Fundamental Mechanisms Underlying the Effectiveness of Nanoparticle Additives to Lubricants: 25 Examples Linking Nano- to Macroscale Friction
Abstract
1. Introduction
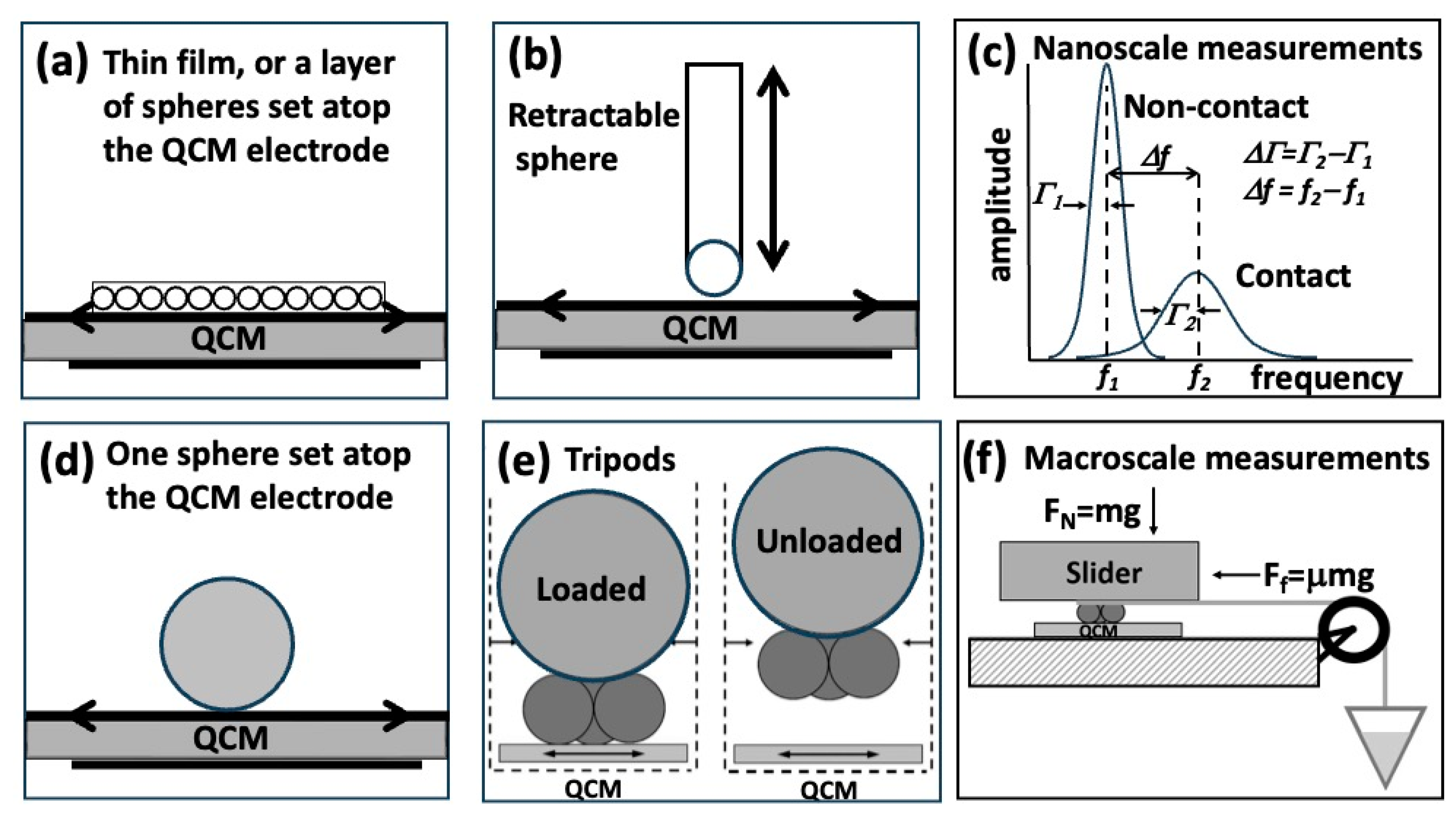
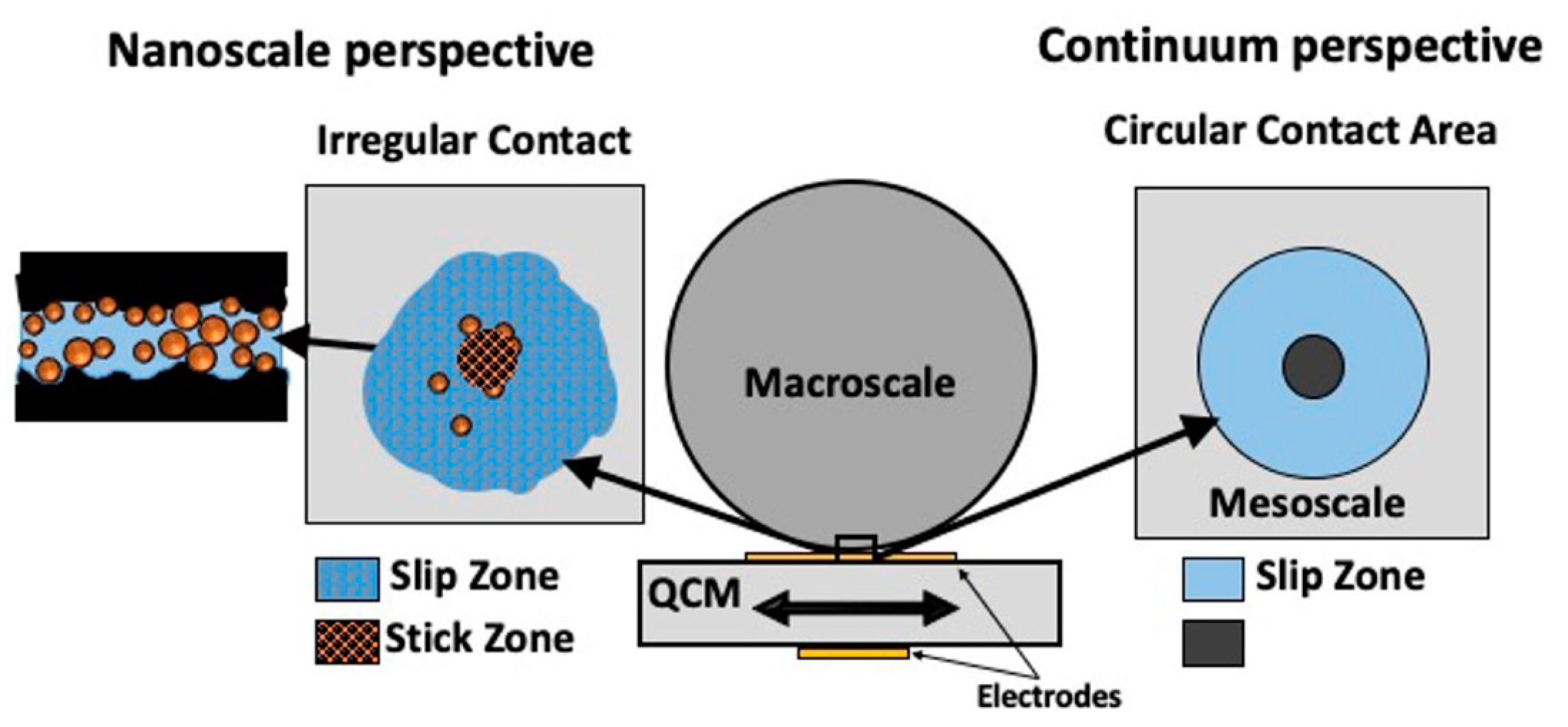
2. Quartz Crystal Microbalance Response to Spherical Contacts Directly Links Nano- and Macroscale Friction
2.1. A Sensitive New Method for the Determination of Adhesive Bonding between a Particle and a Substrate [15]
2.2. High-Frequency Tribological Investigations on Quartz Resonator Surfaces [16]
2.3. An Integrated Force Probe and Quartz Crystal Microbalance for High-Speed Microtribology [17]
2.4. Partial Slip in Mesoscale Contacts: Dependence on Contact Size [18]
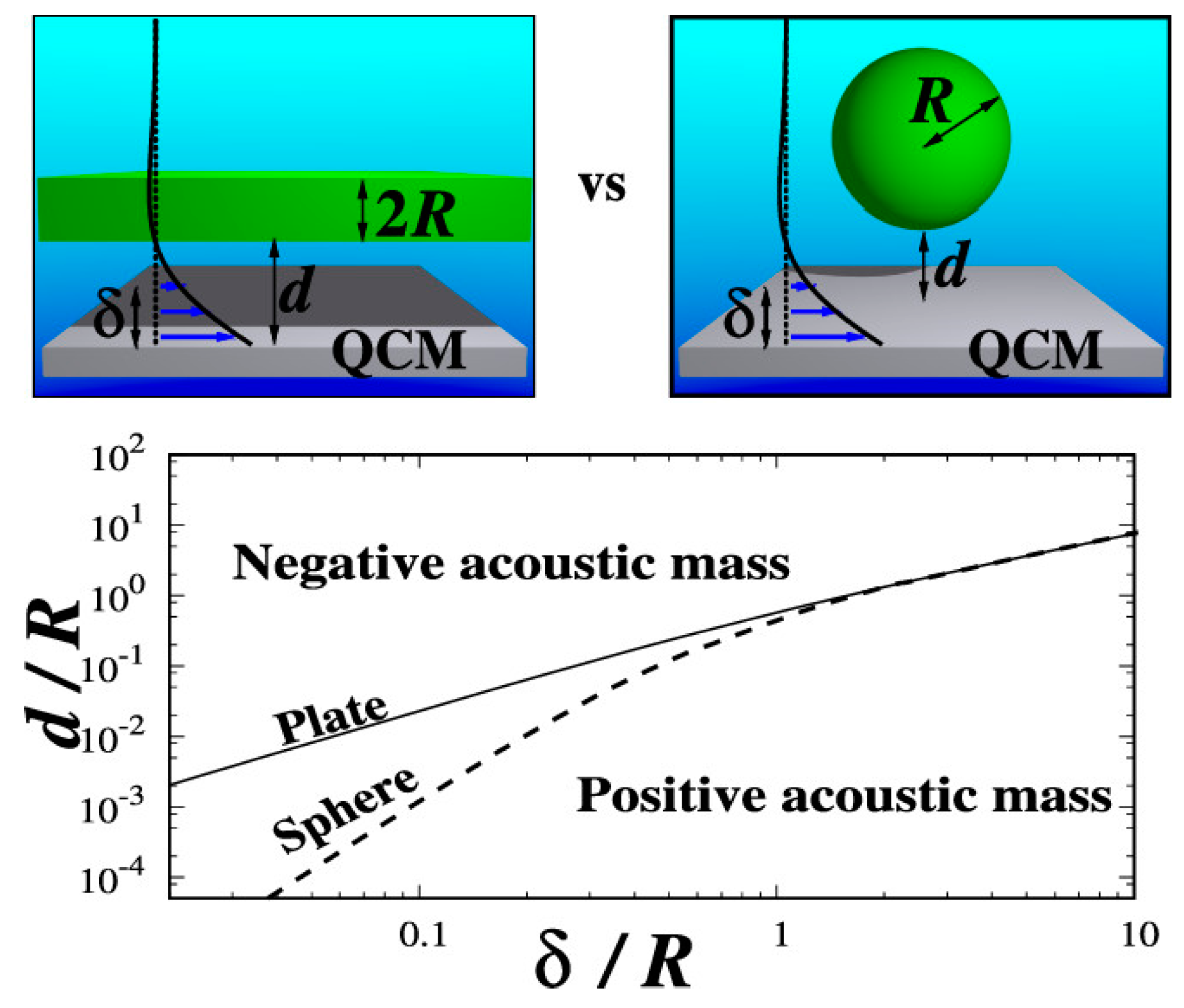
2.5. Correlation of High-Frequency QCM Sphere–Plate Stiffness Measurements with Macroscopic Friction Contacts in Thin Film and Bulk Stainless Steel Materials [19]
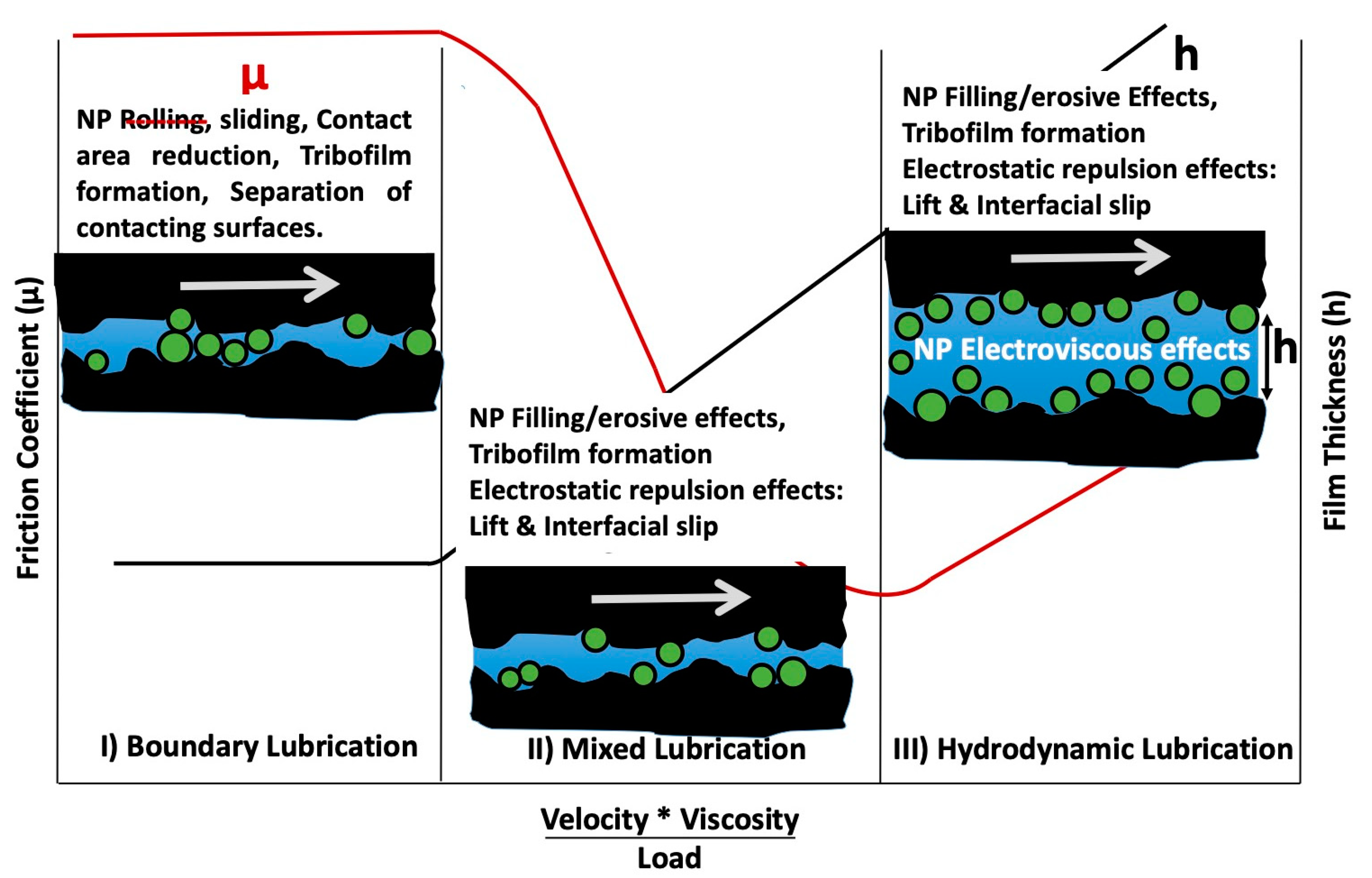
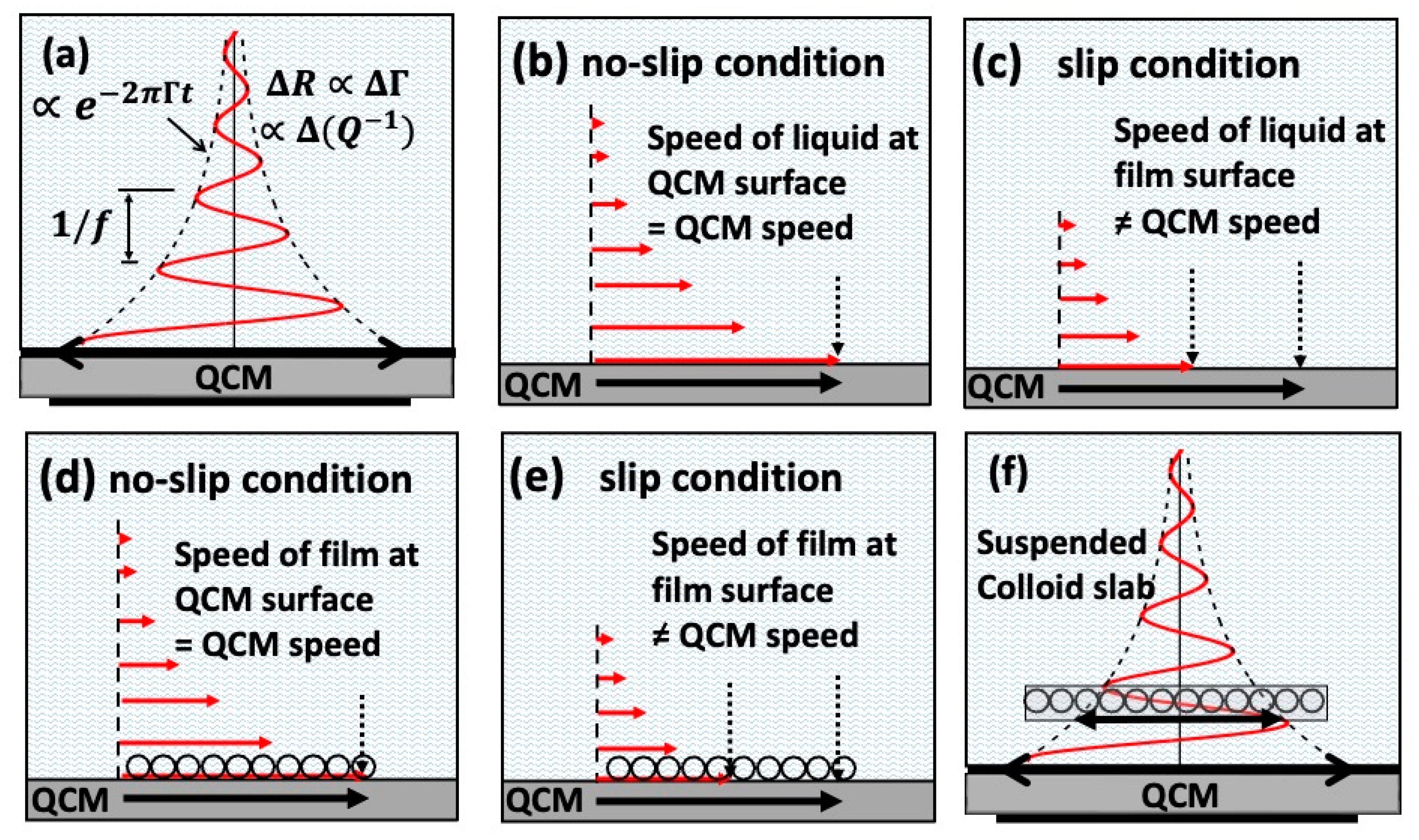
3. Interfacial Slippage Impacts Lubrication in the Mixed and Hydrodynamic Regimes
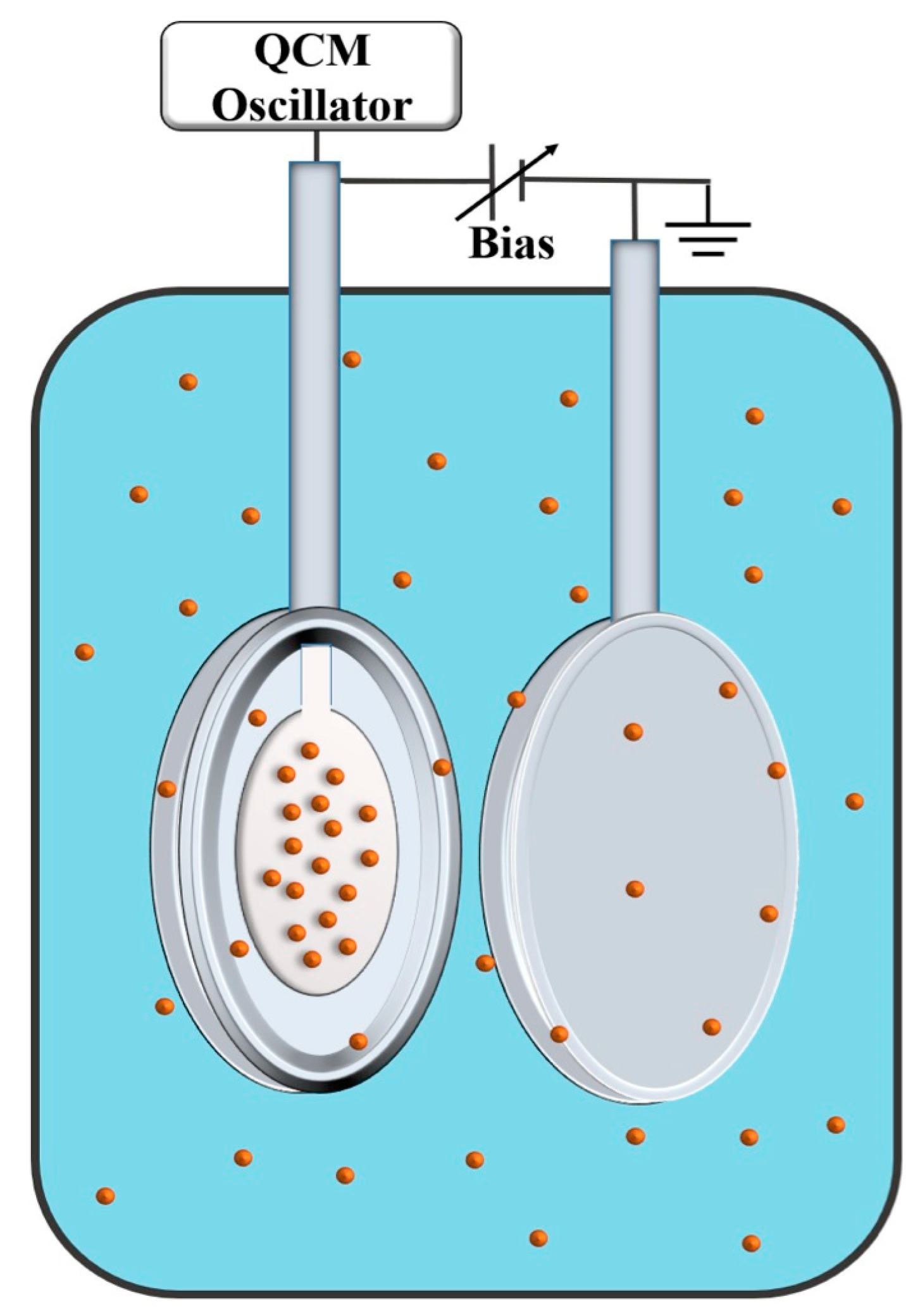
3.1. Slippage at Adsorbate–Electrolyte Interface—Response of Electrochemical Quartz Crystal Microbalance to Adsorption [20]
3.2. A Low Friction Bearing Based on Liquid Slip at the Wall [21]
3.3. A Tribological Study of Aqueous Suspension of γ-Fe2O3 Nanoparticles [22]
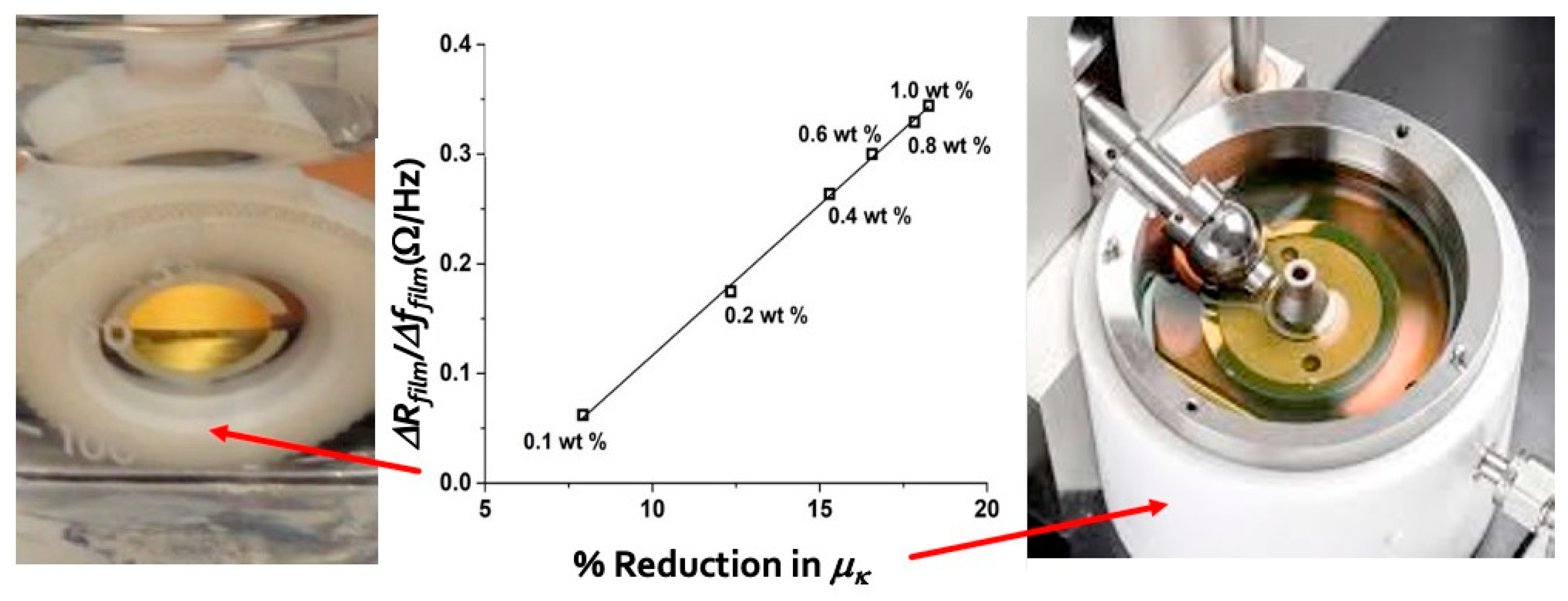
3.4. Nanotribological Performance Factors for Aqueous Suspensions of Oxide Nanoparticles and Their Relation to Macroscale Lubricity [23]
3.5. Load Impedance of Immersed Layers on the Quartz Crystal Microbalance: A Comparison with Colloidal Suspensions of Spheres [24]
4. Nanoparticle Rolling Has Little Influence and When Present May Increase Friction
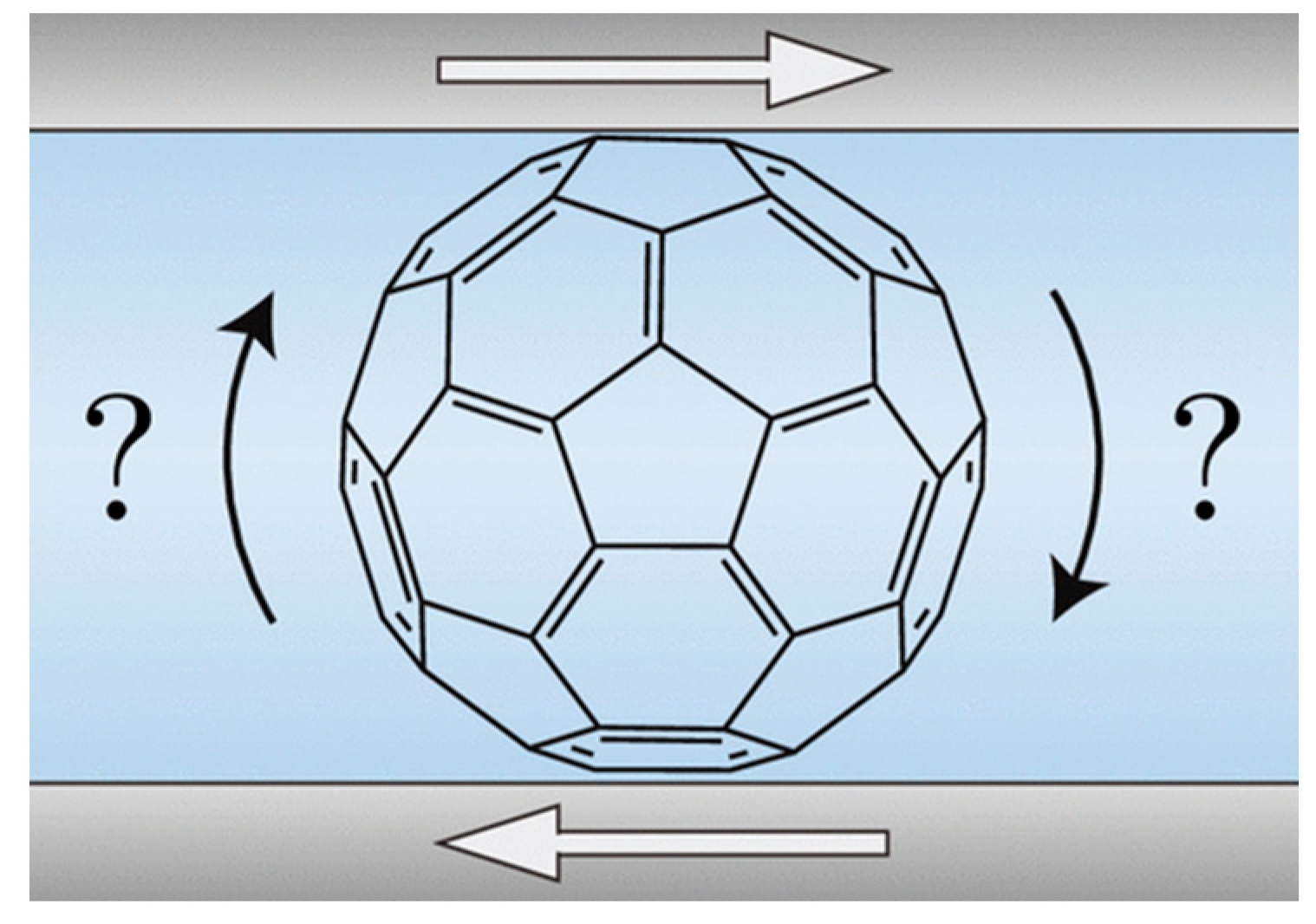
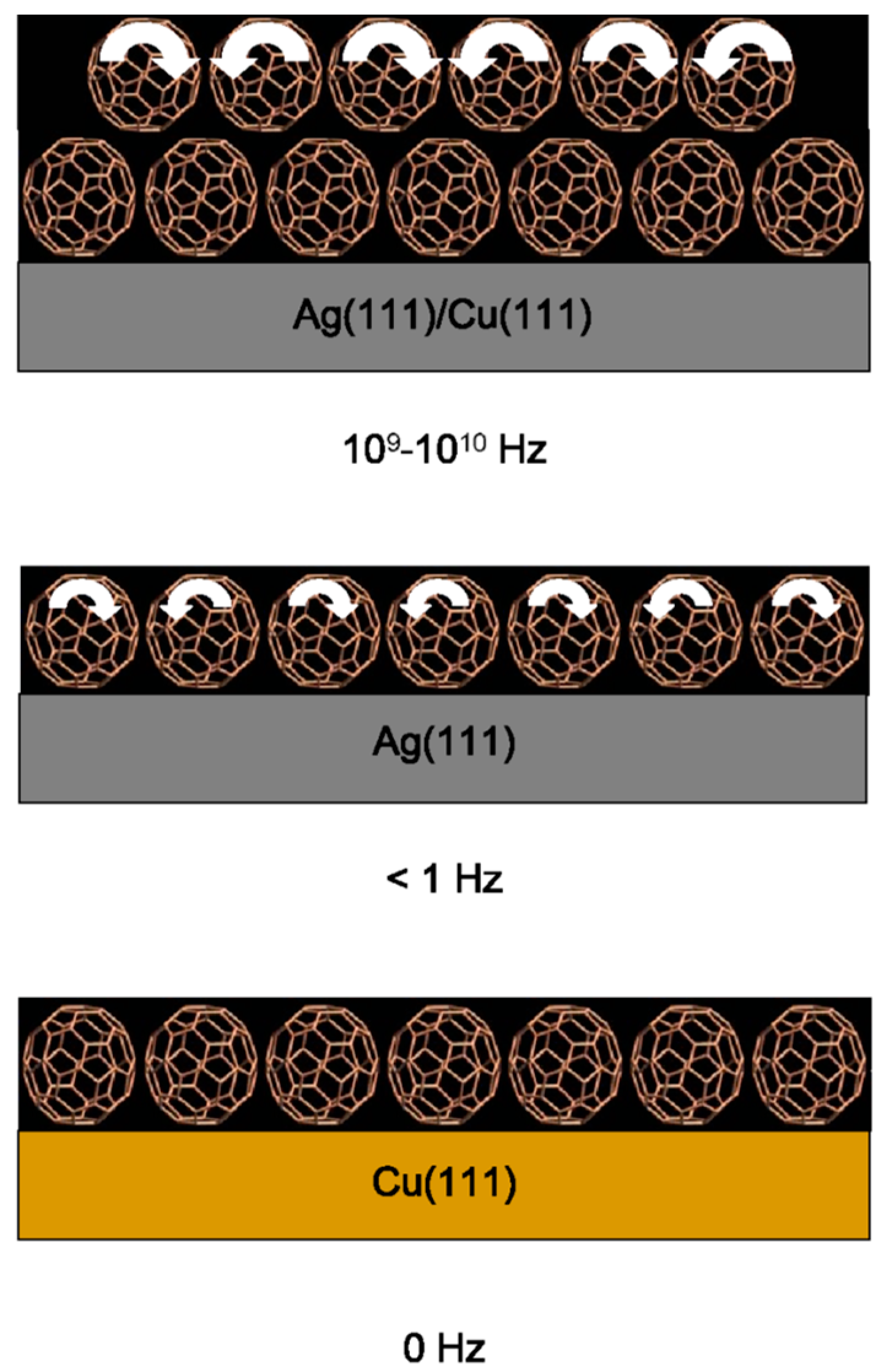
4.1. C60 Molecular Bearings and the Phenomenon of Nanomapping [25]
4.2. Are Buckminsterfullerenes Molecular Ball Bearings [26]?
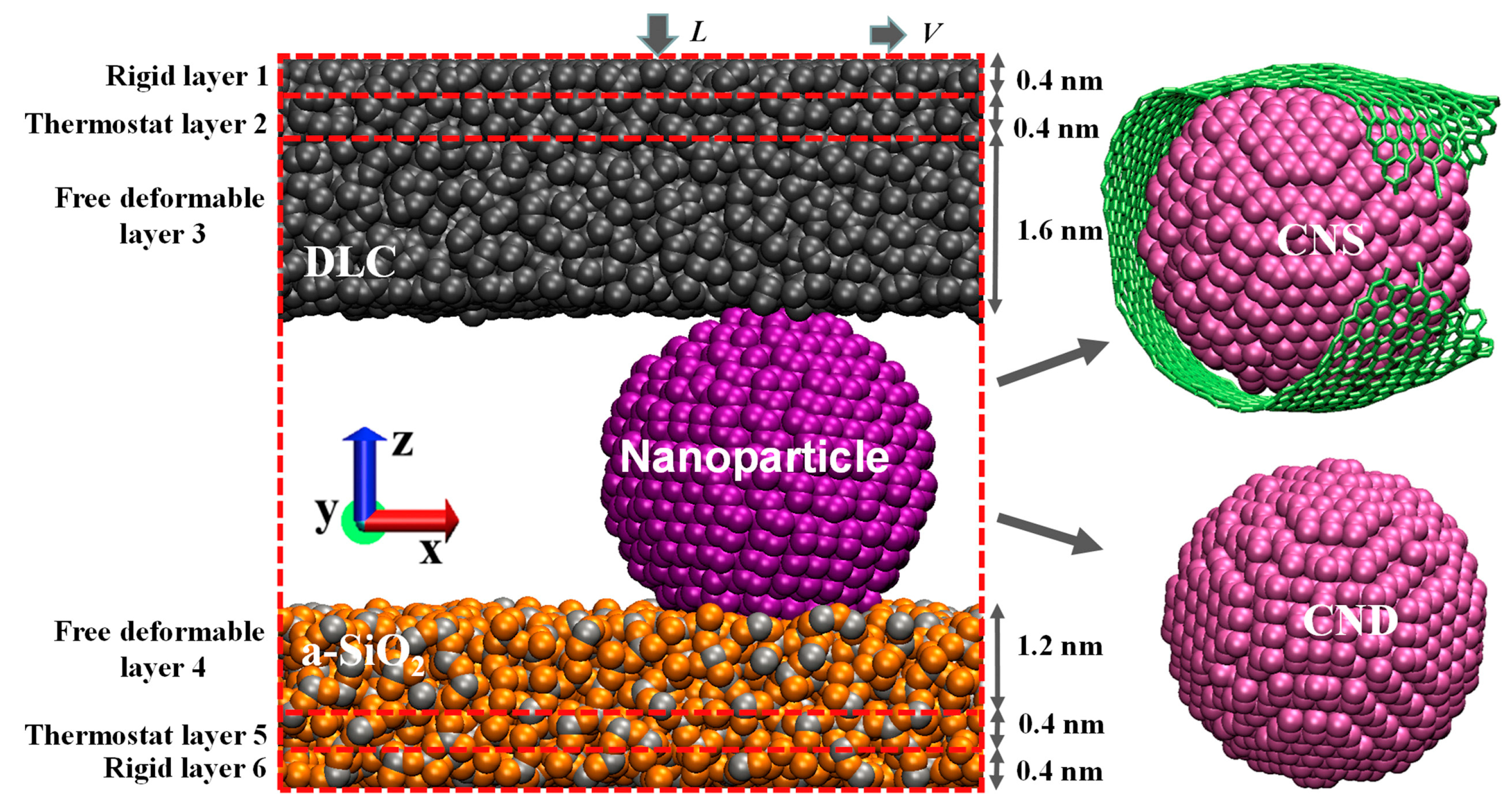
4.3. Friction Properties of Carbon Nanoparticles (Nanodiamonds and Nanoscrolls) Confined between Diamond-like Carbon and a-SiO2 Surfaces [27]
4.4. Addition of Solid Oxide Particles for Friction Reduction [28]
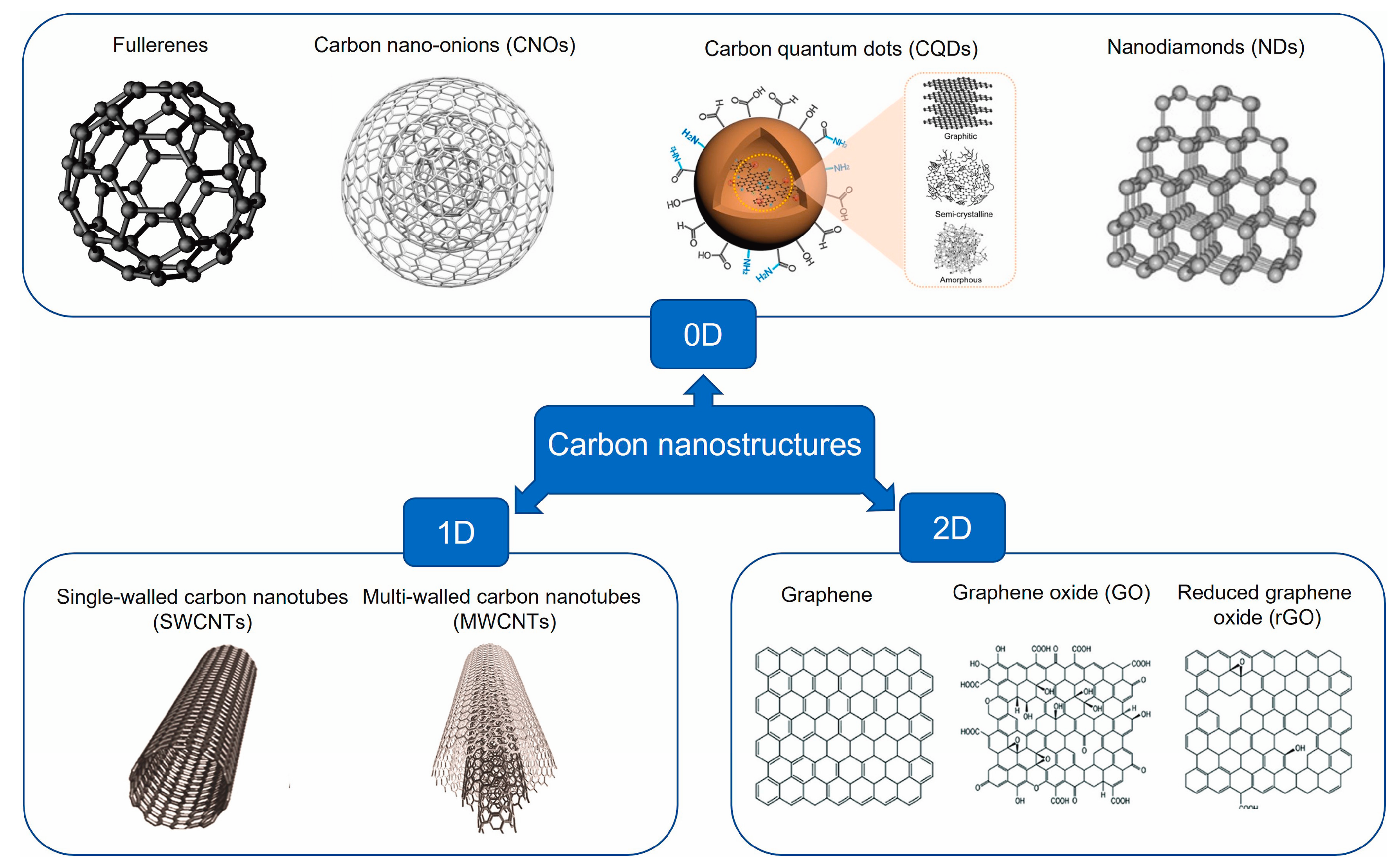
4.5. Functionalized Carbon Nanostructures as Lubricant Additives—A Review [29]
“After critically reviewing the fundamental research available at present date, the roller bearing ability of 1D CNTs is also deemed unlikely for several reasons…, even if all the aforementioned challenges (e.g., entanglement, need for commensurability, structural collapse, etc.) were minimized or eliminated in a way such that rolling motion of CNTs was more plausible, it is still unclear whether this would contribute to friction reduction in any significant way”.[29]
