Biolubricants Based on Epoxidized Vegetable Oils: A Review on Chemical Modifications, Tribological Properties, and Sustainability
Abstract
1. Introduction
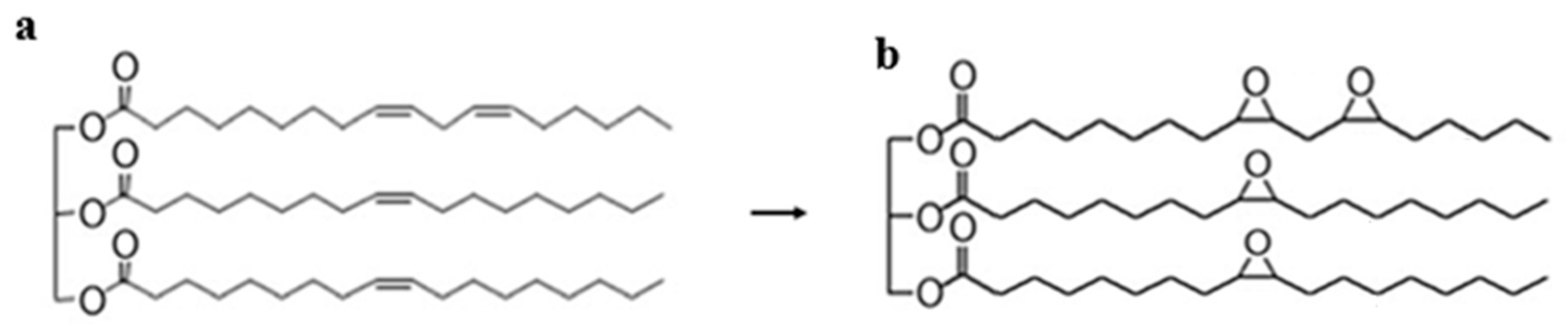
2. Epoxidation of Vegetable Oils: Fundamentals
2.1. Methods of Epoxidation and Influence in Lubrication
2.2. Common Epoxidized Vegetable Oils
2.3. Post-Epoxidation Modifications for Biolubricant Production
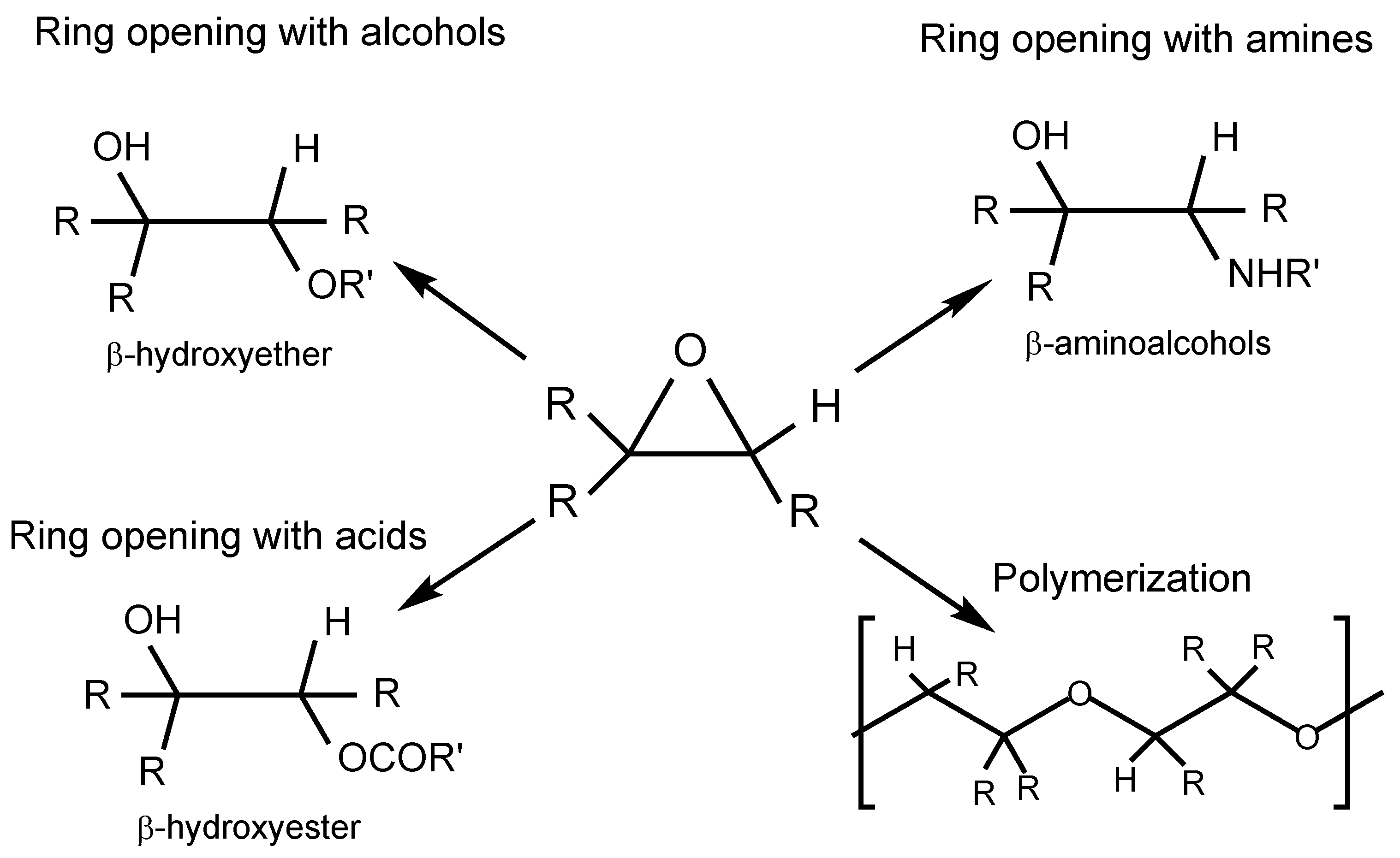
2.3.1. Ring Opening with Alcohols and Polyols
2.3.2. Ring Opening with Amines and Aminoalcohols
2.3.3. Ring Opening with Acids and Anhydrides (Esterification)
2.3.4. Polymerization
- Flowability vs. Network Strength: Increasing the crosslink density enhances viscosity and mechanical integrity; however, excessive structuring can lead to gel-like or solid-like materials that lose the fluidity required for lubrication [58].
