Study on Current-Carrying Friction Characteristics and Corrosion Resistance of Carbon Brush/Collector Ring by Copper–Graphene Electrodeposition Process
Abstract
:1. Introduction
2. Materials and Experimental Section
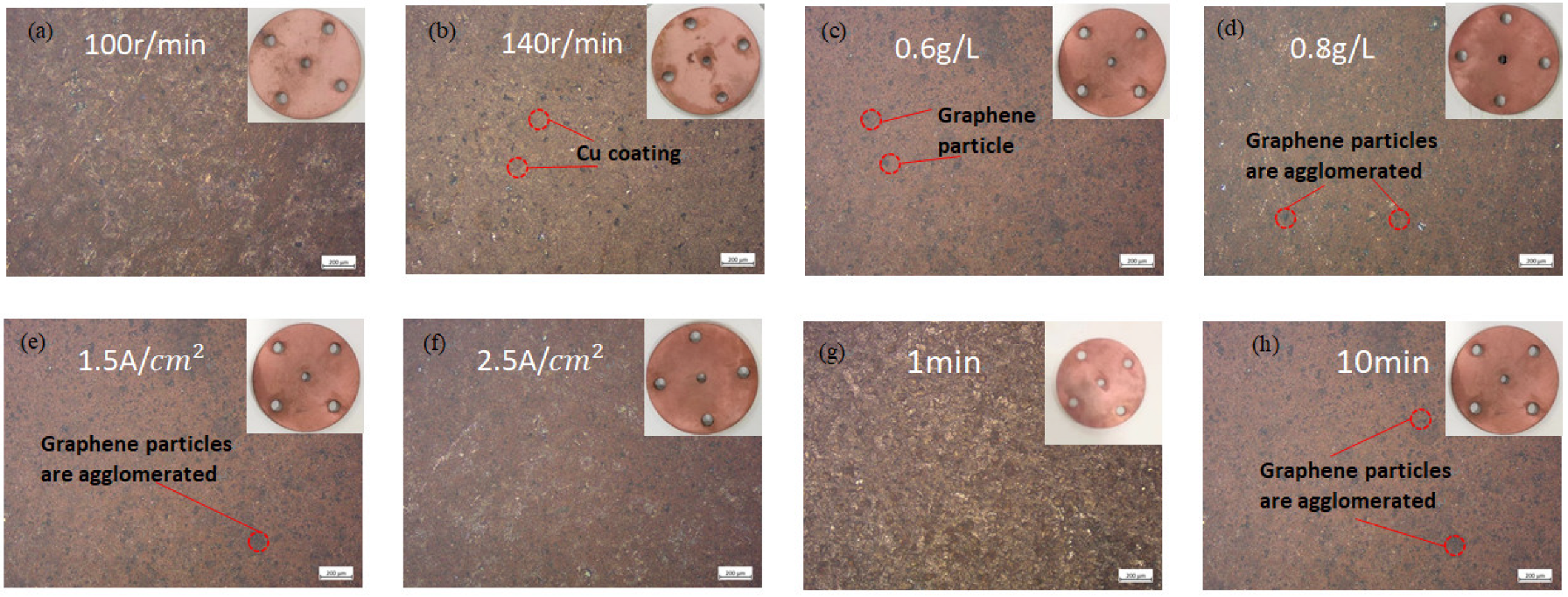
2.1. Production of Coating
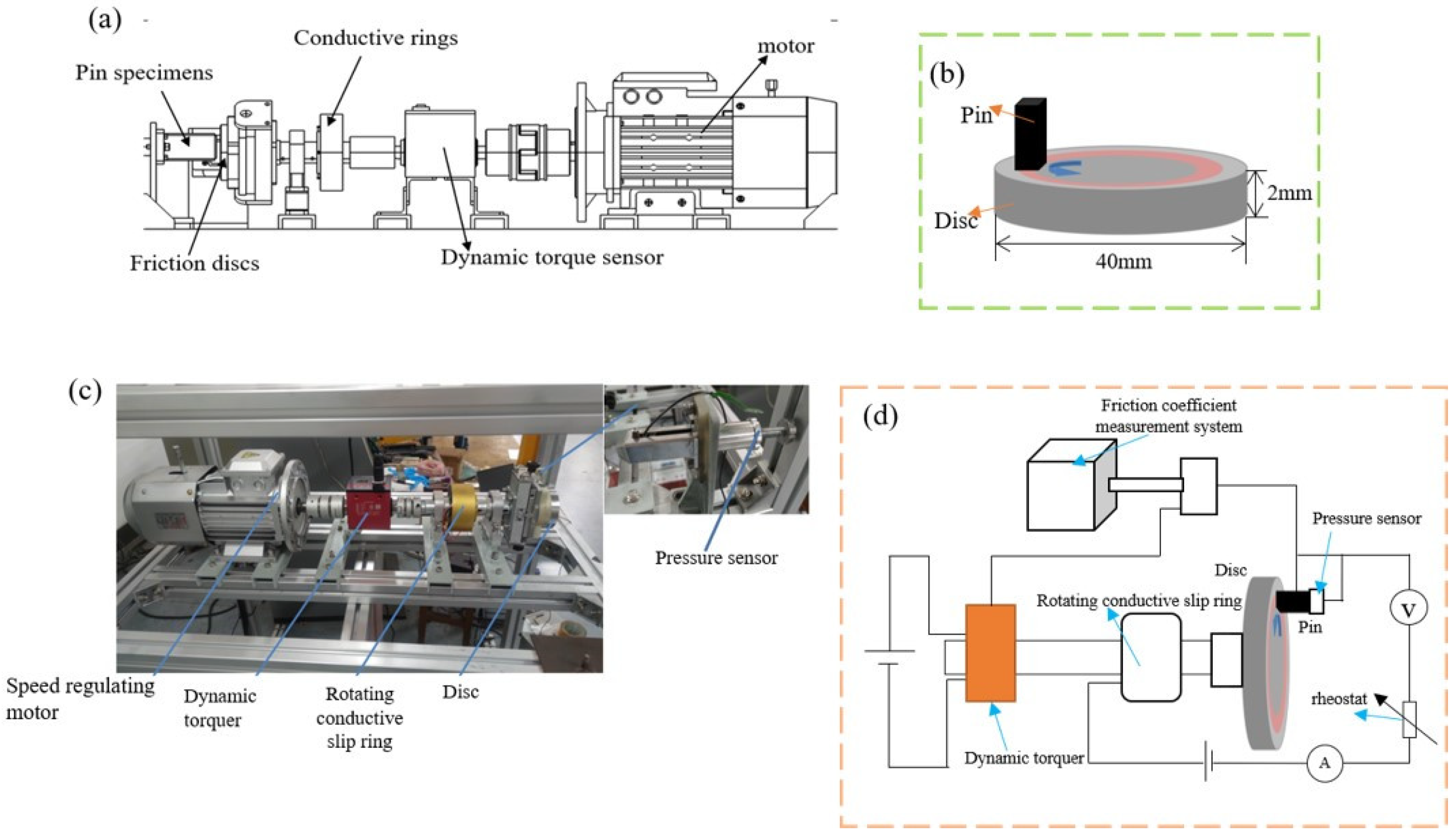
