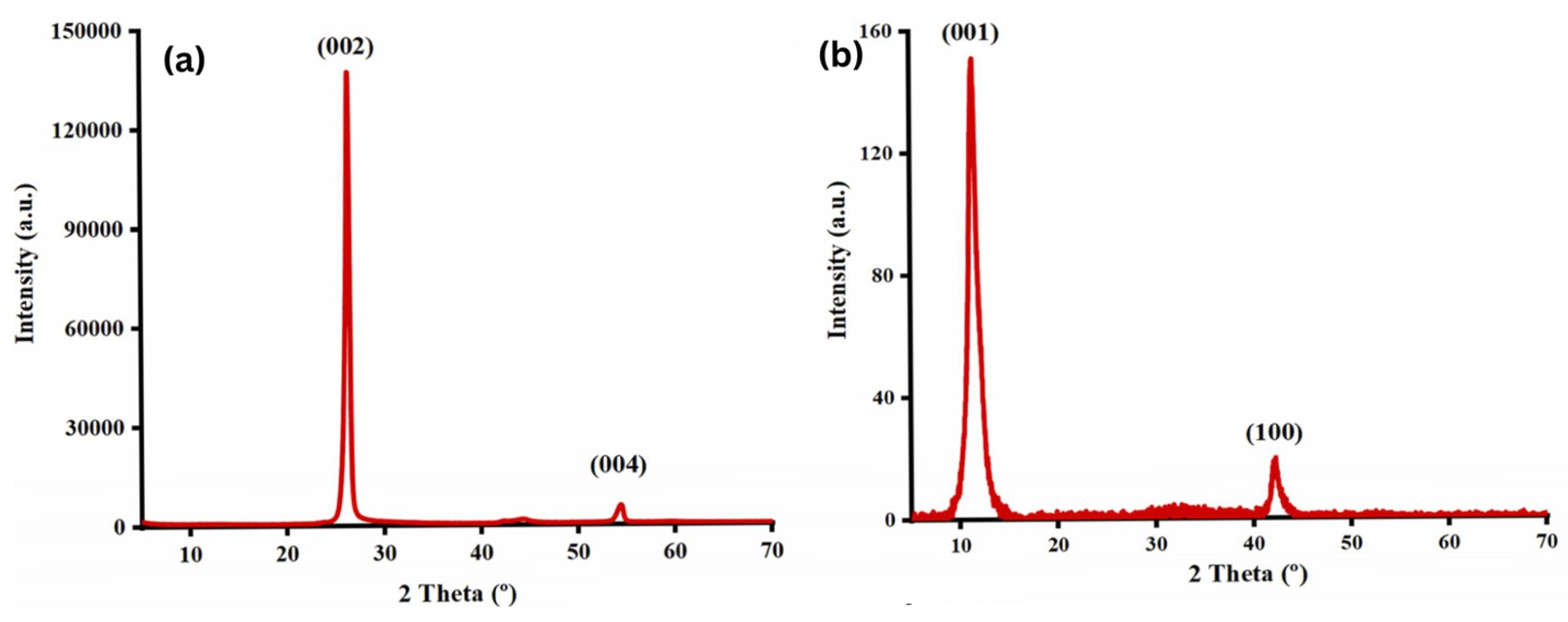
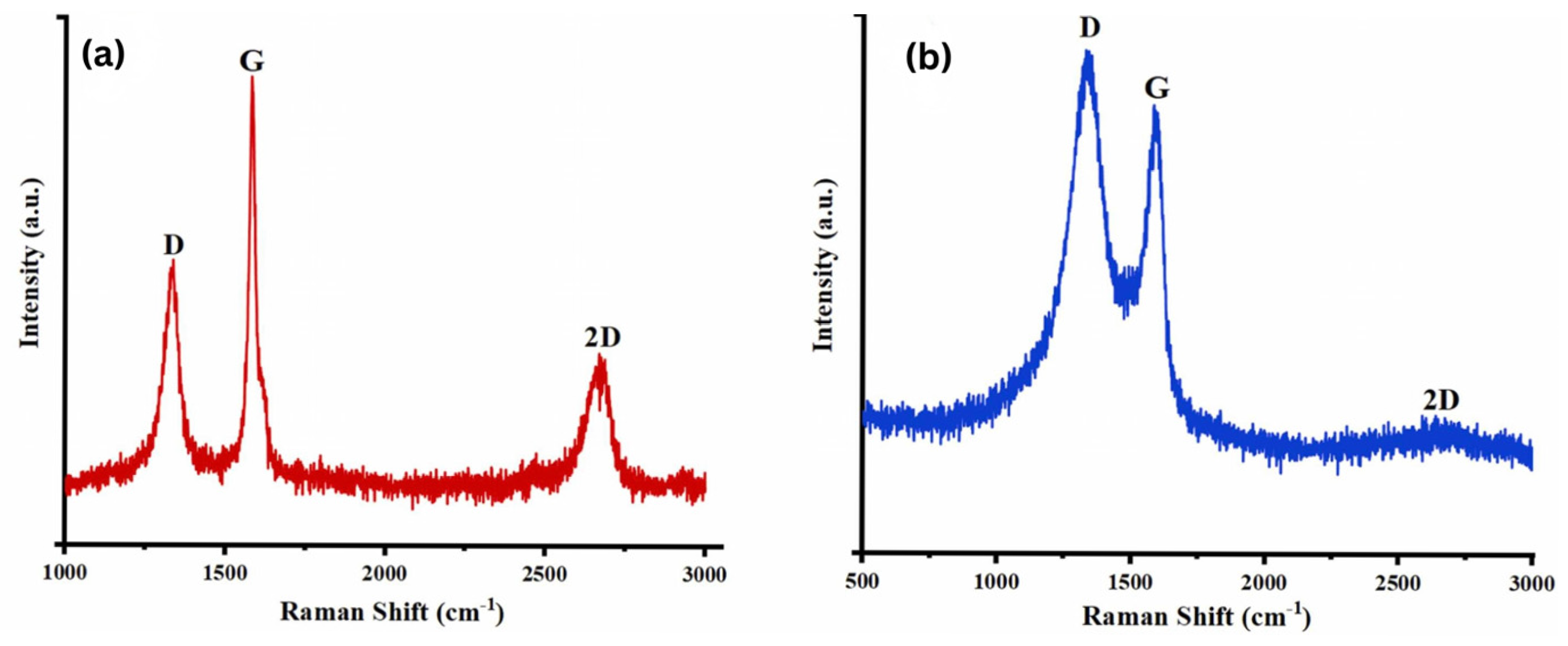
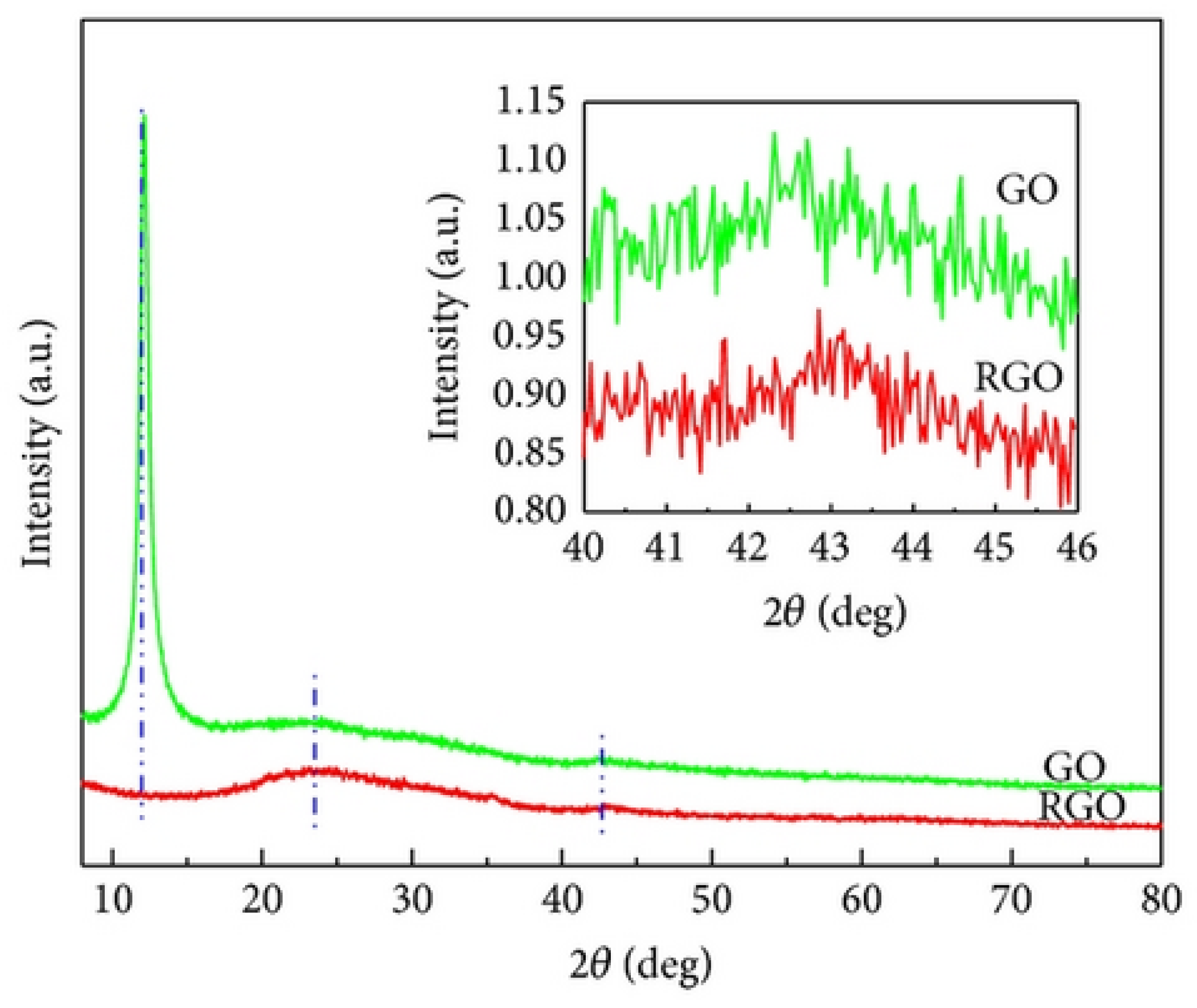
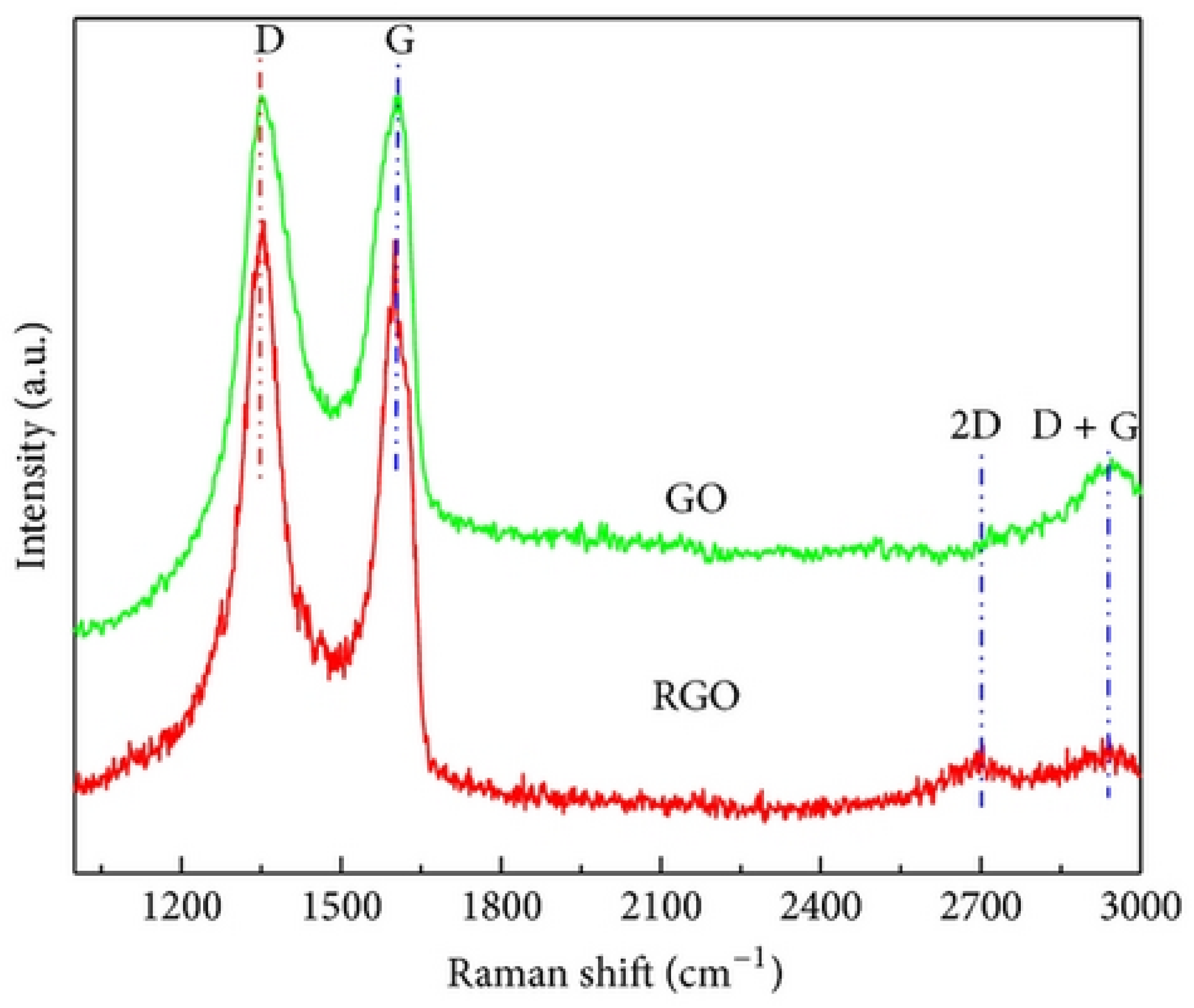
Najari et al. (2025) investigated the use of graphene oxide in four different water-based lubricants as an alternative to traditional oil-based lubricants, which have several disadvantages. In this study, graphene oxide (GO) (GO1: supernatant with 0.06 wt%; GO2: redispersed with 0.06 wt%, no surfactant; GO3: supernatant with 0.06 wt%; GO4: redispersed with 0.06 wt%, concentration of surfactant = 0.5 wt% in water) was synthesized using the Modified Hummers method. The results were analyzed using different techniques, including TEM, XRD, and Raman spectroscopy for material characterization, and a Mini Traction Machine (MTM) tribometer with a ball-on-disc arrangement for tribological testing. The coefficient of friction (COF) was significantly reduced for GO1 and GO2, showing a decrease of 48% and 54%, respectively, compared to DI water. Additionally, when GO3 and GO4 were used as lubricants, adding surfactant further decreased the COF by 72% and 84%, respectively. Moreover, the wear track width was reduced for the samples. XRD analysis (Figure 5) showed that pristine graphite (Figure 5a) exhibited two diffraction peaks corresponding to (002) and (004), with the prominent (002) peak at 2θ ∼ 26.6°, indicating an interlayer distance of 0.34 nm. Graphene oxide (Figure 5b), two peaks are observed: the strong (001) peak at 2θ = 11.4° (d-spacing ∼ 0.78 nm) and the (100) peak at 2θ = 42.2° (d-spacing ∼ 0.213 nm). Raman analysis (Figure 6) showed that the Raman spectrum of graphite (Figure 6a) shows the dominant G band at 1580 cm−1, with the D and 2D bands at 1332 cm−1 and 2670 cm−1, respectively. In contrast, for graphene oxide (Figure 6b), the D band at 1331 cm−1 is the strongest, with the G and 2D bands observed at 1585 cm−1 and 2679 cm−1, respectively [78].