- Processability: Once cured, crosslinked EVO derivatives become insoluble, complicating their blending with other base oils or additives and limiting their applicability in multi-component lubricant systems [59].
- Recyclability and Reversibility: Permanent covalent networks hinder reprocessing and recycling. This is a concern for sustainable lubricant design, where reversible or dynamic crosslinking chemistries (e.g., Diels–Alder and hydrogen bonding) could offer more circular solutions [60].
2.4. Additional Reported Routes Relevant to Biolubricants
- CO2 carbonation: The conversion of epoxides into cyclic carbonates provides a direct route to more polar oils with enhanced oxidative stability and tuneable viscosity [61]. However, these reactions often require high pressures and specific catalysts, which can hinder scalability. Moreover, while cyclic carbonates improve biodegradability, their stability under hydrolytic conditions remains to be explored.
- Thiol–epoxy click chemistry: This reaction efficiently incorporates sulphur-containing groups that enhance the extreme-pressure (EP) performance without relying on conventional ZnDTP-type additives. The method is fast and selective; however, the introduction of sulphur moieties raises concerns about odour, oxidative stability, and possible environmental persistence [62].
- Phosphorus-based ring opening: formation of phosphate esters imparts both anti-wear and fire-resistant properties. Despite their strong performance, their environmental acceptability is debated because phosphorus-containing compounds may contribute to aquatic toxicity and catalyst poisoning in engines [63].
2.5. Hybridization with Other Oils and Additives
- Dispersion and stability: Nanoparticles often aggregate or sediment in polar-modified EVO matrices, reducing their long-term reliability [68].
- Interfacial compatibility: The polarity mismatch between EVOs and certain ILs or nanoparticles can hinder uniform blending, sometimes requiring the use of surfactants or surface modification [69].
- Cost and scalability: ILs and advanced nanomaterials can substantially increase formulation costs, restricting industrial uptake.
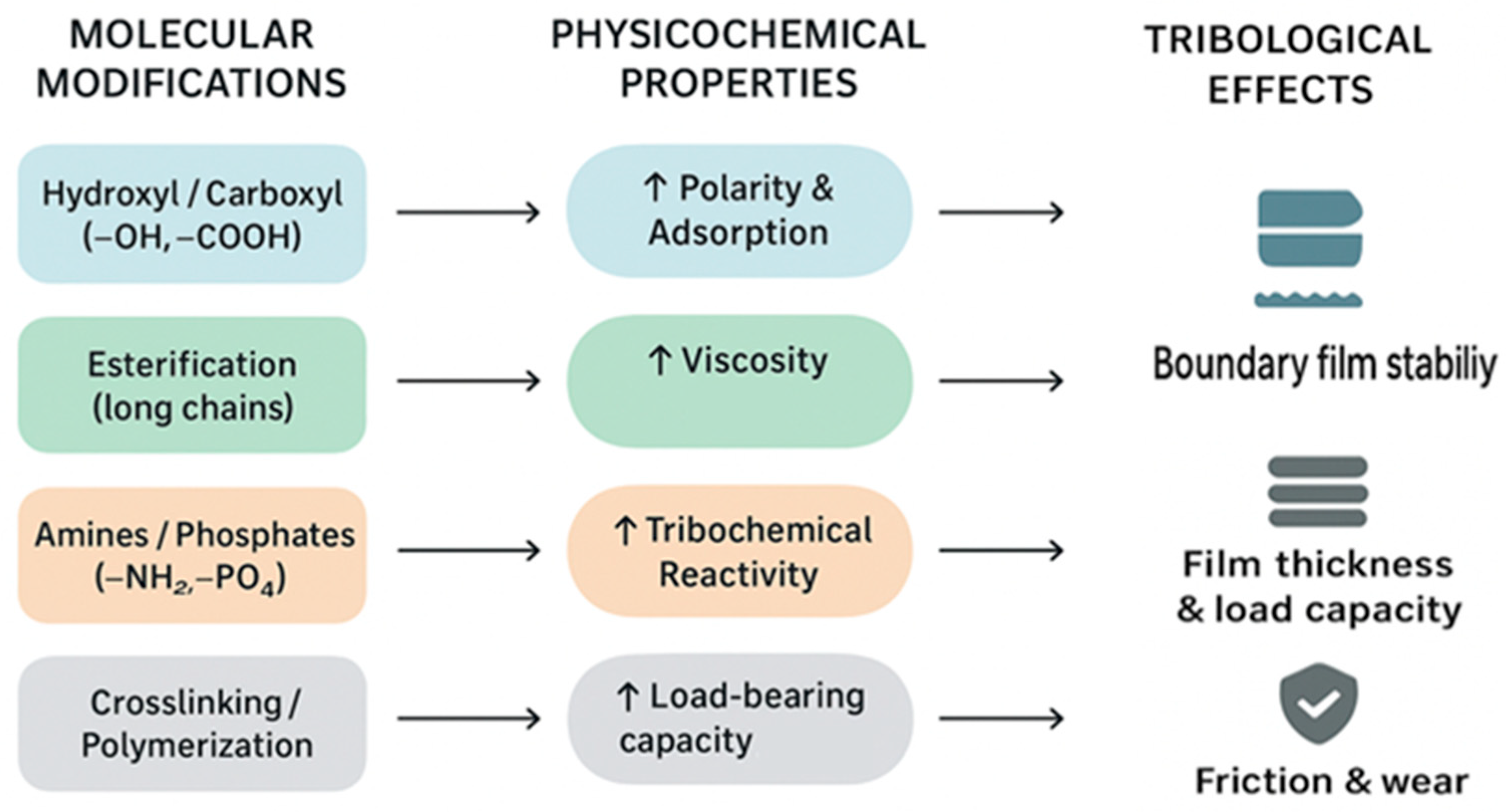
3. Structure–Tribological Property Relationship of Post-Epoxidation Derivatives
3.1. Integrative Summary
- Polar groups (–OH, –NH2, –PO4): enhance boundary lubrication but may reduce the oxidative stability [71].