2.2. Current-Carrying Tribological Experiment
2.3. Coating Corrosion Resistance Test
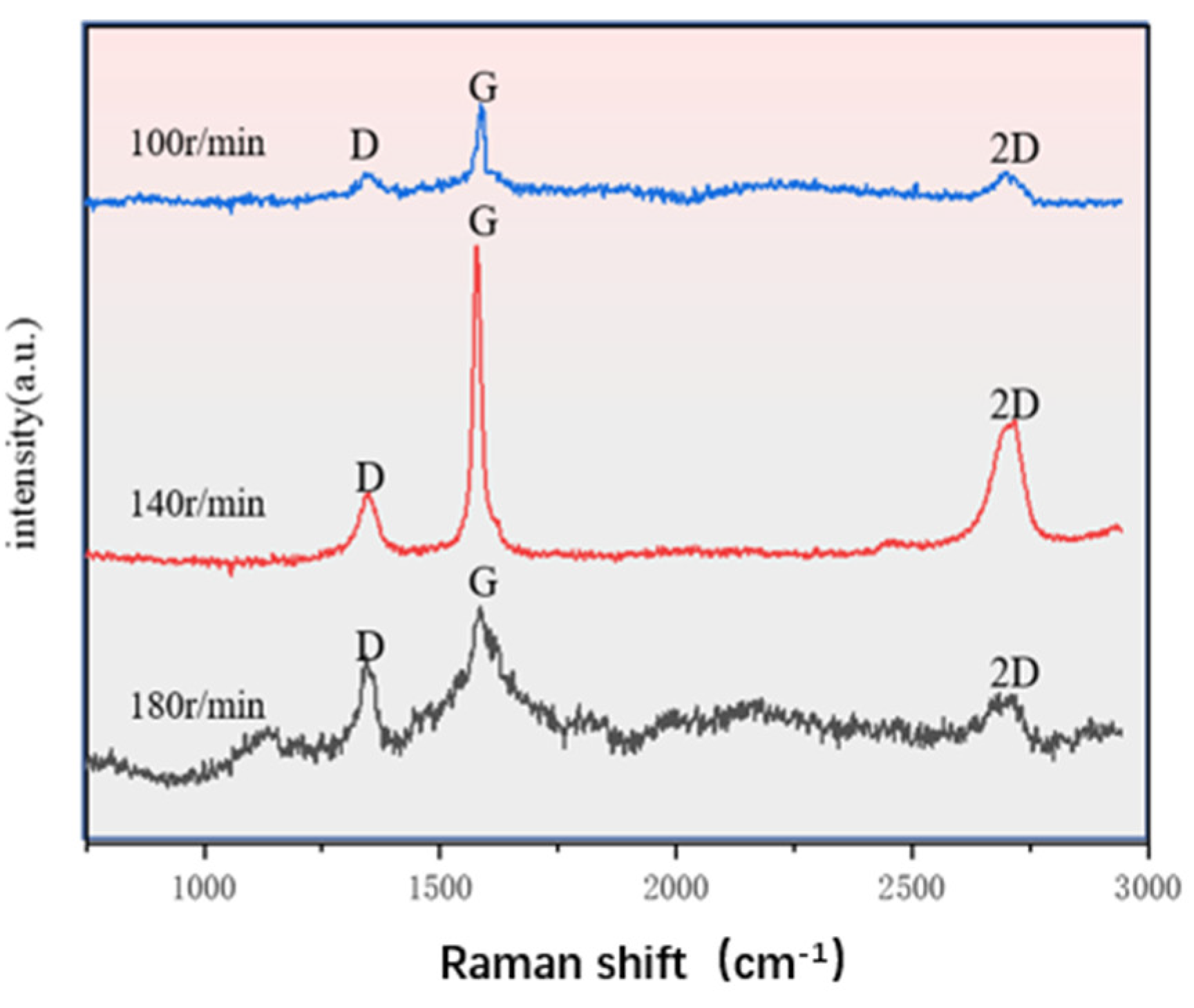
2.4. Raman Spectroscopy Analysis and EDS
3. Results and Discussion
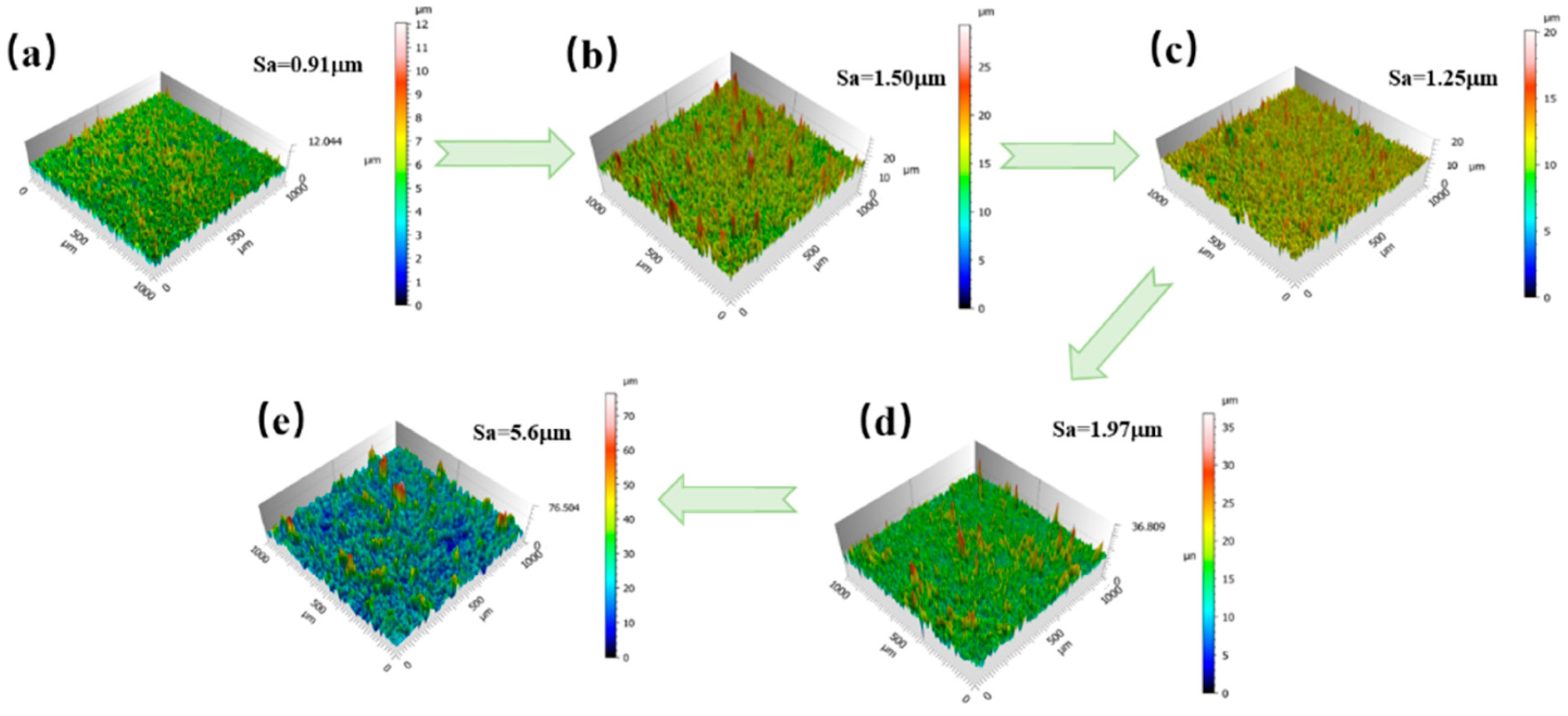
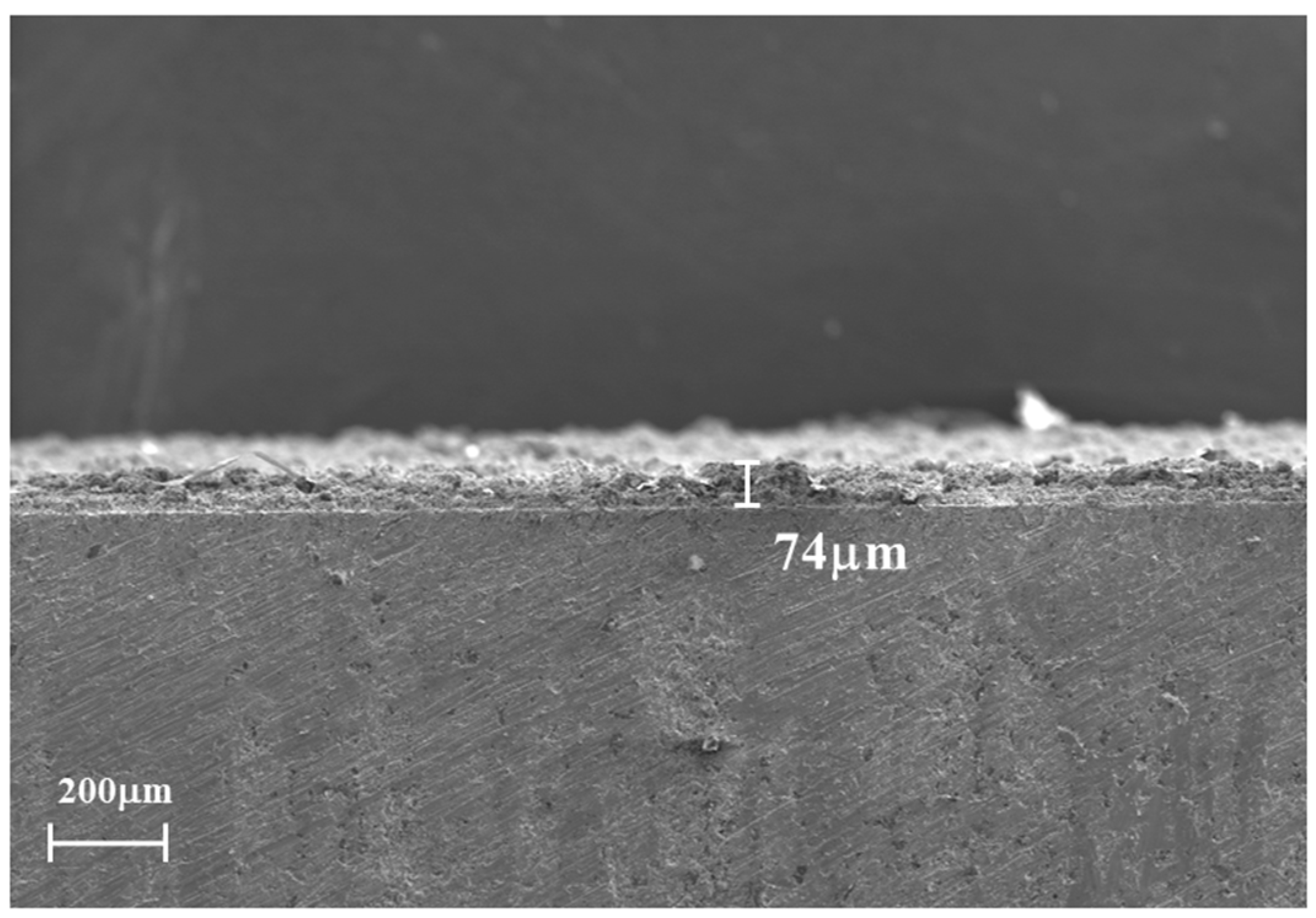
3.1. Composite Coating Characterization
