Abstract
Electrically as well as thermally conductive lubricants have drawn considerable attention and are an emerging research topic because they have unique advantages and advanced lubrication performance over traditional lubricants such as corrosion protection and efficient heat dissipation. For instance, some components of electric vehicles (EVs) such as bearings, seals, pads and gears require conductive lubricants to avoid premature failure and electromagnetic interference (EMI) problems due to induced shaft voltages and currents. This review provides a comprehensive overview of the recent developments in conductive lubricants. The review focuses on the important aspects to enhance the thermal and electrical conductivities as well as the tribological behavior (COF, and wear rate) of conductive solid, semisolid, and liquid lubricants. The lubricants that are electrically and thermally conductive with superior tribological performances have been identified through extensive literature review and presented in tabular form. This review summarizes the effect of various additives used to improve the conductive properties of the lubricants, such as polyalphaolefin oil, hydraulic oil, paraffin oil, and mineral oil. Furthermore, the review discusses the lubricating mechanism of conductive solid and liquid lubricants to facilitate a deeper understanding. Finally, the future perspectives and the research directions for conductive lubricants are also addressed.
1. Introduction
The failure of the majority of engineering components is mostly caused by friction and wear [1]. Because friction accounts for 23–30% of global energy consumption, it is one of the biggest energy consumers in the world [2]. Therefore, lubricants are typically used in traditional systems to reduce friction between mating components, which is essential for the longevity and energy efficiency of mechanical devices [1]. The basic concepts of friction and lubrication, as well as the effect of lubrication on the coefficient of friction between two sliding bodies, were initially explained by Leonardo de Vinci (1452–1519) [3]. Lubricants are materials that are used to reduce friction between two surfaces in relative motion, thereby minimizing wear and tear and increasing the lifespan of the equipment [4]. Lubricants have a variety of functions, including decreasing noise and vibration, preventing corrosion and rust formation, minimizing friction and wear of mating surfaces, cooling and dissipating the heat created during operations, and many more [5]. The lubricants can be more effective and sustainable if they have good tribological qualities, such as a low coefficient of friction and a reduced rate of wear. The coefficient of friction, wear rate, conductivity of the lubricant materials, and additives used to increase lubricity are among the primary variables that influence the tribological performance of lubricants [6,7]. The current global industry demands lubricants with excellent electrical conductivity and thermal conductivity for numerous advance applications such as electric vehicles (EVs) [1,8,9,10], space systems [11,12,13], marine-related applications [14,15,16,17], and modern industrial machineries [18,19,20,21]. Lubricants must be able to operate at a wide variety of temperatures because the space system may lacks in thermal control. In EVs, the lubricating fluid is in contact with the electrical components requires that it has superior electric properties such as electrical conductivity, dielectric constant, and dielectric strength along with the good thermal management, and material adaptability [22]. As there are large number of variations in the type and performance of lubricants, it is crucial to select the right lubricant for the right application.
Conductive lubricants are typically formulated with a combination of conductive particles and a base lubricating fluid [23]. These lubricants may be silicone-based, graphite-based, or contain other conductive additives, depending on the desired properties and compatibility with the target materials and operating conditions [24]. The conductivity of the conductive lubricants can be enhanced by including conductive additives with the base lubricants. These additives offer extra critical features such as thermal conductivity, electrical conductivity along with anti-wear, resistance to extreme pressure, and corrosion prevention, chemical stability, and thermal stability. To meet the particular requirements of the application, the properties of the lubricant can be modified (Figure 1 represents different application fields of conductive lubricants) by using various conductive nanoparticles, base fluids, and additives. Nano-sized titanium dioxide (TiO2) [25], nanocomposite of Ag and graphene [26], nano-Ag/MWCNTs 5–15 nm [27], and carbon nanoballs (CNBs) [28] are the nano-sized additives used to enhance the desired properties of the conductive lubricants. Polyalphaole-fin oil (PAO) [28], Base Oils [29], PEG200 oil [30], hydraulic oil [31], SAE 10 mineral oil [32], paraffin oil [26], engine oil for diesel engine (CD 15W-40) [33] are the base lubricants for achieving desired. Nanoparticles can improve or modify the performance of lubrication in several ways [34]. The performance enhancement achieved by nanoparticles depends on factors such as the type, size, concentration, and dispersion of the nanoparticles added in the lubricant. Lubricants that contain nanoparticles can dramatically reduce wear and friction between surfaces [35]. High-hardness nanoparticles can operate as protective barriers by decreasing surface-to-surface contact and preventing wear. They also have great lubricating qualities. Some nanoparticles have very good heat conductivity. These nanoparticles can help lubricants better dissipate heat produced during operation, improving thermal stability and lowering the chance of lubricant breakdown [36].
