Abstract
Laser clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, 2.0, with x values in molar ratio) high-entropy alloy (HEA) coatings were fabricated on Q345 carbon steel. This study delves into the impact of Nb incorporation on the reciprocating sliding wear resistance of these laser clad coatings against a Φ6 mm silicon nitride ball. The microstructure of the as-clad AlCr2FeCoNiNbx coatings transformed from a single Face-Centered Cubic (FCC) solid solution (when x = 0) to the hypoeutectic state (when x = 0.5) and progressed to the hypereutectic state (when x ≥ 1.0). This evolution was marked by an increase in the Laves phase and a decrease in FCC. Consequently, the HEA coatings exhibited a gradually increasing Vickers hardness, reaching a peak at HV 820. Despite a decline in corrosion resistance, there was a notable enhancement in wear resistance, and the friction of the HEA coating could be reduced by Nb addition. The phase evolution induced by Nb addition led to a shift in the predominant wear mechanism from delamination wear to abrasive wear. The wear rate of Nb0.5 was impressively low, at 6.2 × 10−6 mm N−1 m−1 when reciprocating sliding under 20 N in air. In comparison to Nb0, Nb0.5 showcased 3.6, 7.2, and 6.5 times higher wear resistance at 5 N, 10 N, and 20 N, respectively. Under all applied loads, Nb1.5 has the lowest wear rate among all HEA coatings. This substantiates that the subtle introduction of Laves phase-forming elements to modulate hardness and oxidation ability proves to be an effective strategy for improving the wear resistance of HEA coatings.
1. Introduction
Wear is a basic form of component failure, and improving the wear resistance of materials can improve the service life of components. The industries of metallurgy, mining, electricity, water conservancy, and agricultural machinery have high requirements for the wear resistance of mechanical equipment. It is generally believed that the hardness of materials is closely related to their wear resistance. High-entropy alloys (HEAs), as newly developed alloys, have exhibited lots of outstanding properties, including high hardness, good corrosion resistance, high toughness along with high strength, excellent wear resistance, and exceptional oxidation resistance [1,2,3]. The wear resistance of HEAs and their coatings is attractive and has been widely investigated [4], since they have great potential for engineering applications such as turbine blades and heat exchangers [5]. Compared to traditional alloys, some HEAs have a relatively higher material hardness. HEA with high hardness is expected to have correspondingly high wear resistance. Previous studies on the wear resistance of HEA have shown that, compared to traditional alloy materials, HEA exhibits better wear resistance. For example, the wear resistance of NiCrAlCoMo is higher than that of AISI1050 steel [6]; the wear resistance of Al2CrFeCoxCuNiTi (x = 0.5, 1, 1.5, and 2) is more than three times that of Q235 steel [7]; the wear resistance of Al3CrFeCoNiCu is about four times that of bearing steel [8], and so on. Therefore, HEA has potential value as a wear-resistant coating material. The in-depth study of the influencing factors on the wear resistance of HEA has also become one of the current research hotspots in the field of HEA.
The choice of metal elements and their ratio will directly determine the properties of the HEA itself. The HEA, composed of several transition metals such as Fe, Ni, and Co, along with several other elements such as Al, Cr, Cu, Mn, Ti, etc., first attracted research interest. At present, high-entropy alloys mainly composed of FeCoNi have become the main component series in current HEA research due to their excellent mechanical properties. For the good corrosion resistance comparable to SS304 stainless steel [9] and the high compressive strength of up to 3920 MPa [10], Al-Co-Cr-Fe-Ni high entropy alloy was investigated as a wear-resistant coating. However, the wear resistance of laser clad AlCr2FeCoNi was not as high as expected.
In addition, due to the excellent corrosion resistance and biocompatibility of IIIB and IVB group elements such as Ti and Zr, the TiZr series alloy composition has become a research hotspot for another type of HEA wear-resistant material. Changes in the element content of HEAs cause phase transitions, which have been noted, especially when adding those elements with high mixing enthalpy to other elements. For example, adding Mo to CoCrFeNiMox (x = 0–1.5) leads to the optimum combination of intermetallic compounds and FCC phases, promoting the optimization of wear resistance [11]. Adding Nb to AlCoCrFeNi HEA also resulted in phase evolution and Laves phase formation [12]. With increasing the Nb content in CoCrFeNi HEA, the yield strength is improved accordingly [13]. Furthermore, the eutectic microstructure was found in CoCrFeNi HEA with Nb addition [14]. Unfortunately, how the addition of Nb affects wear resistance is uncertain. The Nb element can directly combine with C at high temperatures to form NbC, which has extremely high hardness. Therefore, adding Nb elements to high-entropy alloys is expected to have a dispersion-strengthening effect, thereby improving wear resistance. Cheng et al. [10] prepared CoNiCuFeCr and CoNiCuFeCrNb coatings using the plasma transfer arc melting method and found that under the same wear test conditions, the addition of Nb can improve the wear resistance of the coating by about 1.5 times. Guo et al. [15] prepared AlCrFeMoNbxTiW high-entropy alloy coatings using laser cladding technology and conducted friction and wear experiments at a load of 200 N and a speed of 200 r·min−1. The results indicate that the average friction coefficient and wear rate of the coating decrease with the increase in Nb content, and the delamination phenomenon caused by adhesive wear of the coating disappears, enhancing the anti-adhesive wearability of the coating. The AlCrFeNi2W0.2Nbx HEA coatings were synthesized on the 304 stainless steel by laser cladding, and the AlCrFeNi2W0.2Nbx (x = 1.5, 2.0) high-entropy alloy coatings exhibit an order of magnitude lower wear than 304 stainless steel [16]. Lin et al. [17] prepared FeCoCrNiAlNbx HEA coatings and found that the friction coefficient and mass loss of the HEA coating first decreased and then increased with the increase in Nb content, and both reached their minimum values at x = 0.5. Therefore, the addition of an appropriate amount of Nb could be an effective way to improve the wear resistance of the HEA coating.