- Long aliphatic chains: improve oxidative resistance and VI but reduce surface adsorption [72].
- Crosslinking: increases load-bearing capacity but reduces biodegradability and flowability [75].
- Castor oil: Best suited for high-load, high-friction, or industrial applications because of its consistently high viscosity and strong tribological behaviour.
- Canola oil: Offers an excellent balance of properties, making it highly versatile for automotive and general-purpose lubricants.
- Soybean oil: A strong general-purpose base oil that is suitable for moderate-load applications after modification.
- Sunflower oil: Limited by moderate oxidative stability and lower tribological performance but is tuneable.
- Palm oil: Highly thermally stable but constrained by poor low-temperature fluidity, restricting its use to high-temperature applications.
3.2. Viscosity and Viscosity Index (VI)
3.3. Coefficient of Friction (COF) Across Lubrication Regimes
3.4. Wear Resistance and Tribofilm Formation
3.5. Effect of Polarity and Surface Chemistry
4. Sustainability and Biodegradability
4.1. Biodegradability Assessment
4.2. Ecotoxicity and Environmental Trade-Offs
4.3. Life Cycle Assessment (LCA)
4.4. Sustainability–Performance Balance
5. Gaps and Future Work
- Developing integrated and standardized protocols linking tribology, biodegradability, and ecotoxicity.
- Advancing bio-based and recyclable catalytic systems for greener EVO modification.
- Exploring dynamic covalent and self-healing lubricants to combine durability and environmental compatibility.
- Leveraging computational and AI-based modeling to predict structure–property–performance relationships.
- Embedding LCA and TEA frameworks to assess sustainability and scalability from the design stage.
6. Conclusions
- Structure–property correlation: Polar functionalities (–OH, –NH2, –PO4) improve boundary lubrication and wear resistance, whereas esterification and long-chain modifications enhance viscosity index (VI) and oxidative stability.
- Performance–sustainability trade-offs: Modifications that increase tribological performance (e.g., crosslinking and amine incorporation) may reduce biodegradability or increase ecotoxicity.
- Versatility of EVOs: They can serve as base oils, reactive intermediates, or components in hybrid systems with nanoparticles, ionic liquids, and multifunctional additives.
- Environmental promise: EVO-based lubricants derived from renewable feedstocks offer a reduced carbon footprint and improved biodegradability compared to petroleum oils.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EVOs | Epoxidized vegetable oils |
| ESBO | Epoxidized soybean oil |
| PAO | Polyalphaolefin |
| VI | Viscosity index |
| OIT | Oxidation induction time |
| EP | Extreme pressure |
| LCA | Life cycle assessment |
| TEA | Techno-economic analysis |
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Zeng, Y.; Shang, Z.; Zheng, Z.; Shi, N.; Yang, B.; Han, S.; Yan, J. A Review of Chemical Modification of Vegetable Oils and Their Applications. Lubricants 2024, 12, 180. [Google Scholar] [CrossRef]
- Panchal, T.M.; Patel, A.; Chauhan, D.D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Woma, T.Y.; Lawal, S.A.; Abdulrahman, A.S.; Olutoye, M.A.; Ojapah, M.M. Vegetable oil based lubricants: Challenges and prospects. Tribol. Online 2019, 14, 60–70. [Google Scholar] [CrossRef]
- Saurabh, T.; Patnaik, M.; Bhagt, S.L.; Renge, V.C. Epoxidation of vegetable oils: A review. Int. J. Adv. Eng. Technol. 2011, 17, 302. [Google Scholar]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B Environ. 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Muszyński, M.; Nowicki, J. Enzymatic synthesis of rapeseed oil-based lubricants. Ind. Crops Prod. 2013, 45, 25–29. [Google Scholar] [CrossRef]
- Kerman, C.O.; Gaber, Y.; Ghani, N.A.; Lämsä, M.; Hatti-Kaul, R. Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts. J. Mol. Catal. B Enzym. 2011, 72, 263–269. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Luo, C.; Xia, X.; Liu, Z.; Zhao, Y.; Du, F.; Jin, X. Opportunities and Emerging Challenges of the Heterogeneous Metal-Based Catalysts for Vegetable Oil Epoxidation. ACS Sustain. Chem. Eng. 2022, 10, 7426–7446. [Google Scholar] [CrossRef]
- Cogliano, T.; Russo, V.; Eränen, K.; Tesser, R.; Di Serio, M.; Salmi, T. Comparison of continuous technologies for vegetable oils epoxidation. Chem. Eng. Sci. 2025, 301, 120726. [Google Scholar] [CrossRef]
- Roslan, A.; Jalil, M.J.; Azmi, I.S.; Aznizam, N.S.; Mustapha, S.A.; Masri, A.N.; Mubarak, N.M.; Hosseini-Bandegharaei, A. In situ epoxidation of hybrid waste cooking oil and oleic acid via peracid mechanism. Sci. Rep. 2025, 15, 19304. [Google Scholar] [CrossRef]
- Janković, M.R.; Govedarica, O.M.; Sinadinović-Fišer, S.V. The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition. Ind. Crops Prod. 2020, 143, 111881. [Google Scholar] [CrossRef]
- Corrêa, F.D.A.; Sutili, F.K.; Miranda, L.S.M.; Leite, S.G.F.; De Souza, R.O.M.A.; Leal, I.C.R. Epoxidation of oleic acid catalyzed by PSCI-Amano lipase optimized by experimental design. J. Mol. Catal. B Enzym. 2012, 81, 7–11. [Google Scholar] [CrossRef]
- Sustaita-Rodríguez, A.; Ramos-Sánchez, V.H.; Camacho-Dávila, A.A.; Zaragoza-Galán, G.; Espinoza-Hicks, J.C.; Chávez-Flores, D. Lipase catalyzed epoxidation of fatty acid methyl esters derived from unsaturated vegetable oils in absence of carboxylic acid. Chem. Cent. J. 2018, 12, 39. [Google Scholar] [CrossRef]
