Tribological Performance of an Automatic Transmission Fluid Additized with a Phosphonium-Based Ionic Liquid Under Electrified Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
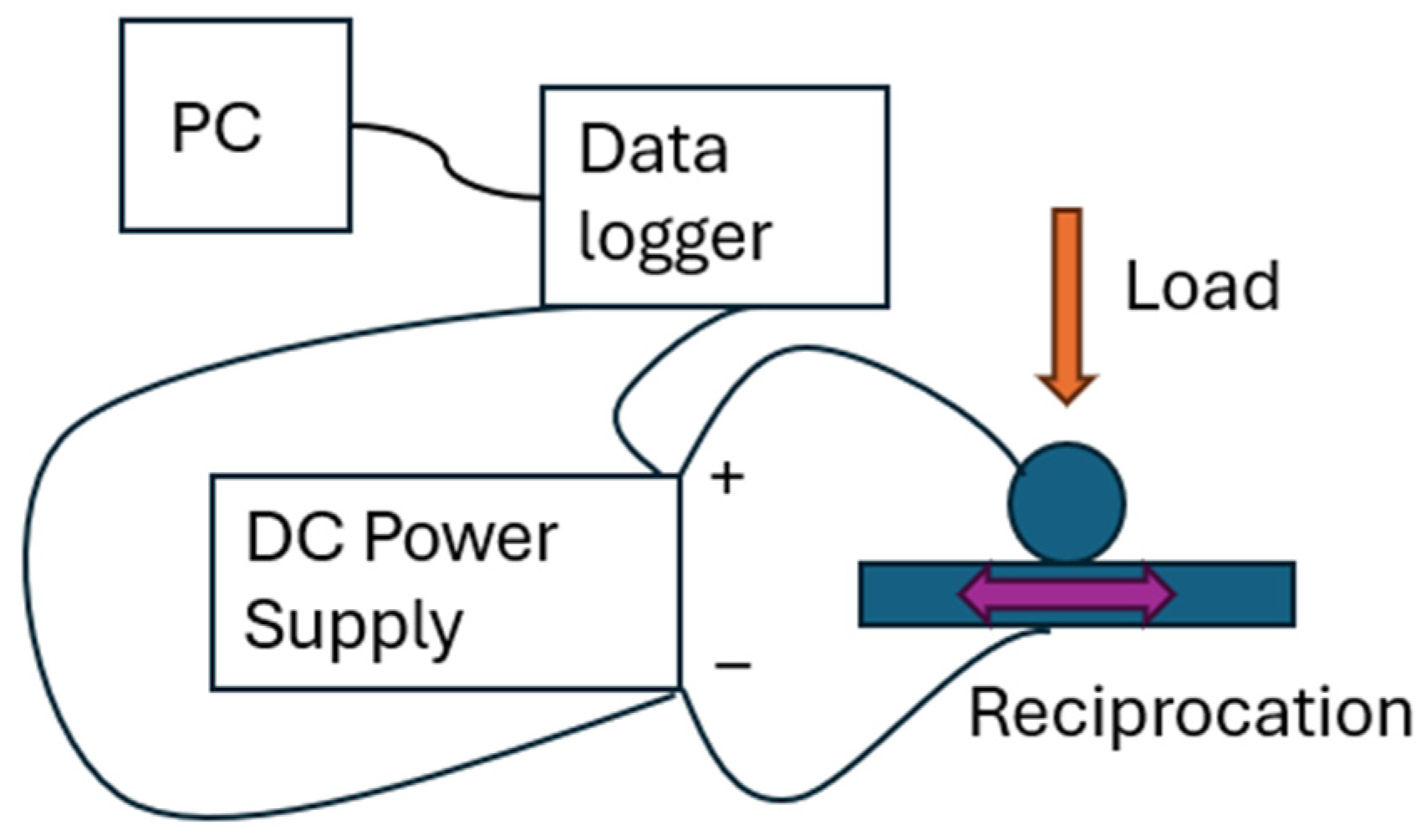
2.2. Tribological Tests
2.3. Surface Analysis
3. Results and Discussion
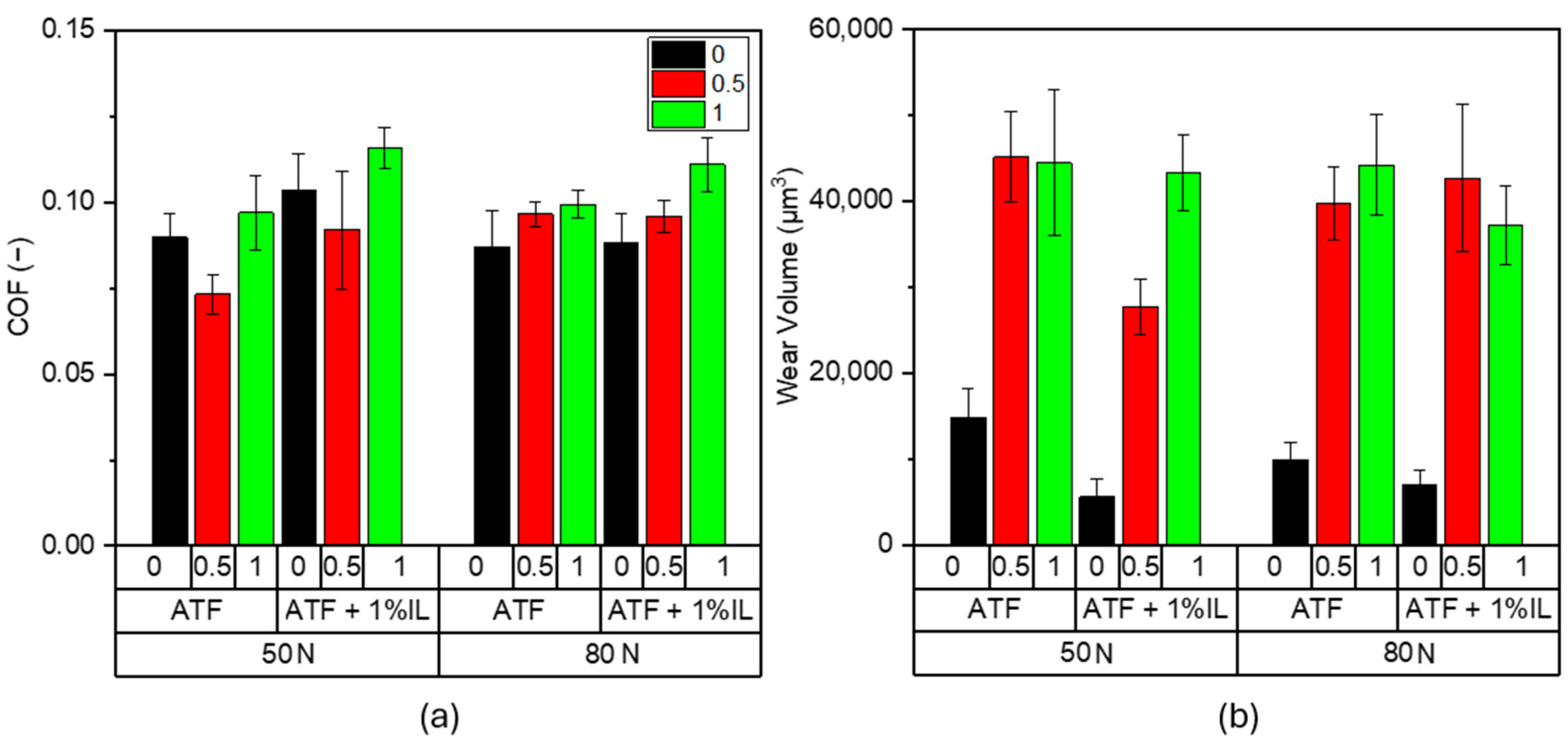
3.1. Friction and Wear
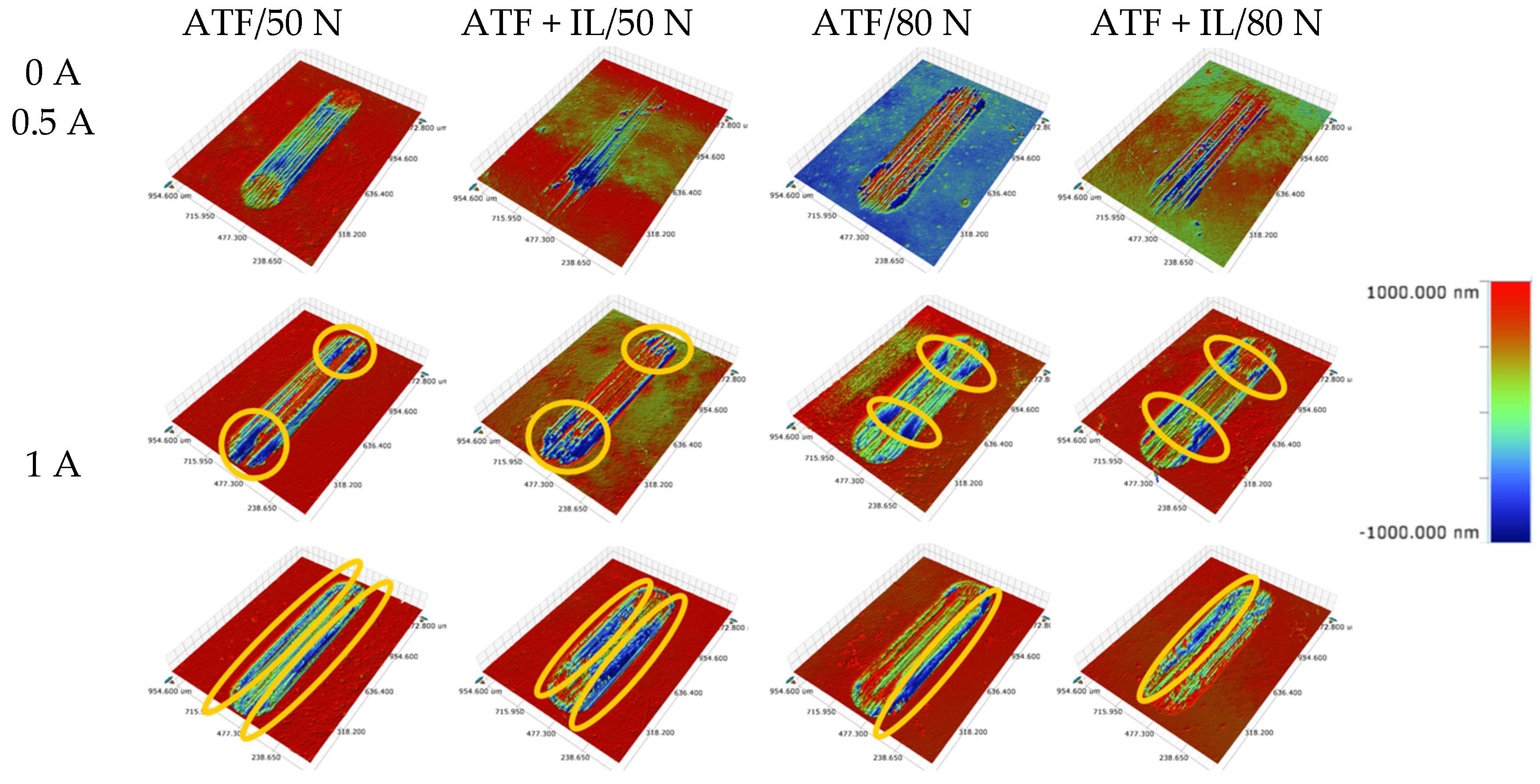
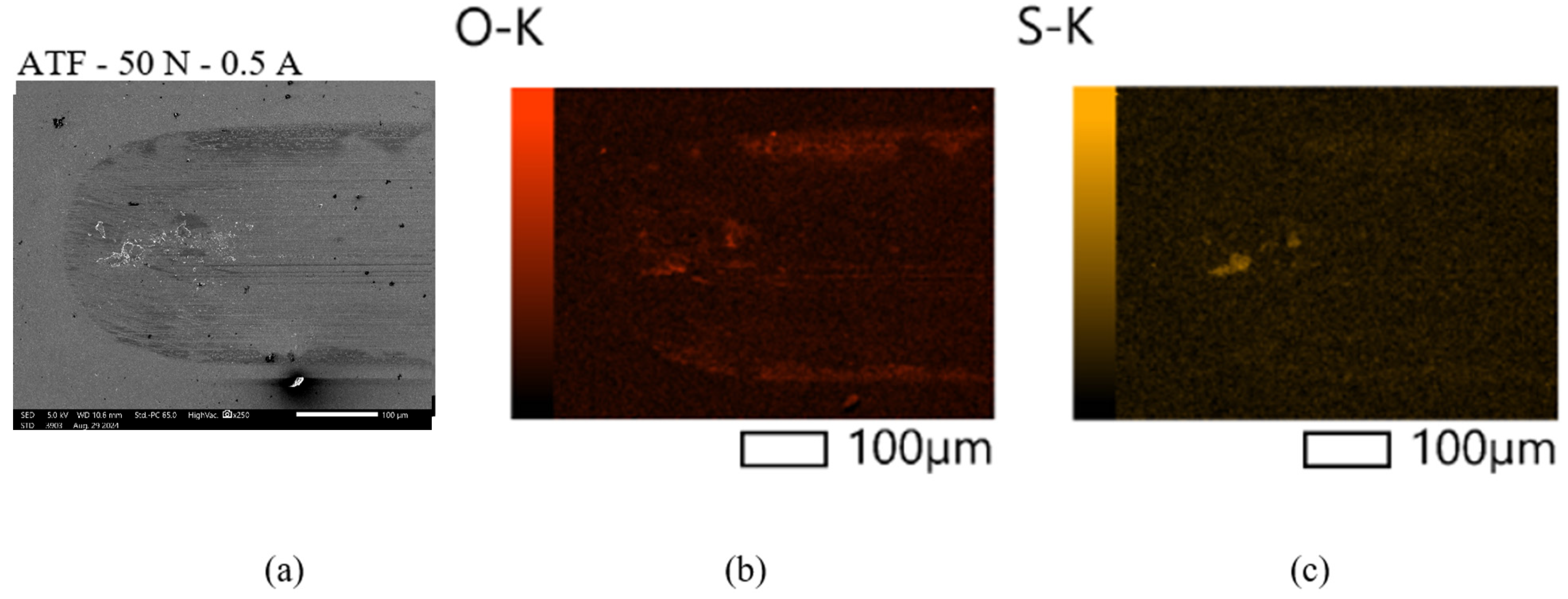
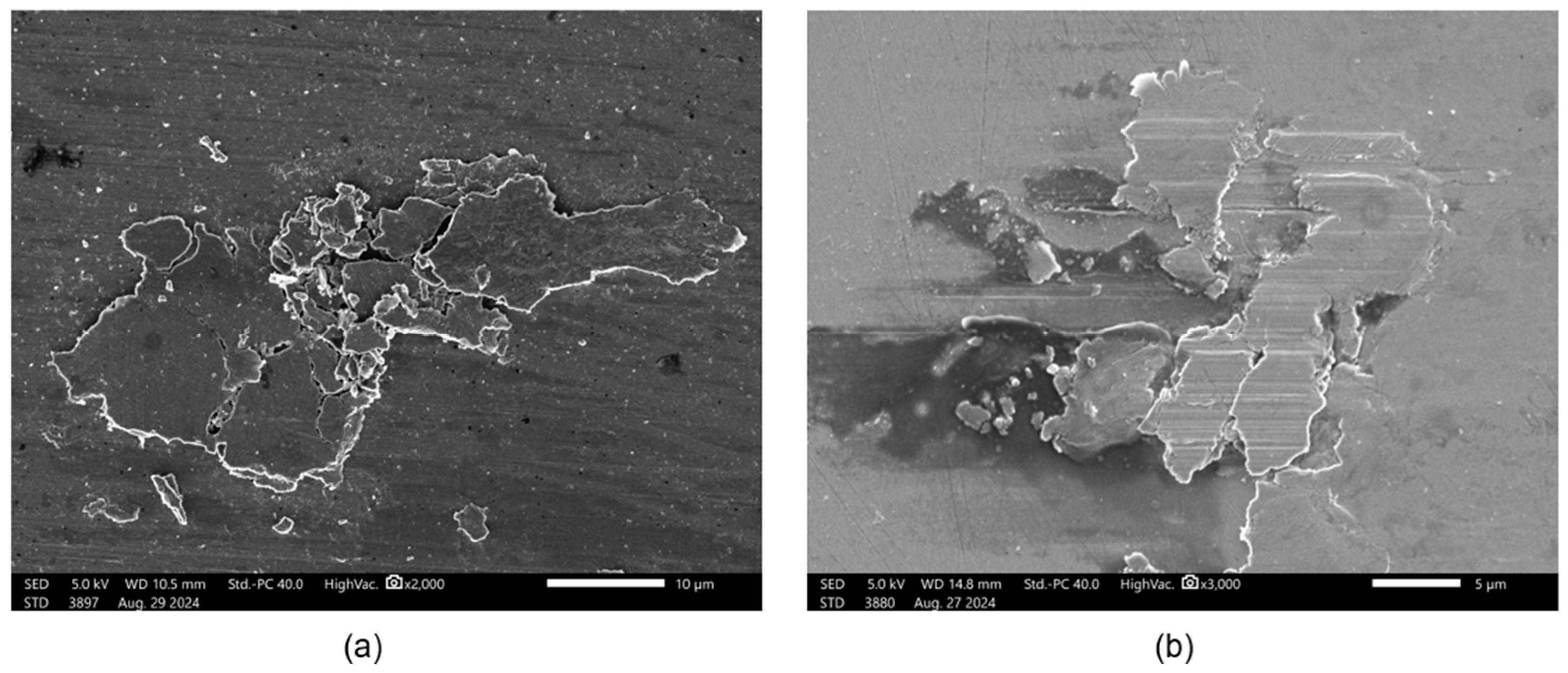
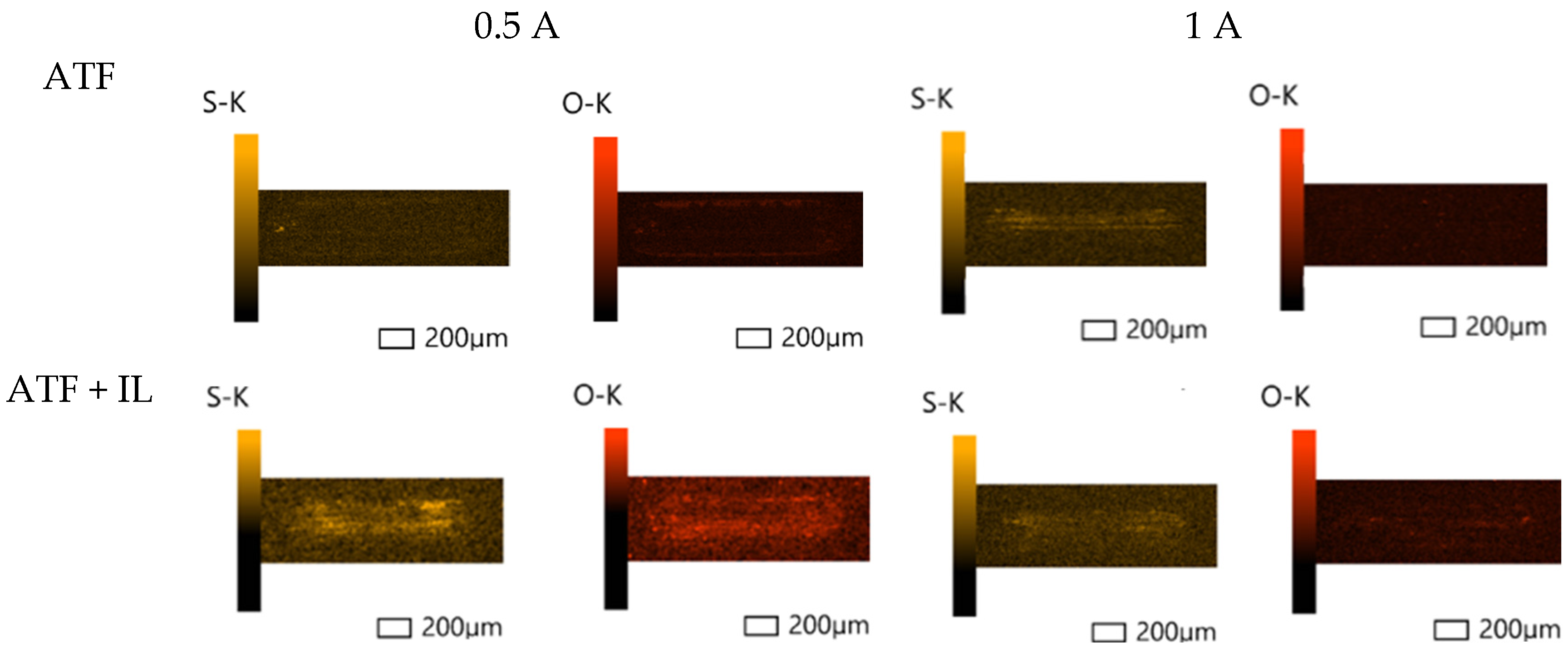
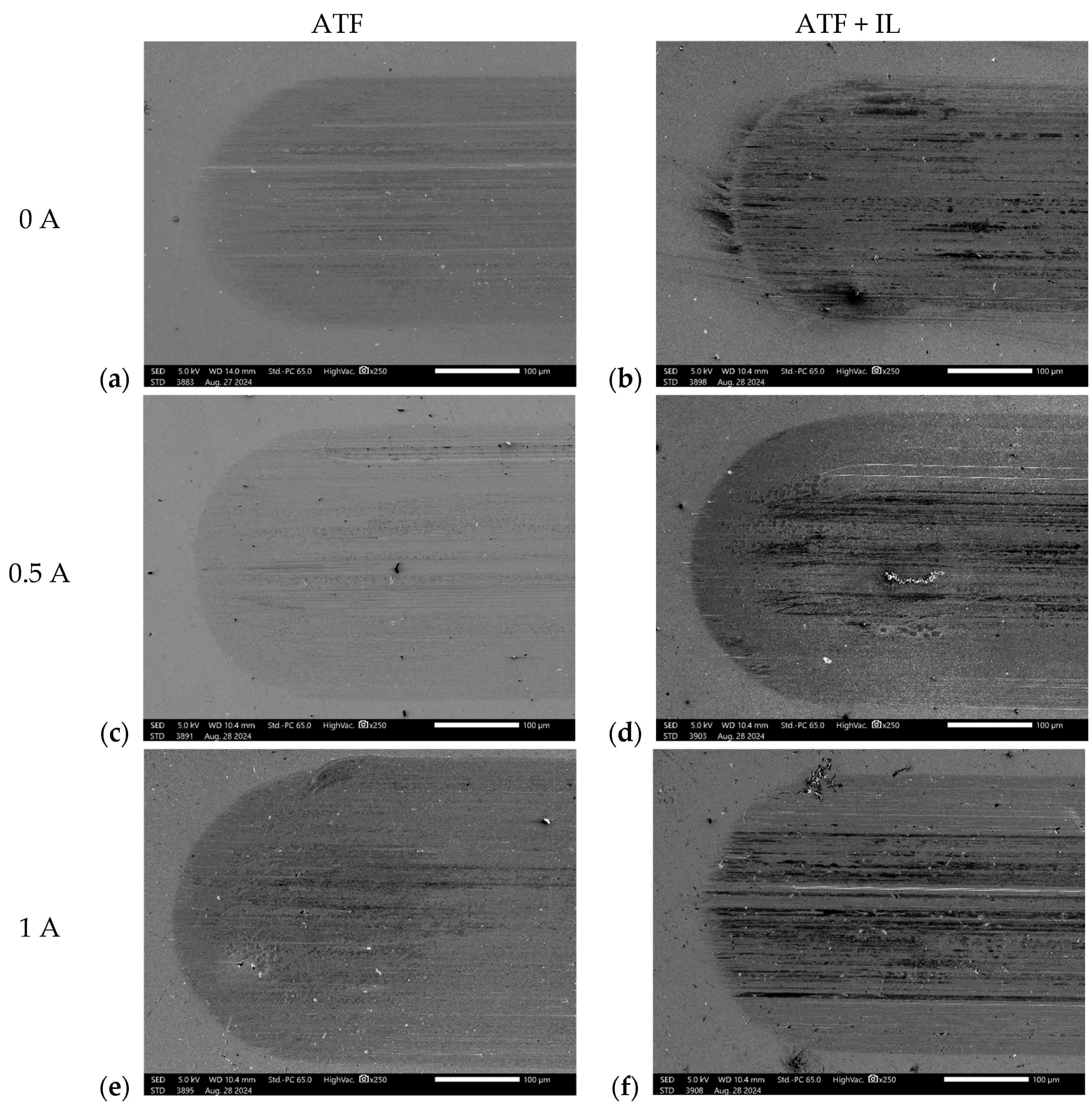
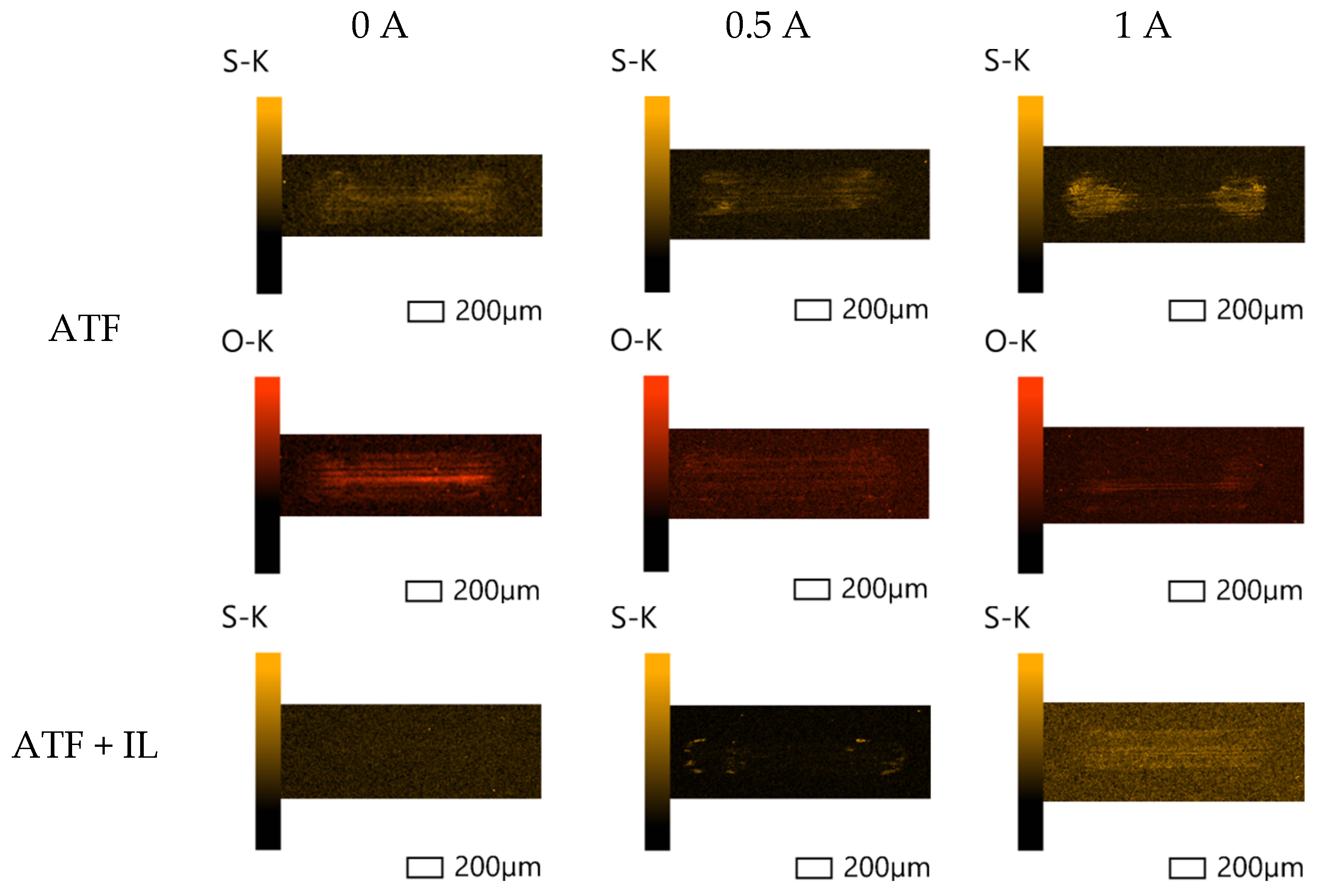
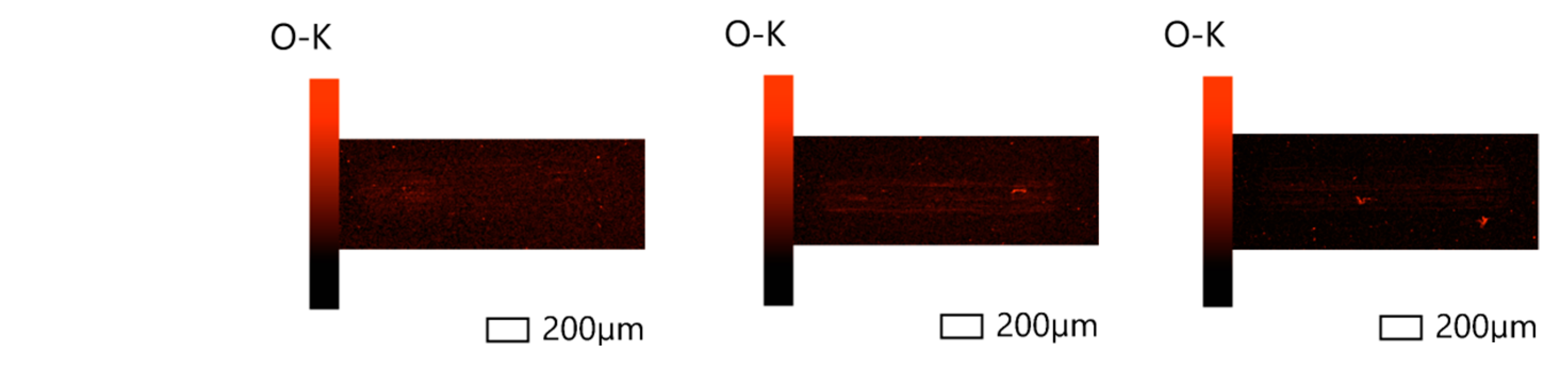
3.2. Surface Analysis
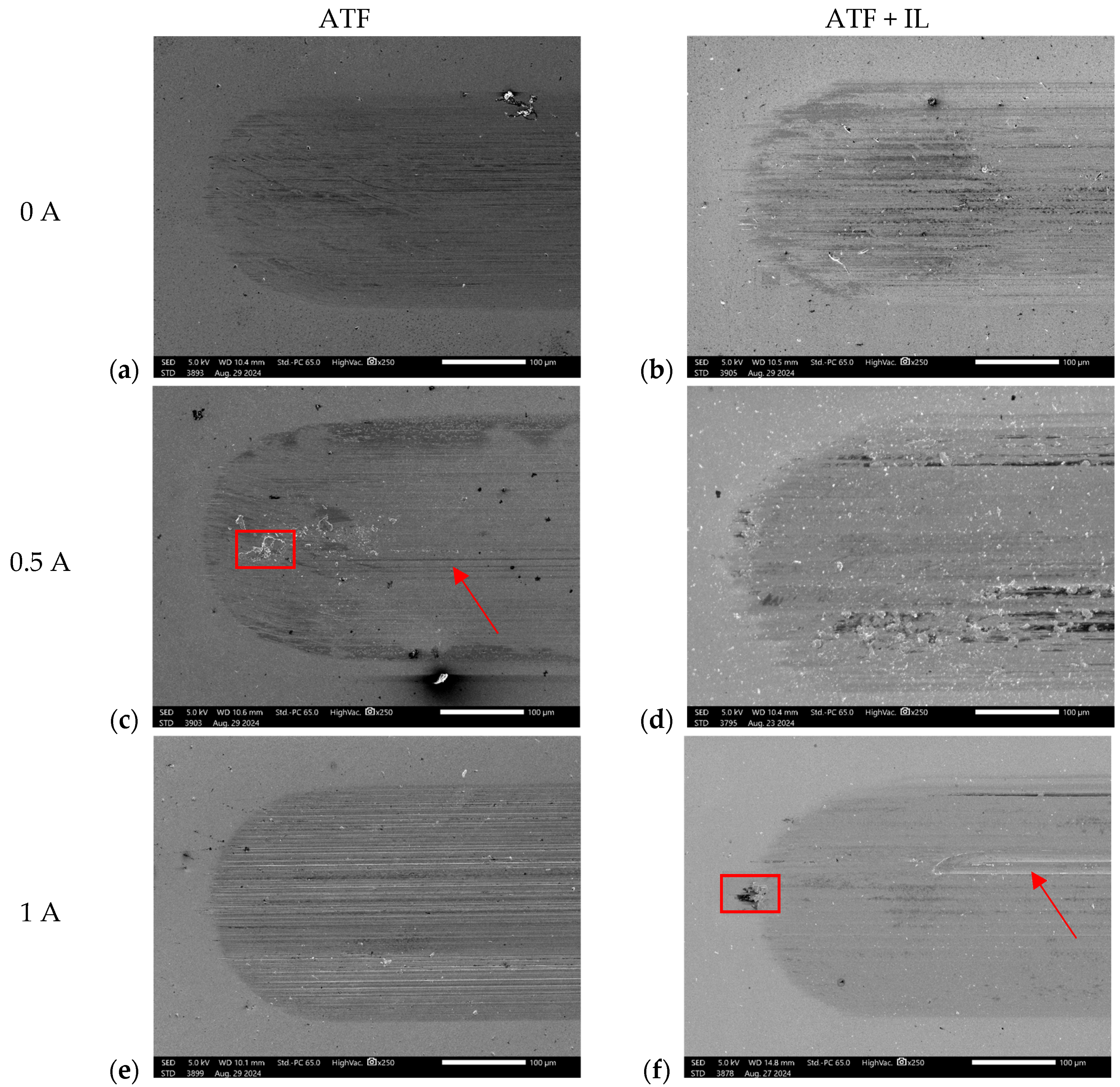