The main preparation methods for HEAs and their coatings include vacuum melting, powder metallurgy, mechanical alloying, laser cladding, thermal spraying, cold spraying, magnetron sputtering, plasma-based ion implantation, electrochemical deposition, etc. Among them, laser cladding is a widely used surface technology, and Ni-based, Fe-based, and Co-based alloys are commonly used as laser clad coatings to improve the surface performance of conventional metal parts [18,19]. Compared with arc melting, laser cladding reduces the economic cost significantly by melting a thin layer of the substrate, which ensures superior metallurgical properties such as supersaturated solid solutions, fast heating and cooling rates, and dense microstructure [19,20,21]. So, fabricating HEA coatings with laser cladding has a wide potential application in strengthening the protective coating market. The former research has proved that a suitable amount of intermetallic compound in the solid solution phases presents a positive effect on the mechanical performance, although many HEAs are designed to prevent their formation [20,22,23]. Consequently, the wear resistance of HEAs may also be strongly influenced by adjusting the phase transformation with a minor addition.
In current work, laser clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) HEAs coatings were manufactured on low carbon steel, and Nb was added to form an intermetallic compound because it has a very negative mixing entropy with other elements. Although our former work on the slurry erosion resistance of AlCr2FeCoNiNbx HEA coatings displayed that the erosion resistance was enhanced by Nb addition [24], the reciprocating sliding wear of this HEA coating has not been studied. Consequently, for the requirement of a protective coating of hydraulic cylinder piston rods, the sliding wear resistance of AlCr2FeCoNiNbx coatings is investigated carefully, as are their microstructure, phase evolution, hardness, and corrosion resistance.
2. Materials and Methods
Laser clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) coatings with different x values in the molar ratio are referred to as Nb0, Nb0.5, Nb1.0, Nb1.5, and Nb2.0, respectively. The nominal alloy compositions of AlCr2FeNiCoNbx HEA coatings are listed in Table 1. The pure metal powders with a purity >99.5 wt.% and an average particle size of 53 μm were mixed by dry ball milling. The ball milling process adopts an ND7-type planetary (Tencan Powder Technology Co., Ltd., Changsha, China) ball mill, an alumina ball milling tank (same manufacturer), and alumina ceramic balls (same manufacturer) with a diameter range of 5–20 mm. The relevant parameters used are frequency 16 Hz (450 r min−1), each running time of 3 min, the following pausing time of 7 min, and the actual ball milling time of 20 h. Then, the mixed powders were paved on the substrate with a 4.5 wt% PVC solution. The thickness of the pre-coating layer is about 0.8 mm. The substrate is low carbon steel (Q345 with 0.12–0.20 wt.% C, 0.20–0.55 wt.% Si, 1.20–1.60 wt.% Mn, <0.045 wt.% P, and <0.045 wt.% S), which is cleaned by sandpaper before pre-coating. The pre-coated layer was dried for 3 h in a vacuum oven. The pulsed laser processing machine (GD-ECYW300, Shenzhen Gd Laser Technology Co., Ltd., Shenzhen, China) was used for laser cladding. The specific processing parameters were: spot diameter D = 1 mm, peak power P = 3.5 kW, pulse frequency F = 19 Hz, pulse width W = 3.0 ms, scanning speed V = 140 mm/min, and an overlap ratio of 50%. For the following tests, the HEA coating samples were cut to a size of 10 × 10 × 10 mm.

Table 1.
Chemical compositions of AlCr2FeNiCoNbx HEA coating (wt.%).