- Sharma, R.V.; Dalai, A.K. Synthesis of bio-lubricant from epoxy canola oil using sulfated Ti-SBA-15 catalyst. Appl. Catal. B Environ. 2013, 142–143, 604–614. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Hwang, H.S.; Adhvaryu, A.; Erhan, S.Z. Preparation and properties of lubricant basestocks from epoxidized soybean oil and 2-ethylhexanol. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 811–815. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.C.F.; do Nascimento, M.R.; Cavalcante, C.L.; de Luna, F.M.T. Synthesis and tribological properties of bio-based lubricants from soybean oil. Biomass Convers. Biorefinery 2024, 14, 20509–20521. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. Study of epoxidized rapeseed oil used as a potential biodegradable lubricant. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Sharma, R.V.; Somidi, A.K.R.; Dalai, A.K. Preparation and properties evaluation of biolubricants derived from canola oil and canola biodiesel. J. Agric. Food Chem. 2015, 63, 3235–3242. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghobadian, B.; Dehghani Soufi, M.; Gorjian, S. Chemical modification of sunflower waste cooking oil for biolubricant production through epoxidation reaction. Mater. Sci. Energy Technol. 2021, 4, 119–127. [Google Scholar] [CrossRef]
- Campanella, A.; Rustoy, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Sriram, G.; Subadhra, L. Synthesis, chemical modification and tribological evaluation of plant oil as bio-degradable low temperature lubricant. Procedia Eng. 2012, 38, 1508–1517. [Google Scholar] [CrossRef]
- Campos Flexa Ribeiro Filho, P.R.; Rocha do Nascimento, M.; Otaviano da Silva, S.S.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Loureiro Cavalcante, C. Synthesis and Frictional Characteristics of Bio-Based Lubricants Obtained from Fatty Acids of Castor Oil. Lubricants 2023, 11, 57. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Zapata-Hernandez, C.; Santa, J.F.; Buitrago-Sierra, R. Palm oil as a biolubricant: Literature review of processing parameters and tribological performance. J. Ind. Eng. Chem. 2022, 107, 31–44. [Google Scholar] [CrossRef]
- Parente, E.J.; Marques, J.P.C.; Rios, I.C.; Cecilia, J.A.; Rodríguez-Castellón, E.; Luna, F.M.T.; Cavalcante, C.L. Production of biolubricants from soybean oil: Studies for an integrated process with the current biodiesel industry. Chem. Eng. Res. Des. 2021, 165, 456–466. [Google Scholar] [CrossRef]
- Antunes, Q.G.; Santos, A.L.A.; Siqueira, J.I.P.; Lima, R.G.S.; da Silva, G.F.; dos Santos, J.P.L. Study of the stability and physicochemical behavior of a novel biolubricant based on castor oil used in cutting operations. Biomass Convers. Biorefinery 2025, 15, 20331–20345. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind. Crops Prod. 2006, 23, 311–317. [Google Scholar] [CrossRef]
- Castro, W.; Perez, J.M.; Erhan, S.Z.; Caputo, F. A study of the oxidation and wear properties of vegetable oils: Soybean oil without additives. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 47–52. [Google Scholar] [CrossRef]
- Madankar, C.S.; Dalai, A.K.; Naik, S.N. Green synthesis of biolubricant base stock from canola oil. Ind. Crops Prod. 2013, 44, 139–144. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, J.; Li, C.; Xu, H.; Sun, M.; Xu, L.; Yu, L. Synthesis and Lubricating Properties of Bio-Based Lubricants from Palm Oil. ChemPlusChem 2025, 90, 202500013. [Google Scholar] [CrossRef]
- Stradolini, P.; Gryczak, M.; Petzhold, C.L. Polyols from castor oil (Ricinus communis) and epoxidized soybean oil (Glycine max) for application as a lubricant base. JAOCS J. Am. Oil Chem. Soc. 2024, 101, 321–334. [Google Scholar] [CrossRef]
- Ramos Moreira, D.; Worman, M.; Nery Ferreira, E.; Roberto Campos Flexa Ribeiro Filho, P.; Morais Ribeiro da Silva, L.; Bezerra Mota Gomes Arruda, T.; Eduardo Arruda Rodrigues, F.; Murilo Tavares de Luna, F.; Liberato Petzhold, C.; Maier, M.E.; et al. Development of polyols analogous to neopentyl glycol and trimethylolpropane for the production of oleic acid-based biolubricants. Fuel 2025, 381, 133156. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crops Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Abdel-Hameed, H.S.; El-Saeed, S.M.; Ahmed, N.S.; Nassar, A.M.; El-Kafrawy, A.F.; Hashem, A.I. Chemical transformation of Jojoba oil and Soybean oil and study of their uses as bio-lubricants. Ind. Crops Prod. 2022, 187, 115256. [Google Scholar] [CrossRef]
- Rios, L.; Echeverri, D.; Cardeño, F. Hydroxylation of vegetable oils using acidic resins as catalysts. Ind. Crops Prod. 2013, 43, 183–187. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants-A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Ribeiro, D.C.M.; Serra, C.; Coelho, J.; Ramalho, A.L. A biolubricant based on epoxidized soybean oil modified with aminoalcohols for enhanced tribological performance. Fuel 2026, 403, 136092. [Google Scholar] [CrossRef]
- Biswas, A.; Adhvaryu, A.; Gordon, S.H.; Erhan, S.Z.; Willett, J.L. Synthesis of diethylamine-functionalized soybean oil. J. Agric. Food Chem. 2005, 53, 9485–9490. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Oxidative Stability of Vegetal Oil-Based Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 1459–1476. [Google Scholar] [CrossRef]