3.2.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
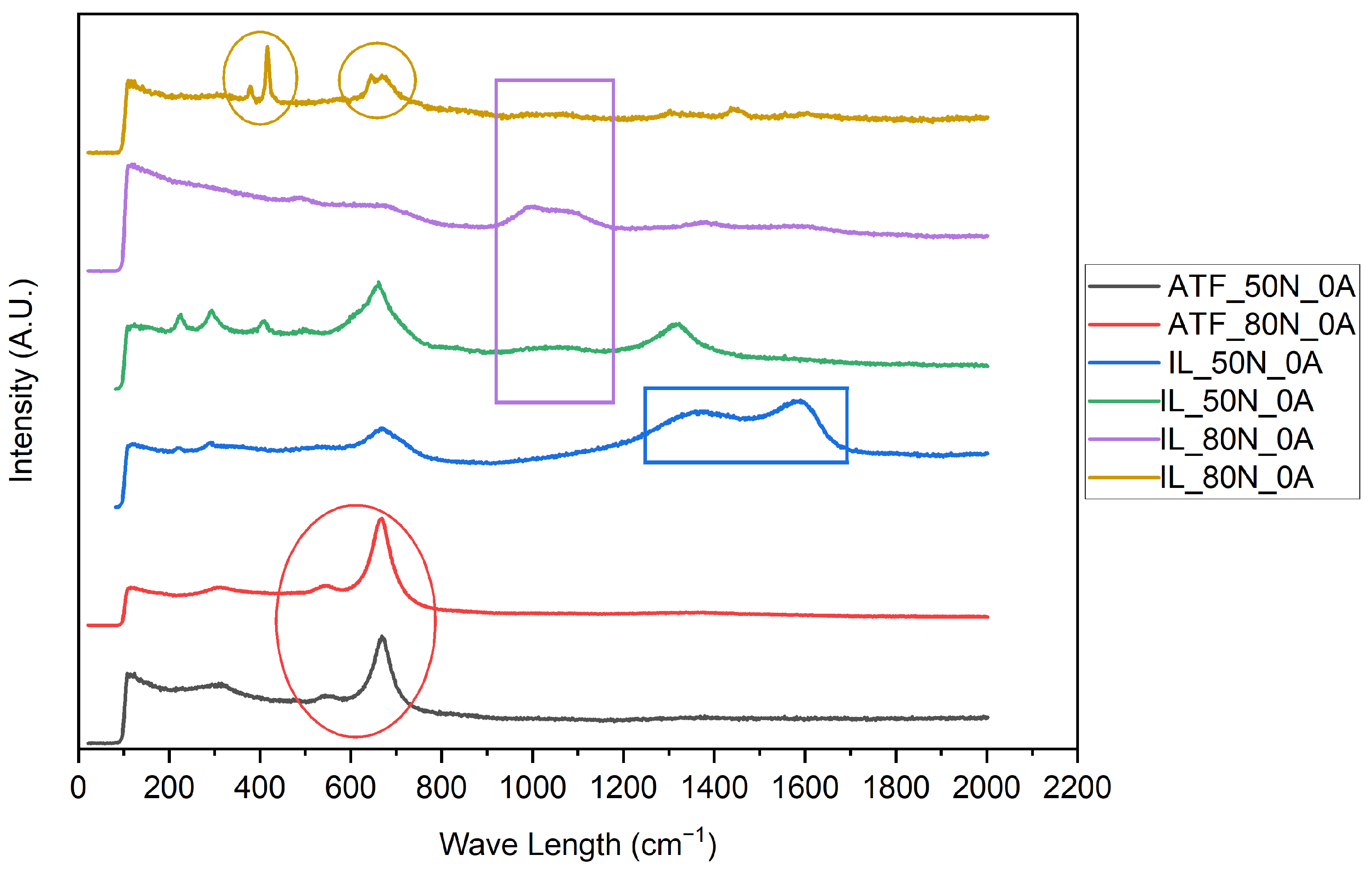
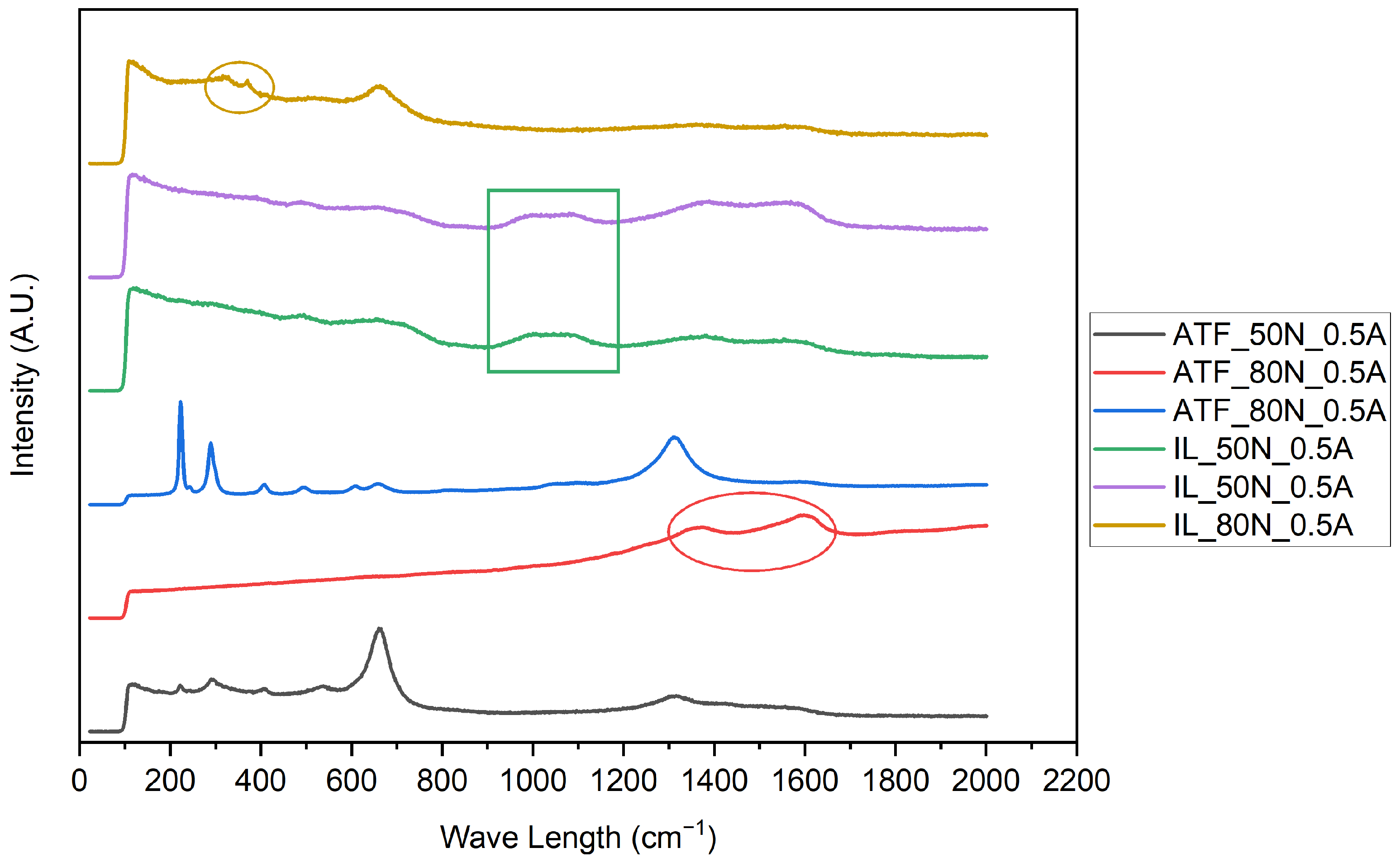
3.2.2. Raman
4. Conclusions
- In general, regardless of the use of the ionic liquid (i.e., trihexyltetradecylphosphonium bis(2-ethylhexyl) phosphate ([P6,6,6,14][BEHP])) as an additive in the ATF, friction coefficients of steel test pairs show a tendency to increase under electrification. The magnitude of increases is somewhat higher under 80 N (creating a Peak Hertz pressure of 1.95 GPa).
- The wear rates of test pairs also increase under electrification, especially in the presence of an IL in ATF, suggesting that existing additives in ATF (even with the additional fortification with an IL) do not