The phase compositions were analyzed by X-ray diffraction (XRD, Rigaku D/max 2500, Akishima, Japan)-using Cu Kα radiation. The microstructure was observed by a scanning electron microscope (SEM, FEI Quanta 200, Thermo Fisher Scientific, Waltham, MA, USA). Through SEM energy dispersive spectrometry (EDS), the chemical compositions of different microareas were studied. The hardness of HEA coatings was tested by a Vickers hardness tester (HXD-1000TC, Shanghai Caikang Optical Instrument Co., Ltd., Shanghai, China) under a loading of 200 g with a holding time of 15 s. Each hardness value was obtained from the average value of five measurements.
After polishing with 240 to 1200 grit SiC paper, the HEA coating samples obtained the same average surface roughness (Ra = 0.1 μm). The anodic polarization curves were performed for the study of corrosion resistance. The measurement starts from −1000 mV (vs. SCE) to 200 mV (vs. SCE) with a sweep rate of 1 mV·s−1. A platinum wire was applied as the auxiliary electrode. The working electrode was the HEA coating sample, which was covered by epoxy resin except for one side surface (1 cm2). The reference electrode was a saturated calomel electrode (SCE).
The sliding wear resistance of the laser clad coating samples was characterized by reciprocating sliding using a tribological testing machine (CFT-I style, Lanzhou Zhongke Kaihua Technology Development Co., Ltd., Lanzhou, China). The sliding speed was 20 mm·s−1, the sliding stroke was 5 mm, and the total sliding duration was 60 min. During sliding against a Φ6 mm silicon nitride ball, the Coefficient of Friction (COF) was continuously recorded. The contact loads were 5 N, 10 N, and 20 N, respectively. After each test, the surface profiles were detected along the wear track. The average values of the cross-sectional area obtained from at least 5 positions along the wear track were used for the calculation of volume loss. The wear rate can be calculated from the following expression: ω = V/(F × L), where V means the wear volume loss (mm3), F represents the applied normal load (N), and L is the total distance of sliding (m).
3. Results and Discussion
3.1. Microstructure and Hardness
3.1.1. Microstructure Evolution
As-clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) HEA coatings were characterized by XRD. The corresponding XRD patterns are presented in Figure 1. Based on the XRD pattern of Nb0, it can be seen that it contains a single FCC solid solution phase. The addition of Nb in the HEA coating Nb0 leads to the emergence of the BCC and Laves phases. Meanwhile, the main crystal planes of the FCC phase transform from (200) to (111). By continuously increasing the content of Nb, the diffraction peak intensity of the FCC phase gradually decreases. When x ≥ 1.0, FCC phase peaks are almost invisible. At the same time, the diffraction peak intensities of BCC and Laves phases gradually increase with the increase in Nb. Therefore, Nb addition is beneficial to the phase evolution from FCC to BCC and the Laves phase. Such phase evolution may greatly enhance the HEA’s wear resistance [25]. However, further addition of Nb causes a decrease in BCC peak intensity and an increase in Laves phase peak intensity. So, when x ≥ 1.0, more Nb content favors the transition from BCC to the Laves phase.
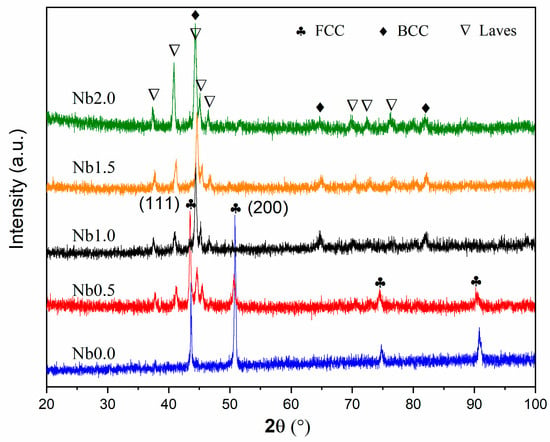
Figure 1.
XRD patterns of as-clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) coatings.
The phase formation of multi-component alloys mainly depends on the competitive outcomes of mixing enthalpy (ΔHmix), mixing entropy (ΔS), the atom-size difference (ΔR), valence electron concentration (VEC), and electro-negativity difference (ΔX) [26]. Here, the Nb element possesses the largest atomic size in the alloying system. Adding Nb will cause severe lattice distortion, which promotes the transition of FCC to higher-density BCC. It results in the FCC being reduced and the BCC phase increasing gradually. On the other hand, following the Miedema model, the ΔHmix in an alloy system is defined as the following [27,28,29]:
where n is the number of components in an alloy system, Ci and Cj are the atomic percentages of the ith and jth elements, and ΔHmix is the mixing enthalpy of binary AB alloys, which can be found in Refs. [30,31]. The calculated values of ΔHmix are −11.00, −14.25, −16.49, −18.03, and −19.06 kJ/mol, respectively, when x = 0, 0.5, 1.0, 1.5, and 2.0, revealing that the increase in Nb content leads to the decrease of ΔHmix. It is well known that the more negative ΔHmix indicates a higher binding energy between atoms. As a result, it favors the formation of the Laves phase. More Nb addition will lead to the disintegration of the solid solution, the failure of the high-entropy effect, and the formation of intermetallic compounds. In other words, due to the significant difference between Nb and other atoms (the very low negative value of ΔHmix), the addition of Nb promotes dense atomic structure and the formation of the Laves phase. In addition, lattice distortion is common in high-entropy alloys with multiple principal components. The strong binding between certain atoms in the lattice may exacerbate lattice distortion and lead to the formation of relatively loose atomic structures, causing the phase transition from FCC to BCC. Therefore, for the greater ΔHmix, the addition of Nb promotes the formation of the Laves phase, and the content of the Laves phase increases with the addition of Nb.