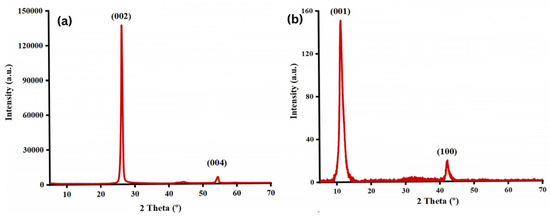
Figure 5.
XRD spectrum of the (a) graphite, (b) graphene oxide.
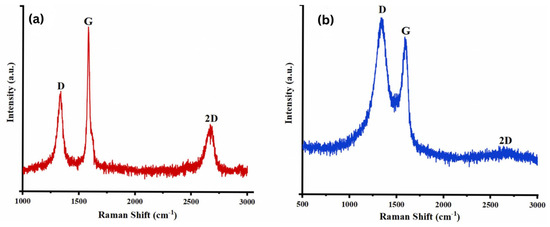
Figure 6.
Raman spectrum of the (a) graphite, (b) graphene oxide.
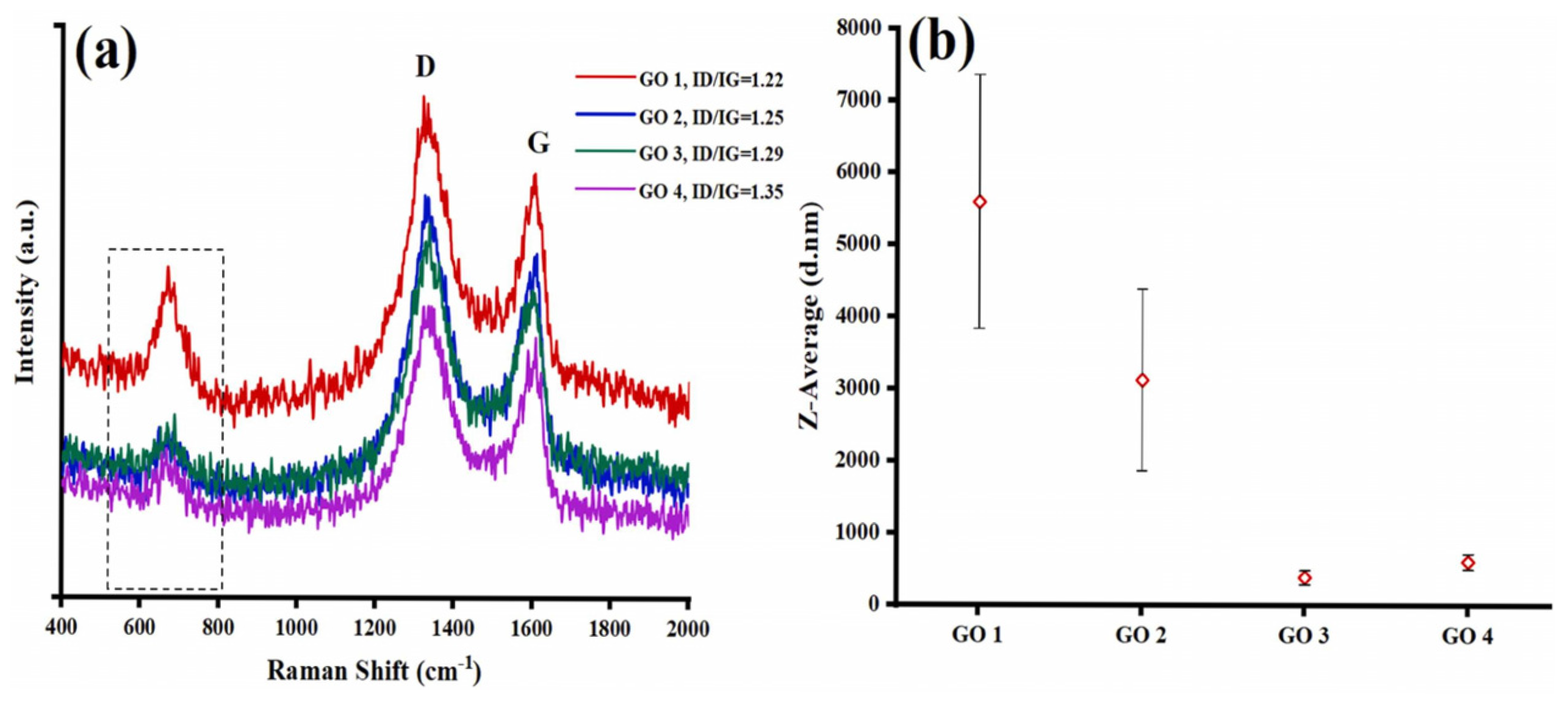
In this study, the formation of the GO tribofilm on discs lubricated with different GO suspensions was observed using Raman spectroscopy (Figure 7). According to the results, the Z-average of pristine GO suspensions was increased for GO1 and GO2 after the tribological test, with values rising to 5602 and 3134 nm, respectively, compared to their initial values of 325 and 2100 nm. In contrast, minimal changes in particle size were exhibited by GO3 and GO4, with values being maintained at 321 and 613 nm. This increase in Z-average for GO1 and GO2 is attributed to the agglomeration of GO nanosheets during the tribological test. On the other hand, the addition of surfactants in GO3 and GO4 effectively prevented agglomeration by inducing electrostatic repulsion, thereby stabilizing the particle size even after the wear test [78].
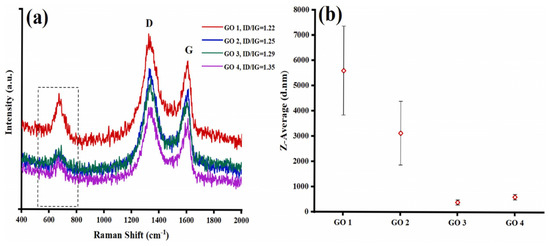
Figure 7.
Raman spectrum of the (a) worn surfaces on discs lubricated with various GO suspensions, (b) Z-average of the GO lubrications after tribological test.
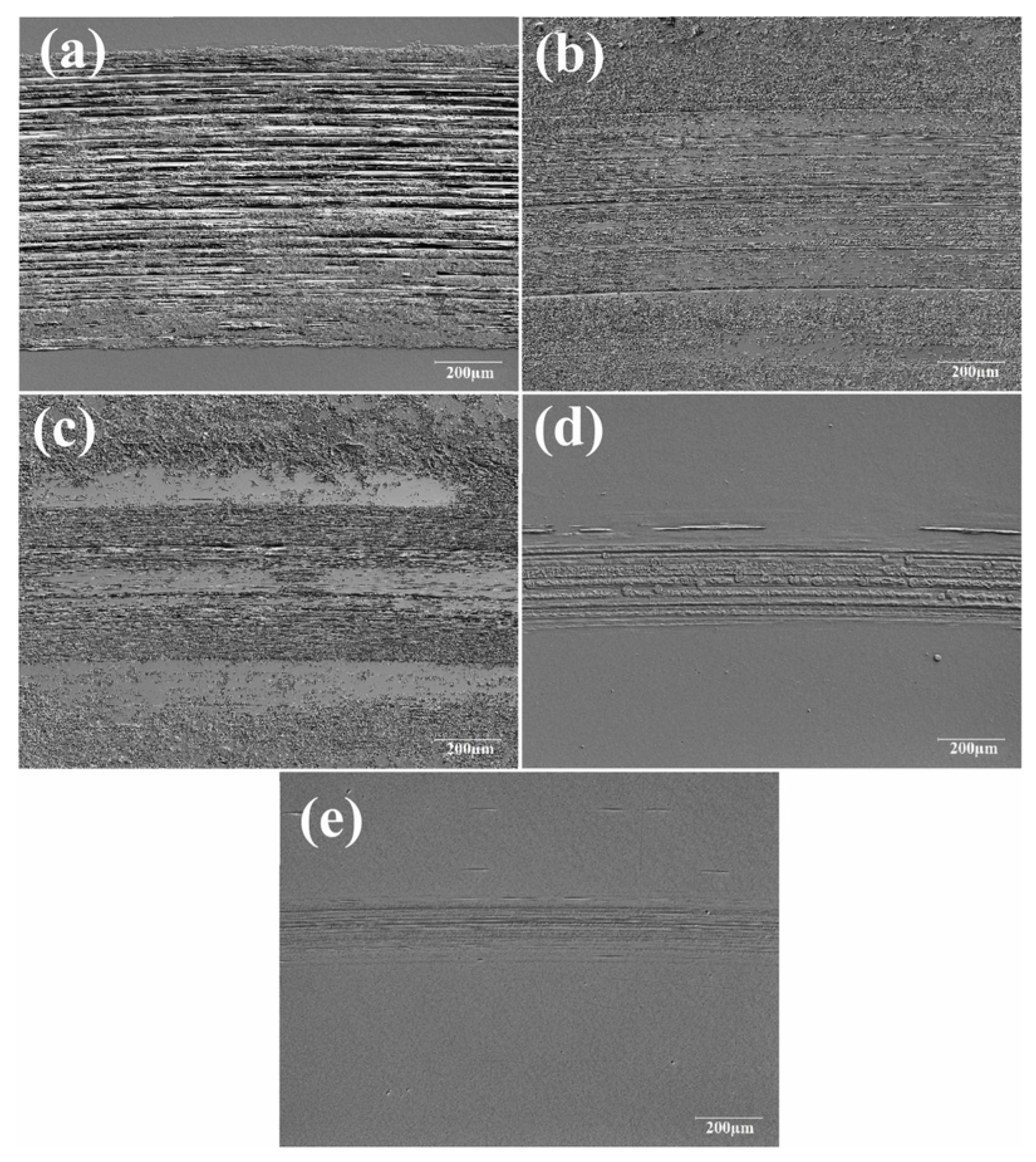
The wear track on disc surfaces lubricated with DI water and different GO suspensions was analyzed (Figure 8). It has been indicated that severe wear scars are formed when pure water (wear track width 902 µm) is used as the lubricant, due to its inability to form a tribofilm or maintain a stable lubricating film, leading to direct contact and boundary interactions between the ball and the disc. The wear track width decreased significantly when GO lubricants were used, with reductions of 74.5% and 80.4% for GO1 and GO2, respectively, compared to water, while discs lubricated with GO3 and GO4 exhibited even greater reductions of 297.4% and 353.8%, respectively [78].
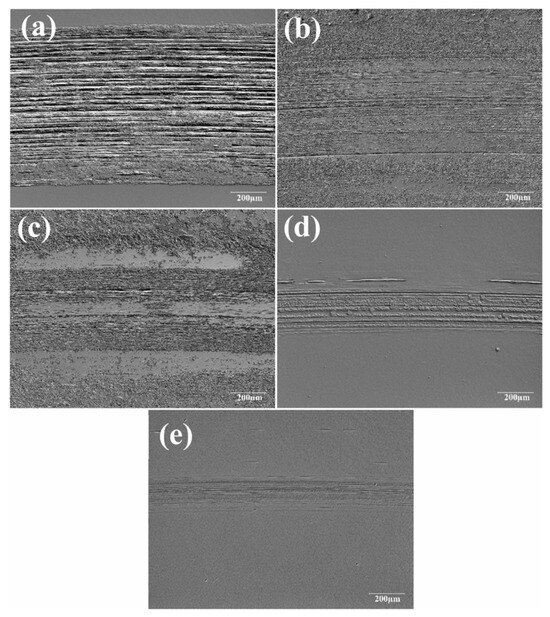
Figure 8.
(a) Microscope images of the worn disc surfaces (b) GO1, (c) GO2, (d) GO3, and (e) GO4.