necessarily provide the level of anti-wear properties needed. This is consistent with the findings reported on other fully formulated conventional oils. The slight decrease in wear in the case of test pairs lubricated by the ATF + 1 wt.% IL is ascribed to the somewhat more enhanced protective capacity of the specific tribofilms formed.
- Based on the microscopic analyses, the use of trihexyltetradecylphosphonium bis(2-ethylhexyl) phosphate ([P6,6,6,14][BEHP]), as an additive to an ATF, suppressed the onset of the abrasive wear mechanism (as the sliding wear tracks were very smooth and lacked deep abrasive wear grooves). Based on the Raman analysis, the formation of a tribofilm that is rich in sulfates and phosphates may have avoided the abrasive wear process in the case of IL-containing fluid.
- Finally, our results point to the need for further improvements in the chemical formulation of the kind of ionic liquid used in our study and others; specifically, their physical (i.e., rheology, electrical, thermal conductivity) and chemical (surface reactivity, stability under electrification, etc.) properties need to be further tailored for electrified contacts. The IL (i.e., [P6,6,6,14][BEHP]) used in our study showed marked improvements in friction and wear under non-electrified sliding conditions (especially under milder loading) but failed under severe loading and higher current conditions. Accordingly, in the future, the knowledge gained from our study and other studies should be used for the development of the next generation of ILs with a capacity to better regulate the electrical conductivity and/or dielectric properties of the lubricants, coupled with superior ability to enhance the protection of the sliding surfaces against the electrically induced wear, thus making them potentially very valuable in future EV applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EEA. Trends and Projections in Europe 2022; EEA Report No 10/2022; European Environment Agency: Copenhagen, Denmark, 2022. [Google Scholar]
- Sources of Greenhouse Gas Emissions|US EPA. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions#transportation (accessed on 19 September 2024).