The best electrical efficiency, reliability, and lifespan of electrical connections depend on the use of conductive lubricants, which also protect against wear and help to dissipate heat [37]. On the hand, thermally conductive lubricants are made to effectively transmit heat while also providing lubrication. To promote more effective heat dissipation, thermally conductive lubricants assist in moving heat away from heat-generating components and distributing it to cooler regions [38]. On the other hand, the electrical continuity between conductive surfaces, such as electrical contacts, connections, and terminals, is established and maintained by electrically conductive lubricants [39]. To ensure optimum electrical performance, reduce resistance, and avoid shortcomings such as voltage drop, arcing, or signal loss, it is essential to have this conductivity [40]. Therefore, it is crucial to increase our understanding of conductive lubricants to fill up the knowledge gap.
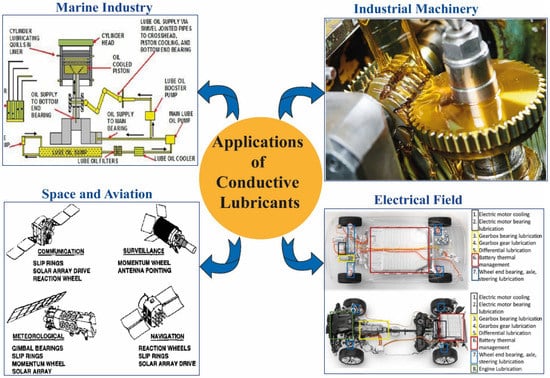
Figure 1.
Different application fields of conductive lubricants. Source: The authors created based on [11,41,42,43].
Due to having outstanding mechanical, thermal, electrical, and tribological properties such as low coefficient of friction, and reduced wear rate enabling the lubricants more efficient and sustainable, one of the recent tribological research’s main focus areas is conductive lubricants [44]. The substantial number of articles on tribological issues related to conductive lubricants that were published between the years 2013 and 2023 are represented in Figure 2. The customary methods have been used to obtain the data from the Elsevier Science Direct database. On the website’s keywords search page, “Electrical and thermal conductivity and tribological property” is typed in as a search query. A total number of 4716 results were displayed for the customized range from 2013 to 2023. The left side of the screen displayed the number of related articles for each year. Then, on 10 June 2023, a graph indicating the number of articles related to the conductivity of lubricants by year was produced. In Figure 2, the number of publications is highest in the year 2022 and it seems to be more at the end of the year 2023 which indicates research on conductive lubricants is a current and burning issue. Moreover, Figure 2 also clarifies the pattern of publications from the year 2013 to the year 2022 is increasing gradually therefore researchers need to focus more on this topic. Therefore, this field attracts tremendous attention from academicians all over the world.
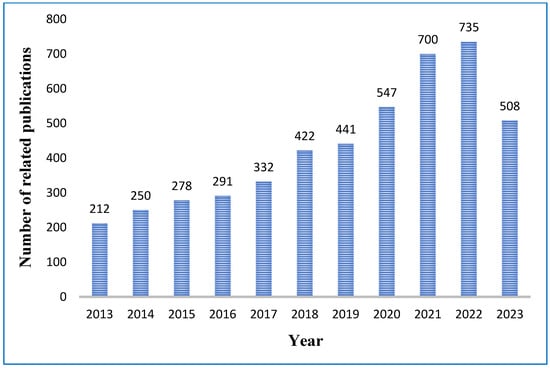
Figure 2.
Statistical data for articles about, “Electrical and thermal conductivity and tribological property” during 2013–2023. The information was extracted on 10 June 2023, from the Elsevier Science Direct database. On the website’s keywords search page, “Electrical and thermal conductivity and tribological property” is typed in as a search query.