3.2. Analysis of Current-Carrying Friction and Wear Characteristics
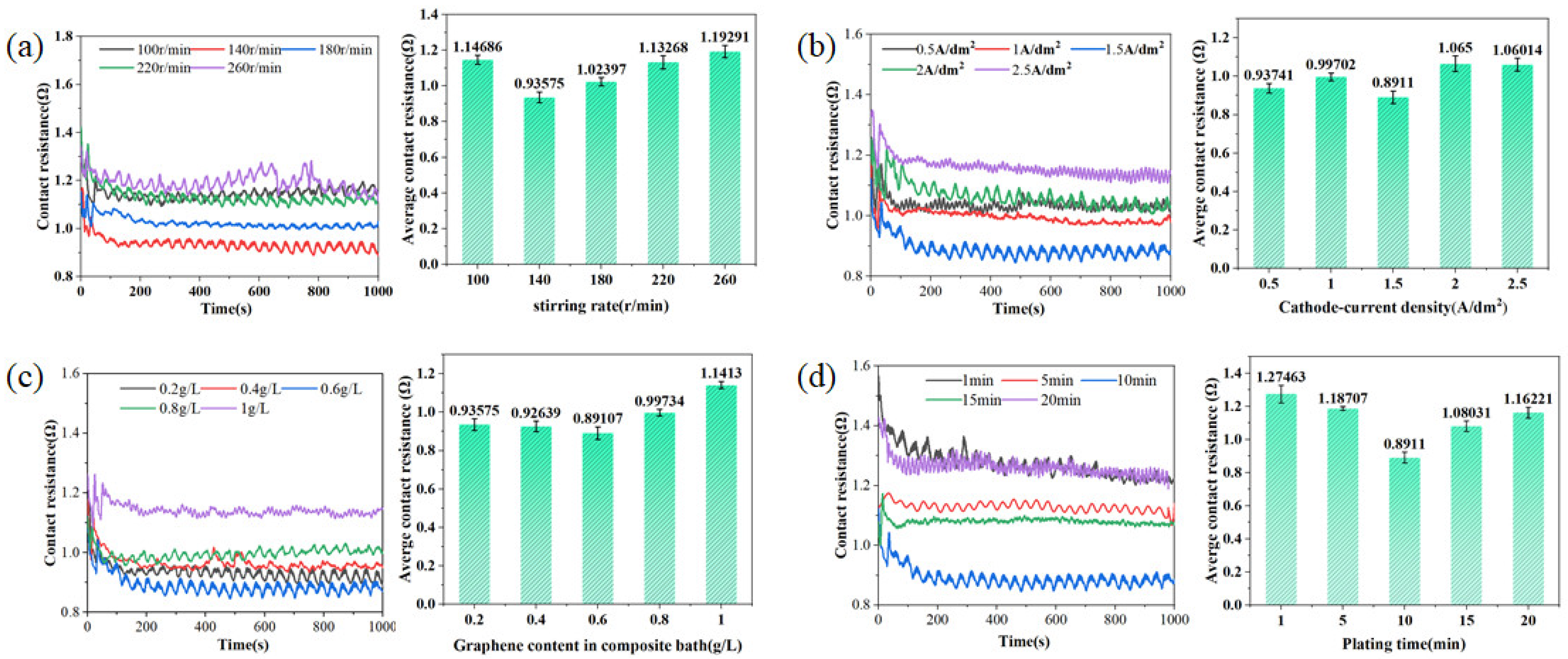
3.2.1. Cu-GNS Coating Contact Resistance Characteristics
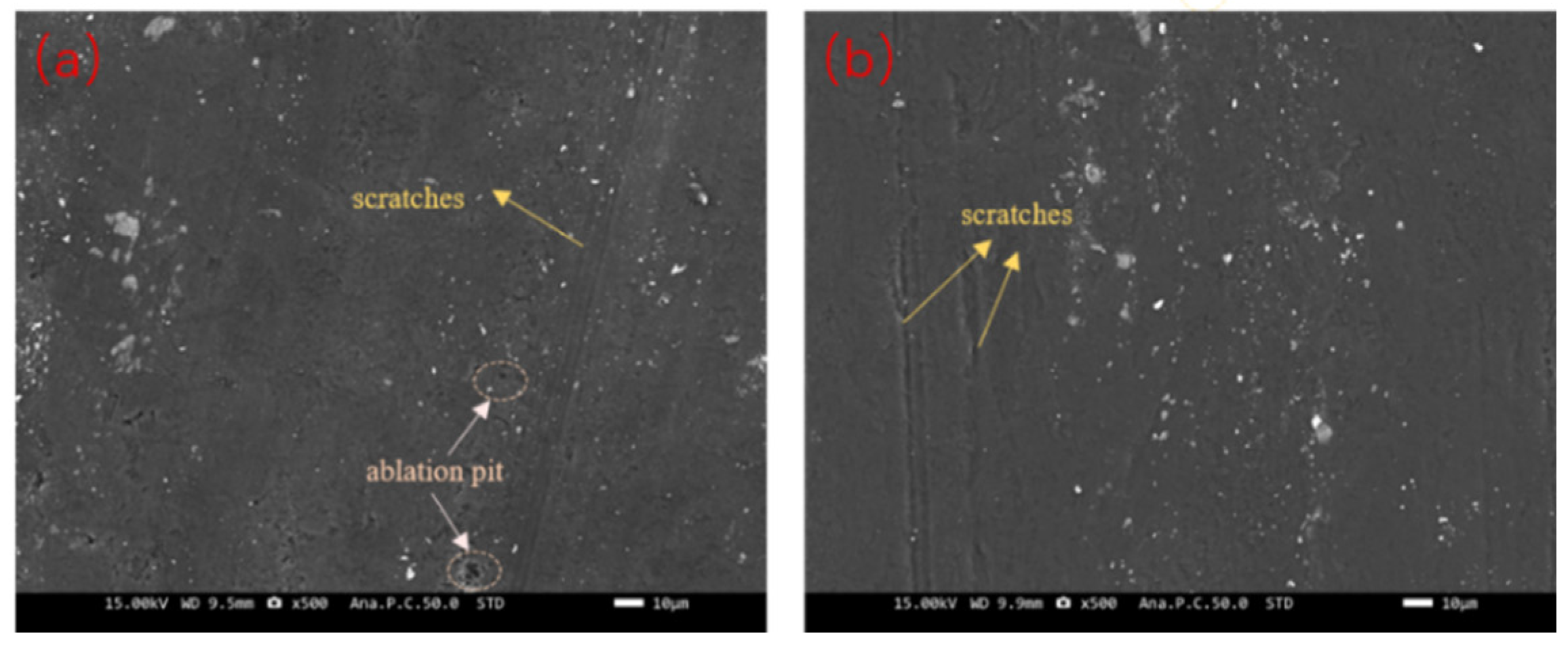
3.2.2. Friction and Wear Characteristics of Cu-GNS Coating
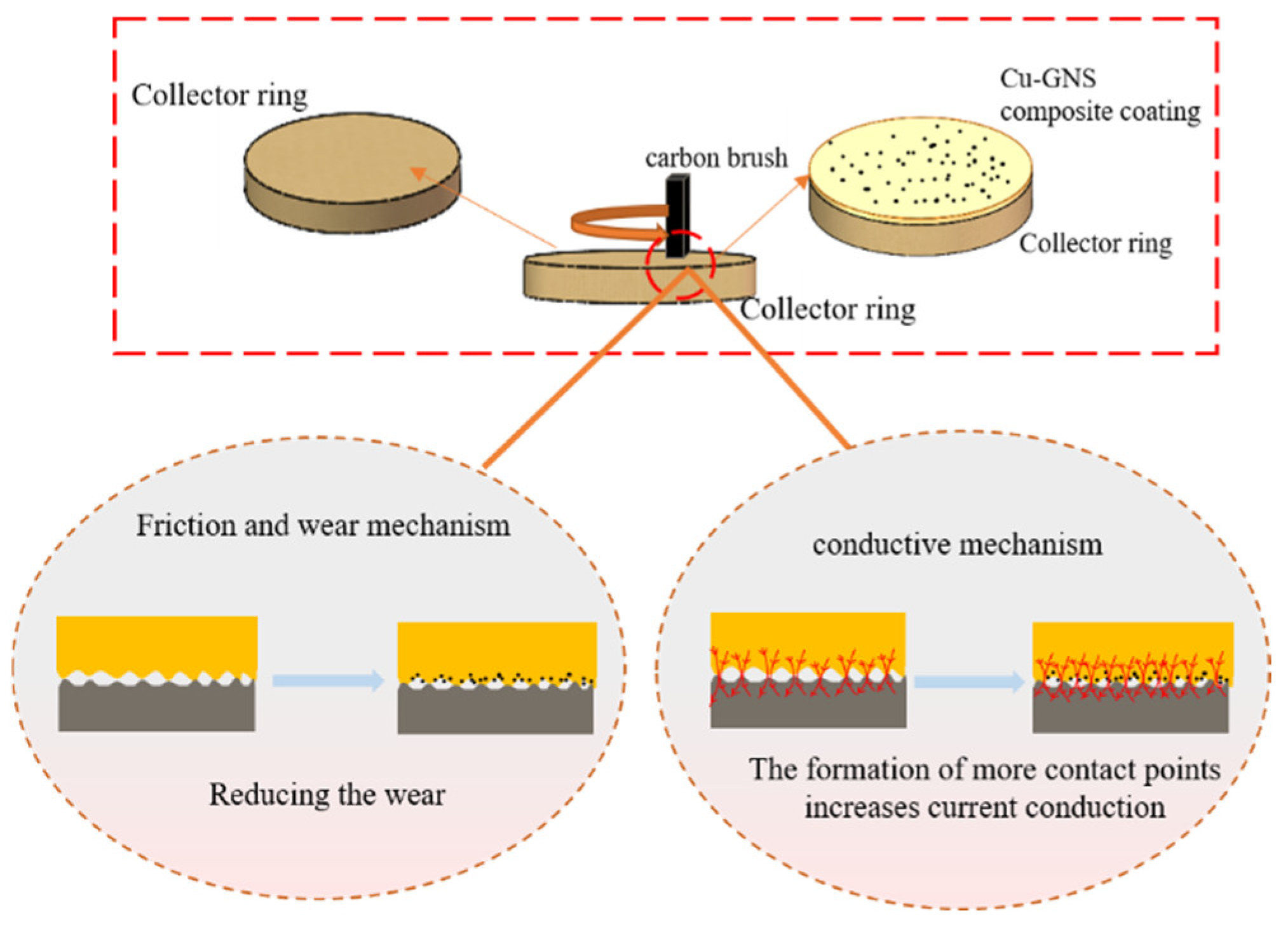
3.3. Study on Tribological Mechanism of Cu/Graphene Composite Coating