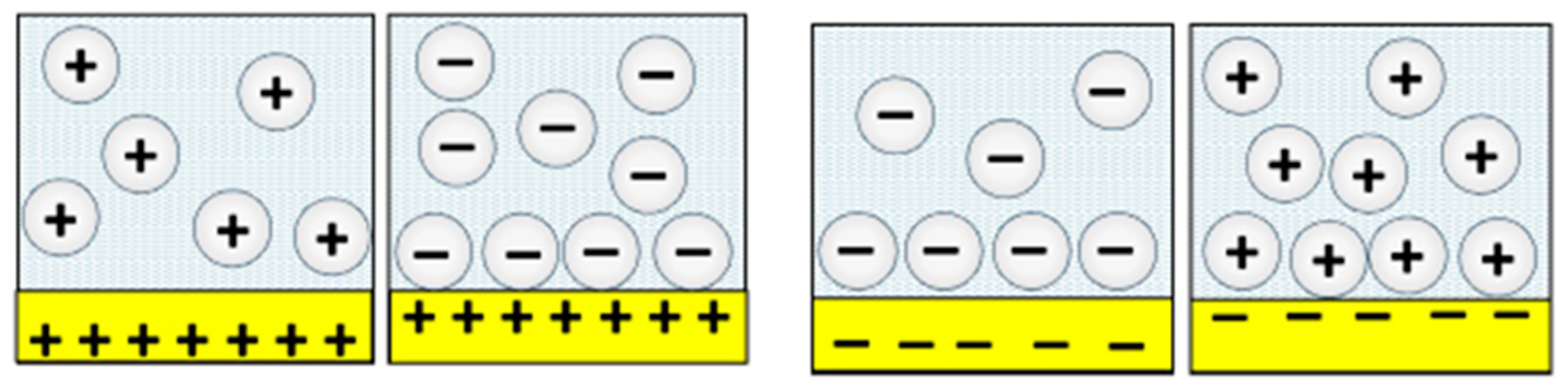
5. Surface Charge on Nanoparticles and Contacting Surfaces and the Surface Groups That Give Rise to the Charge Have an “Astonishing” Impact on Friction
5.1. A Comparative Study of the Nanoscale and Macroscale Tribological Attributes of Alumina and Stainless Steel Surfaces Immersed in Aqueous Suspensions of Positively or Negatively Charged Nanodiamonds [12]
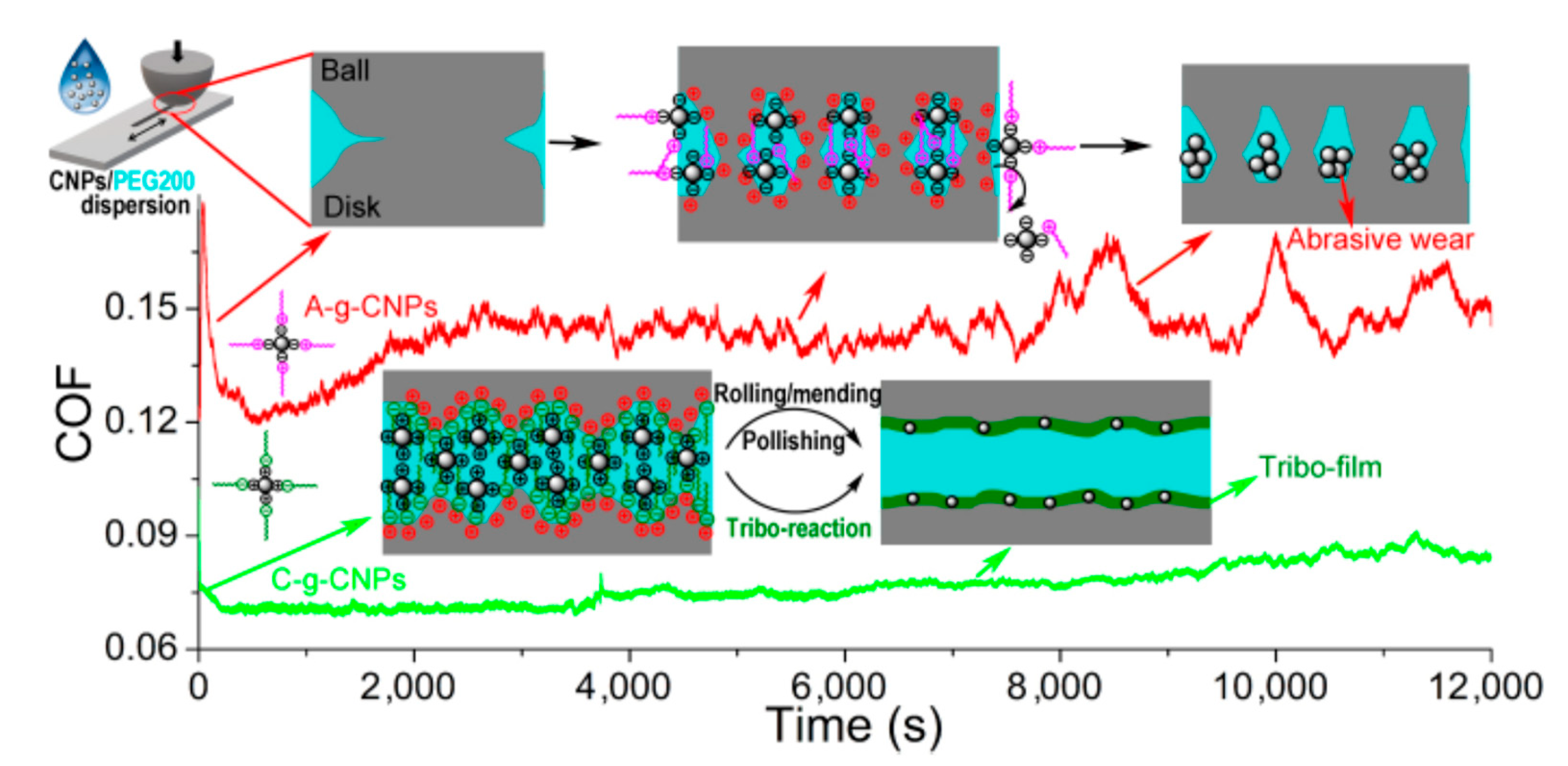
5.2. Astonishingly Distinct Lubricity Difference between the Ionic Liquid Modified Carbon Nanoparticles Grafted by Anion and Cation Moieties [30]
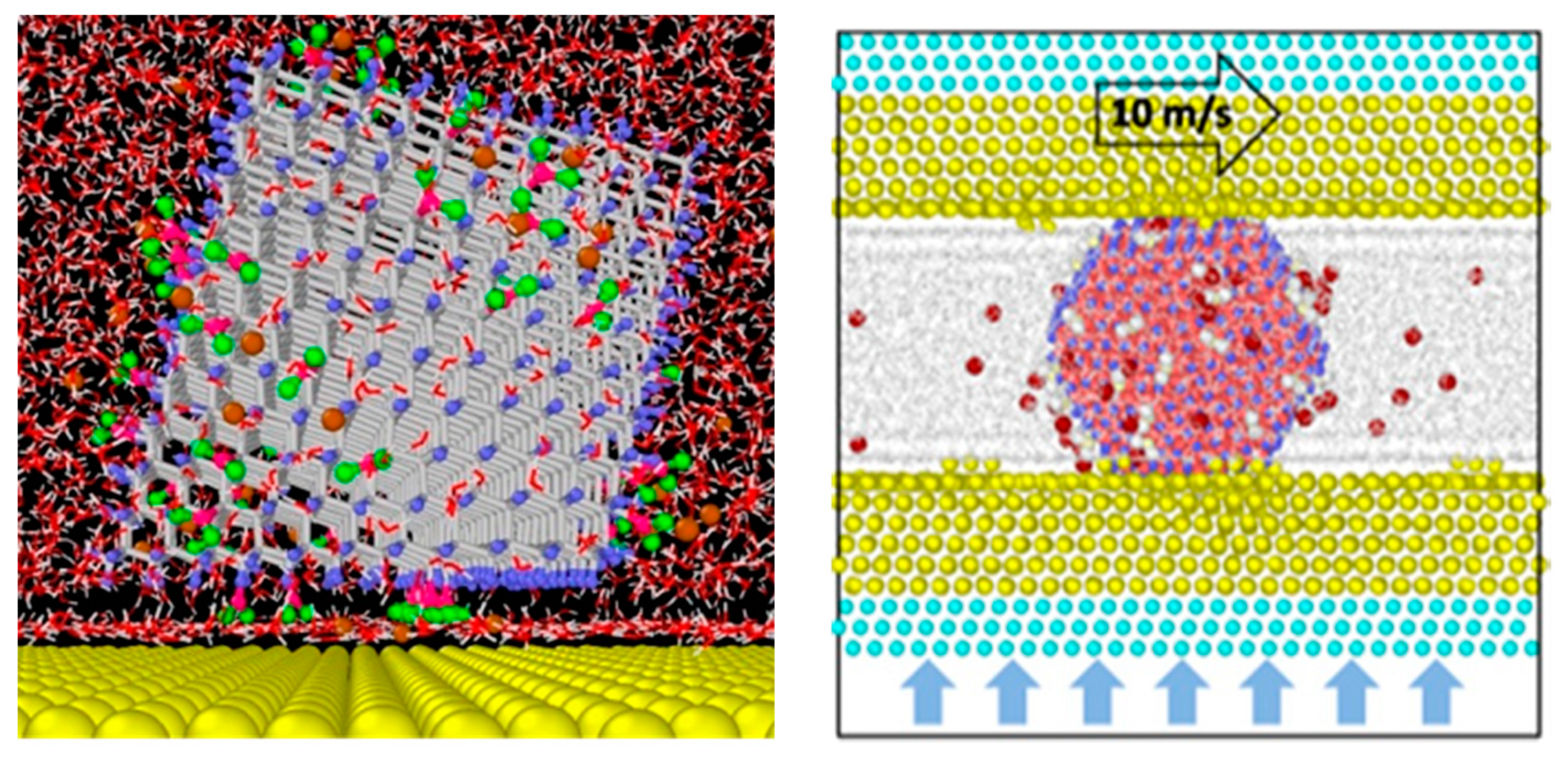
5.3. Interdependent Roles of Electrostatics and Surface Functionalization on the Adhesion Strengths of Nanodiamonds to Gold in Aqueous Environments Revealed via Molecular Dynamics Simulations [31]
5.4. Potential-Controlled Boundary Lubrication Using MoS2 Additives in Diethyl Succinate [32]
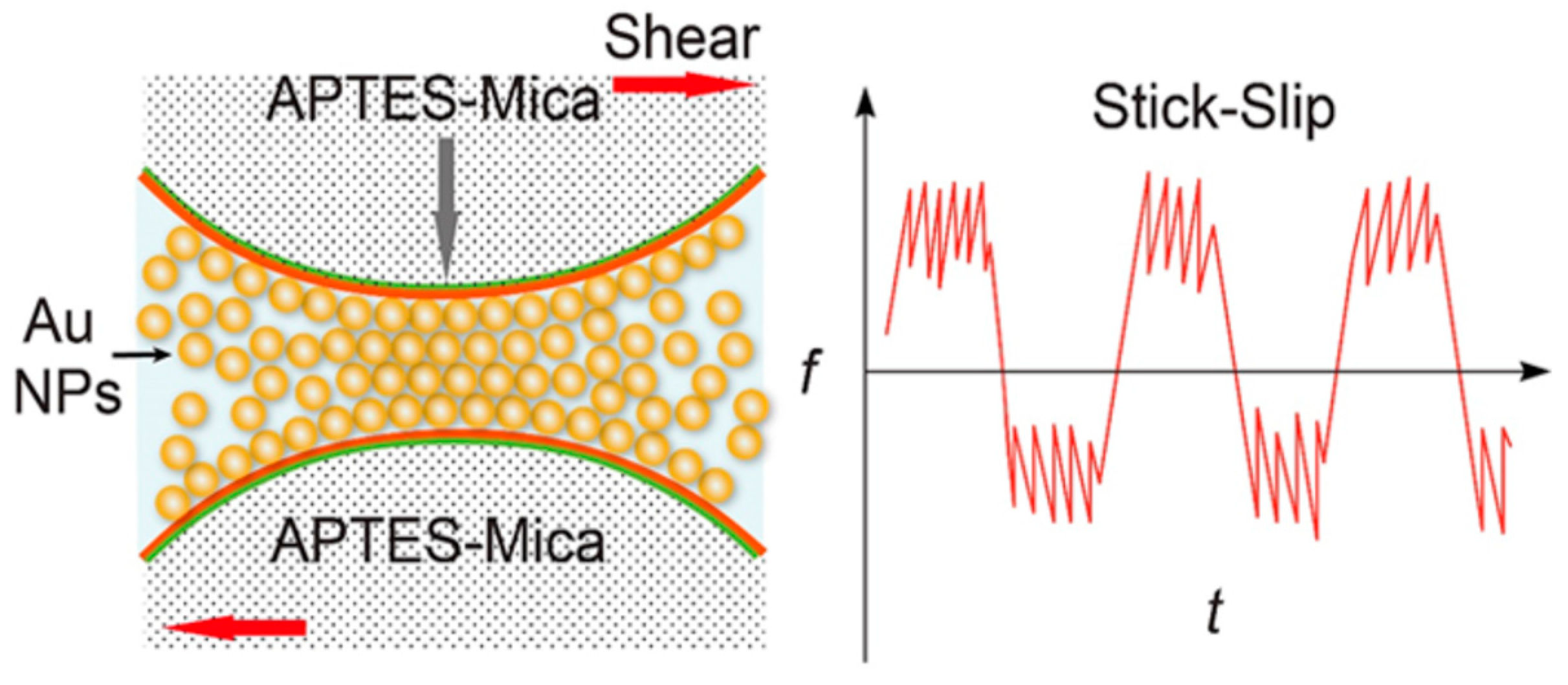
5.5. Probing Self-Assembly and Nonlinear Friction Behavior of Confined Gold Nanoparticles [33]
6. Tuning Friction through Charge and Potential Variations
6.1. Study of Tribotronic Control in Ionic Liquids and Nanoparticle Suspensions [34]
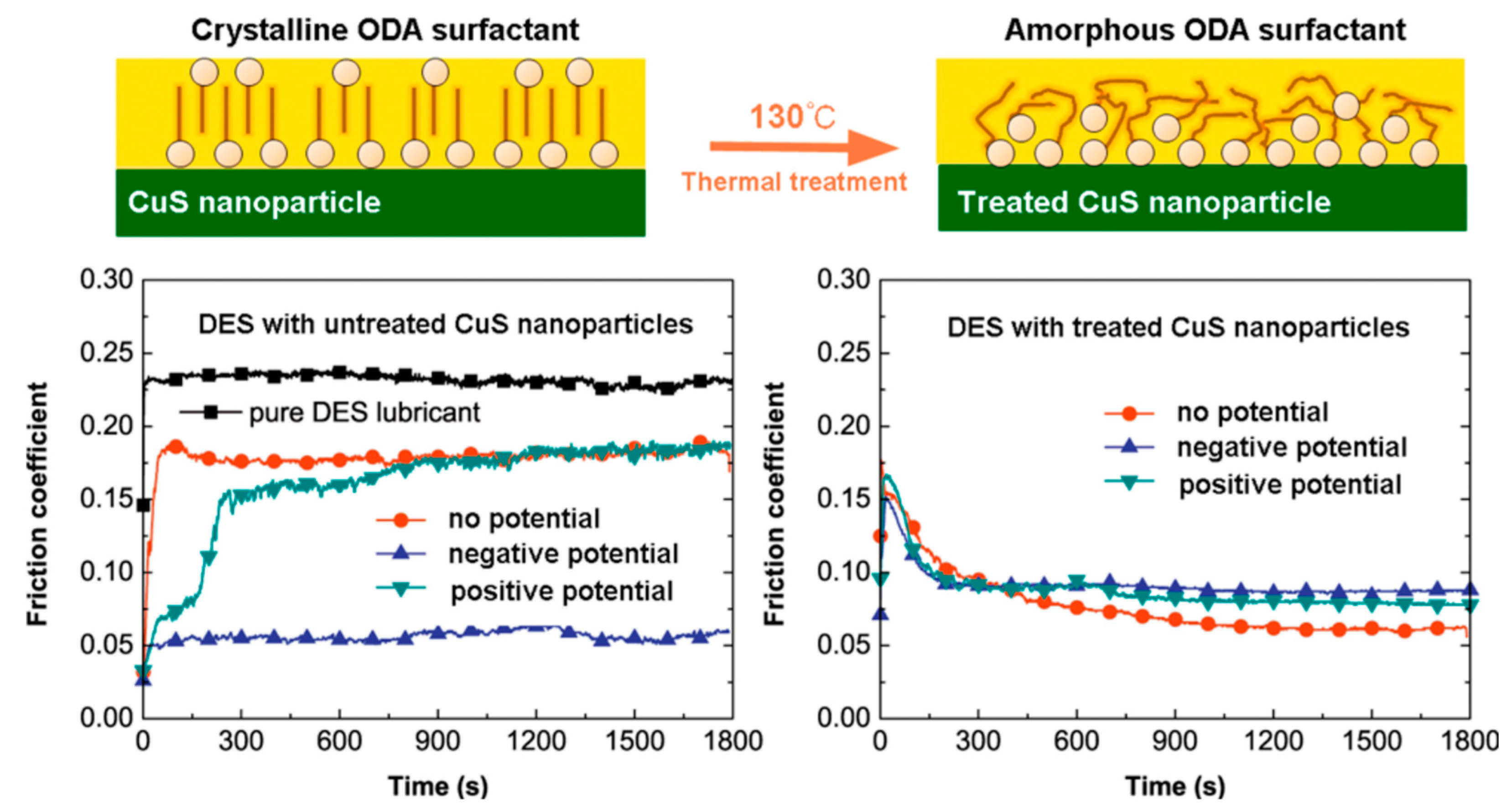
6.2. Electric Response of CuS Nanoparticle Lubricant Additives: The Effect of Crystalline and Amorphous Octadecylamine Surfactant Capping Layers [35]
6.3. Tribotronic and Electrochemical Properties of Platinum–Nanofluid Interfaces Formed by Aqueous Suspensions of 5 and 40 nm TiO2 Nanoparticles [36]
6.4. Friction and Adhesion Control between Adsorbed Layers of Polyelectrolyte Brush-Grafted Nanoparticles via pH-Triggered Bridging Interactions [37]
6.5. High Lubricity and Electrical Responsiveness of Solvent-Free Ionic SiO2 Nanofluids [38]
7. Summary and Concluding Comments
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, S.E.; Luengo, G.; Srdanov, V.I.; Wudl, F.; Israelachvili, J.N. Very low viscosity at the solid–liquid interface induced by adsorbed C60 monolayers. Nature 1996, 382, 520–522. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Al-Janabi, A.S.; Wadzer, Y.; Mamat, H. Review of tribological properties of nanoparticle-based lubricants and their hybrids and composites. Friction 2024, 12, 569–590. [Google Scholar] [CrossRef]
- Dassenoy, F. Nanoparticles as additives for the development of high performance and environmentally friendly engine lubricants. Tribol. Online 2019, 14, 237–253. [Google Scholar] [CrossRef]