Researchers, around the world, are constantly striving to reduce the coefficient of friction and wear rate of lubricants. Recently, Chang Du et al. investigated a study to enhance the performance of lubrication and examine how temperature affects the tribological characteristics of the oils used to lubricate the piston rings in the cylinder liners [45]. Their result reports that the tribological properties of lubricating oils change with the increase in temperature. Hongxiang Yu et al. also conducted a study to explore the impact of functional groups on the tribological performance of lubricants [46]. A study conducted by Corina Birleanu et al. [47] examines the effect of TiO2 nanoparticles on the lubricating properties of oil. Ali et al. [48] used nanoparticles as additives in lubricants to improve their tribological properties. Their review reported that the nanoparticles improved tribological characteristics, exceptionally reduction in friction temperature emissions and minimizing material degradation over time at solid surfaces. Numerous studies have been performed to enhance the tribological performance of lubricants along with different additives. However, very little or no literature has yet examined thoroughly the impacts of lubricant conductivity on tribological characteristics. Therefore, it is a clear-cut study gap to find the impacts of using conductive lubricants for enhancing the tribological performance of the lubricant compounds. Even if there are sufficient amounts of works on lubricants conductivity, these are represented individually, and very few studies have combined thermally and electrically conductive lubricants [49,50,51]. For instance, Yanqiu Xia et al. [52] experimented on the conductivity and tribological properties of ionic liquid-polyaniline/tungsten disulfide (IL-PANI/WS2) composite. Xiaoqiang Fan et al. [53] also accomplished research on the improvement of tribological properties of conductive lubricating greases. Further, combined research on the conductive and tribological characteristics of copper-sliding electrical contacts was carried out by Zhengfeng Cao et al. [49].
Experts are investigating the connections between conductivity and tribological features using different methods. For example, a separate study by M. Kaneta and P. Yang [54] demonstrated the impact of contacting surfaces’ thermal conductivity on elastohydrodynamic lubrication (EHL). Their findings demonstrate that the thermal conductivity of the contact materials controls the temperature in the lubricant layer and, consequently, the viscosity of the lubricant. Md Golam Rasul et al. [55] conducted a study employing functionalized boron nitride nanosheets to primarily increase the heat conductivity and tribological characteristics of polyethylene. Gyorgy Czel et al. [56] investigated how different fillers affected the thermal conductivity and tribological characteristics of polyamide. A study by Shaoli Fu et al. [57] considers only the electrical conductivity along with tribological properties of carbon nanotube-reinforced copper matrix composites. Moreover, Yang Fu et al. [58], in their research, concentrate on how surface roughness and conductive grease filling affect the tribological characteristics and electrical conductivity of carbon brushes. Likewise, Zhengfeng Jia et al. [59] investigated the tribological characteristics and electrical conductivities of the vacuum hot-pressed Cu/reduced graphene oxide composite. A study conducted by Tianhua Chen et al. [60] realized the elastic roll ring’s current-carrying tribological capabilities under various currents.
The creation of innovative lubricant formulations is essential given the rising demand for enhanced lubricant performance to increase energy efficiency, sustainability, and cost reduction. Conducive lubricants are the ideal substitutes for tribological performance because the conventional lubricants used in internal combustion engines (ICE) have some limits for cutting-edge applications including electric vehicles, aerospace, and electromagnetic interference. For accurate and efficient identification of tribological conditions for cutting-edge applications such as EVs and spacecraft, a thorough analysis of the performances of recently produced lubricants is a prerequisite. The effectiveness of several solid, semisolid, and liquid lubricants is examined in this study, and their effectiveness is assessed in terms of electrical compatibility and thermal management. Further, a comprehensive list of conductive lubricant additives to enhance the conductivities of lubricant materials along with their tribological performances have been identified and summarized. Lubricants are also grouped together based on the physical state, organic–inorganic, metallic–nonmetallic, and conductive–nonconductive properties. The goal of this study is to compile the research on formulations of thermally and electrically conductive lubricants and to produce an easily readable, tabular summary. This review study specifically offers the reader a comparative analysis of several additives to enhance thermal and electrical conductivities as well as the performance of the lubricant.