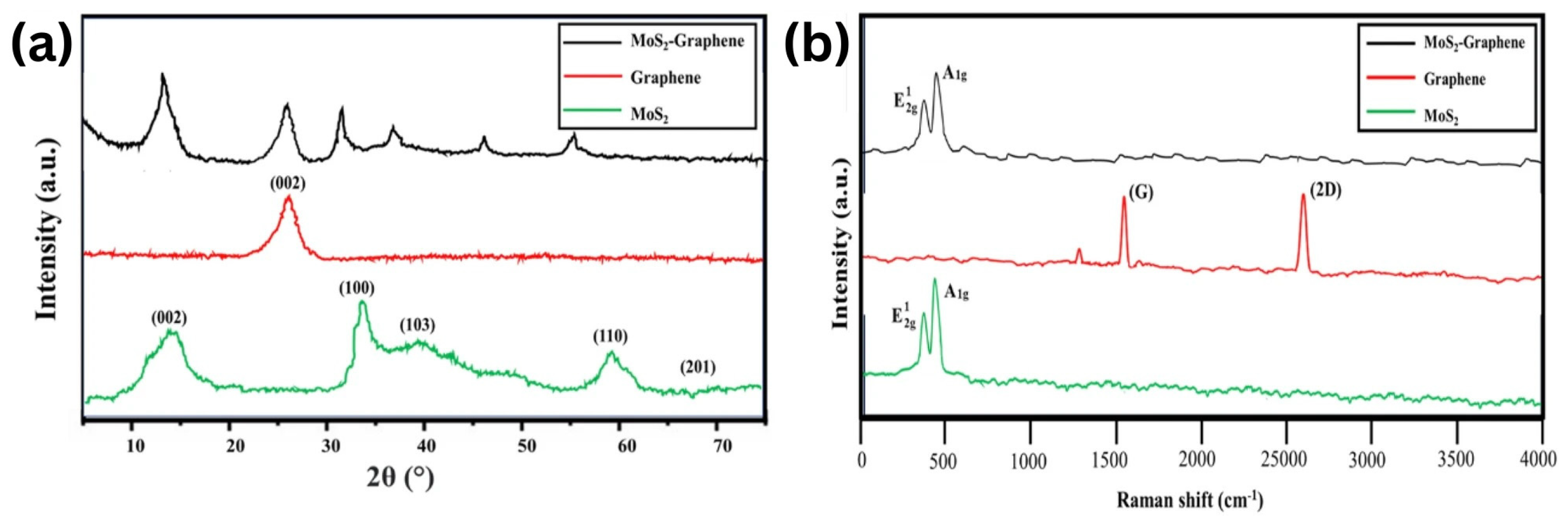
Nagarajan et al. (2023) investigated the enhancement of tribological properties of diesel-based engine oil using a MoS2–graphene additive. In this study, graphene was synthesized using the microwave method. The choice to work with MoS2 was based on its strong interlayer covalent bonds and weak van der Waals forces, which enable the formation of a slippery surface that reduces friction and wear between moving parts. The microwave method demonstrated high efficiency in reducing synthesis time and energy consumption while incorporating 0.05 wt% hybrid MoS2–graphene nanohybrid into SAE 20W40 diesel-based engine oil. Significant improvements were observed in the coefficient of friction and wear scar diameter, with reductions of 58.82% and 36.26%, respectively. Furthermore, when added to the engine oil, the nanolubricant exhibited oxidation resistance and excellent thermal conductivity. As a result, the microwave synthesis of the MoS2–graphene nanohybrid shows exceptional potential as a tribological additive in diesel-based motor lubricants. In addition, the COF of the pure SAE20W40 base oil was measured at 0.0949. COF of the MoS2–graphene nanohybrid with different concentrations (0.1 wt%, 0.05 wt%, 0.01 wt%, and 0.005 wt%) was determined to be 21.69%, 58.82%, 25.98%, and 17.19% lower, respectively. The wear scar diameters of MoS2–graphene nanohybrid in SAE20W40 diesel engine oil were measured (444 µm with the addition of 0.05 wt% MoS2–graphene) with a reduction of 36.26% [79].
According to XRD analysis (Figure 9), diffraction peaks were observed at 2θ = 14.5°, 33.0°, 39.3°, 58.5°, and 69.7° in the pure MoS2 phase, as well as 2θ = 24.6° for graphene. According to the Raman spectroscopy analysis, it is observed that the Raman spectrum of MoS2, 367 cm−1 with the E12g vibrational mode, and 406 cm−1 with the A1g mode were detected. The Raman spectrum is also influenced by the graphene phase, with the characteristic G and 2D peaks of graphene observed at approximately 1580 cm−1 and 2700 cm−1, respectively [79].
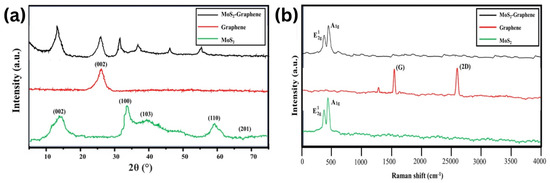
Figure 9.
(a) XRD spectrum of the MoS2–graphene, graphene, and MoS2. (b) Raman spectrum of the MoS2–graphene, graphene, and MoS2.
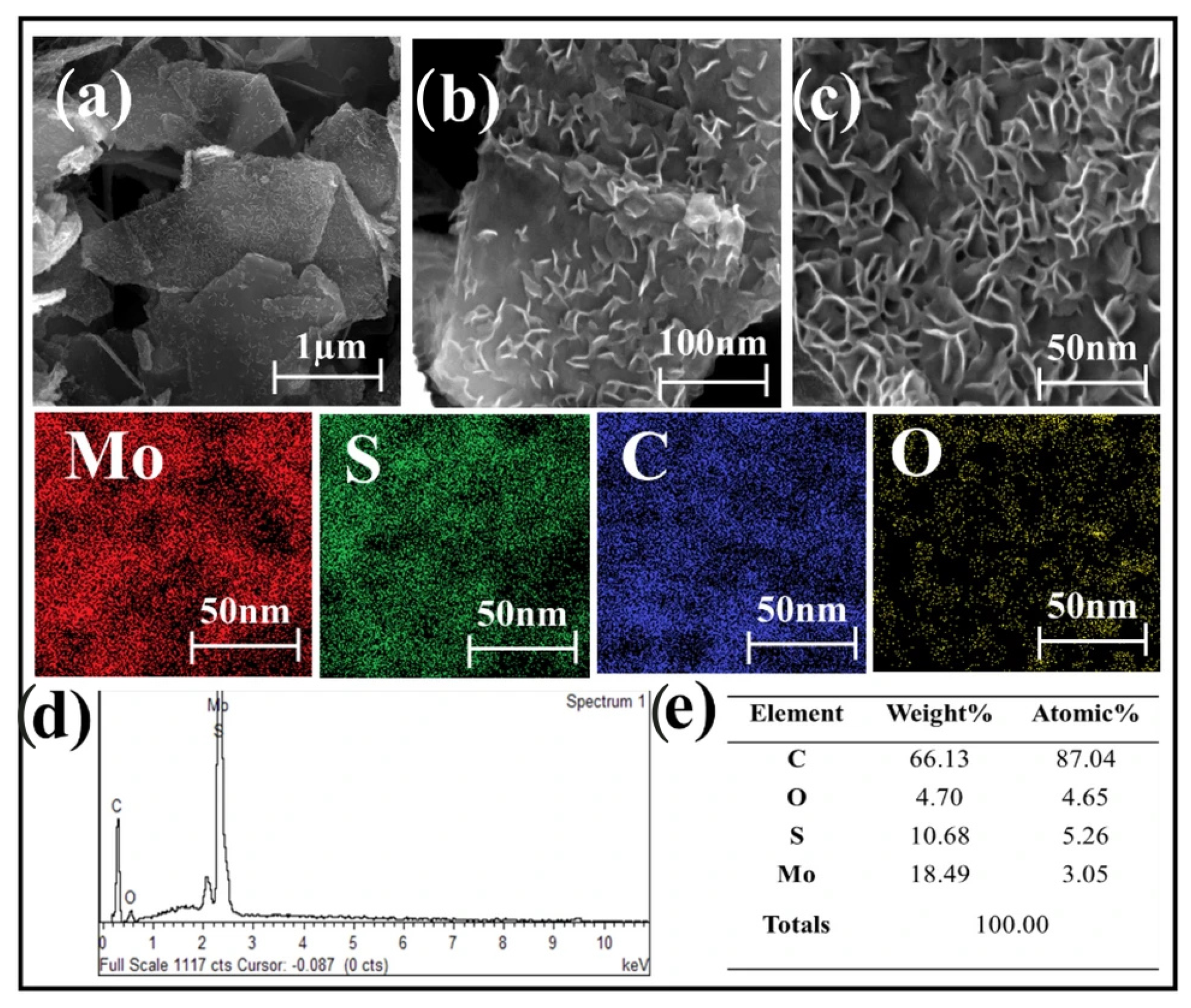
The MoS2 nanoparticles are uniformly grown on graphene surfaces with curved edges, indicating good interfacial interaction between the two phases. The microwave synthesis method proves highly effective in producing this MoS2–graphene nanohybrid, which has flake sizes ranging from 100 to 150 nm, confirming its nanometer-scale structure. This nano-sized feature enhances reaction kinetics and reduces the likelihood of nanoparticle aggregation. The method also allows MoS2 nanoparticles to encapsulate the graphene surface and intercalate between MoS2 layers, creating an interlayer coupling effect. This results in improved structural integrity, enhanced interfacial interactions, and promoted charge transfer between layers. EDS mapping analysis shows a uniform distribution of Molybdenum (Mo), Sulfur (S), Carbon (C), and Oxygen (O) elements across the MoS2–graphene nanosheet, with the EDS spectrum confirming their presence in the hybrid structure (Figure 10) [79].
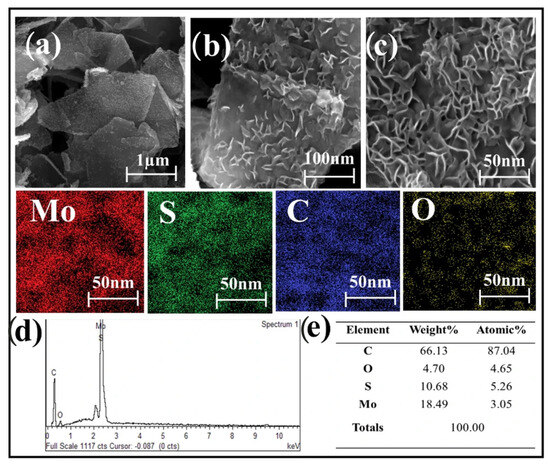
Figure 10.
(a–c) FESEM images of samples, (d) EDS spectrum, and (e) elemental distribution of the MoS2–graphene nanohybrid.
Hettiarachchi et al. (2023) investigated a bio-based lubricant with an Al2O3/graphene additive in coconut oil. In this study, mineral-based engine oil (15W40) was selected as the reference oil for comparison with a synthesized Al2O3/graphene nanocomposite reinforced with coconut oil (CCO). The tribological properties were analyzed using various methods, including a tribometer (LRT), EDX, and SEM. A 28% reduction in the coefficient of friction was observed. EDX analysis indicated the formation of a protective tribofilm on the sliding contact, evidenced by the deposition of C, Ni, Cr, O, Mo, and K, along with Al traces, on the worn surfaces, completely covering the Fe in the underlying material. SEM images further confirmed the presence of a protective layer on the wear surfaces [80].
3.2. Green Reductants
Chemical reduction of graphene oxide is an effective method for removing functional groups from the structure to produce large quantities of reduced graphene oxide and graphene [81]. Recently, environmentally friendly reduction processes have employed natural or non-toxic substances such as non-aromatic amino acids, plant leaf extracts, ethylene glycol, sodium carbonate, sugar, and green tea. However, these chemicals may pose potential risks to human health and the environment, highlighting the need for a reducing agent that can reduce GO without compromising the integrity and flexibility of rGO [82].
Shin et al. employed sodium borohydride, an inorganic compound, to reduce graphene oxide. The comparison between the results of graphene oxide and its reduced form allowed them to identify the changes in the functional groups eliminated during the reduction process [81]. Various chemical reducing agents are used for the reduction process, but it is crucial to prefer non-toxic green agents. These green agents offer advantages, such as the ability to provide high-quality, productive reduced graphene oxide and the capacity to form stable dispersions for a wide range of applications [81].
Although an effective and efficient reductant for synthesizing reduced graphene oxide, hydrazine has significant limitations. Both hydrazine and its by-products pose a threat to living organisms and the environment. If the resulting material contains residues of these hazardous chemicals, it becomes a danger to all living beings, particularly in health applications such as biomedicine. Therefore, environmentally friendly reducing agents are preferred over these toxic chemicals [83]. Green agents, such as organic acids, plant extracts, microorganisms, sugars, antioxidants, amino acids, and proteins, are considered safer alternatives. For instance, ascorbic acid (vitamin C), a non-toxic reagent, is as effective as hydrazine in reduction processes [83]. Figure 11 illustrates various reduction methods, including Brodie, Hummers, Staudenmaier, and Tour for converting graphite to GO and the chemical reductants that reduce GO to rGO [83].
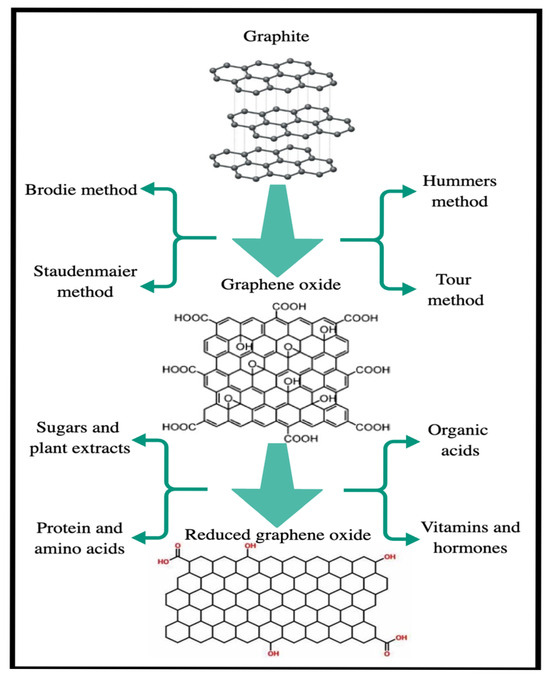
Figure 11.
Reduction of graphite to graphene oxide by various methods and chemical reduction of graphene oxide with some reductants.
Organic Acids: Organic acids and their salts are environmentally friendly reducing agents commonly used for metal nanoparticle synthesis and the reduction of graphene oxide. Wan et al. reported using sodium citrate to reduce graphene oxide films. Additionally, caffeic acid has been utilized in rGO synthesis and shows potential for applications in gas sensors and supercapacitors [83].
Plant Extracts: Various plant extracts, which are easily oxidizable, serve as reducing agents for graphene oxide reduction. Examples include mushroom extracts, green tea, carrot juice, orange peel extracts, coconut water, pomegranate juice, and rose water [83]. Tea, a globally consumed beverage, is rich in polyphenols and is biologically compatible [84]. In a study by Yin et al., green tea was found to react with oxygen derivatives and effectively reduce graphene oxide [84,85].