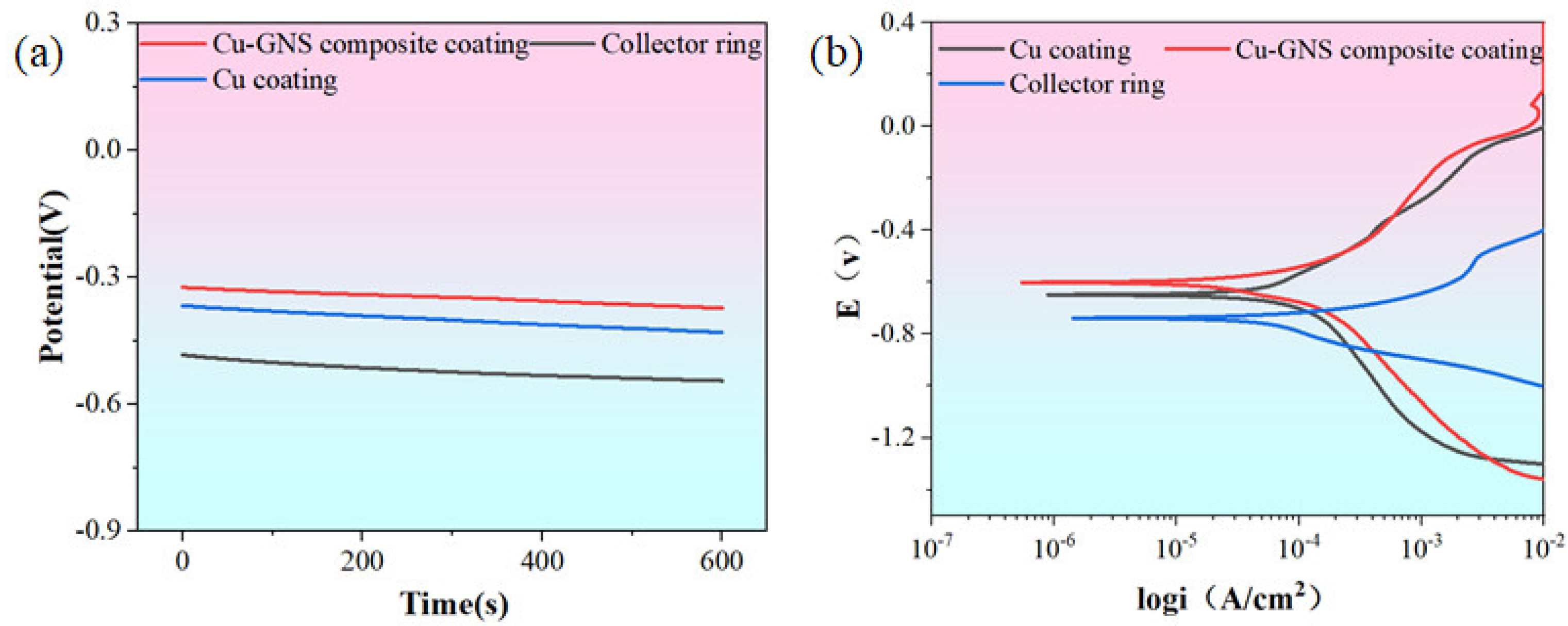
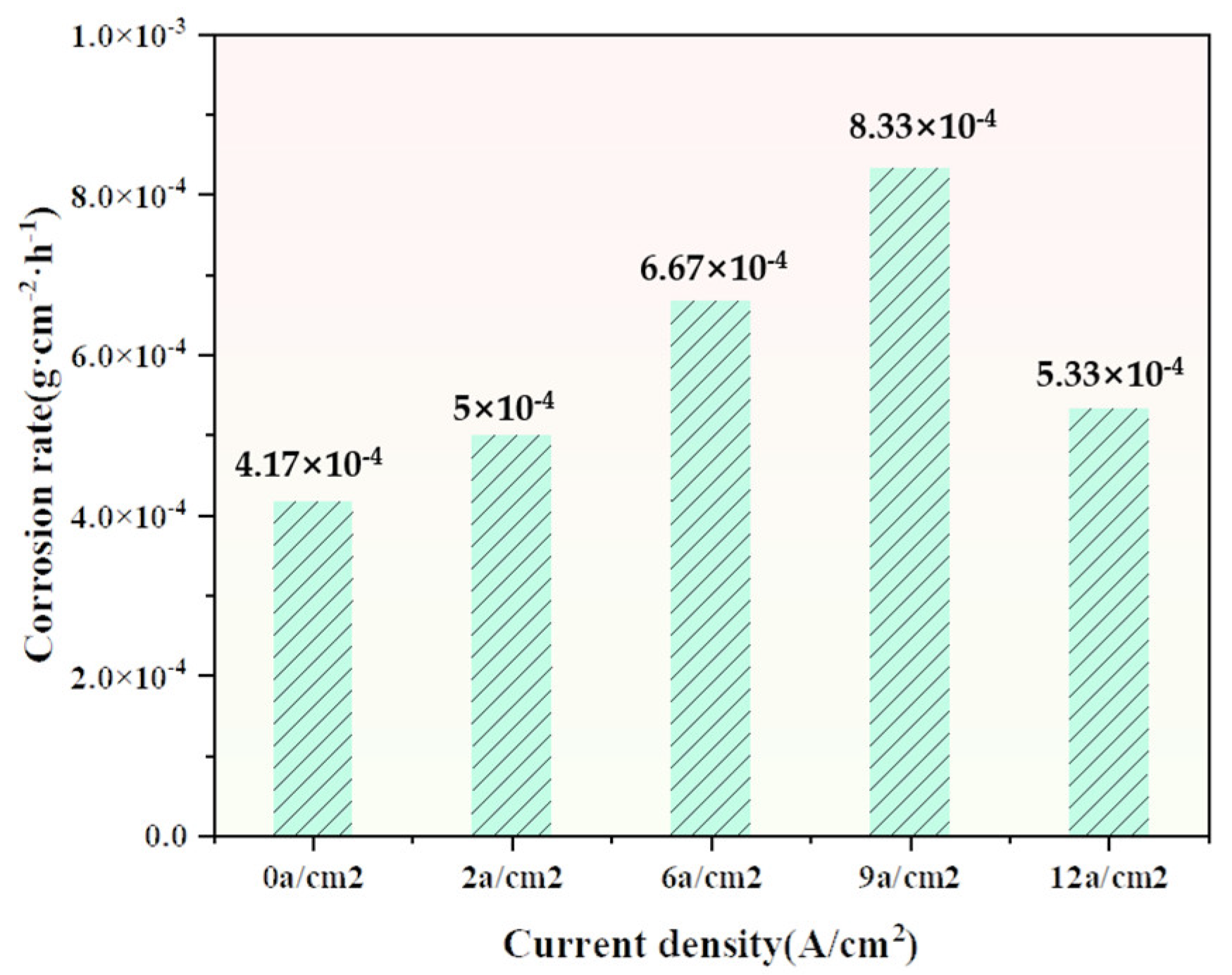
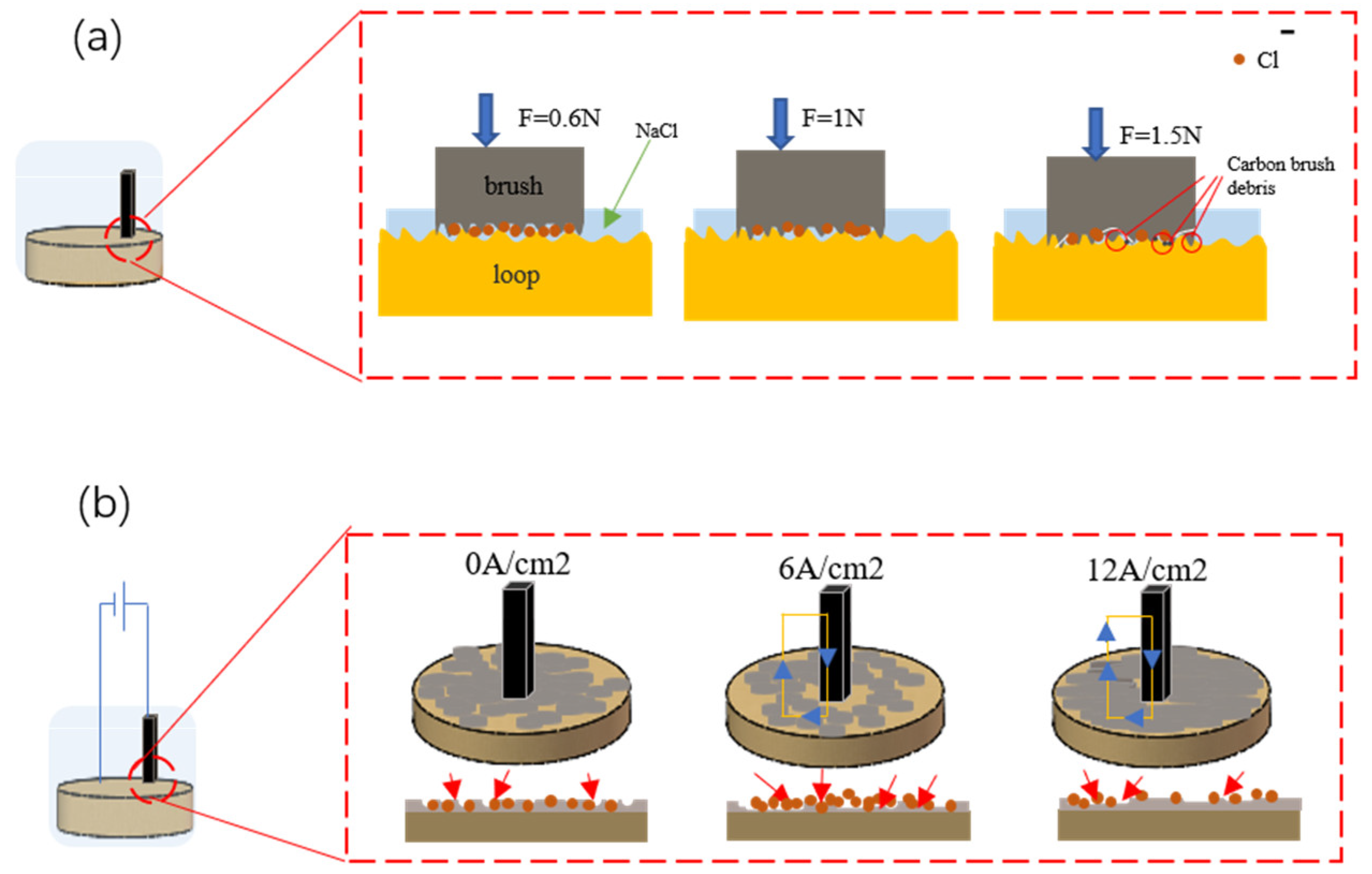
3.4. Research on the Corrosivity of the Collector Ring
3.4.1. Experiment on Erosive Electrochemistry
3.4.2. Immersion Experiment
3.4.3. Corrosion Mechanism Study
4. Conclusions
- In Cu–GNS composite coatings, the deposition rate and uniformity of graphene nanosheets (GNSs) play a crucial role in determining the current-carrying friction characteristics. The optimal electroplating parameters are a stirring speed of 140 r/min, a GNS concentration of 0.6 g/L, a plating time of 10 min, and a current density of 1.5 A/dm2. Under these conditions, the Cu–GNS composite coating demonstrates a 32.59% reduction in contact resistance, a 37.6% decrease in friction coefficient, and an 18.16% reduction in wear rate compared to conventional collector rings. Cu–GNS composite coating can greatly improve the current-carrying friction characteristics.