2. Classification of Lubricants and Lubrication
The lubricants may be conventionally classified into four major classes such as solid lubricants, semisolid lubricants, liquid lubricants, and gaseous lubricants [61]. Solid lubricants are substances that transmit a thin coating of solid material onto the surfaces in contact to reduce friction between them [62]. Solid lubricants do not flow or need a carrier medium, unlike grease or liquid lubricants. They are utilized in situations where conventional lubricants might not be appropriate or effective. Examples of solid lubricants include graphite, chalk, talc, mica, Teflon, soap, way, and gold [63]. Likewise, semisolid lubricants, also known as greases, are a type of lubricating material that combines the properties of a solid and a liquid. They consist of base oil, a thickening agent, and various additives. Semisolid lubricants are characterized by their semisolid or viscous consistency, which allows them to adhere to surfaces and provide long-lasting lubrication [64]. To stick to surfaces and offer long-lasting lubrication, semisolid lubricants have a semisolid or viscous viscosity [65]. Moreover, liquid lubricants are compounds with a liquid condition that are intended to lubricate and minimize friction between two surfaces that are moving relative to one another [66]. These lubricants often flow more freely and can fit into narrow areas since they have a lower viscosity than semisolid lubricants. Liquid lubricants prevent direct metal-to-metal contact and reduce heat generation, wear, and friction by forming a thin film or coating between the moving surfaces [67]. Furthermore, gaseous lubricants, also known as vapor-phase lubricants or lubricating gases, are substances in a gaseous state that are used for lubrication purposes. Unlike liquid or semisolid lubricants, gaseous lubricants do not exist as a liquid film or layer between the moving surfaces [68]. Instead, they function by providing a boundary layer of gas that reduces friction and wear between the surfaces. Depending on the particular application and requirements, the lubrication becomes usually in different forms [69,70]. In boundary lubrication, a small layer of lubricant separates the two surfaces. A layer of protection is created by the lubricant molecules’ adhesion to the surface. The lubricant film, however, may break down under high loads or low speeds, causing metal-to-metal contact and increased friction [71]. The technique of hydrodynamic lubrication involves the development of a fluid film between the surfaces under pressure [72]. The relative motion of the surfaces forces the lubricant into the gap, forming a hydrodynamic wedge. This kind of lubricant works well with fast speeds and large weights. Elastohydrodynamic lubrication (EHL) combines the advantages of boundary lubrication and hydrodynamic lubrication. When the lubricant layer thickness is equal to or less than the surface roughness, this happens [73]. The lubricant can deform elastically due to pressure and viscosity, which improves protection between the surfaces.
On the other hand, lubricants can also be categorized into two major classes based on the conductivity of the lubricant materials. These are conductive and nonconductive lubricants [6,74]. The conductive lubricants can be either electrically conductive or thermally conductive (a detailed classification of lubricants is shown in Figure 3) [75]. Thermally conductive lubricants are formulated to enhance the transfer of heat. When two mating surfaces come in contact produces a high temperature (more than 300 °C). The resultant high temperature breakdowns the lubricants, but the resulting compounds must be lubricants to avoid the occurrence of corrosion or abrasion of mating surfaces [62]. The thermal conductivity of the lubricant materials enables the contacting materials to dissipate heat generated by rubbing [8]. They contain components that improve the lubricant’s capacity to dissipate heat, such as thermally conductive additives such as ceramic particles (such as aluminum oxide, and boron nitride) or metallic fillers (such as silver, and copper) [76]. These additives enhance the lubricant’s thermal conductivity, enabling it to effectively transmit heat away from lubricated surfaces. When heat generation is a concern, such as in electric motors, power electronics, heat sinks, or LED lights, thermally conductive lubricants are utilized. These lubricants help maintain ideal operating temperatures, avoid overheating, and increase the lifespan of the lubricated components by promoting efficient heat dissipation [77].
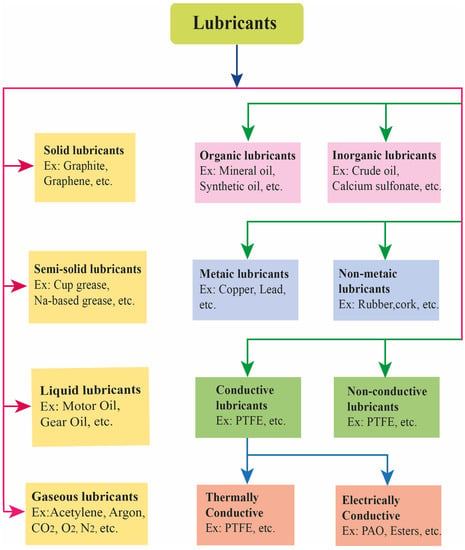
Figure 3.