Microorganisms: Bacteria and yeast can reduce graphene oxide [83]. In a study by Lee et al., parsnip root was used to reduce graphene oxide, with endophytic microorganisms in the root playing a key role in the reduction process [85]. Similarly, Akhavan et al. employed Escherichia coli bacteria to reduce graphene oxide under anaerobic conditions. Graphene oxide and bacterial suspensions were incubated at 37 °C for 48 h, demonstrating reduced capacity [84].
Sugars: Sugars and polysaccharides, recognized as green reductants for graphene oxide, are commonly used. In a study by Zhu et al., glucose, fructose, and sucrose were identified as effective reducing agents for graphene oxide, with glucose exhibiting the highest reducing capacity [83].
Saccharides: They are commonly used as reducing agents for synthesizing various nanomaterials. Monosaccharides like glucose and fructose are particularly notable due to their mild reducing abilities and non-toxic properties. In a study by Dong et al., glucose, fructose, and sucrose were used as reducing agents in an aqueous ammonia solution to reduce graphene oxide (GO). In a basic solution, glucose undergoes oxidation to form aldonic acid, which then converts into lactone. This oxidized product contains several oxygen-containing groups that form hydrogen bonds with residual oxygen functionalities on the reduced graphene oxide (rGO) surface, enhancing dispersion stability. Interestingly, despite not being traditional reducing agents, fructose and sucrose were also found to reduce GO [84].
Proteins and Amino Acids: Bovine serum albumin (BSA), derived from cows, is a complex amphiphilic biopolymer containing hydrophobic and hydrophilic moieties on its surface. BSA has been used not only as a reducing agent but also as a stabilizer due to its ability to adhere to solid surfaces. In a study by Lui et al., rGO was synthesized from GO using BSA. The results indicated that BSA is an effective reducing agent, likely due to the amino acid moiety present in the tyrosine residue [84]. L-cysteine was the first amino acid reported as a reducing agent for graphene oxide. rGO obtained after 72 h of reduction at 26 °C showed a 106-fold increase in conductivity compared to GO [19]. L-cysteine is known for its ability to participate in redox reactions and is also recognized for its antioxidant properties. Additionally, amino acids such as glycine, L-lysine, and gallic acid are also known to exhibit reducing properties [84].
Hormones: Besides regulating biological clocks in animals and plants, melatonin (MLT) is a potent antioxidant. Due to its electron-donating properties, it can act as a scavenger for oxygen and nitrogen derivatives. In a study by Akhavan et al., melatonin was successfully used on rGO. A significant amount of nitrogen absorption was achieved by treating GO with melatonin and ammonia at 80 °C for 3 h. The study found that the high alkalinity of the reaction mixture accelerated the reduction process by preventing the aggregation of graphene sheets [84,85].
Vitamins: L-ascorbic acid, known as vitamin C, is an essential nutrient and a powerful antioxidant in living cells. It has also been identified as a reducing agent for graphene oxide. In the study by Zhang et al., vitamin C was explored for reducing GO, highlighting its advantages. Protons from vitamin C dissociate easily to form an oxygen anion, which acts as a nucleophile in the reduction process, successfully converting GO into rGO [84]. Additionally, Fernandez-Merino et al. found that riboflavin (vitamin B2), phosphate salts, and pyridoxine (vitamin B6) were also effective in reducing graphene oxide [84].
3.3. The Macrotribological Examination of Graphene and Its Derivatives
Macrotribology is the core branch of tribology that focuses on studying larger-scale surfaces and primary interactions [86]. When large normal loads are applied to a small contact area, exceptionally high local pressures can occur, leading to increased wear and a shortened system lifespan. While extensive research has been conducted on the tribological properties of graphene at the nano- and microscales, studies on graphene’s macrotribology remain relatively scarce [87]. However, there has been a growing body of research on graphene’s macroscale tribological behavior in recent years. The relationship between changes in microstructure and tribological properties still requires further investigation to better comprehend the underlying mechanisms of graphene’s tribological performance, which may offer valuable insights into its lubrication capabilities at the macroscale [88].
Qi et al. (2017) focused on developing the macroscale tribological properties of single-layer graphene oxide (GO) coatings on metallic surfaces (316L stainless steel). The study suggested improved bonding through the silane layer, particularly APTES (3-Aminopropyltriethoxysilane), could enhance performance. Additionally, the effects of APTES treatment on the tribological behavior and durability of the GO coating were examined. The results showed that the presence of APTES significantly improved the tribological performance and durability of the GO coating. Chemical surface modification, as demonstrated in this study, may help extend the service life of graphene-based coatings on metallic substrates. This study performed wear tests on four types of samples (316L steel, 316L-A-GO, and 316L-GO). The COF for 316L-A varied significantly during the running-in phase and stabilized at 0.35 after 500 cycles, which is somewhat lower than the value ~0.45 observed for bare 316L as well as the COF for 316-GO at 0.12 for the first 100 cycles. 316L-A performed the highest wear rate, whereas 316L-GO showed medium wear. In addition, a significant further reduction in wear was observed on 316-A-GO, which is attributed to the strengthened bonding between the GO lubricant coating and the steel surface due to the covalent bonds via APTES. On the other hand, the worn 316L-A showed high roughness due to adhesive wear at the steel–steel interface [89].
Nadeem et al. (2023) investigated graphene quantum dots (GQDs) in aqueous glycerol within the boundary lubrication regime. They also examined the lubricating properties of graphene in self-mated steel contacts under reciprocating sliding motion using aqueous glycerol. Adding 0.1 wt% GQDs to the aqueous glycerol improved frictional and anti-wear performance by 72% and 53% under a contact pressure of 206 MPa. Furthermore, the study found that GQDs contributed to forming a durable tribofilm and demonstrated better deformation and crack resistance than traditional 2D graphitic materials. The enhanced tribological performance of the GQDs in boundary lubrication was attributed to the internal shearing of graphene layers within the GQDs [90].
Li et al. (2021) investigated the effect of environmental molecules on the macroscale friction behavior of graphene. This study provides a deeper understanding of the tribological properties of graphite at the macroscale and the lubricating performance of graphene in various atmospheric conditions, emphasizing how environmental molecules influence the tribological properties and microstructure of graphene at the sliding interface [88].
The results showed that graphene exhibits exceptional lubricating properties in air and nitrogen environments. The weak bonding between the graphene nanosheets and their layers also results in a low friction coefficient (0.06–0.07). The weak interaction between the graphene layers and the passivation of edge dangling bonds in nitrogen through conjugation between nitrogen molecules’ lone pair electrons and graphene’s delocalized π bonds contributes to the low macroscale friction coefficient. Moreover, nitrogen molecules were found to passivate the interaction between graphene nanosheets through conjugation [88].
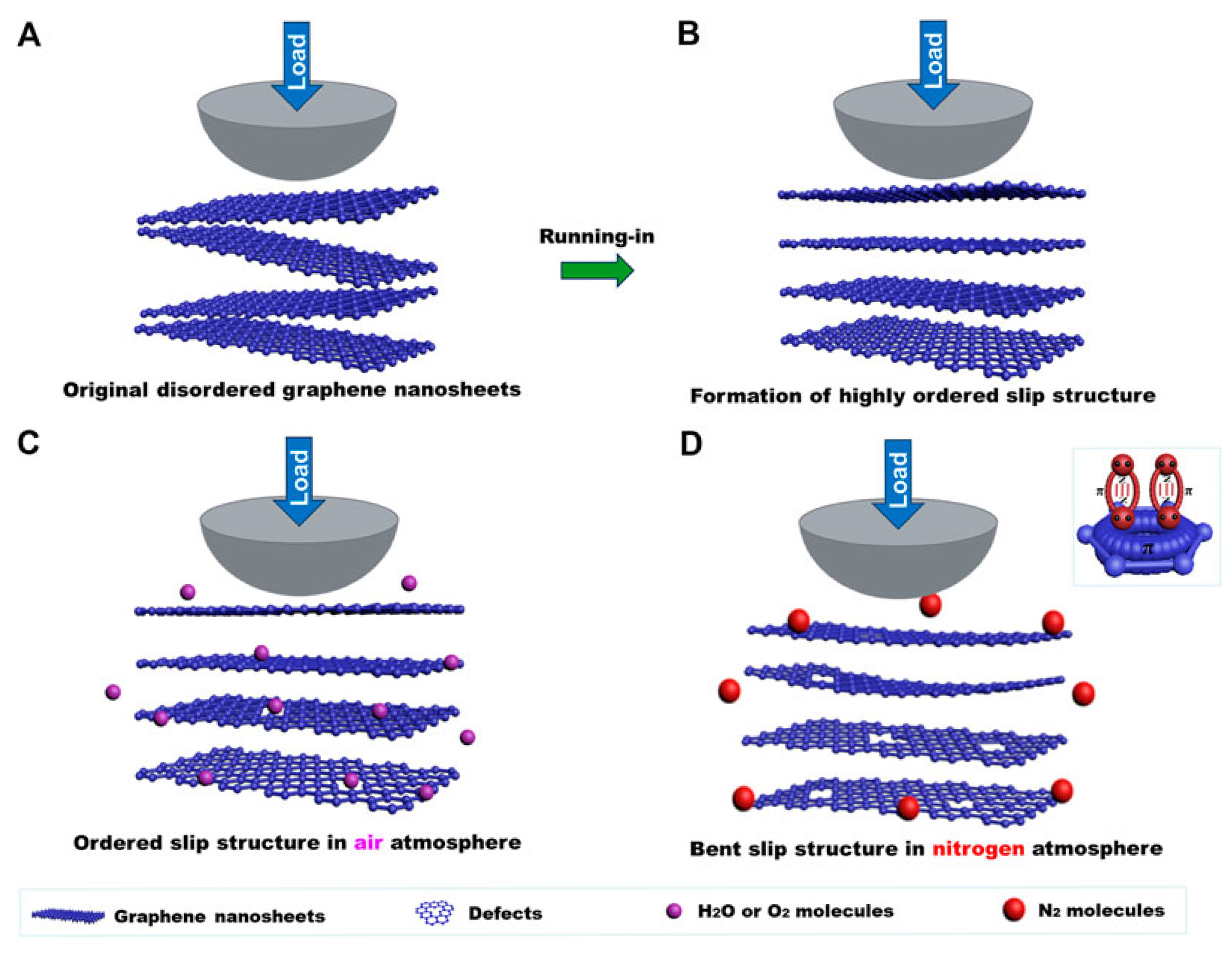
Graphene demonstrates a notable sensitivity to different atmospheric conditions during macroscale friction testing, with its coefficient of friction in air being lower than that in nitrogen. The presence of a uniform transferred and lubricating film indicates that the graphene coating maintains low friction throughout the testing process (Figure 12). Figure 12A,B show that the friction coefficient of the graphene coating is primarily influenced by its microstructure at the sliding interface. In air, a highly ordered, flat layer-by-layer slip structure forms quickly, which is essential for achieving the low friction coefficient of graphene. Moreover, the interaction between the nanosheets and their layers is weak, allowing a flat, ordered layer-by-layer slip structure to form at the sliding interface during the friction process, which leads to damage of the graphene nanosheets (Figure 12C). In addition, nitrogen molecules, with their lone pair electrons, can interact with the π orbital of graphene, passivating the dangling bonds at the graphene edges and weakening the interaction between graphene nanosheets. However, the larger diameter of nitrogen molecules compared to the d-spacing of graphene makes it difficult for them to enter the graphene layers (Figure 12D). During friction testing in nitrogen, defects may form and interact, causing the graphene nanosheets to bend. While the graphene coating still provides lubrication, the friction coefficient increases to about 0.14–0.15, higher than in air [88].
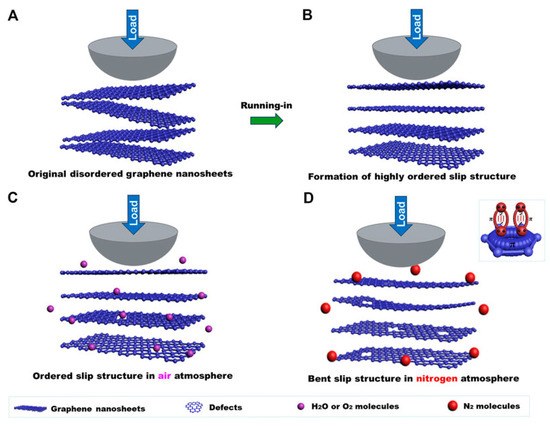
Figure 12.
Macroscale lubricating mechanism of graphene in an atmospheric environment (A) the original disordered graphene nanosheets of its coating, (B) the ordered slip structure formed after a short time in the air atmosphere, (C) the flat ordered layer-by-layer slip structure in the air after friction, (D) the bent graphene nanosheets after friction in nitrogen.
3.4. The Microtribological Examination of Graphene and Its Derivatives
Microtribology is a key area of tribology that focuses on studying friction and wear phenomena at the micrometer scale [91]. Contact area, load, and contact shape significantly influence microtribology, affecting friction, adhesion, and surface tension properties [92]. Additionally, adhesive forces significantly impact friction behavior at low contact stresses and small contact sizes. These adhesive forces are primarily driven by van der Waals interactions and capillary effects between the counter faces [93].
Graphene is often regarded as a solid lubricant in tribology at the microscale and above due to its ability to reduce the coefficient of friction between mating surfaces [55]. In contrast, graphene derivatives have gained significant attention in the scientific community. Graphene oxide (GO), with its nanoscale thickness derived from graphene nanosheets, has attracted considerable interest for its exceptional tribological, anticorrosion, and biocompatibility properties at the micro/nanoscale [94].
White et al. (2018) investigated the microtribological behavior of graphene composites doped with Mo and W under boundary lubrication. In this study, graphene oxide was synthesized using the Modified Hummers method, and graphene was synthesized through a thermal process in a reactor to incorporate Mo and W nanoparticles into the graphene. The results showed that the nanomaterials containing graphene exhibited approximately a 25% reduction in friction compared to the base oil. However, its low load-bearing capacity at high contact pressures limited graphene’s anti-wear properties. As a result, the composites reduced the average wear depth by 30% and 14%, respectively, compared to the base oil and the oil containing only graphene, with Mo2C/G [95].