Furthermore, the chemical compositions of the zones pointed out in Figure 2 are characterized by EDS, as listed in Table 2. As can be seen, all coatings contain a large amount of Fe content. Meanwhile, Al and Ni content is lower than the nominal composition, indicating the surface of Q345 steel is melted partially into the HEA coating during laser melting, resulting in dilution. In addition, Al and Ni may be seriously burned in part by the high-energy laser beam and discharged in the form of scum eventually. The EDS (Table 2) analysis indicates that the FCC contains more Fe, Cr, and Ni, which is consistent with Refs. [13,14]. While the Laves phase and the dendrite are enriched with Nb because of its largest atomic radius. The degree of the supersaturation increased with the addition of Nb, resulting in more Nb-rich BCC and Laves phases being formed.
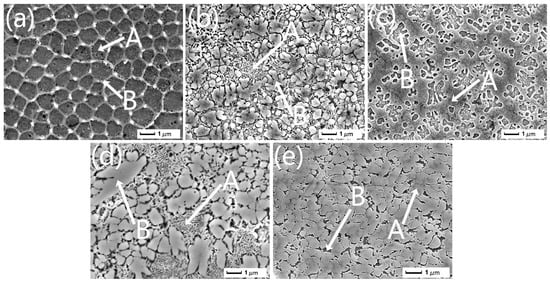
Figure 2.
Microstructure observations of as-clad HEA coatings: (a) Nb0, (b) Nb0.5, (c) Nb1.0, (d) Nb1.5, and (e) Nb2.0. The zones marked as “A” and “B” in each subfigure are measured with EDS, as the results shown in Table 2.

Table 2.
EDS results of as-clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) HEA coatings (at. %).
The microstructure of the as-clad HEA alloy coatings with a different Nb content is presented in Figure 2. The microstructure of Nb0 is relatively simple and exhibits equiaxed crystal morphology (Figure 2a), suggesting the FCC solid solution structure based on the XRD results shown in Figure 1a. A typical hypoeutectic structure with numerous coarse FCC-equiaxed crystals is present in Nb0.5, meaning this HEA alloy belongs to a eutectic high-entropy alloy (EHEA) [32,33]. The eutectic structure is composed of BCC and a network of Laves phases. FCC decreases obviously with the Nb addition. Compared to Nb0 and Nb0.5, Nb1.0 displays a hypereutectic structure, as shown in Figure 2c. The main phase composition of Nb1.0 is the BCC phase, and the eutectic mixture is composed of the BCC and Laves phases. With the addition of Nb, the BCC phase increases continually, becoming a primary phase, as shown in Figure 2d. Meanwhile, the lamellar eutectic structure decreases. Further adding Nb, the coarse BCC phase occupies the majority of the space of Nb2.0, as shown in Figure 2e, and the eutectic structure and Laves phase almost disappear. So, the eutectic point may be located at a chemical composition between Nb0.5 and Nb1.0. The eutectic reaction during solidification may be elucidated as L→FCC + BCC + Laves. Similarly, by adding the Nb element, Ma et al. [12] reported that the binary eutectic point of the AlCoCrFeNbxNi alloy ranges from 0.5 to 0.75, which is slightly different from the ternary eutectic point found in this work.
3.1.2. Hardness Evolution
Figure 3 exhibits the cross-sectional hardness variation curve in laser clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) HEA coatings. Based on the relationship between the hardness values and the distance from the coating surface, it can be inferred that the thickness of the HEA coatings is about 200 μm and the Vickers hardness of HEA coatings with Nb addition is higher than that of Nb0. With the increasing Nb content, the HEA coating’s hardness gradually increases, reaching a maximum of around HV 820. On the other hand, the hardness of Nb0.5 increases by about 135% compared to Nb0, while the hardness of Nb1.0 increases by about 14% compared to Nb0.5. However, the hardness increases in Nb1.5 compared to Nb1.0 and Nb2.0 compared to Nb1.5 is lower, with values around 9%. The increasing hardness of HEA coating can be seen as the result of the increase in BCC and Laves phases. Adding Nb also results in a decrease in the softer FCC content. At the same time, the lattice distortion of the HEA coating is also strengthened by the addition of Nb with larger atomic radii, which severely hinders dislocation movement.