Classification of lubricants based on their physical state, organic–inorganic, metallic–nonmetallic, and conductivity–nonconductivity.
Electrically conductive lubricants are designed to ease the flow of electric current. They have conductive additives such as metallic particles (silver or copper) or carbon-based substances (such as graphite or carbon nanotubes) that help the lubricant forming a conductive network [41]. After the inclusion of additives, the lubricant can function as an electrical conductor. When working with electrical contacts, connections, switches, or grounding points, lubricants that conduct electricity are frequently employed. Electrically conductive lubricants have a staggering potential for electric vehicle (EVs) lubrication. Even if electric vehicles (EVs) are quite energy efficient, there is a challenge to enhance their efficiency even more [68,78]. These lubricants are used to reduce the friction in gears and bearings and to prevent copper corrosion and to cool the electric motor of EVs [74]. These guarantee appropriate electrical continuity, lower resistance, and guard against problems such as electrical arcing and static charge buildup. In fields including electronics, electric vehicles, automotive, aerospace, and power generation these lubricants are frequently used [79]. Recently, electric vehicles (EV) and electric hybrid vehicles (EHV) are attracting enormous attention. Yan Chen and their group reviewed the current requirements and the future proposal of advanced lubricants for EVs, and EHVs [41]. Even then, conventional lubricants for internal combustion engine vehicles (ICEVs) are utilized in EVs with reasonable performance; however, these fluids have not been produced specifically for the evaluation of EV needs [80]. Many different types of lubrication is used to reduce friction: metallic lubrication, soft metals, ionic grease lubrication, and ionic liquid lubrication.
2.1. Metallic Lubrication
To reduce friction and wear between metal surfaces by using metallic materials or compounds as lubricants or lubricant additives is termed as Metallic lubrication. It can withstand higher temperatures and pressures than organic lubricants and are more resistant to oxidation and degradation by providing excellent boundary lubrication that occurs when the metal surfaces come into direct contact inorganic anion [81].
In this paper we have collected several types of metallic lubricants including solid lubricants and lubricants additives Cu-based composites (Cu-Sn-Al- Fe-h-BN graphite-SiC) and Ni-P-h-BN alloy. The COF and WR of Cu-based composites (Cu-Sn-Al- Fe-h-BN graphite-SiC), at 25 °C are approximately 0.5 and 1.3 × 10−5–4.3 × 10−5 mm3 N−1 m−1, respectively [82]. Again, The COF and WR of Ni-P-h-BN alloy at 25 °C are 0.2 and 1.24 × 10−6 N−1 m−1 [83]. So, in alloy composition, when the number of metal is low, the value of COF and WR is low. That means the tribological properties are improved.
2.2. Soft Metals
In Table 1, we can see various soft metals, such as silver, tin, and lead, as self-lubricating films on hard substrates due to their low melting temperatures and shear strength. These soft metals can form a shear-simple tribo-layer and exhibit increased ductility, providing lubrication through mechanisms such as the correction of microstructural faults during sliding. While soft metals such as zinc, lead, tin, and their alloys with low melting points and multi-slip systems are effective for low-temperature and lightly loaded conditions, metals such as silver, gold, and platinum have low hardness and high melting temperatures, limiting their lubricating capabilities. However, coatings of binary alloys such as Sn-Co or ion-plated lead coatings have been developed as alternatives to hard chromium coatings in tribological applications. In recent developments, various techniques and multi-layered approaches involving metals such as Al/Cu/Fe/Cr, Cu/Mo, Zn/W, Ni/Ti, and Au/Cr have been used to create films for tribological applications in turbomachinery parts, fretting interfaces, seals, and bearings, operating at temperatures up to 580 °C. Table 1 displays the tribological behavior of materials that self-lubricate and contain soft metals. The use of different soft metals helps to increase the tribological performance of solid lubricants. From Table 1, we can see Ta-Ag materials where Ag as a soft metals at 6000 C the COF of Ta-Ag alloy is 0.2 and the wear resistance is 5.2 × 10−5. Additionally, for TiN-In alloy the COF is 0.5 and WR is 5.2 × 10−5. For Ni-Cr/Cr3C2-NiCr/h-BN at 20 °C, the COF and WR are 0.65 and 5.3 × 10−5 N−1 m−1, respectively.