- The pressure and current density between the brush and the collector ring have a significant impact on the corrosion resistance of the collector ring. When there is no current or the current density is 12 A/cm2 and the brush pressure on the ring is 1n, the corrosion resistance of the collector ring surface can be effectively improved. Electrochemical tests and immersion experiments confirmed that the addition of graphene greatly improved the corrosion resistance of the material.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Qin, H.; Xu, X.; Zhang, X.; Guo, Z. The effect of surface texture and conductive grease filling on the tribological properties and electrical conductivity of carbon brushes. Tribol. Int. 2021, 153, 106637. [Google Scholar] [CrossRef]
- Huang, Q.; Song, C.; Liu, Z.; Hou, X.; Pang, X.; Sun, C.; Lu, H.; Wang, S.; Zhang, Y. Research progress on the characteristics of current-carrying tribology in electrical transmission. Space Sol. Power Wirel. Transm. 2024, 1, 37–47. [Google Scholar] [CrossRef]
- Yongzhen, Z.; Zhenghai, Y.; Bao, S.G.; Minle, S.; Sanmin, D. Frictional behavior of typical materials carrying current. J. Henan Univ. Sci. Technol. 2012, 33, 9–14. [Google Scholar]
- Tang, Q.; Huang, Y.; Sun, D.; Qunyang, L. Effect of Humidity on the Wear Behavior of Graphene Under Current Carrying Conditions. Tribol. Lett. 2025, 73, 8. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Lv, Y.; Li, Y.; Li, W.; Yang, W. Exploring the Effect of Interface Contact States on Brush/Ring Current-Carrying Friction. Lubricants 2024, 12, 461. [Google Scholar] [CrossRef]
- Deng, H.; Yin, L.; Chengfeng, Z.; Hong, W.; Guoqiang, G.; Juan, W. Effect of Interface Oxidation on the Friction and Wear Performance of Pantograph-Catenary Contact Pairs. Railw. Stand. Des. 2024, 68, 162. [Google Scholar] [CrossRef]
- Omran, H.N.; Eivani, A.R.; Farbakhti, M.; Jafarian, H.R. Tribological properties of copper-graphene (CuG) composite fabricated by accumulative roll bonding. J. Mater. Res. Technol. 2023, 25, 4650–4657. [Google Scholar] [CrossRef]
- Li, X.; Guo, B.; Yu, X.; Yang, C.; Zhou, S.; Cui, S.; Zhang, Z.; Li, W. Particle morphology dependence of the mechanical and electrical properties in the in-situ graphene reinforced Cu matrix composites. Compos. Part A Appl. Sci. Manuf. 2024, 179, 108032. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Liao, T.; Zhang, C.; Wu, X.; Liu, Y.; Qurashi, M.S.; Zheng, F.; Song, Q.; Han, P. Graphene/Cu composites: Electronic and mechanical properties by first-principles calculation. Mater. Chem. Phys. 2019, 231, 188–195. [Google Scholar]
- Asnavandi, M.; Ghorbani, M.; Kahram, M. Production of Cu–Sn–graphite–SiC composite coatings by electrodeposition. Surf. Coat. Technol. 2013, 216, 207–214. [Google Scholar] [CrossRef]
- Pasha, M.; Kaleemulla, M. Erosive wear behavior of sprayed metal matrix composites: An overview. Proc. Inst. Mech. Part J J. Eng. Tribol. 2013, 227, 1063–1075. [Google Scholar]
- Ji, X.; Kunpeng, G.; Yayun, B.; Zhongfa, M.; Fengtao, W.; Dai, H. Effect of Nb addition on the corrosion and wear resistance of laser clad AlCr2FeCoNi high-entropy alloy coatings. Lubricants 2023, 12, 5. [Google Scholar] [CrossRef]
- Deng, C.; Yongzhen, Z.; Fei, Z.; Bao, S.G. Dynamic variation of arc discharge and its effect on corrosion direction under current-carrying sliding. Proc. Inst. Mech. Part J J. Eng. Tribol. 2019, 233, 380–392. [Google Scholar]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs, and emissions. Friction 2017, 5, 263–284. [Google Scholar]
- Zhan, W.; Huang, P. Numerical analysis of time-varying wear with elastic deformation in line contact. Friction 2019, 7, 143–152. [Google Scholar]
- Li, Z.; Lv, X.; Yaru, C.; Xueqiang, L.; Shuilin, X.; Meiyun, Z. To improve the current carrying friction pair tribological characteristics of surface treatment technology review. China Surf. Eng. 2024, 487, 130989. [Google Scholar]
- Chen, W.; Feng, Y.; Wan, Y.; Zhang, L.; Yang, D.; Gao, X.; Yu, Q.; Wang, D. Investigation on anti-wear and corrosion-resistance behavior of steel-steel friction pair enhanced by ionic liquid additives under conducive conditions. Tribol. Int. 2023, 177, 108002. [Google Scholar]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar]
- Xi, H.; He, P.; Liu, S.; Guozheng, M.; Hailou, W.; Zhenlin, L. Research status and prospect of conductive wear-resistant self-lubricating coatings. Surf. Technol. 2019, 48, 353–363. [Google Scholar]
- Zhang, H.; Le, K.; Wang, C.; Sun, J.; Xu, S.; Liu, W. Influence of Deposition Temperature on the Structure and Current-Carrying Friction Performance of Cu Films by DC Magnetron Sputtering Technology. Lubricants 2023, 11, 8. [Google Scholar]
- Xia, Y.; Cao, Y.; Feng, X. A comparative study on the electrical and tribological characteristic of magnetron sputtered Ag, Cu and Al films under current-carrying friction. Ind. Lubr. Tribol. 2021, 73, 1219–1225. [Google Scholar]
- Skrzypek, M.; Wojciechowski, Ł.; Kałużny, J.; Boncel, S.; Marek, A.A.; Runka, T.; Błaszkiewicz, P. Carbon Nanotubes Decorated with Nickel or Copper as Anti-Wear and Extreme-Pressure Additives for Greases. Lubricants 2024, 12, 448. [Google Scholar] [CrossRef]
- Yan, P.; Yuhong, L.; Tongqing, W.; Xinchun, L. Effect of a Cu seed layer on electroplated Cu film. Microelectron. Eng. 2013, 105, 18–24. [Google Scholar]