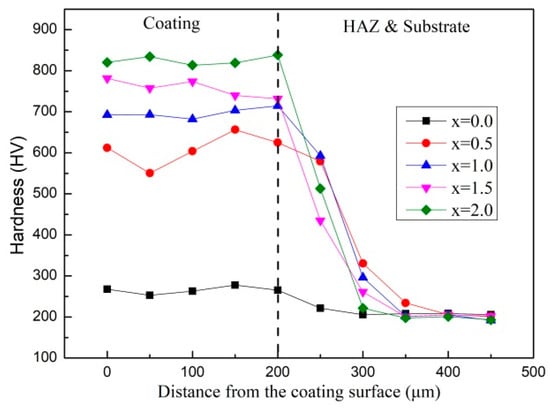
Figure 3.
Cross-sectional hardness values of as-clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) coatings.
3.2. Corrosion Resistance
The corrosion resistance of laser clad AlCr2FeCoNiNbx (x = 0, 0.5, 1.0, 1.5, and 2.0) HEA coatings was analyzed by their polarization curves detected in a 3.5% NaCl salt solution, as shown in Figure 4. By using cathode Tafel line extrapolation to intersect with corrosion potential, relevant data on corrosion potential Ecorr and corrosion current density Icorr can be obtained, as shown in Table 3. Nb0 has the highest corrosion potential and the minimum corrosion current, indicating that Nb0 possesses the best corrosion resistance. Adding the Nb element causes a negative shift in corrosion potential. Meanwhile, the corrosion current significantly increases. Compared with other HEAs, Nb0 only has an FCC phase, which is beneficial for reducing galvanic corrosion between phases. The corrosion potential of Nb0.5 is higher than that of Nb1.0, Nb1.5, and Nb2.0 because the microstructure of Nb0.5 is still dominated by FCC and there is no obvious primary cell formed between phases, resulting in a higher corrosion potential. Due to its most complex multiphase structure containing BCC, Laves, and a small amount of FCC simultaneously, Nb1.0 has the most negative Ecorr and the highest icorr, indicating its worst corrosion resistance. As Nb further increases, the microstructure is simplified to similar BCC and Laves phases, resulting in similar corrosion potentials and corrosion current density. So, a uniform single microstructure is beneficial for improving corrosion resistance, and adding Nb is harmful to corrosion resistance.
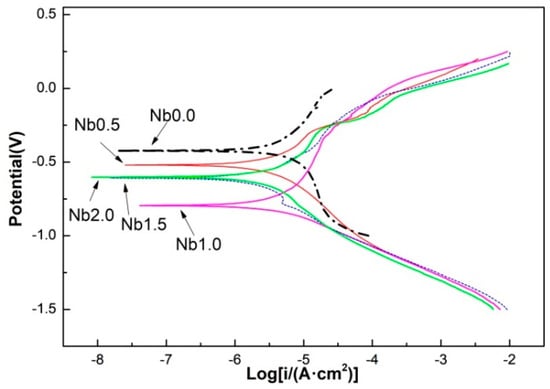
Figure 4.
Polarization curves for as-clad AlCr2FeCoNiNbx HEA coatings in a 3.5% NaCl solution.

Table 3.
Polarization parameters for as-clad AlCr2FeCoNiNbx HEA coatings in a 3.5% NaCl solution.
Due to defects such as cracks and pores, the corrosion resistance of the coating is strongly influenced by both the material and the preparation process. Based on the electrochemical kinetic parameters estimated from the polarization curves after exposure to a 3.5 wt.% NaCl solution, a high-velocity oxy-fuel (HVOF)-prepared 316 L stainless steel coating displays −610 mV corrosion potential and 14.3 μA·cm−2 corrosion current density [34]. Compared with the HVOF 316L coating, Nb0.5 has a nobler corrosion potential and a much lower corrosion current density. Therefore, although the addition of Nb has slightly reduced the corrosion resistance, the HEA coatings studied in this work exhibit considerable corrosion resistance.
3.3. Sliding Wear Resistance
The effect of Nb addition on the wear resistance of the laser clad AlCr2FeCoNiNbx HEA coating was characterized by reciprocating sliding wear against a silicon nitride ball. The detected average Coefficient of Friction (COF) and average wear rate are shown in Figure 5. Except for Nb1.0, the COFs of the HEA coatings generally decreased with the increase in applied load. A very low COF of Nb1.0 coating at 5 N is attributed to its long wear-in stage, which has a COF lower than 0.2. The average COF of Nb1.0 at the stable stage is around 0.74, which is the lowest COF in all the HEA coatings at 5 N. Consequently, compared to Nb0, the average COF of Nb1.0 decreased by 22.7%. However, at 10 N or 20 N loads, the HEA coating Nb1.0 presented the highest COF values, while Nb2.0 possessed the lowest COFs. So, by adding appropriate Nb content, the friction of the HEA coating can be regulated or reduced.