- Han, K.; Zhang, Y.; Song, N.; Yu, L.; Zhang, P.; Zhang, Z.; Qian, L.; Zhang, S. The current situation and future direction of nanoparticles lubricant additives in China. Lubricants 2022, 10, 312. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Cai, M.; Guo, R.; Zhou, F.; Liu, W. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Figure SPM.5; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p, ISBN 978-92-9169-143-2. [Google Scholar]
- United Nations Environment Programme Executive Summary. In Emissions Gap Report 2023: Broken Record—Temperatures Hit New Highs, Yet World Fails to Cut Emissions (Again); United Nations Environment Programme Executive Summary: Nairobi, Kenya, 2023. [CrossRef]
- Li, S.; Macakova, L.; Bełdowski, P.; Claesson, P.M.; Dėdinaitė, A. Phospholipids and Hyaluronan: From Molecular Interactions to Nano- and Macroscale Friction. Colloids Interfaces 2022, 6, 38. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-based lubricants: Development, properties, and performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Ivanov, M.; Shenderova, O. Nanodiamond-based nanolubricants for motor oils. Curr. Opin. Solid State Mater. Sci. 2017, 21, 17–24. [Google Scholar] [CrossRef]
- Curtis, C.K.; Marek, A.; Smirnov, A.I.; Krim, J. A comparative study of the nanoscale and macroscale tribological attributes of alumina and stainless steel surfaces immersed in aqueous suspensions of positively or negatively charged nanodiamonds. Beilstein J. Nanotechnol. 2017, 8, 2045–2059. [Google Scholar] [CrossRef]
- Szabó, Á.I.; Tóth, Á.D.; Abdallah, H.; Hargitai, H. Experimental Wear Analysis of Nano-Sized Titania Particles as Additives in Automotive Lubricants. Micro 2023, 3, 715–727. [Google Scholar] [CrossRef]
- Singh, A.P.; Dwivedi, R.K.; Suhane, A. In the context of nano lubrication, do nanoparticles exhibit favourable impacts on all tribo surfaces? A review. Prot. Met. Phys. Chem. Surf. 2022, 58, 325–338. [Google Scholar] [CrossRef]
- Dybwad, G.L. A sensitive new method for the determination of adhesive bonding between a particle and a substrate. J. Appl. Phys. 1985, 58, 2789–2790. [Google Scholar] [CrossRef]
- Laschitsch, A.; Johannsmann, D. High frequency tribological investigations on quartz resonator surfaces. J. Appl. Phys. 1999, 85, 3759–3765. [Google Scholar] [CrossRef]
- Borovsky, B.P.; Bouxsein, C.; O’Neill, C.; Sletten, L.R. An Integrated Force Probe and Quartz Crystal Microbalance for High-Speed Microtribology. Tribol. Lett. 2017, 65, 148. [Google Scholar] [CrossRef]
- Hanke, S.; Petri, J.; Johannsmann, D. Partial slip in mesoscale contacts: Dependence on contact size. Phys. Rev. E 2013, 88, 032408. [Google Scholar] [CrossRef] [PubMed]
- Seed, C.M.; Acharya, B.; Andrus, R.; Krim, J. Correlation of high frequency QCM sphere-plate stiffness measurements with macroscopic frictional contacts in thin film and bulk stainless-steel materials. Sens. Actuators A Phys. 2020, 306, 111913. [Google Scholar] [CrossRef]
