Thermoxidation Stability of Gear Oils for Electric Vehicles
Abstract
1. Introduction
- (1)
- Initiation—under the influence of temperature, light, and the presence of oxygen, hydrocarbon structures break down and alkyl free radicals are formed.
- (2)
- Propagation—in which chemical reactions of radicals with oxygen occur, leading to the formation of alkyl peroxides, which, when reacting with oxygen, form typical oxidation products, i.e., aldehydes, ketones, and carboxylic acids.
- (3)
- Termination—i.e., the formation of stable end products of the above-mentioned reactions, such as deposits and sludges, polymers, resins, etc. [3].
- Methods are conducted until a defined value of a specific parameter is reached (e.g., an increase in acid number to 2 mg KOH/g, a decrease in oxygen pressure by 10%).
- Oxidation methods are conducted for a specified time.
- To assess the degree of oxidation (oil degradation), the following are used:
- Changes in a specific lubricating oil property (before and after oxidation)—most often, changes in kinematic viscosity, acid number, and coking residue. The amount of insoluble precipitates in pentane and toluene formed during the test is assessed;
- Determination of the induction period, which is defined as the time until the pressure drops by 10% from its maximum value, as this is considered the beginning of the oxidation reaction.
2. Samples and Test Methods
3. Discussion of Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cobb, T.W.; Gatto, V.J.; Moehle, W.E.; Schneller, E.R. Oxidation Fundamentals and its Application to Turbine Oil Testing. J. ASTM Int. 2006, 3, 327–336. [Google Scholar] [CrossRef]
- Skibińska, A. Stabilność termooksydacyjna smarów plastycznych. Nafta-Gaz. 2021, 77, 471–479. [Google Scholar] [CrossRef]
- Xia, D.; Wang, Y.; Liu, H.; Yan, J.; Lin, H.; Han, S. Research Progress of Antioxidant Additives for Lubricating Oils. Lubricants 2024, 12, 115. [Google Scholar] [CrossRef]
- König, T.; Cadau, L.; Steidle, L.; Güney, D.C.; Albrecht, J.; Weber, K.; Kley, M. A concept for comparison of new and aged lubricants in transmissions of electric vehicles and a method of oil aging on a test rig. Forsch Ingenieurwes 2023, 87, 1069–1080. [Google Scholar] [CrossRef]
- Parenago, O.P.; Lyadov, A.S.; Maksimov, A.L. Development of Lubricant Formulations for Modern Electric Vehicles. Russ. J. Appl. Chem. 2022, 95, 765–774. [Google Scholar]
- Rivera, N.; Viesca, J.L.; García, A.; Prado, J.I.; Lugo, L.; Battez, A.H. Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles. Appl. Sci. 2022, 12, 8911. [Google Scholar] [CrossRef]
- Zhmud, B.; Najjari, M.; Brodmann, B. The Effects of the Lubricant Properties and Surface Finish Characteristics on the Tribology of High-Speed Gears for EV Transmissions. Lubricants 2024, 12, 112. [Google Scholar] [CrossRef]
- Shah, R.; Tung, S.; Chen, R.; Miller, R. Grease Performance Requirements and Future Perspectives for Electric and Hybrid Vehicle Applications. Lubricants 2021, 9, 40. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.J.; Ren, N.; England, R. Lubrication subjected to effects of electric and magnetic fields: Recent research progress and a generalized MEMT-field Reynolds equation. Front. Mech. Eng. 2024, 9, 1334814. Available online: https://www.frontiersin.org/journals/mechanical-engineering/articles/10.3389/fmech.2023.1334814/pdf (accessed on 28 July 2025). [CrossRef]
- ASTM D5704-20; Standard Test Method for Evaluation of the Thermal and Oxidative Stability of Lubricating Oils Used for Manual Transmissions and Final Drive Axles. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D8206-18; Standard Test Method for Oxidation Stability of Lubricating Greases—Rapid Small-Scale Oxidation Test (RSSOT). ASTM International: West Conshohocken, PA, USA, 2024.
- PN-EN 16091:2011; Ciekłe Przetwory Naftowe—Paliwa i Mieszaniny ze Średnich Destylatów Naftowych i Estrów Metylowych Kwasów Tłuszczowych (FAME)—Oznaczanie Stabilności Oksydacyjnej Metodą Szybkiego Utleniania w Małej Skali. Polski Komitet Normalizacyjny: Warszawa, Poland, 2023.