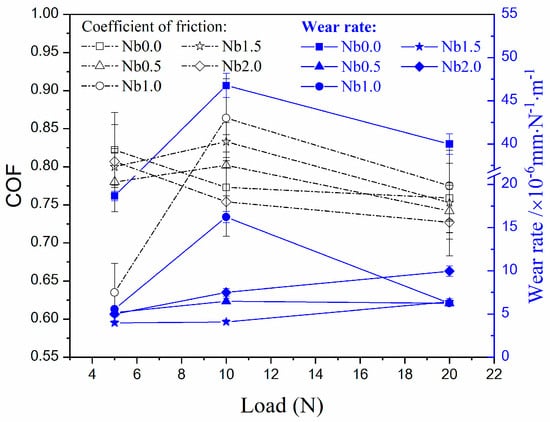
Figure 5.
Average COFs and average volume loss rates of laser clad AlCr2FeCoNiNbx HEA coatings after reciprocating sliding wear against silicon nitride balls under 5, 10, and 20 N, respectively.
The average volume loss rates of laser clad AlCr2FeCoNiNbx HEA coatings significantly decreased after Nb addition. At 5 N load, the average wear rate of Nb0 was 18.7 × 10−6 mm N−1 m−1, and the corresponding value of Nb0.5 was 5.2 × 10−6 mm N−1 m−1, which is about a 72% decrease. At high applied loads, the effect of Nb addition is more significant on the reduction of wear rate. At 10 N and 20 N, the average wear rate of Nb0 was 46.8 and 40 × 10−6 mm N−1 m−1, respectively. Accordingly, the corresponding values of Nb0.5 were 6.5 and 6.2 × 10−6 mm N−1 m−1, respectively. So, Nb addition leads to a more than 84% wear rate decrease in the laser clad HEA coating at high sliding applied loads. Because of Nb addition, the hard Laves phase is synthesized in the HEA coating, leading to an increase in hardness and the ability to resist plastic deformation. However, more Nb additions did not result in an obvious decrease in wear rate. Nb1.5 presented the lowest wear rate of all HEA coatings. At 20 N load, Nb0.5, Nb1.0, and Nb1.5 show almost the same low wear rate. The wear rate in Nb2.0 increased with the applied load, indicating this HEA coating possesses the characterization of high strength materials. Compared with the hardness of the HEA coatings (Figure 3) with and without adding Nb, it can be seen that the high hardness is the decisive factor for high wear resistance. However, too high a level of hardness is harmful to wear resistance, especially under high applied loads. Nb1.5 has the lowest wear rate in the applied load range of 5 N to 20 N. So, achieving a strong and tough combination, with the majority of hard phases and a small amount of dispersed soft phases, is an effective way to obtain the lowest wear rate.
3.4. Wear Mechanism
To analyze the wear mechanism, the worn surface morphologies of the HEA coatings were observed, as illustrated in Figure 6. Although the volume loss rate of the HEA coatings can be connected with their hardness, the reason causing the wear rate of Nb1.0 to be much higher than others is not clear. As can be seen in Figure 6a, the worn surface of Nb0 is covered by lots of black tribo-layers, which contain cracks and may be formed from metal oxides and debris. Meanwhile, there are some deep craters with limited deformation resistance, indicating delamination is the primary wear mechanism of Nb0.
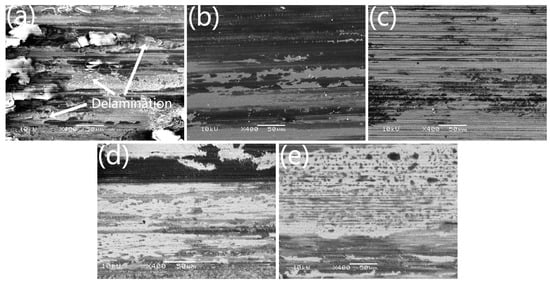
Figure 6.
Worn surface morphologies of laser clad AlCr2FeCoNiNbx HEA coatings after reciprocating sliding wear against a silicon nitride ball under 10 N: (a) Nb0, (b) Nb0.5, (c) Nb1.0, (d) Nb1.5, and (e) Nb2.0.
As we all know, oxidation of the metal contact surface is inevitable due to the presence of oxygen and frictional heat when sliding in the atmospheric environment. The worn surface of Nb0.5 presents large-area oxidation layers, as shown in Figure 6b. However, on the worn surface of Nb1.0, the oxidation layers are substituted by many abrasive grooves, as shown in Figure 6c. Oxidation-generated tribo-layer is beneficial to decreasing COF and wear rate because the tribo-layer can inhibit further oxidation and abrasion. Similarly, the low friction and wear of VAlTiCrMo1.6 are attributed to the tribo-oxidation-induced layered oxidic surface [35]. Without the protection of the tribal-layer, Nb1.0 is suffering serious abrasive wear, which leads to a higher COF and a worse wear rate, as shown in Figure 5. With more Nb addition, the HEA coatings Nb1.5 and Nb2.0 present many scratched grooves, as shown in Figure 6d,e, which are shallow when compared with those on the surface of Nb1.0. However, similar to Nb1.0, Nb1.5, and Nb2.0 have limited oxidation layers, which indicates that the FCC phase tends to form an oxidation layer. So, the HEA coating with the Nb addition is mainly controlled by abrasive wear.