Hu et al. (2015) studied the microtribological properties of graphene oxide (GO) and graphene films. Hydrazine hydrate was used in the liquid phase to reduce the as-prepared GO films and create graphene films. Various characterization techniques, including X-ray photoelectron spectroscopy, Raman spectroscopy, X-ray diffraction, UV–vis absorption spectroscopy, water contact angle measurement, and atomic force microscopy, were employed to analyze the microstructures and microtribological characteristics of the samples. According to XRD analysis (Figure 13), the XRD pattern of GO shows a diffraction peak at 12.1°, corresponding to water-bound intercalated oxidized graphite. In contrast, the XRD pattern of RGO exhibits diffraction peaks at 24.5° and 42.8°. According to Raman spectroscopy analysis (Figure 14), the Raman spectra of GO and rGO show two peaks at 1328 cm−1 (D band) and 1595 cm−1 (G band). The G band is associated with sp2-hybridized carbon vibrations, while the D band corresponds to defects like vacancies and disorder [96].
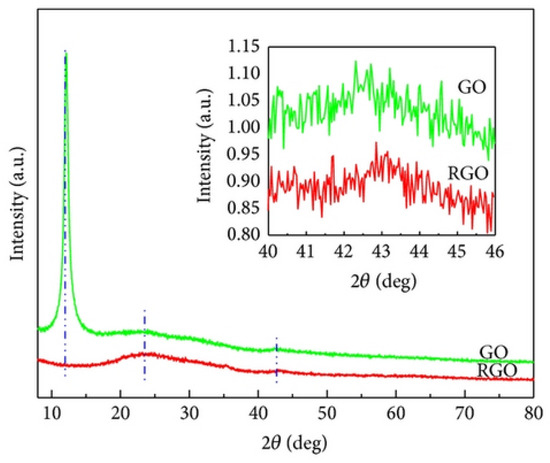
Figure 13.
XRD spectrum of the GO and rGO.
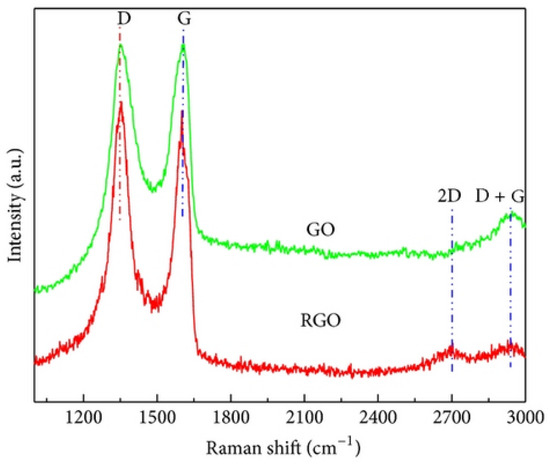
Figure 14.
Raman spectrum of the GO and rGO.
This study identifies several forces contributing to the adhesive interaction between an AFM tip and a sample surface, including capillary force (Fc), solid–solid contact, van der Waals force (FvdW), electrostatic force (FE), and chemical bonding force (FB). Furthermore, due to the oxidation of graphite, the surface of graphene oxide (GO) contains more oxygen functional groups than reduced graphene oxide (rGO), leading to stronger van der Waals forces between the edges of the GO sheets and the AFM tip. These differences in adhesion force between GO and rGO can be attributed to the variation in the number of surface functional groups [96].
Wang et al. (2019) investigated the microtribological properties of Ti-6Al-4V alloy coated with GO and self-assembled dopamine, aiming to enhance anticorrosion and tribological performance for biomedical micro/nano-electromechanical applications under both dry and simulated body fluid (SBF) lubrication conditions. The study found that the GO-coated sample with a dopamine (DA) adhesive layer (APTES-DA-GO) demonstrated superior properties compared to the APTES-GO coating, which utilized a 3-aminopropyltriethoxysilane (APTES) transition layer. Various analytical techniques were employed, including adhesion testing with an automatic scratch tester, electrochemical corrosion analysis using an electrochemical workstation, and microtribological evaluation through a ball-on-plate method. Characterization was further performed using XPS, Raman spectroscopy, and SEM [94].
The results indicated that APTES-DA-GO exhibited superior anticorrosion and microtribological properties compared to APTES-GO. When compared to the uncoated Ti-6Al-4V alloy, both GO-coated samples (APTES-GO and APTES-DA-GO) showed improved anti-wear properties and lower coefficients of friction (COFs) under both dry and simulated body fluid (SBF) lubrication conditions, as demonstrated by the microtribological performance data [94].
3.5. The Nanotribological Examination of Graphene and Its Derivatives
Nanotribology is a critical field within tribology that focuses on friction, wear, and lubrication at the atomic and molecular scale. In this context, nano lubricants must possess various properties, from controlling friction at varying speeds to ensuring long-lasting performance [97]. Conversely, macroscopic evaluations of friction between large objects often pose challenges in understanding the underlying mechanisms of the system [98]. This highlights the importance of studying nanotribology.
Carbon-based materials significantly influence tribological performance, particularly through the distribution of carbon hybridization, atomic hydrogen content, and additives such as Si, F, N, B, and O. These factors can play a crucial role in surface energy, mechanical properties, and electrical conductivity [99]. C60, a carbon allotrope, reduces friction due to weak van der Waals forces. Previous studies have shown that the coefficient of friction (COF) is affected by various parameters, including the environment, surface properties, and applied force. Another notable carbon-based material is carbon nanotubes (CNTs), which exhibit exceptional properties like high tensile strength and elastic modulus [97].
According to a study, the tribological properties of OLC were investigated in a synthetic lubricant oil and compared with those of C60, kish graphite (KG), and CNTs. The friction coefficient of PAO was evaluated with varying concentrations of OLC under contact pressures of 0.51, 0.70, and 0.94 GPa, showing an initial decrease followed by an increase as the additive concentration rose. This indicates that the addition of OLC effectively reduces the friction coefficient up to an optimal concentration, beyond which the effect diminishes. The friction coefficients of PAO (PAO400, PAO30, and PAO02) containing 0.1 wt.% of CNT, C60, and KG additives were evaluated. A concentration of just 0.005 wt.% OLC was enough to lower the friction coefficient of PAO at a contact pressure of 0.70 GPa. For example, it was detected that the COF for PAO400, CNT, OLC, C60, and KG under a 0.70 GPa contact pressure (0.1 wt%) was approximately 0.064, 0.072, 0.063, 0.062, and 0.07, respectively [97].
Graphene, a carbon-based material, exhibits nanotribological properties influenced by its production methods [100]. Due to its lubricious characteristics, graphene is valuable in various applications, including contact-based microelectromechanical systems, sensing devices, and storage [101]. Although GO and rGO possess structural defects, they are still widely used in water filtration membranes, anti-corrosion coatings, lubrication additives, and tribological coatings [102]. Additionally, chemical functionalization alters the surface composition of graphene, which can inhibit the beneficial layer-to-layer interactions between carbon atoms, resulting in increased friction. However, studies show that graphene and graphene oxide, when used as lubricants, effectively reduce friction [55].
Sierros et al. (2014) investigated the adhesive properties of nanoscale graphene, doped SiO2 monolayers, and multilayer graphene synthesized using the mechanical exfoliation method. Regardless of the material type and AFM tip radius, doped graphene exhibited significantly lower friction than the bare SiO2 surface. Furthermore, the doped graphene structures demonstrated the highest pull-off forces, highlighting the combined effects of van der Waals forces and in-plane elasticity between the AFM tip, sample surface, and underlying substrate [101].
In this study, lateral force microscopy friction measurements were used on both T1 (top-down) and T2 (bottom-up). The T1 surfaces, obtained through liquid exfoliation, show a coefficient of friction (COF) of approximately 0.03, while the COF of the T2 surfaces, grown from the bottom-up, is around 0.04. The slightly higher COF observed for T2 can be attributed to its relatively greater concentration of defects than the T1 surface. The shearing force exerted by the sliding tip on these defects results in an increased COF [101].
Furthermore, adhesion measurements from the center to the edge of the FLG are shown to decrease from around 24 to 22 nN, except at point g, where the adhesion is increased above 25 nN due to interaction with the edge. This behavior is believed to be attributed to the varying density of atoms, with a more consistent tip/surface interaction provided by the central region. Stronger short-range forces are exerted on the tip by the higher density of atoms in the center, while weaker adhesion is observed at the edges, influenced by defects and disorder [101].
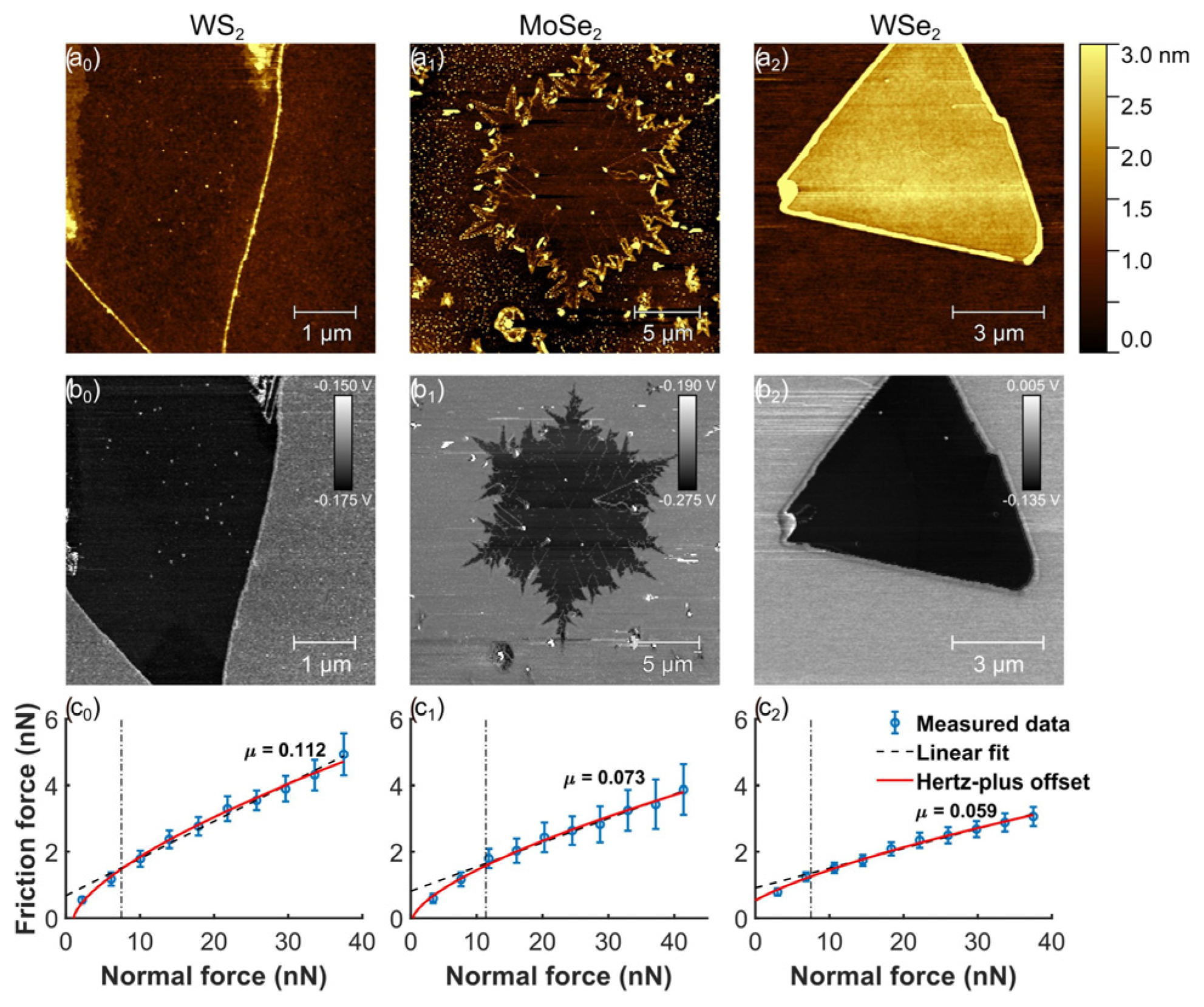
Rapuc et al. (2021) investigated graphene synthesized via the chemical vapor deposition method to examine how chemical composition and sliding speed affect the nanoscale friction of monolayers. The study used transition metal dichalcogenide (TMD) monolayers, including WS2, MoSe2, and WSe2. Various analytical techniques were employed, such as atomic force microscopy (AFM) for nanoscale friction analysis, Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) to characterize the monolayers. The frictional behavior of each sample was evaluated by measuring its load-dependent friction at different sliding speeds, with the lowest friction observed for the WSe2 monolayer [102].
The surface topography of the selected flakes was acquired immediately before conducting each friction experiment (Figure 15). Triangular crystals were observed for WS₂ and WSe₂, suggesting that crystal growth was well-controlled. In contrast, MoSe₂ exhibited a snowflake-like morphology. Nonetheless, triangular grain boundaries were identified within the MoSe₂ flake, indicating that the individual grains were originally formed in a triangular shape. In addition, A high friction contrast between the flakes and the SiO₂ substrate is revealed in the friction maps. A significantly lower friction was observed for all the analyzed TMD flakes in comparison to the substrate, highlighting their superior frictional properties. This suggests that coating a single surface in MEMS/NEMS contacts with a TMD monolayer could lead to a considerable enhancement in performance. Stick-slip motion was not observed in any of the samples. The measurements remained consistent across three different WS₂ and WSe₂ flakes, as well as two separate locations on the MoSe₂ flake. Significant differences were not observed between the loading and unloading curves for any of the samples, which is expected for an elastic contact where the layer adheres well to the substrate [102].
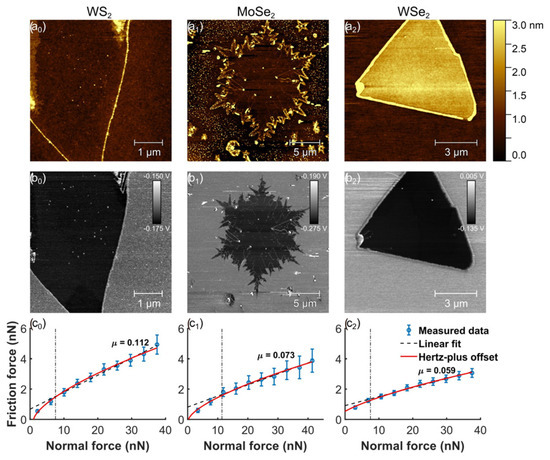
Figure 15.