- Cañellas, G.; Emeric, A.; Combarros, M.; Navarro, A.; Beltran, L.; Vilaseca, M.; Vives, J. Tribological Performance of Esters, Friction Modifier and Antiwear Additives for Electric Vehicle Applications. Lubricants 2023, 11, 109. [Google Scholar] [CrossRef]
- Boyde, S. Hydrolytic stability of synthetic ester lubricants. J. Synth. Lubr. 2000, 16, 297–312. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, J.; Ni, H.; Wang, X.; Zhu, Y.; Chen, L. Study on the coupling relationship of low temperature fluidity and oxidation stability of biodiesel. Appl. Sci. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Hu, C.; Ai, J.; Ma, L.; Wen, P.; Fan, M.; Zhou, F.; Liu, W. Ester Oils Prepared from Fully Renewable Resources and Their Lubricant Base Oil Properties. ACS Omega 2021, 6, 16343–16355. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coatings 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Vermiglio, A.L.; Alarcon, R.T.; Cavalheiro, É.T.G.; Bannach, G.; Farmer, T.J.; North, M. Highly crosslinked polyesters prepared by ring-opening copolymerization of epoxidized baru nut and macaw palm oils with cyclic anhydrides. RSC Sustain. 2023, 1, 987–993. [Google Scholar] [CrossRef]
- Barros, A.; Nepomuceno, N.; Nicácio, P.; Souza, M.; Silva, I.; Luna, C.; Fook, M.; Araújo, E.; Wellen, R. Enhancement of Biobased Epoxy Through the Curing and Thermal Stability Control with Carboxylic Acids. J. Polym. Environ. 2024, 32, 2431–2447. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Property analysis of triglyceride-based thermosets. Polymer 2005, 46, 61–69. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Perez-Sena, W.Y.; Ciccarelli, F.; Eränen, K.; Di Serio, M.; Russo, V.; Salmi, T. A pathway to cyclic carbonates: Cycloaddition of carbon dioxide to epoxidized methyl oleate on grafted heterogeneous catalysts. J. CO2 Util. 2025, 91, 103005. [Google Scholar] [CrossRef]
- Sammaiah, A.; Padmaja, K.V.; Prasad, R.B.N. Tribology and oxidation studies of fatty acid sulfide derivatives synthesized via thiol-ene “Click” additions. Eur. J. Lipid Sci. Technol. 2016, 118, 495–502. [Google Scholar] [CrossRef]
- Illy, N.; Fache, M.; Ménard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of bio-based compounds: The state of the art. Polym. Chem. 2015, 6, 6257–6291. [Google Scholar] [CrossRef]
- Avilés, M.D.; Mostaza, P.; Bermúdez, M.D.; Carrión-Vilches, F.J. Epoxidized vegetable lubricant enhanced by ionic liquid. Wear 2025, 564–565, 205740. [Google Scholar] [CrossRef]
- Lai, C.M.; How, H.G.; Jason, Y.J.J.; Teoh, Y.H.; Yaqoob, H.; Zhang, S.; Rafe Hatshan, M.; Sher, F. Tribological characterisation of graphene hybrid nanolubricants in biofuel engines. Fuel 2024, 357, 129654. [Google Scholar] [CrossRef]
- Kulkarni, T.; Toksha, B.; Chatterjee, A.; Naik, J.; Autee, A. Anti-wear (AW) and extreme-pressure (EP) behavior of jojoba oil dispersed with green additive CaCO3 nanoparticles. J. Eng. Appl. Sci. 2023, 70, 29. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Extreme pressure and antiwear additives for lubricant: Academic insights and perspectives. Int. J. Adv. Manuf. Technol. 2022, 120, 1–27. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmed, A.; Abid, M.; Mufti, R.A.; Ferreira, F.; Bashir, M.N.; Shah, A.U.R.; Jafry, A.T.; Zulkifli, N.W.; Fattah, I.M.R. Ionic liquids as lubricants: An overview of recent developments. J. Mol. Struct. 2024, 1301, 137307. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crops Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
- He, X.; Lu, J.; Desanker, M.; Invergo, A.M.; Lohr, T.L.; Ren, N.; Lockwood, F.E.; Marks, T.J.; Chung, Y.W.; Wang, Q.J. Boundary Lubrication Mechanisms for High-Performance Friction Modifiers. ACS Appl. Mater. Interfaces 2018, 10, 40203–40211. [Google Scholar] [CrossRef]
- Gusain, R.; Khan, A.; Khatri, O.P. Fatty acid-derived ionic liquids as renewable lubricant additives: Effect of chain length and unsaturation. J. Mol. Liq. 2020, 301, 112322. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Wang, J.; Yao, X.; Xiong, X.; Huang, H. Synergistic Tribological Performance of Phosphorus- and Sulfur-Based Extreme Pressure and Anti-Wear Additives. Lubricants 2025, 13, 55. [Google Scholar] [CrossRef]
- Luiz, J.F.; Spikes, H. Tribofilm Formation, Friction and Wear-Reducing Properties of Some Phosphorus-Containing Antiwear Additives. Tribol. Lett. 2020, 68, 1–24. [Google Scholar] [CrossRef]
- Najman, M.N.; Kasrai, M.; Bancroft, G.M.; Miller, A. Study of the chemistry of films generated from phosphate ester additives on 52100 steel using X-ray absorption spectroscopy. Tribol. Lett. 2002, 13, 209–218. [Google Scholar] [CrossRef]
- Turco, R.; Tesser, R.; Vitiello, R.; Russo, V.; Andini, S.; Di Serio, M. Synthesis of biolubricant basestocks from epoxidized soybean oil. Catalysts 2017, 7, 309. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Y.; Zeng, X.; Li, J. Alkyl-Ethylene Amines as Effective Organic Friction Modifiers for the Boundary Lubrication Regime. Langmuir 2020, 36. [Google Scholar] [CrossRef]
- Lasch, G.; Stradolini, P.; Gehlen, G.S.; Barros, L.Y.; Poletto, J.C.; Ramalho, A.; Fernandes, C.M.C.G.; Romio, P.C.; Petzhold, C.L.; Ferreira, N.F.; et al. Comparative tribological investigation of castor oil and its transesterified and aminolyzed derivatives. Tribol. Int. 2024, 196. [Google Scholar] [CrossRef]