- Miranda, J.J.; Scholz, I.; Agard, J.; Bobylev, S.N.; Dube, O.P.; Hathie, I.; Kanie, N.; Madise, N.J.; Malekpour, S.; Montoya, J.C.; et al. Global Susutainable Developement Report 2023. Times of Crisis, Times of Change; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Holmberg, K.; Erdemir, A. The Impact of Tribology on Energy Use and CO2 Emission Globally and in Combustion Engine and Electric Cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Woydt, M. The Importance of Tribology for Reducing CO2 Emissions and for Sustainability. Wear 2021, 474–475, 203768. [Google Scholar] [CrossRef]
- COP26 Transport Declaration. Available online: https://cop26transportdeclaration.org/en/?contextKey=en#fulldeclaration (accessed on 15 June 2022).
- Kooshian, C.; Posada, F.; Yang, Z.; Blumberg, K.; Weber, F.; Füssler, J.; Winkelman, S. Compendium on Greenhouse Gas Baselines and Monitoring. Passenger and Freight Transport; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Lubes’n’Greases. Perspective on Electric Vehicles. 2019 Annual Report; Lubes’n’Greases: West Falls Church, VA, USA, 2019. [Google Scholar]
- Gangopadhyay, A.; Hanumalagutti, P.D. Challenges and Opportunities with Lubricants for HEV/EV Vehicles. In Proceedings of the STLE Annual Meeting, Nashville, TN, USA, 19–23 May 2019. [Google Scholar]
- Farfan-Cabrera, L.I. Tribology of Electric Vehicles: A Review of Critical Components, Current State and Future Improvement Trends. Tribol. Int. 2019, 138, 473–486. [Google Scholar] [CrossRef]
- Kaufman, H.N.; Boyd, J. The Conduction of Current in Bearings. ASLE Trans. 1959, 2, 67–77. [Google Scholar] [CrossRef]
- Prashad, H. Investigation of Damaged Rolling-Element Bearings and Deterioration of Lubricants under the Influence of Electric Fields. Wear 1994, 176, 151–161. [Google Scholar] [CrossRef]
- Chiou, Y.-C.; Lee, R.-T.; Lin, C.-M. Formation Criterion and Mechanism of Electrical Pitting on the Lubricated Surface under AC Electric Field. Wear 1999, 236, 62–72. [Google Scholar] [CrossRef]
- Beyer, M.; Brown, G.; Gahagan, M.; Higuchi, T.; Hunt, G.; Huston, M.; Jayne, D.; McFadden, C.; Newcomb, T.; Patterson, S.; et al. Lubricant Concepts for Electrified Vehicle Transmissions and Axles. Tribol. Online 2019, 14, 428–437. [Google Scholar] [CrossRef]
- Magdum, O.; Gemeinder, Y.; Binder, A. Investigation of Influence of Bearing Load and Bearing Temperature on EDM Bearing Currents. In Proceedings of the 2010 IEEE Energy Conversion Congress and Exposition, Atlanta, GA, USA, 12–16 September 2010; pp. 2733–2738. [Google Scholar]
- Aguilar-Rosas, O.A.; Alvis-Sánchez, J.A.; Tormos, B.; Marín-Santibáñez, B.M.; Pérez-González, J.; Farfan-Cabrera, L.I. Enhancement of Low-Viscosity Synthetic Oil Using Graphene Nanoparticles as Additives for Enduring Electrified Tribological Environments. Tribol. Int. 2023, 188, 108848. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Zhang, C.; Yu, Q.; Sun, Y.; Zhou, F.; Liu, W. Exploring Tribo-Electro-Chemistry Mechanisms of h-BNO@PDA as Lubricant Additives under Electrified Conditions for Electrical Vehicles Powertrain. Tribol. Int. 2024, 200, 110092. [Google Scholar] [CrossRef]
- Aguilar-Rosas, O.A.; Farfan-Cabrera, L.I.; Erdemir, A.; Cao-Romero-Gallegos, J.A. Electrified Four-Ball Testing—A Potential Alternative for Assessing Lubricants (E-Fluids) for Electric Vehicles. Wear 2023, 522, 204676. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Erdemir, A.; Cao-Romero-Gallegos, J.A.; Alam, I.; Lee, S. Electrification Effects on Dry and Lubricated Sliding Wear of Bearing Steel Interfaces. Wear 2023, 516–517, 204592. [Google Scholar] [CrossRef]
- ASTM D4172; Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid (Four-Ball Method). ASTM International: West Conshohocken, PA, USA, 2016.
- Cao-Romero-Gallegos, J.A.; Taghizadeh, S.; Aguilar-Rosas, O.A.; Dwyer-Joyce, R.S.; Farfan-Cabrera, L.I. The Effect of Electrical Current on Lubricant Film Thickness in Boundary and Mixed Lubrication Contacts Measured with Ultrasound. Friction 2024, 12, 1882–1896. [Google Scholar] [CrossRef]
- Cao-Romero-Gallegos, J.A.; Farfan-Cabrera, L.I.; Erdemir, A.; Pascual-Francisco, J.B. Lubricated Sliding Wear of Gear Material under Electrification—A New Approach to Understanding of the Influence of Shaft Currents in the Wear of EV Transmissions. Wear 2023, 523, 204782. [Google Scholar] [CrossRef]
- Xu, X.; Spikes, H. Study of Zinc Dialkyldithiophosphate in Di-Ethylhexyl Sebacate Using Electrochemical Techniques. Tribol. Lett. 2007, 25, 141–148. [Google Scholar] [CrossRef]
- Flores-Torres, S.; Holt, D.G.L.; Carey, J.T. Method for Preventing or Minimizing Electrostatic Discharge and Dielectric Breakdown in Electric Vehicle Powertrains. WO 2018/067905 A1, 12 April 2018. [Google Scholar]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-Temperature Ionic Liquids: A Novel Versatile Lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Bermúdez, M.-D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic Liquid Lubricants: Designed Chemistry for Engineering Applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- Blanco, D.; Battez, A.H.; Viesca, J.L.; González, R.; Fernández-González, A. Lubrication of CrN Coating with Ethyl-Dimethyl-2-Methoxyethylammonium Tris(Pentafluoroethyl)Trifluorophosphate Ionic Liquid as Additive to PAO 6. Tribol. Lett. 2011, 41, 295–302. [Google Scholar] [CrossRef]
- Viesca, J.L.; García, A.; Hernández Battez, A.; González, R.; Monge, R.; Fernández-González, A.; Hadfield, M. FAP- Anion Ionic Liquids Used in the Lubrication of a Steel-Steel Contact. Tribol. Lett. 2013, 52, 431–437. [Google Scholar] [CrossRef]
- Otero, I.; López, E.R.; Reichelt, M.; Fernández, J. Friction and Anti-Wear Properties of Two Tris(Pentafluoroethyl) Trifluorophosphate Ionic Liquids as Neat Lubricants. Tribol. Int. 2014, 70, 104–111. [Google Scholar] [CrossRef]
- Qu, J.; Truhan, J.J.; Dai, S.; Luo, H.; Blau, P.J. Ionic Liquids with Ammonium Cations as Lubricants or Additives. Tribol. Lett. 2006, 22, 207–214. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-Alkyl -3-Methylimidazolium Ionic Liquids as Neat Lubricants and Lubricant Additives in Steel-Aluminium Contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Battez, A.H.; González, R.; Viesca, J.L.; Blanco, D.; Asedegbega, E.; Osorio, A. Tribological Behaviour of Two Imidazolium Ionic Liquids as Lubricant Additives for Steel/Steel Contacts. Wear 2009, 266, 1224–1228. [Google Scholar] [CrossRef]