- Daikhin, L.; Gileadi, E.; Tsionsky, V.; Urbakh, M.; Zilberman, G. Slippage at adsorbate–electrolyte interface. Response of electrochemical quartz crystal microbalance to adsorption. Electrochim. Acta 2000, 45, 3615–3621. [Google Scholar] [CrossRef]
- Choo, J.H.; Glovnea, R.P.; Forrest, A.K.; Spikes, H.A. A low friction bearing based on liquid slip at the wall. J. Tribol. 2007, 129, 611–620. [Google Scholar] [CrossRef]
- Pardue, T.N.; Acharya, B.; Curtis, C.K.; Krim, J. A Tribological Study of γ-Fe2O3 Nanoparticles in Aqueous Suspension. Tribol. Lett. 2018, 66, 130. [Google Scholar] [CrossRef]
- Acharya, B.; Pardue, T.N.; Su, L.; Smirnov, A.I.; Brenner, D.W.; Krim, J. Nanotribological performance factors for aqueous suspensions of oxide nanoparticles and their relation to macroscale lubricity. Lubricants 2019, 7, 49. [Google Scholar] [CrossRef]
- Melendez, M.; Vázquez-Quesada, A.; Delgado-Buscalioni, R. Load impedance of immersed layers on the quartz crystal microbalance: A comparison with colloidal suspensions of spheres. Langmuir 2020, 36, 9225–9234. [Google Scholar] [CrossRef] [PubMed]
- Coffey, T.; Krim, J. C60 Molecular Bearings and the Phenomenon of Nanomapping. Phys. Rev. Lett. 2006, 96, 186104. [Google Scholar] [CrossRef] [PubMed]
- Lhermerout, R.; Diederichs, C.; Sinha, S.; Porfyrakis, K.; Perkin, S. Are buckminsterfullerenes molecular ball bearings? J. Phys. Chem. B 2018, 123, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Duan, F. Friction properties of carbon nanoparticles (nanodiamond and nanoscroll) confined between DLC and a-SiO2 surfaces. Tribol. Int. 2020, 145, 106153. [Google Scholar] [CrossRef]
- Van Sang, L.; Yano, A.; Osaka, A.I.; Sugimura, N.; Washizu, H. Addition of Solid Oxide Particles for Friction Reduction. Tribol. Lett. 2022, 70, 60. [Google Scholar] [CrossRef]
- Nyholm, N.; Espallargas, N. Functionalized carbon nanostructures as lubricant additives—A review. Carbon 2023, 201, 1200–1228. [Google Scholar] [CrossRef]
- Wang, B.; Yao, L.; Dai, S.; Lu, H. Astonishingly distinct lubricity difference between the ionic liquid modified carbon nanoparticles grafted by anion and cation moieties. Friction 2023, 11, 949–965. [Google Scholar] [CrossRef]
- Su, L.; Krim, J.; Brenner, D.W. Interdependent roles of electrostatics and surface functionalization on the adhesion strengths of nanodiamonds to gold in aqueous environments revealed by molecular dynamics simulations. J. Phys. Chem. Lett. 2018, 9, 4396–4400. [Google Scholar] [CrossRef]