- de Andrade Souza, L.; Moreira, D.R.; Ricardo, N.M.P.S.; Maier, M.E.; Filho, P.R.C.F.R.; Luna, F.M.T.; Petzhold, C.L. Esters from castor oil functionalized with aromatic amines as a potential lubricant. JAOCS J. Am. Oil Chem. Soc. 2023, 100, 175–184. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Viscosity modification of high-oleic sunflower oil with polymeric additives for the design of new biolubricant formulations. Environ. Sci. Technol. 2009, 43, 2060–2065. [Google Scholar] [CrossRef]
- Zulkifli, W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- Loza, K.; Epple, M.; Maskos, M. Stability of nanoparticle dispersions and particle agglomeration. In Biological Responses to Nanoscale Particles; NanoScience and Technology; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 85–100. [Google Scholar] [CrossRef]
- Guegan, J.; Southby, M.; Spikes, H. Friction Modifier Additives, Synergies and Antagonisms. Tribol. Lett. 2019, 67, 83. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Melo Neta, M.M.F.; Lima, G.R.; Tavares, P.D.O.; Figueredo, I.D.M.; Rocha, W.D.S.; Ribeiro Filho, P.R.; Cavalcante, C.L., Jr.; Luna, F.M.T. Thermo-Oxidative Stability and Tribological Properties of Biolubricants Obtained from Castor Oil Fatty Acids and Isoamyl Alcohol. Lubricants 2023, 11, 490. [Google Scholar] [CrossRef]
- Acevedo-Serrano, M.; Nogales-Delgado, S.; González, J.F.G. Catalytic Biolubricant Production from Canola Oil Through Double Transesterification with Methanol and Neopentyl Glycol. Catalysts 2024, 14, 748. [Google Scholar] [CrossRef]
- Saka, A.; Abor, T.K.; Okafor, A.C.; Okoronkwo, M.U. Thermo-rheological and tribological properties of low- and high-oleic vegetable oils as sustainable bio-based lubricants. RSC Sustain. 2025, 3, 1461–1476. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, Y.; Li, B.; Yang, M.; Zhang, X.; Guo, S.; Liu, G.; Zhai, M. Comparative evaluation of the lubricating properties of vegetable-oil-based nanofluids between frictional test and grinding experiment. J. Manuf. Process. 2017, 26, 94–104. [Google Scholar] [CrossRef]
- Filho, P.R.C.F.R.; Santos, L.D.S.E. The influence of unsaturation modifications on the tribological characteristics of bio-based lubricants obtained from vegetable oils: A review. J. Brazilian Soc. Mech. Sci. Eng. 2025, 47, 1–14. [Google Scholar] [CrossRef]
- de Melo Neta, M.M.F.; de Oliveira Tavares, P.; Filho, P.R.C.F.R.; Cavalcante, C.L.; de Luna, F.M.T. Lubricants basestock oil obtained from residual fatty acids: Evaluation of tribological properties and thermo-oxidative stability. J. Brazilian Soc. Mech. Sci. Eng. 2025, 47, 1–12. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Benyajati, C.N.; Sritham, W.; Soparat, J.; Limprayoon, N.; Seetapan, N.; Fuongfuchat, A. Roles of viscosity, applied load and surface wettability on the lubrication behaviour of model liquid/semi-solid foods: Measurements with a bespoke tribo-cell fixture and rotational rheometer. Curr. Res. Food Sci. 2022, 5, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Biboulet, N.; Venner, C.H.; Lubrecht, A.A. Prediction of the Stribeck curve under full-film Elastohydrodynamic Lubrication. Tribol. Int. 2020, 149, 105569. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Hsu, S.M.; Gates, R.S. Boundary lubricating films: Formation and lubrication mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Havet, L.; Blouet, J.; Valloire, F.R.; Brasseur, E.; Slomka, D. Tribological characteristics of some environmentally friendly lubricants. Wear 2001, 248, 140–146. [Google Scholar] [CrossRef]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 1–26. [Google Scholar] [CrossRef]
- Spikes, H. Mechanisms of ZDDP—An Update. Tribol. Lett. 2025, 73. [Google Scholar] [CrossRef]
- Nalam, P.C.; Pham, A.; Castillo, R.V.; Espinosa-Marzal, R.M. Adsorption Behavior and Nanotribology of Amine-Based Friction Modifiers on Steel Surfaces. J. Phys. Chem. C 2019, 123, 13672–13680. [Google Scholar] [CrossRef]
- Ali, Z.A.; Takhakh, A.M.; Al-Waily, M. A review of use of nanoparticle additives in lubricants to improve its tribological properties. Mater. Today Proc. 2022, 52, 1442–1450. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Yusop, R.M.; Salih, N. Synthesis, reactivity and application studies for different biolubricants. Chem. Cent. J. 2014, 8, 1–11. [Google Scholar] [CrossRef]
- Raimondi, A.; Girotti, G.; Blengini, G.A.; Fino, D. LCA of petroleum-based lubricants: State of art and inclusion of additives. Int. J. Life Cycle Assess. 2012, 17, 987–996. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Kalam, M.A.; Mujtaba, M.A.; Almomani, F. A review of recent advances in the synthesis of environmentally friendly, sustainable, and nontoxic bio-lubricants: Recommendations for the future implementations. Environ. Technol. Innov. 2023, 32, 103366. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Biresaw, G.; Sharma, B.K.; Erhan, S.Z. Friction behavior of some seed oils: Biobased lubricant applications. Ind. Eng. Chem. Res. 2006, 45, 3735–3740. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. OECD Guideline 301-Ready Biodegrability; Organisation for Economic Co-operation and Development: Paris, France, 1992. [Google Scholar]
- ISO 9439; Water Quality-Evaluation of Ultimate Aerobic Biodegradability of Organic Compounds in Aqueous Medium-Method by Analysis of Released Carbon Dioxide. International Organization for Standardization: Geneva, Switerland, 1999.