4. Conclusions
The microstructure of as-clad AlCr2FeCoNiNbx HEA coatings underwent notable changes with increasing Nb content, transitioning from a singular FCC structure (Nb0) to a hypoeutectic microstructure (Nb0.5), and eventually to a hypereutectic configuration. The eutectic point, potentially situated between Nb0.5 and Nb1.0, manifested a reaction expressed as L→BCC + FCC + Laves. Correspondingly, the volume fraction of BCC and Laves phases increased, paralleled by a rise in hardness. The addition of Nb propelled the Vickers hardness of the HEA coatings to a peak at HV 820, surpassing that of the Nb-free HEA coating by more than threefold. Consequently, the corrosion resistance of the HEA coating declined, while wear resistance exhibited a significant improvement. The reciprocating sliding wear tests showed that the friction of the HEA coating can be reduced by adding appropriate Nb content. Achieving a strong and tough combined microstructure is an effective way to obtain the lowest wear rate. With Nb incorporation, the primary wear mechanism shifted from delamination wear to abrasive wear. Based on the wear rate values of Nb0.5, when an Nb element is added, the wear rate of Nb0 can be reduced by 72% at 5 N and by more than 84% at 10 N or 20 N. This underscores that the adjustment of FCC and BCC contents, coupled with the formation of the Laves phase, significantly enhances the wear resistance of laser clad HEA coatings through Nb addition.
Author Contributions
Conceptualization, X.J. and F.W.; methodology, X.J. and Y.B.; validation, K.G., Y.B. and Z.M.; formal analysis, X.J.; investigation, K.G. and Y.B.; resources, Y.B. and H.D.; data curation, K.G.; writing—original draft preparation, X.J. and Y.B.; writing—review and editing, X.J.; visualization, Z.M. and H.D.; supervision, X.J.; project administration, F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the STU Scientific Research Foundation for Talents (NTF21011). F.W. would like to acknowledge the support from the 2020 Li Ka Shing Foundation Cross-Disciplinary Research (2020LKSFG01D) and Guangdong Provincial University Innovation Team Project (2020KCXTD012).
Data Availability Statement
The authors ensure that the data supporting the results of the research are included in the article.
Acknowledgments
The author, X.J., would like to express his gratitude to MDPI Publishing and the Lubricants editorial department.
Conflicts of Interest
Yayun Bao was employed by Bomag (Changzhou) Construction Machinery Co., Ltd., but he participated in the research when he was a student at Hohai University. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, Z.; Chen, W.P.; Wu, B.Y.; Cao, X.Y.; Liu, L.S.; Fu, Z.Q. Effects of Co and Ti on microstructure and mechanical behavior of Al0.75FeNiCrCo high entropy alloy prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A-Struct. 2015, 648, 217–224. [Google Scholar] [CrossRef]
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Kumar, D. Recent advances in tribology of high entropy alloys: A critical review. Prog. Mater. Sci. 2023, 136, 101106. [Google Scholar] [CrossRef]
- Wu, W.H.; Yang, C.C.; Yeh, J.W. Industrial development of high-entropy alloys. In Annales de Chimie-Science des Materiaux; Masson: Paris, NY, USA, 2006; Volume 31, pp. 737–747. [Google Scholar]
- Lin, Y.C.; Cho, Y.H. Elucidating the microstructural and tribological characteristics of NiCrAlCoCu and NiCrAlCoMo multicomponent alloy clad layers synthesized. Surf. Coat. Technol. 2009, 203, 1694–1701. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of Al2CrFeCoxCuNiTi high-entropy alloy coating prepared by laser cladding. Mater. Sci. Eng. Powder Met. 2013, 18, 735–740. [Google Scholar]
- Ji, X.; Alavi, S.H.; Harimkar, S.P.; Zhang, Y. Sliding Wear of Spark Plasma Sintered CrFeCoNiCu High-Entropy Alloy Coatings: Effect of Aluminum Addition. J. Mater. Eng. Perform. 2018, 27, 5815–5822. [Google Scholar] [CrossRef]
- Kar, S.; Meena, L.K.; Srivastava, V.C.; Mandal, G.K. Microstructural, Mechanical, and Corrosion Behaviour of Ni-Fe-Cr-Al and Ni-Fe-Cr-Al-Co Alloys. Trans. Indian Inst. Met. 2023. [Google Scholar] [CrossRef]