- ASTM D2272-22; Standard Test Method for Oxidation Stability of Steam Turbine Oils by Rotating Pressure Vessel. ASTM International: West Conshohocken, PA, USA, 2022.
- PN-67/C-04080; Przetwory Naftowe—Badania Odporności Olejów na Utlenianie. Wydawnictwa Normalizacyjne: Warszawa, Poland, 1976.
- DIN 51451:2004; Testing of Petroleum Products and Related Products—Analysis by Infrared Spectrometry—General Working Principles. Deutsches Institut für Normung: Berlin, Germany, 2004.
- PN-EN ISO 3104:2024; Przetwory Naftowe—Ciecze Przezroczyste i Nieprzezroczyste—Oznaczanie Lepkości Kinematycznej i Obliczanie Lepkości Dynamicznej. Polski Komitet Normalizacyjny: Warszawa, Poland, 2024.
- PN-ISO 6618:2011; Przetwory Naftowe i Środki Smarowe—Oznaczanie Liczby Kwasowej i Zasadowej—Metoda Miareczkowania Wobec Wskaźników Barwnych. Polski Komitet Normalizacyjny: Warszawa, Poland, 2011.
- Krasodomski, M.; Krasodomski, W.; Skibińska, A.; Żółty, M. Badania porównawcze w zakresie metod oznaczania stabilności termooksydacyjnej olejów bazowych. Przemysł Chem. 2019, 4, 563–568. [Google Scholar] [CrossRef]
| Test Method | Method Specific to the Product | Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | Catalyst | Steam | Sample Quantity | Medium /Gas | Test Time, h, | Result | ||
| ASTM D5704 | Automotive gear oils | 162.8 | Copper plate | none | 120 mL | air, flow 22.08 mg/min | 50 | kinematic viscosity (at 40 and 100 °C) change, acid number change, catalyst mass change, insoluble matter content |