(a0–a2) Surface characterization of the examined monolayer WS2, MoSe2, and WSe2 flakes includes topography, (b0–b2) corresponding friction maps, and (c0–c2) load-dependent friction measurements.
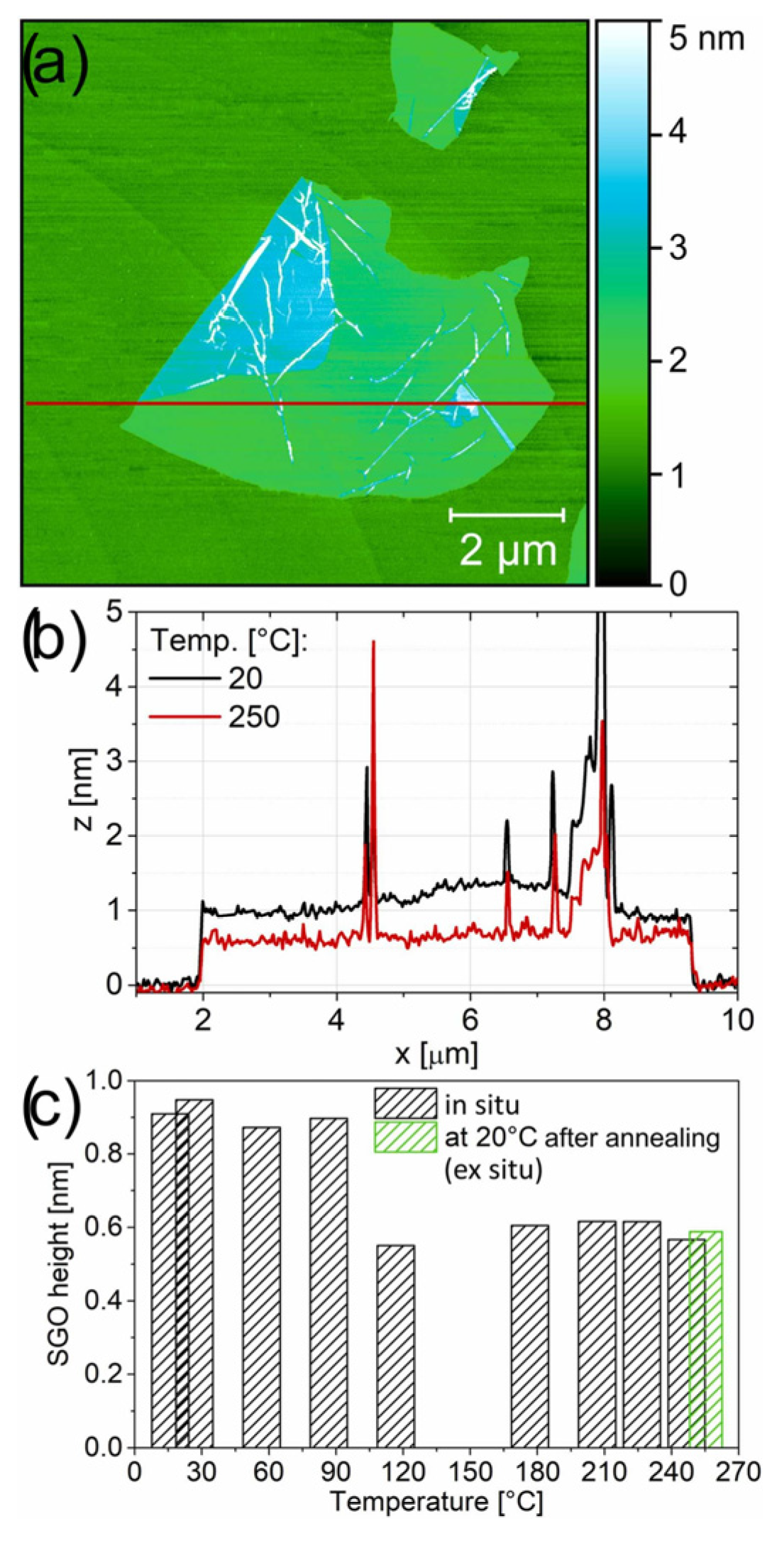
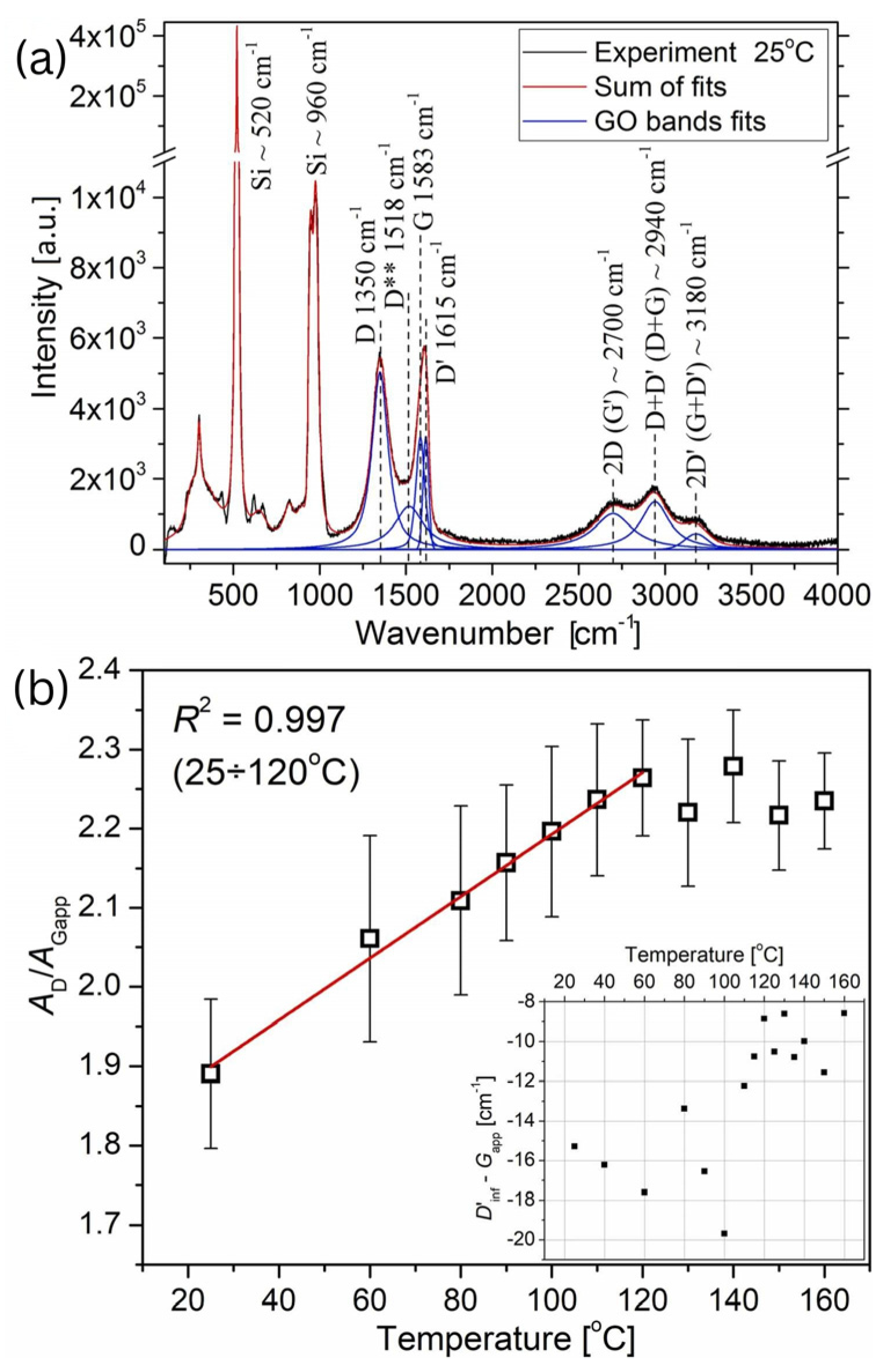
Weiss et al. (2025) investigated the tribological and conductive properties of single-layer graphene oxide (GO). GO was synthesized using the Modified Hummers method and analyzed through atomic force microscopy (AFM), Fourier-transform infrared (FTIR), and Raman spectroscopy. The study identified the temperature threshold at which structural and morphological changes occur, finding that the conversion of GO’s properties, including conductivity, adhesion, and friction, takes place at 130 °C. By controlling the thermal reduction process of GO, one can influence its adhesion, friction, and electrical conductivity, which can enhance the performance of materials in various applications, including MEMS devices [64].
In this study, the changes in the morphology of a single-layer flake on the mica were analyzed (Figure 16). During the heating process, the in situ topography of the same GO flake (Figure 16a,b) was captured. AFM images were obtained at temperature intervals of 30 °C. The flake’s shape and lateral dimensions remained unchanged. On the other hand, a notable reduction in height was observed in the SGO flake between 90 °C and 120 °C (Figure 16c). The height of the pristine SGO before the transition was measured to be 0.9 nm, which is in good agreement with the height of GO under dry conditions. At temperatures above 120 °C was about 0.6 nm. Additionally, the lowest COF values are observed for untreated GO (0.210 at 1 µm/s and 0.158 at 100 µm/s), while a general increase is noted in samples reduced at temperatures between 60 and 150 °C. For rGO prepared at 150 °C, the COF nearly doubles (0.407 and 0.357) compared to the untreated GO in argon gas conditions [64].
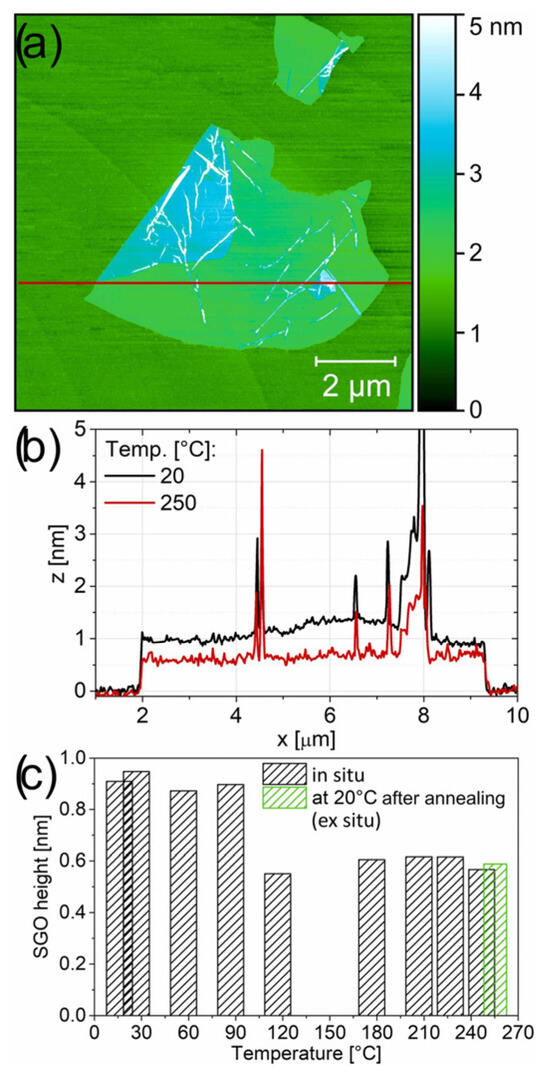
Figure 16.
(a) AFM topography of an SGO flake deposited on freshly cleaved mica; (b) corresponding cross-sectional profile through the flake region, captured at 20 °C and during annealing at 250 °C; (c) the variation in SGO height as a function of reduction (annealing) temperature is shown, with the green bar indicating the measurement taken post-annealing at 20 °C.
According to the Raman spectroscopy analysis (Figure 17), it was observed that the peaks at 1350 cm−1 and 1583 cm−1 correspond to the D and G bands, respectively. Furthermore, the band at 1615 cm−1, referred to as the D’ band, lies beside the G peak and is responsible for broadening the G (Gapp) peak and shifting its position to higher wavenumbers. The peak at 1518 cm−1, D** band, is linked to C–H vibrations in hydrogenated carbon or phonon density states in finite-sized graphitic crystals. The D* band was not detected in this study. Another set of bands between 2500 and 3300 cm−1 includes three peaks: the 2700 cm−1 peak, attributed to the 2D (G’) Raman mode; the 2940 cm−1 peak, assigned to either the D+D’ or D+G mode; and the 3180 cm−1 peak, associated with either the 2D’ or G+D’ mode [64].
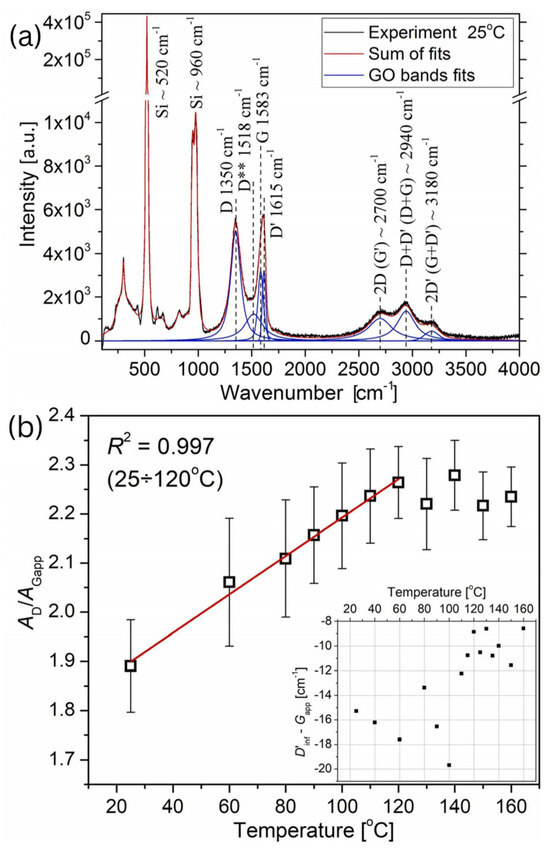
Figure 17.
(a) Raman spectrum of the SGO flake at 25 °C, (b) the temperature-dependent difference between the half-energy of the 2D’ (D’inf) and Gapp modes during the annealing process.