- Rogal, L.; Szklarz, Z.; Bobrowski, P.; Kalita, D.; Garzel, G.; Tarasek, A.; Kot, M.; Szlezynger, M. Microstructure and Mechanical Properties of Al-Co-Cr-Fe-Ni Base High Entropy Alloys Obtained Using Powder Metallurgy. Met. Mater. Int. 2019, 25, 930–945. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Y.X.; Cui, S.G.; Yi, Y.L.; Xing, X.W.; Wang, X.J.; Li, W. Effect of Mo Element on the Mechanical Properties and Tribological Responses of CoCrFeNiMo High-Entropy Alloys. Metals 2021, 11, 486. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A-Struct. 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNbxNi High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q.; Zhou, F. Microsturcture and wear resistance of high-melting-point AlCrFeMoNbxTiW high-entropy alloy coating by laser cladding. Rare Met. 2017, 41, 1327–1332. [Google Scholar] [CrossRef]
- Liang, H.; Yao, H.; Qiao, D.; Nie, S.; Lu, Y.; Deng, D.; Cao, Z.; Wang, T. Microstructures and Wear Resistance of AlCrFeNi2W0.2Nbx High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Therm. Spray Technol. 2019, 28, 1318–1329. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, N.; He, B.; Gong, X.; Zhang, Y.; Li, D.; Dong, F. Structural Evolution and Performance Changes in FeCoCrNiAlNbx High-Entropy Alloy Coatings Cladded by Laser. J. Therm. Spray Technol. 2017, 26, 2005–2012. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, W. Laser surface cladding: The state of the art and challenges. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1041–1060. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, T.; Kovacevic, R. Erosion and corrosion resistance of laser cladded AISI 420 stainless steel reinforced with VC. Appl. Surf. Sci. 2017, 410, 225–240. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Fu, H.Z. Solid Solution or Intermetallics in a High-Entropy Alloy. Adv. Eng. Mater. 2009, 11, 641–644. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Liu, W.J.; Chen, Z.Y.; Xue, H.D.; He, J.C. Effect of elemental interaction on microstructure and mechanical properties of FeCoNiCuAl alloys. Mater. Sci. Eng. A-Struct. 2012, 556, 395–399. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Chen, W.P.; Chen, Z.; Wen, H.M.; Lavernia, E.J. Influence of Ti addition and sintering method on microstructure and mechanical behavior of a medium-entropy Al0.6CoNiFe alloy. Mater. Sci. Eng. A-Struct. 2014, 619, 137–145. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Ji, X.; Bao, Y.; Zhao, J.; Gu, P. Erosion wear resistance of laser cladding AlCr2FeCoNiNbx high-entropy alloy coatings. In Asia International Conference on Tribology 2018; Abdollah, M.F.B., Ed.; Malaysia Tribology Society: Kuching, Malaysia, 2018; pp. 3–5. [Google Scholar]
- Yang, W.; Luo, J.; Fu, H.; Cheung, C.F.; Ruan, H.; Yang, X.-S. bcc → hcp phase transition significantly enhancing the wear resistance of metastable refractory high-entropy alloy. Scr. Mater. 2022, 221, 114966. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloys Compd. 2017, 698, 761–770. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Raghavan, R.; Kumar, K.C.H.; Murty, B.S. Analysis of phase formation in multi-component alloys. J. Alloys Compd. 2012, 544, 152–158. [Google Scholar] [CrossRef]
- Pi, J.H.; Pan, Y. Thermodynamic Analysis for Microstructure of High-Entropy Alloys. Rare Met. Mater. Eng. 2013, 42, 232–237. [Google Scholar] [CrossRef]
- Praveen, S.; Murty, B.S.; Kottada, R.S. Alloying behavior in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys. Mater. Sci. Eng. A-Struct. 2012, 534, 83–89. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.M.; Gao, X.X.; Lu, Y.P.; Cao, Z.Q.; Gao, M.C.; Hawk, J.A.; Li, T.J. A new strategy to design eutectic high-entropy alloys using simple mixture method. Mater. Des. 2018, 142, 101–105. [Google Scholar] [CrossRef]
- Lu, Y.P.; Jiang, H.; Guo, S.; Wang, T.M.; Cao, Z.Q.; Li, T.J. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, A.; Sarkar, K.; Pathak, A.; Banerjee, A.; Laha, T. A Study on the Corrosion Inhibition of Fe-Based Amorphous/Nanocrystalline Coating Synthesized by High-Velocity Oxy-Fuel Spraying in an Extreme Environment. J. Therm. Spray Technol. 2019, 28, 1433–1447. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Pu, J.; Shi, Y. Anti-friction mechanism of VAlTiCrMo high-entropy alloy coatings through tribo-oxidation inducing layered oxidic surface. Tribol. Int. 2022, 171, 107523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).