| ASTM D8206 | Greases | 160.0 | None | none | 4 g | oxygen, 700 kPa | - | time (in min.) until oxygen pressure drops by 10% from maximum pressure |
| PN-EN 16091 | Lubricating oils | 140.0 | None | none | 5 mL | oxygen, 800 kPa | - | time (in min.) until oxygen pressure drops by 10% from maximum pressure |
| ASTM D2272 | Steam turbine oils | 150.0 | Copper wire coil | present | 50 g | oxygen, 620 kPa | - | time (in min.) until oxygen pressure drops by 175 kPa |
| PN-C-04080 | Lubricating oils | 160.0 | Copper plate | none | 75 g | air, flow 3 dm3/h | 24 | kinematic viscosity (at 40 °C) change, acid number change |
| Test Method | Results | |
|---|---|---|
| Oil A | Oil B | |
| ASTM D5704 | Kinematic viscosity change: - at 40 °C: increase by 35.50% - at 100 °C: decrease by 24.13% Acid number increase by 3.97% Catalyst mass loss: 0.0006 g Amount of insoluble matter: - in pentane: 0.038% (m/m), - in toluene: 0.108% (m/m) | Kinematic viscosity change: - at 40 °C: increase by 14.31% - at 100 °C: decrease by 8.98% Acid number increase by 56.00% Catalyst mass loss: 0.0259 g Amount of insoluble matter: - in pentane: 0.075% (m/m), - in toluene: 0.194% (m/m) |
| ASTM D8206 | 808 min | 778 min |
| PN-EN 16091 | 878 min | 748 min |
| ASTM D2272 | 812 min | 584 min |
| PN-C-04080 | Kinematic viscosity change: - at 40 °C: decrease by 2.47% - at 100 °C: decrease by 0.51% Acid number increase by 32.16% | Kinematic viscosity change: - at 40 °C: decrease by 2.03% - at 100 °C: decrease by 10.60% Acid number increase by 64.03% |