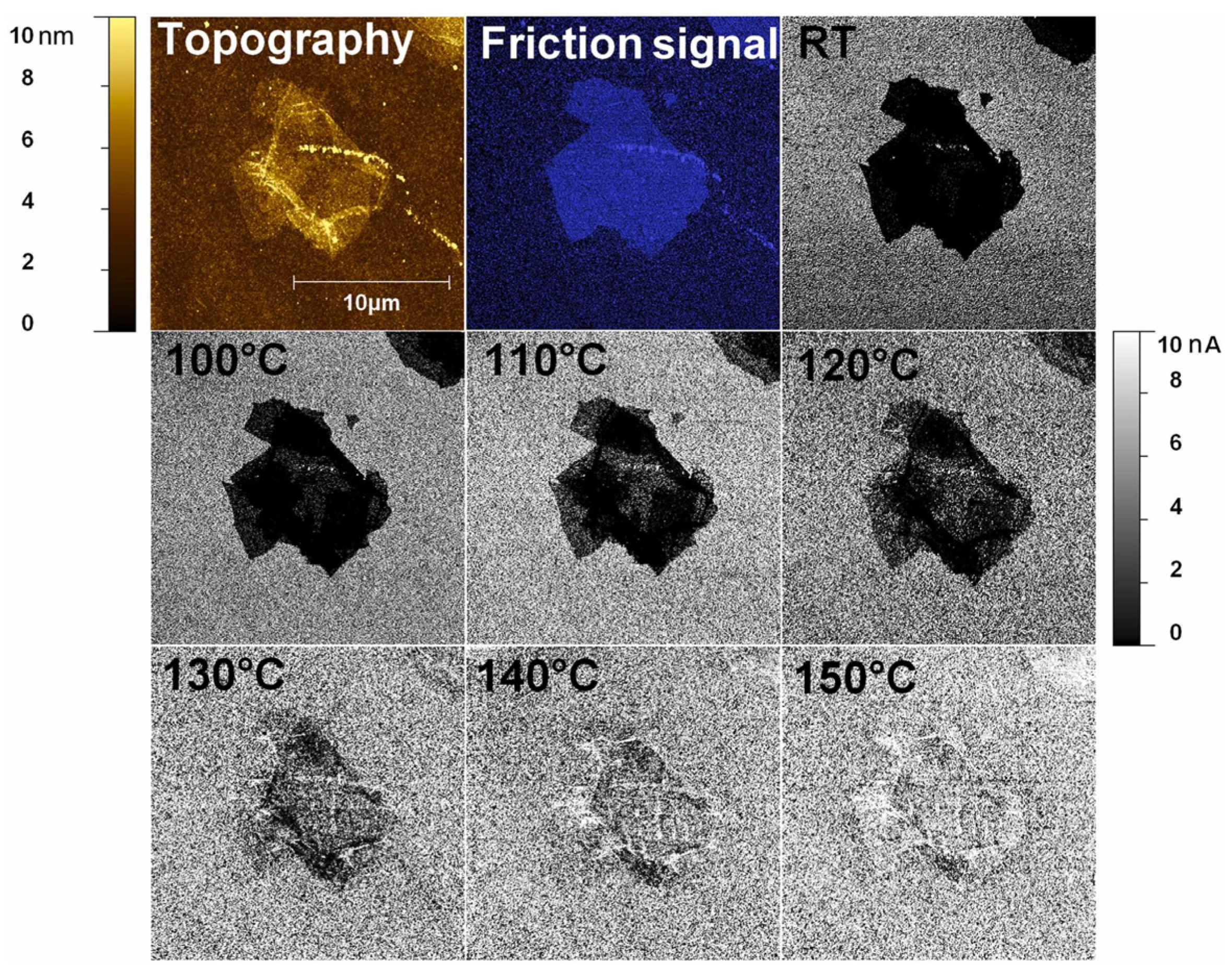
It is widely recognized that the reduction of GO results in the transformation from the insulating sp3 carbon phase to the conductive sp2 network. It was stated that the initial sp2 conductive network can grow and connect during the reduction process, leading to the formation of a lateral conductive network that exceeds the percolation threshold. The vertical local conductivity of rGO depends on the reduction state. Figure 18 shows C-AFM images at room temperature, as well as thermal reduction in temperatures incremented from 100 °C to 150 °C with a step of 10 °C. Thermal reduction between 100 °C and 120 °C develops vertical conductivity in regions of a single-layer flat GO flake. At 130 °C, conductivity begins to increase across the entire flake, completing at 150 °C. An annealing temperature of 150 °C leads to the formation of conductive clusters that are large enough to dominate electrical transport through percolating paths. This suggests that the desorption of H₂O from GO begins at an annealing temperature of 70 °C, which is attributed to the removal of water from the GO surface and interlayered spacing [64].
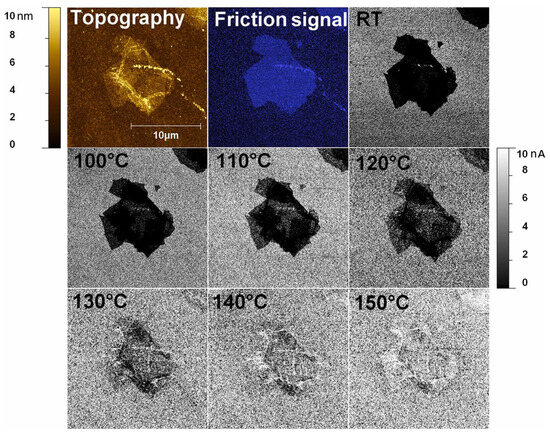
Figure 18.
Ex situ C-AFM vertical conductivity maps at room temperature (RT) between 100 and 150 °C, along with the topographical and friction contrast images of the SGO flake.
4. Conclusions
- Graphene and its derivatives offer exceptional specific tribological properties, including low friction coefficients and self-lubricating capabilities, making them ideal additives for complex lubrication systems. For example, graphene’s mechanical stability renders it an excellent material for preventing surface degradation, while the weak van der Waals forces between graphene sheets facilitate smooth sliding over surfaces. Additionally, the one-atom-thick, two-dimensional carbon lattice of graphene enables the formation of highly stable, ultra-thin lubrication layers at the interface between moving surfaces. Furthermore, graphene’s high surface area significantly contributes to its exceptional wear resistance. These materials, therefore, exhibit both anti-wear and low-friction properties, which further enhance their performance.
- Graphene-based materials are applicable in various industries, such as automotive, aerospace, and electronics, and are a promising material in energy-efficient systems, self-healing coatings, adaptive lubrication systems, and wearable biomedical devices. The study of graphene family materials in tribology gains valuable insight into how these materials can be engineered and utilized for the application areas, ultimately contributing to more efficient, durable, eco-friendly, and sustainable next-generation systems. Additionally, graphene derivatives (GO and rGO) offer substantial potential in tribological applications, which have unique properties such as low friction, high wear resistance, and tunable surface chemistry. These features make them ideal candidates for developing the performance and sustainability of modern tribological systems.
- Despite graphene’s significant role in tribology, further research is needed to gain a deeper understanding. Therefore, graphene and its derivatives require further development regarding scalability and cost-effectiveness, integration with other materials, long-term performance and stability, and eco-friendly synthesis methods. On the other hand, graphene derivatives require further evaluation concerning synthesis methods using green reductants, and comprehending how these approaches may affect systems at various scales (macro, micro, nano) and across different application fields, such as the development of eco-friendly lubricants, advanced coatings, and composite materials.
- Gaining insights into how these materials perform under small-scale contacts, particularly regarding adhesion and friction, will be crucial for developing new materials and creating innovative parts and systems for practical applications. For example, understanding the hydrophilic nature of graphene oxide and the hydrophobic nature of reduced graphene oxide, as well as the in-depth examination of the functional groups within their structures from a tribological perspective, could open the door to new applications. The functional groups in the structure of GO and rGO should be examined with different surfaces, such as metals, polymers, and ceramics. Furthermore, exploring graphene oxide (GO) and reduced graphene oxide (rGO) under varying parameters, concentrations, and modifications is essential to understanding their tribological behavior across macroscale, microscale, and nanoscale systems.
Author Contributions
Conceptualization, Ç.G.A. and A.Ž.; methodology, Ç.G.A.; software, Ç.G.A.; validation, Ç.G.A. and A.Ž.; formal analysis, Ç.G.A.; investigation, Ç.G.A.; resources, Ç.G.A. and A.Ž.; data curation, Ç.G.A. and A.Ž.; writing—original draft preparation, Ç.G.A.; writing—review and editing, Ç.G.A. and A.Ž.; visualization, Ç.G.A. and A.Ž.; supervision, A.Ž.; project administration, A.Ž. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin, B.; Chen, G.; He, Y.; Zhang, C.; Luo, J. Lubrication properties of graphene under harsh working conditions. Mater. Adv. 2023, 18, 100369. [Google Scholar] [CrossRef]
- Eregie, S.B.; Sanusi, I.A.; Olaniran, A.O. Bibliometric review of transcriptomic microalgae-based biodegradation of lubricant oil waste hydrocarbon: Current research outlook. Bioresour. Technol. Rep. 2025, 29, 102074. [Google Scholar] [CrossRef]
- Wang, K.; Qi, C.; Li, Y.; Zhang, D.; Sun, H.; Wan, Y. Macroscopic superlubricity achieved by water-based lubricant with green lycium barbarum extract as additives. J. Mol. Liq. 2025, 427, 127414. [Google Scholar] [CrossRef]
- Korkmaz, M.E.; Gupta, M.K. Nano lubricants in machining and tribology applications: A state of the art review on challenges and future trend. J. Mol. Liq. 2024, 407, 125261. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Thien, G.S.H.; Shahid, M.; Lind, T.Y.; Hashem, A.; Chan, K.; Johan, M.R. Graphene as a lubricant additive for reducing friction and wear in its liquid-based form. Lubricants 2023, 11, 29. [Google Scholar] [CrossRef]
- Wagh, V.P.; Saboo, N.; Gupta, A. Tribology as emerging science for warm mix technology: A review. Constr. Build. Mater. 2022, 359, 129445. [Google Scholar] [CrossRef]
- Sun, J.; Du, S. Application of graphene derivatives and their nanocomposites in tribology and lubrication: A review. RSC Adv. 2019, 9, 40642–40661. [Google Scholar] [CrossRef]
- Jafari, A.; Majdoub, M.; Sengottuvelu, D.; Ucak-Astarlioglu, M.G.; Al-Ostaz, A.; Nouranian, S. Tribological properties of synthetic and biosourced lubricants enhanced by graphene and its derivatives: A review. ACS Omega 2024, 9, 50868–50893. [Google Scholar] [CrossRef]
- Tomanik, E.; Christinelli, W.; Souza, R.M.; Oliveira, V.L.; Ferreira, F.; Zhmud, B. Review of graphene-based materials for tribological engineering applications. Eng 2023, 4, 2764–2811. [Google Scholar] [CrossRef]
- Mariño, F.; Río, J.M.L.; López, E.E.; Fernández, J. Chemically modified nanomaterials as lubricant additive: Time stability, friction, and wear. J. Mol. Liq. 2023, 382, 121913. [Google Scholar] [CrossRef]
- Nugroho, A.; Kozin, M.; Mamat, R.; Bo, Z.; Ghazali, M.F.; Kâmil, M.P.; Puranto, P.; Fitriani, D.A.; Azahra, S.A.; Suwondo, K.P.; et al. Enhancing tribological performance of electric vehicle lubricants: Nanoparticle-enriched palm oil. Heliyon 2024, 10, e39742. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, X.; Chang, C. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2, 276143. [Google Scholar] [CrossRef]
- Jankovský, O.; Marvan, P.; Nováček, M.; Luxa, J.; Mazánek, V.; Klímová, K.; Sedmidubský, D.; Sofer, Z. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl. Mater. Today 2016, 4, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Jin, Y.; Chen, G.; Zhang, X. Microwave-assisted solvothermal synthesis of sulfur-doped graphene for electrochemical sensing. J. Electroanal. Chem. 2015, 739, 172–177. [Google Scholar] [CrossRef]
- Viana, M.M.; Lima, M.C.F.S.; Forsythe, J.C.; Gangoli, V.S.; Cho, M.; Cheng, Y.; Silva, G.G.; Wong, M.S.; Caliman, V. Facile graphene oxide preparation by microwave-assisted acid method. J. Braz. Chem. Soc. 2015, 26, 978–984. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Topçu, A.A. A Green Pathway for the Production of Chemically Exfoliated Graphene Sheets with the Assistance of Microwave Irradiation. Master’s Thesis, Koç University, Istanbul, Turkey, 2012. [Google Scholar]
- Kozal, B. Karbon Tabanlı Petek Örgülerin Elektronik Özellikleri. Ph.D. Thesis, Ankara University, Ankara, Turkey, 2014. [Google Scholar]
- Spyrou, K.; Rudolf, P. An Introduction to Graphene; Wiley-VCH: Weinheim, Germany; Hoboken, NJ, USA, 2014; pp. 1–18. [Google Scholar]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Er, E. Grafen Oksitin Sülfürik ve Fosforik Asit Varlığında Indirgenmesi ve Elektroanalitik Uygulamalarının Araştırılması. Master’s Thesis, Gazi University, Ankara, Turkey, 2013. [Google Scholar]
- Dalkılıç, Z. Dikey Karbon Nanotüp Üretimi ve Çeşitli Uygulamalar Için Yüzey Özelliklerinin Incelenmesi. Master’s Thesis, Teknik University, Istanbul, Turkey, 2014. [Google Scholar]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Akın, D. Glukoza Duyarlı Grafen Esaslı Yeni Bir Biyosensör Hazırlanması. Master’s Thesis, Gazi University, Ankara, Turkey, 2017. [Google Scholar]
- Tiyek, İ.; Dönmez, U.; Yıldırım, B.; Alma, M.; Ersoy, M.S.; Karataş, Ş.; Yazıcı, M. Kimyasal yöntem ile indirgenmiş grafen oksit sentezi ve karakterizasyonu. Sak. Univ. J. Sci. 2016, 20, 349–357. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Gao, X. Synthesis, Characterization, Chemical Reduction and Biological Application of Graphene Oxide. Master’s Thesis, University of Waterloo, Waterloo, Canada, 2013. [Google Scholar]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Bedeloğlu, A.; Taş, M. Grafen ve grafen üretim yöntemleri. Afyon Kocatepe Univ. J. Sci. Eng. 2016, 16, 544–554. [Google Scholar]
- Özcan, Ş. Kimyasal Yöntemlerle Grafen ve Grafen Oksit Üretimi ve Li-Hava Pil Uygulamaları. Master’s Thesis, Sakarya University, Sakarya, Turkey, 2015. [Google Scholar]
- Warner, J.H.; Schaffel, F.; Rummeli, M.H.; Bachmatiuk, A. Graphene: Fundamentals and Emergent Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Khenfouch, M.; Buttner, U.; Baitoul, M.; Maaza, M. Synthesis and characterization of mass produced high quality few layered graphene sheets via a chemical method. Graphene 2014, 3, 7–13. [Google Scholar] [CrossRef]
- Lalire, T.; Longuet, C.; Taguet, A. Electrical properties of graphene/multiphase polymer nanocomposites: A review. Carbon 2014, 225, 119055. [Google Scholar] [CrossRef]
- Elibol, K. Grafit Soyma ve Epitaksiyel Yöntemlerle Elde Edilmiş Grafenlerde Manyetoiletim ve Yüzey Özellikleri Incelemeleri. Master’s Thesis, Gazi University, Ankara, Turkey, 2012. [Google Scholar]
- Babayiğit, M. Polikristalin Bakır Folyo Üzerinde Büyütülmüş Grafenin Elektriksel Karakterizasyonu. Master’s Thesis, Hacettepe University, Ankara, Turkey, 2013. [Google Scholar]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of raphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Wang, Y.; Zhou, J.; Song, L. Separating nano graphene oxide from the residual strong-acid filtrate of the modified hummers method with alkaline solution. Appl. Surf. Sci. 2015, 329, 83–86. [Google Scholar] [CrossRef]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- Musa, N.; Halim, N.F.A.; Ahmad, M.N.; Zakaria, Z.; Hashim, U. Electrical characterization of reduced graphene oxide (rGO) on organic thin film transistor (OTFT). In Proceedings of the 11th Asian Conference on Chemical Sensors, Penang, Malaysia, 13 March 2017. [Google Scholar]