- Liu, C.; Meng, Y.; Tian, Y. Potential-Controlled Boundary Lubrication Using MoS2Additives in Diethyl Succinate. Tribol. Lett. 2020, 68, 72. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Xie, L.; Liu, H.; Huang, C.; Lu, Q.; Qiu, X.; Zeng, H. Probing the self-assembly and nonlinear friction behavior of confined gold nano-particles. Langmuir 2019, 35, 15701–15709. [Google Scholar] [CrossRef] [PubMed]
- Seed, C.M.; Acharya, B.; Krim, J. QCM Study of Tribotronic Control in Ionic Liquids and Nanoparticle Suspensions. Tribol. Lett. 2021, 69, 83. [Google Scholar] [CrossRef]
- Liu, C.; Friedman, O.; Li, Y.; Li, S.; Tian, Y.; Golan, Y.; Meng, Y. Electric response of CuS nanoparticle lubricant additives: The effect of crystalline and amorphous octadecylamine surfactant capping layers. Langmuir 2019, 35, 15825–15833. [Google Scholar] [CrossRef]
- Seed, C.M.; Acharya, B.; Nunn, N.; Smirnov, A.I.; Krim, J. Tribotronic and electrochemical properties of platinum–nanofluid interfaces formed by aqueous suspensions of 5 and 40 nm TiO2 nanoparticles. J. Chem. Phys. 2023, 159, 11. [Google Scholar] [CrossRef]
- Riley, J.K.; Matyjaszewski, K.; Tilton, R.D. Friction and adhesion control between adsorbed layers of polyelectrolyte brush-grafted nanoparticles via pH-triggered bridging interactions. J. Colloid Interface Sci. 2018, 526, 114–123. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Zhang, G.; Wang, D.; Wang, T.; Wang, Q. High lubricity and electrical responsiveness of solvent-free ionic SiO2 nanofluids. J. Mater. Chem. A 2018, 6, 2817–2827. [Google Scholar] [CrossRef]
- Taylor, R.I. Rough surface contact modelling—A review. Lubricants 2022, 10, 98. [Google Scholar] [CrossRef]
- Falvo, M.R.; Superfine, R. Mechanics and Friction at the Nanometer Scale. J. Nanoparticle Res. 2000, 2, 237–248. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, mechanical, and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef]
- Liamas, E.; Connell, S.D.; Ramakrishna, S.N.; Sarkar, A. Probing the frictional properties of soft materials at the nanoscale. Nanoscale 2020, 12, 2292–2308. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, X.; Li, W.; Qin, X.; Li, Y.; Wang, K.; Guo, Y.; Li, X.; Zhang, W.; Wang, Z. Interfacial friction at action: Interactions, regulation, and applications. Friction 2023, 11, 2153–2180. [Google Scholar] [CrossRef]
- Shu, J.; Bin Melvin Teo, J.; Kong Chan, W. Fluid Velocity Slip and Temperature Jump at a Solid Surface. Appl. Mech. Rev. 2017, 69, 020801. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Liu, Q.; Masliyah, J.; Xu, Z. QCM-D study of nanoparticle interactions. Adv. Colloid Interface Sci. 2016, 233, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Urbakh, M.; Tsionsky, V.; Gileadi, E.; Daikhin, L. Probing the solid/liquid interface with the quartz crystal microbalance. Piezoelectric Sens. 2007, 111–149. [Google Scholar] [CrossRef]