| Test Method | Sample | Kinematic Viscosity at 40 °C, mm/s2 | Kinematic Viscosity at 100 °C, mm/s2 | Acid Number, mg KOH/g | |||
|---|---|---|---|---|---|---|---|
| Before the Test | After the Test | Before the Test | After the Test | Before the Test | After the Test | ||
| ASTM D5704 | Oil A | 32.39 | 43.89 | 6422 | 4872 | 2068 | 2150 |
| ASTM D8206 | - * | - * | 3568 | ||||
| PN-EN 16091 | - * | - * | 3783 | ||||
| ASTM D2272 | 30.20 | 6261 | 4279 | ||||
| PN-C-04080 | 31.59 | 6455 | 2733 | ||||
| ASTM D5704 | Oli B | 32.06 | 36.68 | 7625 | 6947 | 1743 | 2719 |
| ASTM D8206 | - * | - * | 2733 | ||||
| PN-EN 16091 | - * | - * | 2701 | ||||
| ASTM D2272 | 30.10 | 6319 | 5582 | ||||
| PN-C-04080 | 31.41 | 6817 | 2859 | ||||
| Test Method | Sample | Physicochemical Property Change [%] | ||
|---|---|---|---|---|
| Kinematic Viscosity at 40 °C | Kinematic Viscosity at 100 °C | Acid Number | ||
| ASTM D5704 | Oil A | 35.50 | −24.13 | 3.97 |
| ASTM D8206 | - * | - * | 72.53 | |
| PN-EN 16091 | - * | - * | 82.93 | |
| ASTM D2272 | −6.76 | −2.50 | 106.91 | |
| PN-C-04080 | −2.47 | −0.51 | 32.16 | |
| ASTM D5704 | Oil B | 14.41 | −8.89 | 56.00 |
| ASTM D8206 | - * | - * | 56.80 | |
| PN-EN 16091 | - * | - * | 54.96 | |
| ASTM D2272 | −6.11 | −17.13 | 220.25 | |
| PN-C-04080 | −2.03 | −10.60 | 64.03 | |
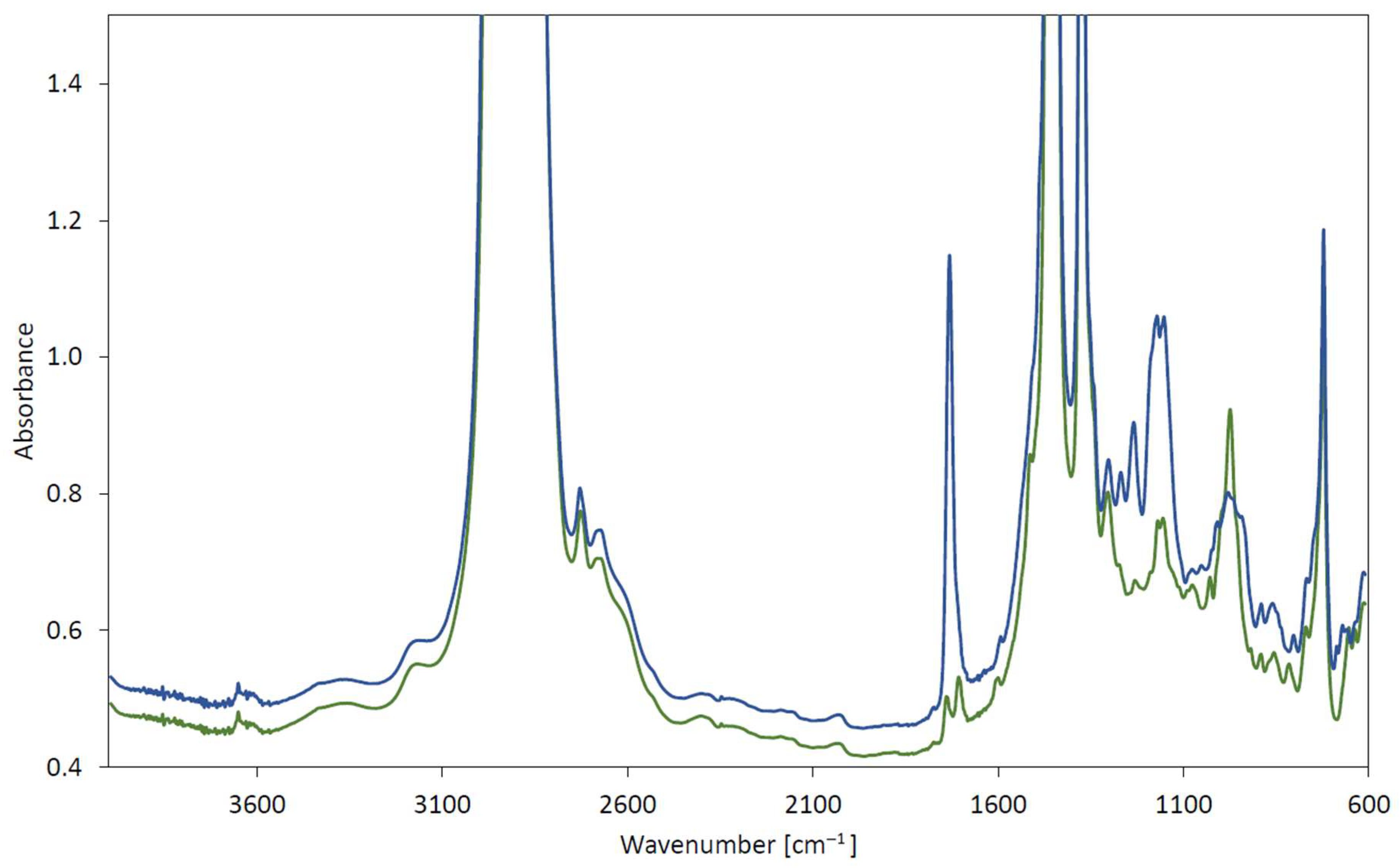
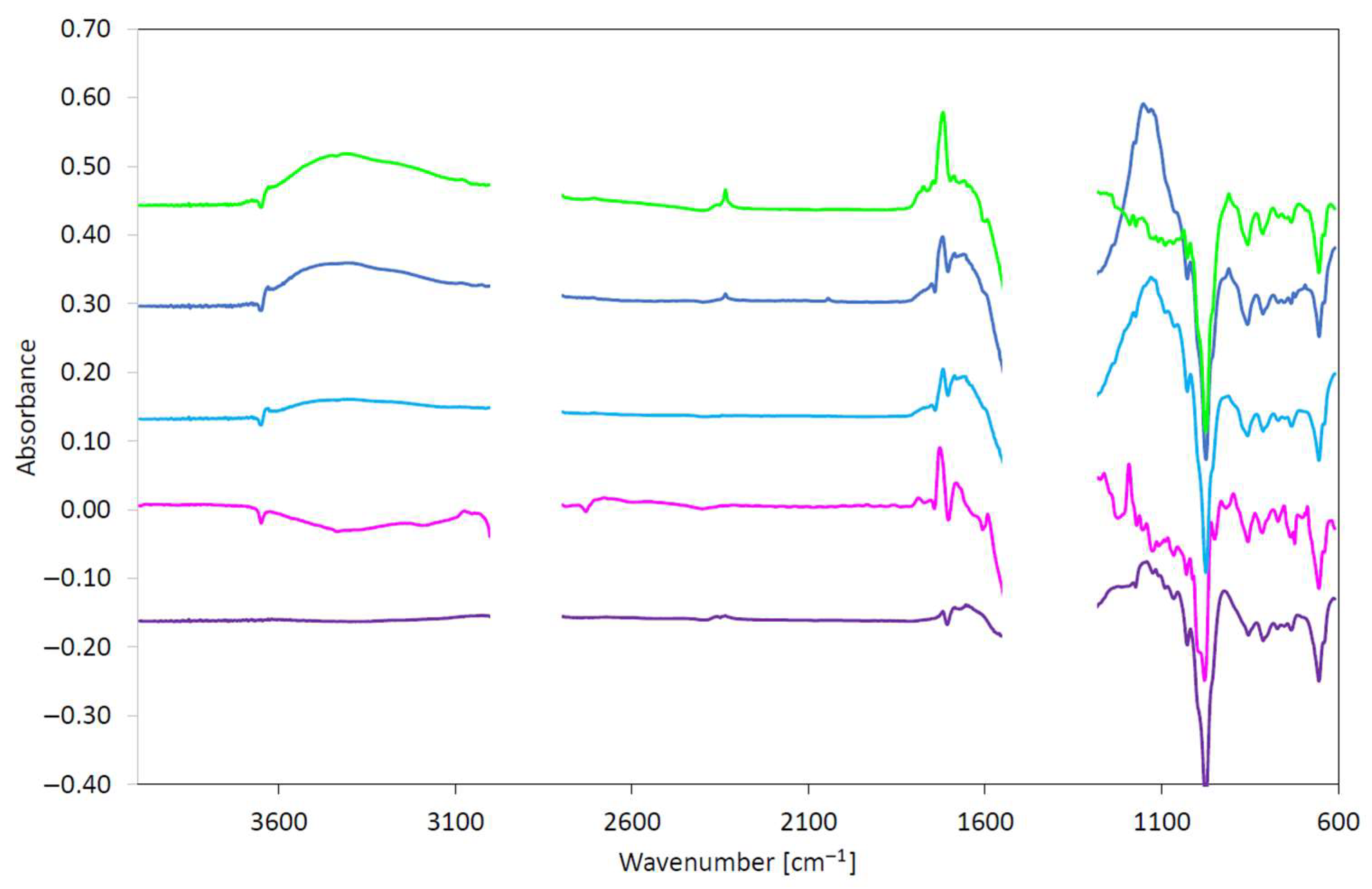