Table 1.
Tribological data of different conductive solid lubricants.
2.3. Ionic Grease Lubrication
Ionic grease is type of specialized lubricant that is generally electrical conductive containing conductive particle or additives that allow the grease to conduct electricity with reducing friction; it is primarily used in electrical and electronics application. Grease is often use with ionic liquids as additives. Ionic liquids have been demonstrated as effective additives to promote lubrication in base oils and greases. Using benzotriazole group grafted imidazolium IL as additive in poly (ethylene glycol) (PEG) and polyurea grease, which effectively reduced friction/wear of steel pairs that outperforms commercial zincdiakyldithiophosphate-based additive (T204). Synthesizing lubricating grease by using 1-octyl-3-methylimidazolium hexafluorophosphate and 1-octyl-3-methylimidazolium tetrafluoroborate as base oil and the polytetrafluoroethylene as thickener to reduce friction and wear on steel/steel contacts. In Table 2, we have used several types of ionic grease lubricants such as [Li(PAG)]BF4 grease, [Li(PAG)]PF6 grease, [Li(PAG)]NTf2 grease, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide (L-F106). For example, in Table 2, we can see for [Li (PAG)]PF6 grease the COF becomes 0.092 and also for [Li(PAG)]NTf2 grease the COF becomes 0.095. Thus, the COF and wear resistance is increased after using conductive particles as additives.

Table 2.
Tribological data of different conductive semisolid lubricants.
2.4. Ionic Liquid Lubrication
Ionic liquids (ILs) consist of large, asymmetric organic cations and usually an inorganic anion [81]. Ionic liquid lubricant has high thermal stability, low volatility, reduce friction, nonflammability, low melting point and good at stability. It has been discovered that the most significant mechanism controlling the molecular behavior of ionic liquids in the gap in the dynamic state is their contact with the solid wall. So, the use of ionic lubricant as boundary is getting more and more.
Liu et al. [98] indicates that, in alcohol-based ionic liquid with the increase in alkyl chains, the wear resistance and friction reduction properties of the liquids were improved. As a comparison, in Table 3, the wear scar of 1- ethyl-3-hexylimidazolium tetrafluorobo-rate (L206) and 1-ethyl-3-octylimidazolium tetrafluorobo-rate (L208) is 0.30 mm and 0.27 mm, respectively. Again, COF and WR of 1-octyl, 3-methyl (L106) and 1-octyl, 3-methyl (L108) at 100 °C are, 0.08, 0.04 and 7.27 × 10−4 mm3/m, 5.22 × 10−4 mm3/m, respectively. So, the wear resistance and friction reduction properties of the liquids were improved [98].
Acid-based ionic liquids improved the anti-corrosion. Additionally, act as a good lubricant. Wang et al. [99] indicates that, with the increase in alkyl chain length, the COF of the acid-based ionic liquid gradually decrease. As a comparison, in Table 3, the COF of choline-based lauric acid ([Ch][DA], choline-based palmitic acid ([Ch][PA]) and choline-based stearic acid ([Ch][SA]) is 0.35, 0.30 and 0.11, respectively.

Table 3.
Tribological data of different conductive liquid lubricants.
Table 3.
Tribological data of different conductive liquid lubricants.