- Johannsmann, D.; Langhoff, A.; Leppin, C. Studying Soft Interfaces with Shear Waves: Principles and Applications of the Quartz Crystal Microbalance (QCM). Sensors 2021, 21, 3490. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.; Evans, D.R.; Bonaccurso, E.; Butt, H.J.; Craig, V.S. Boundary slip in Newtonian liquids: A review of experimental studies. Rep. Prog. Phys. 2005, 68, 2859. [Google Scholar] [CrossRef]
- Baroncini, M.; Silvi, S.; Credi, A. Photo-and redox-driven artificial molecular motors. Chem. Rev. 2019, 120, 200–268. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, S. Application of graphene derivatives and their nanocomposites in tribology and lubrication: A review. RSC Adv. 2019, 9, 40642–40661. [Google Scholar] [CrossRef]
- Ali, I.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, E.; Grachev, V.; Tkachev, A. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, J.; Gong, L.; Zeng, H. Surface forces and interaction mechanisms of soft thin films under confinement: A short review. Soft Matter 2020, 16, 6697–6719. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Klein, J. Direct measurement of surface forces: Recent advances and insights. Appl. Phys. Rev. 2021, 8, 3. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; He, W.; Zhang, J.; Qiao, X.; Li, J.; Xiang, D.; Qian, G.; Bai, P.; Meng, Y.; et al. A Review of Electric Potential-Controlled Boundary Lubrication. Lubricants 2023, 11, 467. [Google Scholar] [CrossRef]
- Bresme, F.; Kornyshev, A.A.; Perkin, S.; Urbakh, M. Electrotunable friction with ionic liquid lubricants. Nat. Mater. 2022, 21, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Spikes, H.A. Triboelectrochemistry: Influence of Applied Electrical Potentials on Friction and Wear of Lubricated Contacts. Tribol. Lett. 2020, 68, 90. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. [Google Scholar] [CrossRef]
- Horton, J.W.; Marrison, W.A. Precision determination of frequency. Proc. Inst. Radio Eng. 1928, 16, 137–154. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Na Songkhla, S.; Nakamoto, T. Overview of quartz crystal microbalance behavior analysis and measurement. Chemosensors 2021, 9, 350. [Google Scholar] [CrossRef]
- Berg, S.; Johannsmann, D. High speed microtribology with quartz crystal resonators. Phys. Rev. Lett. 2003, 91, 145505. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Prellberg, T.; Johannsmann, D. Nonlinear contact mechanics based on ring-down experiments with quartz crystal resonators. Rev. Sci. Instrum. 2003, 74, 118–126. [Google Scholar] [CrossRef][Green Version]