| Test Method | Phenolic Antioxidant Degradation | Carboxyl Structure Changes/Degree of Oxidation | Structure C-O Changes | EP Additive Degradation (1st Band) | EP Additive Degradation (2nd Band) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range 3600–3700 cm−1 | Range 1800–1640 cm−1 | Range 1200–1000 cm−1 | Range 1000–900 cm−1 | Range 700–650 cm−1 | ||||||
| Band | Absorbance | Band | Absorbance | Band | Absorbance | Band | Absorbance | Band | Absorbance | |
| Unit | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm |
| ASTM D5704 | 3651 | −0.020 | 1724 | 0.085 | 1190 | 0.060 | 981 | −0.247 | 657 | −0.121 |
| ASTM D8206 | 3651 | −0.018 | 1718 | 0.069 | 1132 | 0.203 | 977 | −0.226 | 653 | −0.063 |
| PN-EN 16091 | 3651 | −0.020 | 1721 | 0.095 | 1153 | 0.288 | 976 | −0.228 | 656 | −0.049 |
| ASTM D2272 | 3650 | −0.020 | 1721 | 0.142 | 1150 | 0.013 | 977 | −0.324 | 655 | −0.091 |
| PN-C-04080 | - | - | 1689 | 0.018 | 1140 | 0.086 | 977 | −0.258 | 656 | −0.073 |
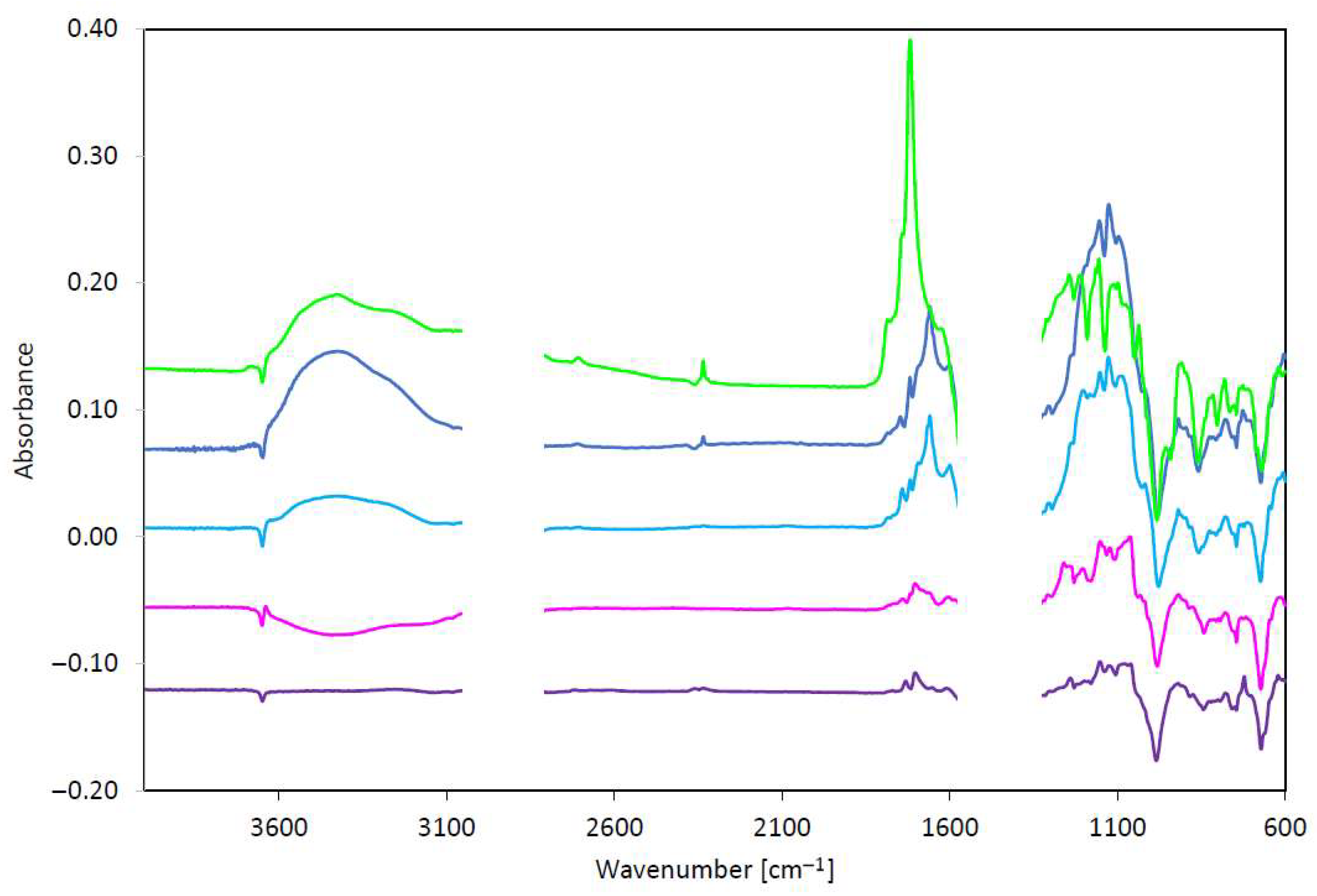
| Test Method | Phenolic Antioxidant Degradation | Carboxyl Structure Changes/Degree of Oxidation | Structure C-O Changes | EP Additive Degradation (1st Band) | EP Additive Degradation (2nd Band) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range 3600–3700 cm−1 | Range 1800–1640 cm−1 | Range 1200–1000 cm−1 | Range 1000–900 cm−1 | Range 700–650 cm−1 | ||||||
| Band | Absorbance | Band | Absorbance | Band | Absorbance | Band | Absorbance | Band | Absorbance | |