- ASTM D5864; Standard Test Method for Determining Aerobic Aquatic Biodegradation of Lubricants. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Uppar, R.; Dinesha, P.; Kumar, S. A Critical Review on Vegetable Oil-Based Bio-Lubricants: Preparation, Characterization, and Challenges; Springer: Dordrecht, Netherlands, 2023; Volume 25. [Google Scholar]
- Vag, C.; Marby, A.; Kopp, M.; Furberg, L.; Norrby, T. A comparative life cycle assessment of the manufacture of base fluids for lubricants. J. Synth. Lubr. 2002, 19, 39–57. [Google Scholar] [CrossRef]
- Patel, J.R.; Chauhan, K.V.; Rawal, S.; Patel, N.P.; Subhedar, D.G. Advances and Challenges in Bio-Based Lubricants for Sustainable Tribological Applications: A Comprehensive Review of Trends, Additives, and Performance Evaluation. Lubricants 2025, 13, 440. Available online: https://www.preprints.org/manuscript/202509.0365/v1 (accessed on 20 October 2025).
- Moussa, H. Life Cycle Assessment of a Hybrid Poly Butylene Succinate Composite. 2014. Available online: https://core.ac.uk/reader/144147184 (accessed on 20 October 2025).
- Thanahiranya, P.; Sadhukhan, J.; Charoensuppanimit, P.; Vacharanukrauh, T.; Chuetor, S.; Chanthanumataporn, M.; Assabumrungrat, S. Design and assessment of biolubricant production processes utilizing various feedstocks in palm oil-based biodiesel industry. J. Clean. Prod. 2025, 518, 145949. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Deleanu, L.; Georgescu, C.; Cristea, G.C.; Ionescu, T.F.; Dionis, G.; Ojoc, G.G.; Dima, D.; Paduraru, I. Standardization in Tribology. Tribol. Ind. 2024, 46, 398–417. [Google Scholar] [CrossRef]
- Liu, S.; Saha, B.; Vlachos, D.G. Catalytic production of renewable lubricant base oils from bio-based 2-alkylfurans and enals. Green Chem. 2019, 21, 3606–3614. [Google Scholar] [CrossRef]
- Xue, H.; Wang, C.; Liang, F.; He, J.; He, Q.; Cai, M.; Tian, Q.; Chang, G.; Huang, Q.; Siddiq, M.; et al. Dynamic Covalent Polymer-Nanoparticle Networks as High-Performance Green Lubricants: Synergetic Effect in Load-Bearing Capacity. Macromolecules 2024, 57, 8383–8391. [Google Scholar] [CrossRef]

| Method | Description | Advantages | Challenges | Epoxide Conversion (%) | Relevance for Lubricants | References |
|---|---|---|---|---|---|---|
| In situ peracid epoxidation | Organic acid (formic/acetic) + H2O2 generates peracid in situ, which attacks C=C bonds. | Simple, cost-effective, high conversion, scalable. | Corrosive medium, formation of by-products (diols, acids), risk of ring-opening, environmental concerns. | 80–95 | Produces stable base oils with improved oxidation resistance and reactive epoxides for further functionalization. | [15,16] |
| Enzymatic epoxidation | Lipase catalyzes in situ peracid formation under mild aqueous/organic conditions. | High selectivity, minimal side reactions, environmentally benign, mild temperature. | High enzyme cost, low reaction rates, limited scalability. | 50–70 | Green approach suitable for specialty or high-value lubricants, though limited for bulk production. | [17,18] |
| Heterogeneous catalytic epoxidation | Solid catalysts (Ti/SiO2, zeolites, metal oxides) with H2O2 as oxidant. | Catalyst recyclability, easy separation, less effluent, tunable selectivity. | Catalyst deactivation, mass-transfer limitations, higher cost, scale-up complexity. | 70–90 | Environmentally friendly and promising for continuous, greener large-scale lubricant production. | [19,20] |
| Peroxyacid-assisted epoxidation | Preformed peroxyacids react directly with C=C bonds. | Rapid reaction, high conversion efficiency. | Handling hazards, avoiding formation of secondary products. | 80–95 | Produces epoxidized intermediates for subsequent ring-opening or reactions. | [21] |
| Vegetable Oil | Fatty Acid Profile | Lubricant Advantages | Lubricant Limitations | Effect of Epoxidation | References |
|---|---|---|---|---|---|
| Soybean | Moderate unsaturation (~54% linoleic acid), medium viscosity | Renewable, biodegradable; effective friction reduction | Moderate oxidative stability; limited high-temperature performance | Increases oxidative and thermal stability; enhances polarity for better film formation and high-temperature performance | [34,35] |
| Canola | Lower unsaturation (~28% linoleic acid), higher oleic acid content | Good low-temperature fluidity; good oxidative stability | Low polarity; moderate viscosity | Improves oxidative stability; epoxide groups increase polarity and adhesion to metal surfaces | [25,36] |
| Sunflower | High unsaturation (~60–65% linoleic acid) | Excellent lubricity under moderate loads | Poor oxidative and thermal stability due to high C=C content | Significantly enhances oxidative and thermal stability; epoxide groups improve interaction with metal surfaces | [28] |
| Castor | Rich in ricinoleic acid (hydroxyl group + one double bond), naturally viscous | High viscosity: polar hydroxyl groups enhance film formation; excellent low-temperature lubricity | Moderate oxidative stability: high viscosity may limit high-speed performance | Further increases oxidative stability and polarity; improves film formation and thermal performance | [30] |