- Somers, A.E.; Khemchandani, B.; Howlett, P.C.; Sun, J.; Macfarlane, D.R.; Forsyth, M. Ionic Liquids as Antiwear Additives in Base Oils: Influence of Structure on Miscibility and Antiwear Performance for Steel on Aluminum. ACS Appl. Mater. Interfaces 2013, 5, 11544–11553. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Yao, M.; Xia, Y.; Zhou, F.; Liu, W. Imidazolium Ionic Liquids as Antiwear and Antioxidant Additive in Poly(Ethylene Glycol) for Steel/Steel Contacts. ACS Appl. Mater. Interfaces 2010, 2, 870–876. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Short Alkyl Chain Imidazolium Ionic Liquid Additives in Lubrication of Three Aluminium Alloys with Synthetic Ester Oil. Tribol.-Mater. Surf. Interfaces 2012, 6, 109–115. [Google Scholar] [CrossRef]
- Pejaković, V.; Kronberger, M.; Kalin, M. Influence of Temperature on Tribological Behaviour of Ionic Liquids as Lubricants and Lubricant Additives. Lubr. Sci. 2014, 26, 107–115. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-Organophosphate Ionic Liquids as Lubricant Additives: Effects of Cation Structure on Physicochemical and Tribological Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef]
- Qu, J.; Luo, H.; Chi, M.; Ma, C.; Blau, P.J.; Dai, S.; Viola, M.B. Comparison of an Oil-Miscible Ionic Liquid and ZDDP as a Lubricant Anti-Wear Additive. Tribol. Int. 2014, 71, 88–97. [Google Scholar] [CrossRef]
- Zhou, Y.; Dyck, J.; Graham, T.W.; Luo, H.; Leonard, D.N.; Qu, J. Ionic Liquids Composed of Phosphonium Cations and Organophosphate, Carboxylate, and Sulfonate Anions as Lubricant Antiwear Additives. Langmuir 2014, 30, 13301–13311. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Bartolomé, M.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; Battez, A.H. Effectiveness of Phosphonium Cation-Based Ionic Liquids as Lubricant Additive. Tribol. Int. 2016, 98, 82–93. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Hernández Battez, A.; Fernandes, C.M.C.G.; Martins, R.C.; Bartolomé, M.; González, R.; Seabra, J.H.O. Two Phosphonium Cation-Based Ionic Liquids Used as Lubricant Additive: Part I: Film Thickness and Friction Characteristics. Tribol. Int. 2017, 107, 233–239. [Google Scholar] [CrossRef]
- Hernández Battez, A.; Fernandes, C.M.C.G.; Martins, R.C.; Graça, B.M.; Anand, M.; Blanco, D.; Seabra, J.H.O. Two Phosphonium Cation-Based Ionic Liquids Used as Lubricant Additive. Part II: Tribofilm Analysis and Friction Torque Loss in Cylindrical Roller Thrust Bearings at Constant Temperature. Tribol. Int. 2017, 109, 496–504. [Google Scholar] [CrossRef]
- González, R.; Viesca, J.L.; Battez, A.H.; Hadfield, M.; Fernández-González, A.; Bartolomé, M. Two Phosphonium Cation-Based Ionic Liquids as Lubricant Additive to a Polyalphaolefin Base Oil. J. Mol. Liq. 2019, 293, 111536. [Google Scholar] [CrossRef]
- Dold, C.; Amann, T.; Kailer, A. Influence of Electric Potentials on Friction of Sliding Contacts Lubricated by an Ionic Liquid. Phys. Chem. Chem. Phys. 2015, 17, 10339–10342. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Tian, Y. Effect of Imidazolium Ionic Liquid Additives on Lubrication Performance of Propylene Carbonate under Different Electrical Potentials. Tribol. Lett. 2014, 56, 161–169. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Tian, Y. Controllable Friction and Wear of Nitrided Steel under the Lubrication of [DMIm]PF6/PC Solution via Electrochemical Potential. Wear 2016, 360–361, 104–113. [Google Scholar] [CrossRef]
- García Tuero, A.; Sanjurjo, C.; Rivera, N.; Viesca, J.L.; González, R.; Battez, A.H. Electrical Conductivity and Tribological Behavior of an Automatic Transmission Fluid Additised with a Phosphonium-Based Ionic Liquid. J. Mol. Liq. 2022, 367, 120581. [Google Scholar] [CrossRef]
- Rodríguez, E.; Rivera, N.; Fernández-González, A.; Pérez, T.; González, R.; Battez, A.H. Electrical Compatibility of Transmission Fluids in Electric Vehicles. Tribol. Int. 2022, 171, 107544. [Google Scholar] [CrossRef]
- García, A.; Valbuena, G.D.; García-Tuero, A.; Fernández-González, A.; Viesca, J.L.; Battez, A.H. Compatibility of Automatic Transmission Fluids with Structural Polymers Used in Electrified Transmissions. Appl. Sci. 2022, 12, 3608. [Google Scholar] [CrossRef]
- García-Tuero, A.; Ramajo, B.; Valbuena, G.D.; Fernández-González, A.; Mendoza, R.; García, A.; Hernández Battez, A. Automatic Transmission Fluids in Electrified Transmissions: Compatibility with Elastomers. Appl. Sci. 2022, 12, 6213. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Andriušis, A.; Treinytė, J.; Kupčinskas, A.; Jankauskas, V. Investigation of the Lubricating Conditions in a Reciprocating Sliding Tribotest with Applied Electric Voltage. Lubricants 2024, 12, 104. [Google Scholar] [CrossRef]
- Deshpande, P.; Yelkarasi, C.; Lee, S.; Farfan-Cabrera, L.I.; Erdemir, A. Electrotribochemical Formation of Abrasive Nano-Carbon Particles under Electrified Conditions on Lubricated Sliding Contacts. Carbon 2024, 228, 119425. [Google Scholar] [CrossRef]
- Dorgham, A.; Azam, A.; Parsaeian, P.; Wang, C.; Morina, A.; Neville, A. An Assessment of the Effect of Relative Humidity on the Decomposition of the ZDDP Antiwear Additive. Tribol. Lett. 2021, 69, 72. [Google Scholar] [CrossRef]
- Okubo, H.; Tadokoro, C.; Sasaki, S. In Situ Raman-SLIM Monitoring for the Formation Processes of MoDTC and ZDDP Tribofilms at Steel/Steel Contacts under Boundary Lubrication. Tribol. Online 2020, 15, 105–116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Shi, G.; Wang, G.; Zhang, H.; Zhang, Q.; Jiang, X.; Li, X.; Luo, F.; Hu, Y.; et al. Preparation and Electrochemical Properties of Electrospun FeS/Carbon Nanofiber Composites. Ionics 2020, 26, 3051–3060. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Z.; Qiu, H.; Chen, D.; Li, W.; Li, S.; Tu, X. Nano Serpentine Powders as Lubricant Additive: Tribological Behaviors and Self-Repairing Performance on Worn Surface. Nanomater 2020, 10, 922. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-Based Tribofilms from Lubricating Oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Pasternak, M.P.; Rozenberg, G.K.; Milner, A.P.; Amanowicz, M.; Zhou, T.; Schwarz, U.; Syassen, K.; Dean Taylor, R.; Hanfland, M.; Brister, K. Pressure-Induced Concurrent Transformation to an Amorphous and Crystalline Phase in Berlinite-Type FePO4. Phys. Rev. Lett. 1997, 79, 4409. [Google Scholar] [CrossRef]
- Sharma, V.; Gabler, C.; Doerr, N.; Aswath, P.B. Mechanism of Tribofilm Formation with P and S Containing Ionic Liquids. Tribol. Int. 2015, 92, 353–364. [Google Scholar] [CrossRef]
- Litasov, K.D.; Podgornykh, N.M. Raman Spectroscopy of Various Phosphate Minerals and Occurrence of Tuite in the Elga IIE Iron Meteorite. J. Raman Spectrosc. 2017, 48, 1518–1527. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; 347p. [Google Scholar]
- Montagnac, G. Raman Experiments for Astrobiology and Planetology. SSHADE (OSUG Data Center). Service/Database. Available online: https://www.sshade.eu/db/reap (accessed on 9 November 2024).