| Unit | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm | cm−1 | abs/0.1 mm |
| ASTM D5704 | 3652 | −0.010 | 1703 | 0.022 | 1152 1130 | 0.057 0.062 | 983 | −0.048 | 675 | −0.067 |
| ASTM D8206 | 3649 | −0.020 | 1660 | 0.094 | 1201 1151 1129 1086 | 0.117 0.132 0.144 0.129 | 976 | −0.051 | 673 | −0.046 |
| PN-EN 16091 | 3652 | −0.016 | 1660 | 0.111 | 1156 1124 | 0.188 0.203 | 981 | −0.061 | 674 | −0.031 |
| ASTM D2272 | 3649 | −0.021 | 1744 1718 | 0.129 0.295 | 1155 | 0.108 | 981 | −0.114 | 668 | −0.072 |
| PN-C-04080 | 3648 | −0.009 | 1704 | 0.017 | 1155 | 0.026 | 986 | −0.056 | 675 | −0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibińska, A.; Barglik, E.; Krasodomski, W.; Żółty, M.; Biernat, K. Thermoxidation Stability of Gear Oils for Electric Vehicles. Lubricants 2025, 13, 337. https://doi.org/10.3390/lubricants13080337
Skibińska A, Barglik E, Krasodomski W, Żółty M, Biernat K. Thermoxidation Stability of Gear Oils for Electric Vehicles. Lubricants. 2025; 13(8):337. https://doi.org/10.3390/lubricants13080337
Chicago/Turabian StyleSkibińska, Agnieszka, Ewa Barglik, Wojciech Krasodomski, Magdalena Żółty, and Krzysztof Biernat. 2025. "Thermoxidation Stability of Gear Oils for Electric Vehicles" Lubricants 13, no. 8: 337. https://doi.org/10.3390/lubricants13080337
APA StyleSkibińska, A., Barglik, E., Krasodomski, W., Żółty, M., & Biernat, K. (2025). Thermoxidation Stability of Gear Oils for Electric Vehicles. Lubricants, 13(8), 337. https://doi.org/10.3390/lubricants13080337