| Palm | High saturation (~50% palmitic acid), low unsaturation | Thermally stable; good oxidative stability | Low polarity; limited lubricity; poor low-temperature fluidity | Minor improvements in polarity and lubricity; limited effect on oxidative stability due to low unsaturation | [37] |
| Reaction Type | Key Lubricant Properties | Applications |
|---|---|---|
| Alcohol/polyol ring opening | Increased hydroxyl content, polarity, and viscosity; strong surface affinity; good biodegradability | Hydraulic fluids, gear oils, greases |
| Amine/aminoalcohol ring opening | Improved thermal stability and antiwear performance via strong tribofilm; but reduced oxidation stability | Engine oils, gear/chain lubricants |
| Acid/anhydride esterification | Tuneable viscosity and pour point; improved oxidative stability; good lubricity and biodegradability | Hydraulic and gear lubricants |
| Polymerization/crosslinking | Large viscosity increases up to gels/greases; enhanced load capacity and structural stability | Biodegradable greases, heavy-duty lubricants |
| Hybridization with additives | Synergistic friction reduction, improved antiwear and oxidation resistance | Automotive and industrial lubricants |
| CO2 carbonation | Increased polarity and oxidative stability; cyclic carbonates provide reactive sites | Functionalized eco-lubricants |
| Thiol–epoxy click | Introduces sulphur functionalities; improved extreme-pressure (EP) behaviour | EP-enhanced biodegradable oils |
| Phosphorus-based opening | Produces phosphate esters with strong antiwear and fire resistance | AW/EP additives, fire-resistant fluids |
| Molecular Modification | Typical Viscosity (40 °C, cSt) | COF Range | Wear Reduction (%) | Dominant Regime | References |
|---|---|---|---|---|---|
| Epoxide ring-opening (alcohol/polyol) | 150–500 | 0.08–0.12 | 40–60 | Mixed | [9,36,38,44,76] |
| Amine-functionalized EVO | 150–300 | 0.05–0.08 | - | Boundary | [46,77,78,79] |
| Long-chain esterified EVO | 300–600 | 0.08–0.10 | 20–50 | Mixed–Hydrodynamic | [39,80,81] |
| EVO + nanoparticles (graphene, MoS2, clay) | — | 0.04–0.08 | +10–20 over base oil | Boundary–Mixed | [82,83] |
| Oil | Stage | Viscosity | Oxidative Stability | Low-Temp Fluidity | Tribological Performance | Notes |
|---|---|---|---|---|---|---|
| Castor | Pure | 5 | 3 | 4 | 5 | High polarity and viscosity; good boundary lubrication [85] |
| Epoxidized | 5 | 4 | 4 | 5 | Oxirane ring improves stability and film formation [85] | |
| Post-Mod. | 5 | 5 | 4 | 5 | Excellent for high load/industrial applications [38] | |
| Canola | Pure | 3 | 4 | 4 | 3 | Balanced properties for general applications [86] |
| Epoxidized | 4 | 4 | 4 | 4 | Epoxidation enhances tribology and viscosity [36] | |
| Post-Mod. | 4–5 | 5 | 4–5 | 4 | Tuneable for automotive or moderate-load use [86] | |
| Soybean | Pure | 3 | 3 | 3 | 3 | Moderate properties; requires enhancement at high temperature [32] |
| Epoxidized | 3 | 4 | 3 | 3 | Oxidative stability improved [23] | |
| Post-Mod. | 4 | 4–5 | 4 | 4 | Good lubricity achievable via modifications [48] | |
| Sunflower | Pure | 2 | 2 | 3 | 3 | High unsaturation limits oxidative stability [87] |
| Epoxidized | 3 | 3 | 3 | 3 | Slight improvement in stability [28] | |
| Post-Mod. | 3–4 | 4 | 3–4 | 3–4 | Moderate tribology and low-temp properties [27] | |
| Palm | Pure | 3 | 5 | 2 | 2 | Thermally stable, poor low-temp fluidity [88] |
| Epoxidized | 4 | 5 | 2 | 3 | Slight improvement in viscosity and tribology [75] | |
| Post-Mod. | 4 | 5 | 2–3 | 3 | Best for high-temperature applications [53] |
| Modification Type | Biodegradability (OECD 301, 28 Days) | GWP vs. Mineral Oil (% Reduction) | GWP (kg CO2-eq/kg Lubricant) | Reference |
|---|---|---|---|---|
| Native triglycerides | 65–90% (readily biodegradable) | ~65–75% | 1.5–1.8 | [105,106] |
| Epoxidation (EVO) | 50–70% | ~50–60% | 2.0–2.3 | [106,110] |
| Esterification (long-chain/polyol) | 60–85% | ~60–65% | 1.6–1.9 | [109] |
| Hydroxylation/Amination | 40–60% | ~30–40% | n.d. | [106,111] |
| Crosslinking/Polyesters | <30% | ~40% | 2.5–3.0 | [101] |
| Aromatic substitution | <20% | ~20–30% | 3.0–3.2 | [102] |
| TMP esters | >70% | ~80–85% | 6.0 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.C.M.; Ramalho, A.; Serra, A.C.; Coelho, J. Biolubricants Based on Epoxidized Vegetable Oils: A Review on Chemical Modifications, Tribological Properties, and Sustainability. Lubricants 2025, 13, 510. https://doi.org/10.3390/lubricants13120510
Ribeiro DCM, Ramalho A, Serra AC, Coelho J. Biolubricants Based on Epoxidized Vegetable Oils: A Review on Chemical Modifications, Tribological Properties, and Sustainability. Lubricants. 2025; 13(12):510. https://doi.org/10.3390/lubricants13120510
Chicago/Turabian StyleRibeiro, Diana C. M., Amílcar Ramalho, Arménio C. Serra, and Jorge Coelho. 2025. "Biolubricants Based on Epoxidized Vegetable Oils: A Review on Chemical Modifications, Tribological Properties, and Sustainability" Lubricants 13, no. 12: 510. https://doi.org/10.3390/lubricants13120510
APA StyleRibeiro, D. C. M., Ramalho, A., Serra, A. C., & Coelho, J. (2025). Biolubricants Based on Epoxidized Vegetable Oils: A Review on Chemical Modifications, Tribological Properties, and Sustainability. Lubricants, 13(12), 510. https://doi.org/10.3390/lubricants13120510