- Vlachová, J.; König, R.; Johannsmann, D. Stiffness of sphere-plate contacts at MHz frequencies: Dependence on normal load, oscillation amplitude, and ambient medium. Beilstein J. Nanotechnol. 2015, 6, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Seed, C.M.; Acharya, B.; Krim, J. Continuum Model Analysis of QCM Nanotribological Data to Obtain Friction Coefficients for 304SS Contacts Lubricated by Water and TiO2 Nanoparticle Suspensions. Front. Mech. Eng. 2020, 6, 72. [Google Scholar] [CrossRef]
- Leopoldes, J.; Jia, X. Transverse shear oscillator investigation of boundary lubrication in weakly adhered films. Phys. Rev. Lett. 2010, 105, 266101. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Seed, C.M.; Brenner, D.W.; Smirnov, A.I.; Krim, J. Tuning friction and slip at solid-nanoparticle suspension interfaces by electric fields. Sci. Rep. 2019, 9, 18584. [Google Scholar] [CrossRef] [PubMed]
- Spikes, H.A. The half-wetted bearing. Part 1: Extended Reynolds equation. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2003, 217, 1–14. [Google Scholar] [CrossRef]
- Spikes, H.A. The half-wetted bearing. Part 2: Potential application in low load contacts. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2003, 217, 15–26. [Google Scholar] [CrossRef]
- Payam, A.F.; Kim, B.; Lee, D.; Bhalla, N. Unraveling the liquid gliding on vibrating solid liquid interfaces with dynamic nanoslip enactment. Nat. Commun. 2022, 13, 6608. [Google Scholar] [CrossRef]
- Liang, Q.; Tsui, O.K.C.; Xu, Y.; Li, H.; Xiao, X. Effect of c 60 molecular rotation on nanotribology. Phys. Rev. Lett. 2003, 90, 146102. [Google Scholar] [CrossRef] [PubMed]
- Benassi, A.; Vanossi, A.; Pignedoli, C.A.; Passerone, D.; Tosatti, E. Does rotational melting make molecular crystal surfaces more slippery? Nanoscale 2014, 6, 13163–13168. [Google Scholar] [CrossRef]
- Lhermerout, R.; Perkin, S. A new methodology for a detailed investigation of quantized friction in ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 455–466. [Google Scholar] [CrossRef]
- He, J.; Tang, H.; Wang, C. Advances of molecular dynamics simulation in tribochemistry and lubrication investigations: A review. J. Ind. Eng. Chem. 2023, 126, 1–19. [Google Scholar] [CrossRef]
- Grewal, M.S.; Abe, H.; Matsuo, Y.; Yabu, H. Aqueous dispersion and tuning surface charges of polytetrafluoroethylene particles by bioinspired polydopamine–polyethyleneimine coating via one-step method. R. Soc. Open Sci. 2021, 8, 210582. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Dai, S.; Lu, H. Carbon Dot-Based Nanofluids as Additives for Mechanical Lubrication. ACS Appl. Nano Mater. 2024, 7, 7925–7933. [Google Scholar] [CrossRef]