- Hui, Y.; Liu, G.; Yan, T.; Liu, M.; Du, L. Influence of sliding frequency on current-carrying friction and wear properties of supersonic plasma sprayed Mo coatings. China Surf. Eng. 2019, 32, 109–119. [Google Scholar]
- Xu, J.; Zhou, L.; Ma, G.; Li, G.; Zhao, H.; Li, Y.; Tan, N.; Wang, H. Laser cladding Mo-based coatings on copper alloys to improve the current-carrying tribological properties of Cu/Al friction pairs. Surf. Coat. Technol. 2024, 487, 130989. [Google Scholar]
- Baiocco, G.; Menna, E.; Mingione, E.; Rubino, G.; Ucciardello, N. Effect of process parameters and film stratification on morphology and performance of auto-lubricating Ni-GnP electroplated coated steel. Eng. Fail. Anal. 2024, 161, 108223. [Google Scholar]
- Pritima, D.; Ravichandran, M.; Veerappan, G.; Mohanavel, V. Mechanical properties and corrosion behavior of SiC reinforced high entropy alloy (Ni-Co-Fe-Ti) matrix composites produced by microwave and conventional sintering techniques. Surf. Topogr. Metrol. Prop. 2024, 12, 045002. [Google Scholar]
- Wen, X.; Qu, R.; Yuwen, F.; Yanan, Q.; Tingmei, W.; Ruiqing, Y.; Jinjun, L. Genesis and Robust Tribological Property of Friction-Induced Cu-Graphene Self-lubricating Composite Under Electrical Contact. Tribol. Lett. 2023, 71, 82. [Google Scholar]
- Zhang, L.; Shi, Q.; Ge, X. Comparative Study of the Friction Behavior of Functionalized Graphene Oxide Additives Under Electric Stimulations. Lubricants 2024, 12, 455. [Google Scholar] [CrossRef]
- Hou, X.; Tang, H.; Leyang, D.; Xianglin, L.; Guanglin, L.; Zhiqiang, A.; Yanhua, J.; Chenxing, S.; Hong, W. Potassium borate/graphene nanocomposite lubricant additive with anti-friction/wear and anti-corrosion functions for marine diesel engine burning low sulfur fuel. Wear 2024, 550, 205395. [Google Scholar]
- Cho, K.H.; Hong, U.S.; Lee, K.S.; Ho, J. Tribological properties and electrical signal transmission of copper–graphite composites. Tribol. Lett. 2007, 27, 301–306. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, R.; Zuo, X.; Wenxin, X. Tribo-electrical behaviors of CNTs-MoS2/Cu composites under sliding electrical contact with brass. Tribol. Int. 2023, 180, 108207. [Google Scholar] [CrossRef]
- Cao, Z.; Li, R.; Shou, M.; Luo, R.; Wei, B.; Wang, T. Mechanical properties and tribological behaviors of Ag/graphene composite coating under sliding friction and current-carrying fretting. Tribol. Int. 2024, 197, 109811. [Google Scholar] [CrossRef]
- Almonti, D.; Salvi, D.; Ucciardello, N.; Vesco, S. Enhanced Wear Resistance and Thermal Dissipation of Copper–Graphene Composite Coatings via Pulsed Electrodeposition for Circuit Breaker Applications. Materials 2024, 17, 6017. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Xiong, D.; He, Y.; Ji, Y.; Qin, Y. Preparation and tribological behavior of Ni-graphene composite coating under room temperature. Appl. Surf. Sci. 2016, 361, 49–56. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, D.; Chaudhry, V. Tribological and corrosion behavior of hydrided zirconium alloy. Tribol. Int. 2024, 195, 109660. [Google Scholar]
- Hu, N.; Dong, X.; He, X.; Browning, J.F.; Schaefer, D.W. Effect of sealing on the morphology of anodized aluminum oxide. Corros. Sci. 2015, 97, 17–24. [Google Scholar] [CrossRef]









| Pressure/N | Open Circuit Potential/v |
|---|---|
| 0 | −0.59772 |
| 0.3 | −0.58631 |
| 0.6 | −0.5233 |
| 1 | −0.52057 |
| 1.5 | −0.52629 |
| Pressure/N | Ecorr (v) | Icorr (A/cm2) |
|---|---|---|
| 0 | −1.0341 | 9.56 × 10–5 |
| 0.3 | −0.8871 | 6.17 × 10−5 |
| 0.6 | −0.8115 | 5.05 × 10−5 |
| 1 | −0.7662 | 2.47 × 10−5 |
| 1.5 | −0.9594 | 6.84 × 10−5 |
| Sample | Open Circuit Potential/v |
|---|---|
| Collector ring | −0.52057 |
| Cu–GNS composite coating | −0.3484 |
| Sample | Ecorr (v) | Icorr (A/cm2) |
|---|---|---|
| Collector ring | −0.7662 ± 0.012 | (2.47 ± 0.09) × 10−5 |
| Cu–GNS composite coating | −0.6195 ± 0.008 | (2.21 ± 0.007) × 10−5 |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | Qdl (S∙sⁿ/cm2) | n1 | Qbl (S∙sⁿ/cm2) | Rbl (Ω·cm2) | n2 |
|---|---|---|---|---|---|---|---|
| Collector ring | ) × 10−3 | 0.75 | - | - | - | ||
| Cu–GNS composite coating | (4.98 ± 0.22) × 10−3 | 0.72 | (1.61 ± 0.08) × 10−3 | 6.101 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, J.; Li, C.; Li, Y.; Zhang, X. Study on Current-Carrying Friction Characteristics and Corrosion Resistance of Carbon Brush/Collector Ring by Copper–Graphene Electrodeposition Process. Lubricants 2025, 13, 162. https://doi.org/10.3390/lubricants13040162
Zhao M, Li J, Li C, Li Y, Zhang X. Study on Current-Carrying Friction Characteristics and Corrosion Resistance of Carbon Brush/Collector Ring by Copper–Graphene Electrodeposition Process. Lubricants. 2025; 13(4):162. https://doi.org/10.3390/lubricants13040162
Chicago/Turabian StyleZhao, Meiyun, Jianwen Li, Chenshi Li, Yangyang Li, and Xiaolong Zhang. 2025. "Study on Current-Carrying Friction Characteristics and Corrosion Resistance of Carbon Brush/Collector Ring by Copper–Graphene Electrodeposition Process" Lubricants 13, no. 4: 162. https://doi.org/10.3390/lubricants13040162
APA StyleZhao, M., Li, J., Li, C., Li, Y., & Zhang, X. (2025). Study on Current-Carrying Friction Characteristics and Corrosion Resistance of Carbon Brush/Collector Ring by Copper–Graphene Electrodeposition Process. Lubricants, 13(4), 162. https://doi.org/10.3390/lubricants13040162