- Liu, Y.; Wang, D.; Zhang, K.; Wu, H.; Yu, G.; Zhang, Q.; Zhou, Y.; Ma, T.; Song, A. Superlubricating Electrical Contact between Graphite Layers. Friction 2025, 13, 9440989. [Google Scholar] [CrossRef]
- Cusati, T.; Fiori, G.; Gahoi, A.; Passi, V.; Lemme, M.C.; Fortunelli, A.; Iannaccone, G. Electrical Properties of Graphene-Metal Contacts. Sci. Rep. 2017, 7, 5109. [Google Scholar] [CrossRef]
- Kumar Das, P.; Kumar, N.; Chakraborti, P. Loading Effect on Friction Behavior of Ordered/Disordered Graphite in Ambient and Inert Condition. Indian J. Eng. Mater. Sci. 2018, 25, 19–25. [Google Scholar]
- Burgo, T.A.L.; Silva, C.A.; Balestrin, L.B.S.; Galembeck, F. Friction Coefficient Dependence on Electrostatic Tribocharging. Sci. Rep. 2013, 3, 2384. [Google Scholar] [CrossRef]
- Gatti, F.; Amann, T.; Kailer, A.; Baltes, N.; Rühe, J.; Gumbsch, P. Towards Programmable Friction: Control of Lubrication with Ionic Liquid Mixtures by Automated Electrical Regulation. Sci. Rep. 2020, 10, 17634. [Google Scholar] [CrossRef]
- Haider, N.; Moneeb Butt, M.; Zahid, R.; Ali, A.; Aslam, J.; Mufti, R.A.; Usman Bhutta, M. Friction and Wear Properties of Phosphonium Based Ionic Liquid Used as Additive in Synthetic and Bio Based Lubricants Keywords: Bio-Lubricants Ionic Liquids Polyalphaolefin Rattan Jot Oil Cotton Seed Oil Waste Cooking Oil Coefficient. Tribol. Ind. 2024, 46, 611–623. [Google Scholar] [CrossRef]


| Properties | Additive Elements | ||
|---|---|---|---|
| Density at 15 °C (g/cm3) | 0.847 | Ca (ppm) | - |
| Kinematic Viscosity at 40 °C (mm2/s) | 29.8 | B (ppm) | 59–88 |
| Kinematic Viscosity at 100 °C (mm2/s) | 5.8 | P (ppm) | 136–194 |
| Viscosity Index (VI) | 144 | Zn (ppm) | 20 |
| Flash Point (°C) | 216 | S (%) | 0.192 |
| Pour Point (°C) | −49 | N (%) | - |
| IL | Cation | Anion |
|---|---|---|
| Trihexyltetradecylphosphonium bis(2-ethylhexyl)phosphate [P6,6,6,14][BEHP] Empirical Formula: C48H102O4P2 Purity: 98% Molecular Weight: 805.29 | Trihexyltetradecylphosphonium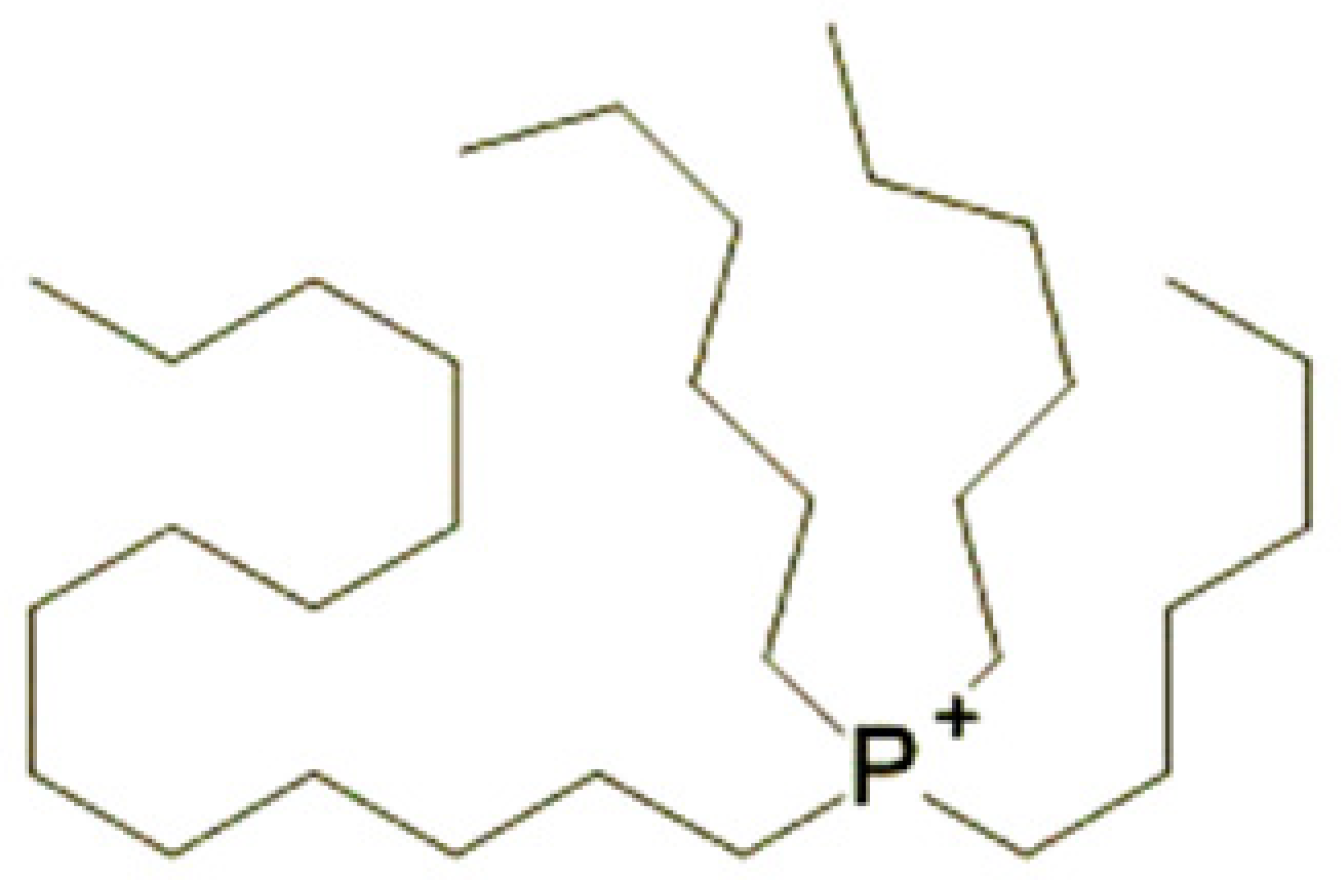 | Bis(2-ethylhexyl)phosphate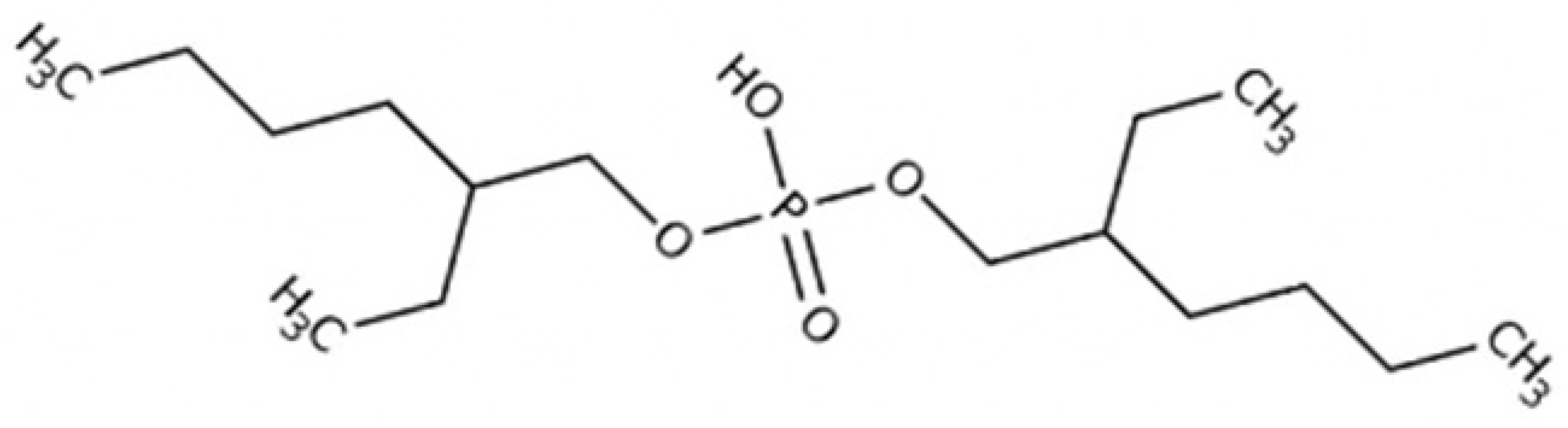 |
| Kinematic Viscosity at 40 °C (mm2/s) | 528 | |
| Kinematic Viscosity at 100 °C (mm2/s) | 59 | |
| Viscosity Index | 181 | |
| Electrical Conductivity at 27 °C (µS/cm) | 0.19 | |
| Temp. (°C) | ATF 10−4 κ (μS/cm) | ATF + IL 10−4 κ (μS/cm) |
|---|---|---|
| 25 | 0 | 1.3 |
| 40 | 0 | 3.8 |
| 80 | 2.5 | 6.9 |
| 100 | 4.87 | 11.3 |
| 125 | 7.5 | 16.7 |
| Temp. (°C) | ATF μ (mPa·s) | ATF + IL μ (mPa·s) | ATF ν (mm2/s) | ATF + IL ν (mm2/s) |
|---|---|---|---|---|
| 20 | 56.25 | 56.162 | 66.931 | 66.84 |
| 40 | 23.6 | 23.446 | 28.515 | 28.334 |
| 60 | 11.987 | 11.897 | 14.708 | 14.6 |
| 80 | 7.0264 | 6.9689 | 8.7571 | 8.6867 |
| 100 | 4.5706 | 4.5309 | 5.787 | 5.7379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Tuero, A.; Lee, S.; Hernández Battez, A.; Erdemir, A. Tribological Performance of an Automatic Transmission Fluid Additized with a Phosphonium-Based Ionic Liquid Under Electrified Conditions. Lubricants 2025, 13, 209. https://doi.org/10.3390/lubricants13050209
García Tuero A, Lee S, Hernández Battez A, Erdemir A. Tribological Performance of an Automatic Transmission Fluid Additized with a Phosphonium-Based Ionic Liquid Under Electrified Conditions. Lubricants. 2025; 13(5):209. https://doi.org/10.3390/lubricants13050209
Chicago/Turabian StyleGarcía Tuero, Alejandro, Seungjoo Lee, Antolin Hernández Battez, and Ali Erdemir. 2025. "Tribological Performance of an Automatic Transmission Fluid Additized with a Phosphonium-Based Ionic Liquid Under Electrified Conditions" Lubricants 13, no. 5: 209. https://doi.org/10.3390/lubricants13050209
APA StyleGarcía Tuero, A., Lee, S., Hernández Battez, A., & Erdemir, A. (2025). Tribological Performance of an Automatic Transmission Fluid Additized with a Phosphonium-Based Ionic Liquid Under Electrified Conditions. Lubricants, 13(5), 209. https://doi.org/10.3390/lubricants13050209