- Su, L.; Krim, J.; Brenner, D.W. Dynamics of Neutral and charged nanodiamonds in aqueous media confined between gold surfaces under normal and shear loading. ACS Omega 2020, 5, 10349–10358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, X.; Duan, F.; Meng, Y. An efficient lubrication approach to mitigate soot-induced wear: Synergistic repair effect of magnetic MoS2 composites and magnetic field. Wear 2022, 488, 204182. [Google Scholar] [CrossRef]
- Fajardo, O.Y.; Bresme, F.; Kornyshev, A.A.; Urbakh, M. Electrotunable friction with ionic liquid lubricants: How important is the molecular structure of the ions? J. Phys. Chem. Lett. 2015, 6, 3998–4004. [Google Scholar] [CrossRef]
- Liu, C.; Friedman, O.; Meng, Y.; Tian, Y.; Golan, Y. CuS nanoparticle additives for enhanced ester lubricant performance. ACS Appl. Nano Mater. 2018, 1, 7060–7065. [Google Scholar] [CrossRef]
- Riley, J.K.; Junxue, A.; Tilton, R.D. Ionic Surfactant Binding to pH-Responsive Polyelectrolyte Brush-Grafted Nanoparticles in Suspension and on Charged Surfaces. Langmuir 2015, 31, 13680–13689. [Google Scholar] [CrossRef] [PubMed]
- Notley, S.M.; Biggs, S.; Craig, V.S.; Wågberg, L. Adsorbed layer structure of a weak polyelectrolyte studied by colloidal probe microscopy and QCM-D as a function of pH and ionic strength. Phys. Chem. Chem. Phys. 2004, 6, 2379–2386. [Google Scholar] [CrossRef]
- Cuddy, M.F.; Poda, A.R.; Brantley, L.N. Determination of isoelectric points and the role of pH for common quartz crystal microbalance sensors. ACS Appl. Mater. Interfaces 2013, 5, 3514–3518. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Ray Chowdhury, S.; Herrera, R.; Jiang, D.D.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. Functionalized nanostructures with liquid-like behavior: Expanding the gallery of available nanostructures. Adv. Funct. Mater. 2005, 15, 1285–1290. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krim, J.; Smirnov, A.I. Fundamental Mechanisms Underlying the Effectiveness of Nanoparticle Additives to Lubricants: 25 Examples Linking Nano- to Macroscale Friction. Lubricants 2024, 12, 225. https://doi.org/10.3390/lubricants12060225
Krim J, Smirnov AI. Fundamental Mechanisms Underlying the Effectiveness of Nanoparticle Additives to Lubricants: 25 Examples Linking Nano- to Macroscale Friction. Lubricants. 2024; 12(6):225. https://doi.org/10.3390/lubricants12060225
Chicago/Turabian StyleKrim, Jacqueline, and Alex I. Smirnov. 2024. "Fundamental Mechanisms Underlying the Effectiveness of Nanoparticle Additives to Lubricants: 25 Examples Linking Nano- to Macroscale Friction" Lubricants 12, no. 6: 225. https://doi.org/10.3390/lubricants12060225
APA StyleKrim, J., & Smirnov, A. I. (2024). Fundamental Mechanisms Underlying the Effectiveness of Nanoparticle Additives to Lubricants: 25 Examples Linking Nano- to Macroscale Friction. Lubricants, 12(6), 225. https://doi.org/10.3390/lubricants12060225