- Zhu, Y.; James, D.K.; Tour, J.M. New routes to graphene, graphene oxide and their related applications. Adv. Mater. 2012, 24, 4924–4955. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Aziz, M.; İsmail, A.F.; Rahman, M.A.; Othman, M.H.D. Synthesis of graphene oxide nanosheets via modified hummers’ method and its physicochemical properties. J. Teknol. 2015, 74, 195–198. [Google Scholar] [CrossRef]
- Kayhan, E. Graphene: Synthesis, Characterization, Properties and Functional Behavior as Catalyst Support and Gas Sensor. Master’s Thesis, Technical University of Darmstadt, Darmstadt, Germany, 2013. [Google Scholar]
- Yazıcı, M.; Tiyek, İ.; Ersoy, S.M.; Alma, H.M.; Dönmez, U.; Yıldırım, B.; Salan, T.; Karataş, Ş.; Uruş, S.; Karteri, İ.; et al. Modifiye Hummers yöntemiyle grafen oksit sentezi ve karakterizasyonu. Gazi Univ. J. Science. 2016, 4, 41–48. [Google Scholar]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, Q.; Wang, J. A simple and fast microwave assisted approach for the reduction of graphene oxide. Ceram. Int. 2016, 42, 3007–3013. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Abdelsayed, V.; Khder, S.A.E.R.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Sharma, B.; Shekhar, S.; Malik, P.; Jain, P. Study of mechanism involved in synthesis of graphene oxide and reduced graphene oxide from graphene nanoplatelets. Mater. Res. Express 2018, 5, 065012. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Aroor, G.; Khan, M.A.; Shetty, A.R.; Rai, R.; Ganesha, H.; Navada, M.K. From chemistry to performance: How nano additives are transforming bio-lubricants for enhanced tribological applications. J. Mol. Liq. 2025, 425, 127242. [Google Scholar] [CrossRef]
- Berman, D.; Farfan-Cabrera, L.I.; Rosenkranz, A.; Erdemir, A. Advancing the frontiers of EV tribology with 2D materials—A critical perspective. Mater. Sci. Eng. R Rep. 2024, 161, 100855. [Google Scholar] [CrossRef]
- Venturi, F. Carbon-based films: A review on mechanical and tribology properties. Mater. Chem. Phys. 2024, 325, 129716. [Google Scholar] [CrossRef]
- Baiocco, G.; Salvi, D.; Ucciardello, N. Optimizing graphene nanoplatelet coating for enhanced wear resistance on copper through electrophoretic deposition parameters. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Mingione, E.; Salvi, D.; Almonti, D.; Ponticelli, G.S. Improvement of thermal, electrical, and tribological performances of GnPs composites produced by selective laser sintering. Polym. Compos. 2025. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Al-Janabi, A.S.; Wadzer, Y.; Mamat, H. Review of tribological properties of nanoparticle-based lubricants and their hybrids and composites. Friction 2024, 12, 569–590. [Google Scholar] [CrossRef]
- Kaleli, H.; Demirtaş, S.; Uysal, S.; Karnis, I.; Stylianakis, M.M.; Anastasiadis, S.H.; Kim, D.-E. Tribological performance investigation of a commercial engine oil incorporating reduced graphene oxide as additive. Nanomaterials 2021, 11, 386. [Google Scholar] [CrossRef]
- Kumar, S.S.A.; Bashir, S.; Ramesh, K.; Ramesh, S. A comprehensive review: Super hydrophobic graphene nanocomposite coatings for underwater and wet applications to enhance corrosion resistance. FlatChem 2022, 31, 100326. [Google Scholar] [CrossRef]
- Genna, S.; Salvi, D.; Ucciardello, N. Copper-graphene coatings for improving thermal shielding of CFRPs through electrodeposition techniques. Polym. Compos. 2024, 46, 2030–2046. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Graphene-family lubricant additives: Recent developments and future perspectives. Lubricants 2022, 10, 215. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, C.; Fan, X.; Dienwiebel, M.; Wang, S.; Li, Y. Enhancement of polyurea grease performance through graphene oxide-functionalized polyurea thickeners: A novel hydrogen-bond network approach. Tribol. Int. 2025, 201, 110123. [Google Scholar] [CrossRef]
- Weiss, M.; Majchrzycki, L.; Skonieczny, R.; Florjan, D.; Ptak, A. Influence of the thermal reduction process on the tribological and conductive properties of single-layer graphene oxide. Tribol. Int. 2025, 201, 110203. [Google Scholar] [CrossRef]
- Cai, Z.-B.; Zhao, L.; Zhang, X.; Yue, W.; Zhu, M.-H. Combined effect of textured patterns and graphene flake additives on tribological behavior under boundary lubrication. PLoS ONE 2016, 11, 0152143. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Friction and wear behaviour of hypereutectic Al-Si alloy/steel tribopair under dry and lubricated conditions. J. Tribol. 2017, 15, 21–49. [Google Scholar]
- Zhou, Q.; Huang, J.; Wang, J.; Yang, Z.; Liu, S.; Wang, Z.; Yang, S. Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive. RSC Adv. 2015, 5, 91802–91812. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L.; Li, W. Multilayer graphene as a lubricating additive in bentone grease. Tribol. Lett. 2014, 55, 455–464. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Z.; Zhao, H.; Yang, H.; Li, X.; Ni, J. Effect of the grease thickener on tribological properties of Si3N4/GCr15 contact interface and the performance in hybrid ceramic ball bearing. Ceram. Int. 2023, 49, 16857–16867. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Zhu, M. Amine-terminated ionic liquid modified graphene oxide/copper nanocomposite toward efficient lubrication. Appl. Surf. Sci. 2019, 491, 105–115. [Google Scholar] [CrossRef]
- Uniyal, P.; Gaur, P.; Yadav, J.; Khan, T.; Ahmed, O.S. A Review on the effect of metal oxide nanoparticles on tribological properties of biolubricants. ACS Publ. 2024, 9, 12436–12456. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Kalam, M.A.; Mujtaba, M.A.; Almomani, F. A review of recent advances in the synthesis of environmentally friendly, sustainable, and nontoxic bio-lubricants: Recommendations for the future implementations. Environ. Technol. Innov. 2023, 32, 103366. [Google Scholar] [CrossRef]
- Rahmani, A.; Ravazi, H.K.; Dehghani-Soufi, M. Green tribology assessment: A Comprehensive review of bio-lubricants and nano enhancers. Energy Convers. Manag. X 2024, 24, 9. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, B.; Xiong, C.; Liu, Y.; Zhao, Q. Study on the lubricating characteristics of graphene lubricants. Lubricants 2023, 11, 506. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, Z.; Li, D.; Wang, L.; Lu, Z.; Zhang, G. An investigation on the tribological properties of graphene and ZDDP as additives in PAO4 oil. Diam. Relat. Mater. 2021, 120, 108635. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.; Li, W.; Fan, M. Two-dimension layered nanomaterial as lubricant additives: Covalent organic frameworks beyond oxide graphene and reduced oxide graphene. Tribol. Int. 2020, 143, 106051. [Google Scholar] [CrossRef]
- Fuadi, Z.; Rahmadiawan, D.; Kurniawan, R.; Mulana, F.; Abral, H.; Nasruddin, N.; Khalid, M. Effect of graphene nanoplatelets on tribological properties of bacterial cellulose/polyolester oil bio-lubricant. Front. Mech. Eng. 2022, 8, 810847. [Google Scholar] [CrossRef]
- Najari, M.R.; Mohammadpour, M.; Saremi-Yarahmadi, S. Graphene oxide as an additive in aqueous lubricants for electric drive units: Synthesis, preparation, and tribological performance. Tribol. Int. 2025, 205, 110554. [Google Scholar] [CrossRef]
- Nagarajan, T.; Sridewi, N.; Wong, W.P.; Walvekar, R.; Khalid, M. Enhanced tribological properties of diesel-based engine oil through synergistic MoS2-graphene nanohybrid additive. Sci. Rep. 2023, 13, 17424. [Google Scholar] [CrossRef]
- Hettiarachchi, S.J.; Bowen, J.; Kershaw, M.; Baragau, I.; Nicolaev, A.; Kellici, S. Nanostructured Al2O3/graphene additive in bio-based lubricant: A novel approach to improve engine performance. Tribol. Int. 2023, 186, 108619. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Tewatia, K.; Sharma, A.; Sharma, M.; Kumar, A. Synthesis of graphene oxide and its reduction by green reducing agent. Mater. Proc. 2021, 44, 3933–3938. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Thakur, S.; Karaj, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon 2015, 94, 224–242. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, X.; Yang, C.; Chen, G.; Meng, Y.; Zhou, H.; Zhang, S. Insights into robust carbon nanotubes in tribology: From nano to macro. Mater. Today 2024, 74, 203–234. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Ji, L.; Li, H.; Chen, L.; Liu, X.; Zhou, H.; Chen, J. Environmental molecular effect on the macroscale fricction behaviors of graphene. Front. Chem. 2021, 9, 679417. [Google Scholar]
- Qi, S.; Li, X.; Dong, H. Improving the macro-scale tribology of monolayer graphene oxide coating on stainless steel by a silane bonding layer. Mater. Lett. 2017, 209, 15–18. [Google Scholar] [CrossRef]
- Nadeem, I.; Malok, M.; Kovač, J.; Yaqub, T.B.; Cavaleiro, A.; Kalin, M. Superior macro-scale tribological performance of steel contacts based on graphene quantum dots in aqueous glycerol. Tribol. Int. 2023, 181, 108328. [Google Scholar] [CrossRef]
- Komvopoulus, K. Surface engineering and microtribology for microelectromechanical systems. Wear 1996, 200, 305–327. [Google Scholar] [CrossRef]
- Stoyanov, P.; Chromik, R.R. Scaling Effects on Materials Tribology: From macro to micro scale. Materials 2006, 10, 550. [Google Scholar] [CrossRef]
- Achanta, S.; Liskiewicz, T.; Drees, D.; Celis, J. Friction mechanisms at the micro-scale. Tribol. Int. 2009, 42, 1792–1799. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Li, Z.; Xu, Y.; Zeng, X.; Zhao, S.; Deng, J.; Hu, H.; Zhang, Y.; Ren, T. Microtribological properties of Ti 6Al 4V alloy treated with self-assembled dopamine and graphene oxide coatings. Tribol. Int. 2019, 137, 46–58. [Google Scholar] [CrossRef]
- White, D.; Chen, M.; Xiao, C.; Huang, W.; Sundararajan, S. Microtribological behavior of MO and W nanoparticle/graphene composites. Wear 2018, 414–415, 310–316. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, H.; Liu, W.; Lin, Q.; Liu, B. Preparation and investigation of the microtribological properties of graphene oxide and graphene films via electrostatic layer-by-layer self-assembly. J. Nanomater. 2015, 19, 282369. [Google Scholar] [CrossRef]
- Ohmae, N. Nanotribology and Nanoscale Materials Coatings for Lubricants; Elsevier: Kobe, Japan, 2011; pp. 419–443. [Google Scholar]
- Cihan, E.; Özoğul, A.; Baykara, M.Z. Structure and nanotribology of thermally deposited gold nanoparticles on graphite. Appl. Surf. Sci. 2015, 354, 429–436. [Google Scholar] [CrossRef]
- Grierson, D.S.; Carpick, R.W. Nanotribology of carbon-based materials. Nano Today 2007, 2, 12–21. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, Y.; Lang, H.; Liu, L. Controllable nanotribological properties of graphene nanosheets. Sci. Rep. 2017, 7, 41891. [Google Scholar] [CrossRef]
- Sierros, K.A.; Ramayanam, S.S.; Stinespring, C.D. Nanotribological properties of few layer graphene surfaces, prepared by bottom-up and top-down methods, in ambient air and liquid environments. J. Mater. Res. 2016, 31, 1924–1931. [Google Scholar] [CrossRef]
- Rapuc, A.; Wang, H.; Polcar, T. Nanotribology of transition metal dichalcogenide flakes deposited by chemical vapour deposition: The influence of chemical composition and sliding speed on nanoscale friction of monolayers. Appl. Surf. Sci. 2021, 556, 149762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).