| Lubricants | Lubricant Material | Application | Tribological Behavior | Ref. | |
|---|---|---|---|---|---|
| COF | WR | ||||
| Automatic transmission fluid (ATF) with 1% N-hexyl-N-methylpiperidinium bis(2-ethylhexyl)phosphate ([P6,6,6,14][BEHP]) at load 5N | Ionic liquid | Power steering systems | 0.15 | Wear volume 1.9 µm3 | [100] |
| Choline-based stearic acid ([Ch][SA]) | Ionic liquid | Industrial lubrications, bio-based lubricants, automotive lubrication, aerospace | 0.11 | Wear volume is 11 × 10−4 mm3 | [99,101,102] |
| Choline-based palmitic acid ([Ch][PA]) | Ionic liquid | Metalworking and cutting Fluids, forming and stamping, anti-seize and assembly lubrications | 0.30 | Wear volume is 14 × 10−4 mm3 | [99,102,103] |
| Choline-based lauric acid ([Ch][DA]) | Ionic liquid | Metalworking and cutting fluids, industrial lubrication | 0.35 | Wear volume is 9 × 10−4 mm3 | [99,102] |
| Combination of black carbon and 1% alkyl-phosphonium-based IL trihexyltetradecyl-phosphonium docusate ([][DOC]-[CB]) | Ionic liquid | Bearings and electrically loaded bearings, self-lubrication | 0.55 | Wear volume 0.05 mm3 | [7] |
| Combination of carbon nanotubes and 1% alkyl-phosphonium-based IL trihexyltetradecyl-phosphonium docusate ([][DOC]-[CTN]) | Ionic liquid | To improved creep resistances and strength, applied in electrically loaded bearings, self-lubricating | 0.59 | Wear volume 0.059 mm3 | |
| 1,2-dimethyl-3-propylimidazolium tetrafluoroborate ([im]BF4) | Ionic liquid | Metalworking and cutting fluids, corrosion protection, electrical contacts, energy storage systems, applicable in industries such as aerospace and automotive | 0.07 | 0.05/10−4 mm | [66] |
| 1-ethyl-3-hexylimidazolium tetrafluoroborate (L206) | Ionic liquid | Used in gears, bearings, and chains | 0.039 | Wear scar 0.30 mm | [98] |
| 1-ethyl-3-octylimidazolium tetrafluoroborate (L208) | Ionic liquid | Used as electrolytes, used in gears, bearings, and chains | 0.039 | Wear scar 0.27 mm | [98] |
| 1-ethyl-3-hexylimidazolium-bis(trifluoromethylsulfonyl)-imide (L-F206) | Ionic liquid | Pump, membranes | 0.08 | Wear volume 0.1 × 10−4 mm3 | [104,105] |
| 1-ethyl-3-hexylimidazolium tetrafluoroborate (L-B206) | Ionic liquid | Aviation, space technology, automobile industry | 0.045 | Wear volume 0.2 × 10−4 mm3 | [104,106] |
| 1-hexyl-3-methylimidazolium tetrafluoroborate ([hmim][PF6]) | Ionic liquid | Tribological applications, electrical contacts, gears, bearings, and chains | 0.056 | 1.8 × 1010 mm3/mm | [107] |
| 1-hexyl-3-methylimidazoliumhexafluorophosphate ([hmim][BF4]) | Ionic liquid | Applied for separation of organic compounds, electrochemical cells | 0.048 | 2.4 × 1010 mm3/mm | [107] |
| Motor oil SAE 30 with 0.3% Cu additive | liquid | High-temperature applications, marine and aerospace applications | 0.023 | Wear path is 33 m | [108,109] |
| 1-octyl, 3-methyl (L106) at 100 °C | Ionic liquid | High-temperature turbine and space applications, solar energy | 0.08 | 7.27 × 10−4 mm3/m | [110,111] |
| 1-octyl, 3-methyl (L108) at 100 °C | Ionic liquid | Automotive lubrication, electrical contacts | 0.04 | 5.22 × 10−4 mm3/m | [110] |
| Polyethylene glycol (PEG)-based 1-ethyl-3-methylimidazolium cations (([C1imC10imC1]) and bis(trifluoromethylsulfonyl)imide anions (NTf2) [2,2′-methyl-[C1imC10imC1](NTf2)2]) | Ionic liquid | Lubricants and tribology, biocompatible lubricants, energy storage systems, extraction and separation processes, green chemistry and catalysis | 0.12 | 1 × 10−8 mm3/Nm | [66] |
| Polyethylene glycol (PEG)-based 1-ethyl-3-methylimidazolium cations ([C1imC10imC1]) with methyl substitution | Ionic liquid | High-temperature lubrication, metalworking and cutting fluids, corrosion protection | 0.13 | 25 × 10−8 mm3/Nm | |
| Polyethylene glycol (PEG)-based 1-ethyl-3-methylimidazolium cations ([C1imC10imC1]) with methyl substitution at the 2 and 2′ positions, and tetrafluoroborate anions (BF4-) ([2,2′-methyl-[C1imC10imC1](BF4)2]) | Ionic liquid | Electrical contacts, corrosion protection, lubricants and tribology | 0.11 | 6 × 10−8 mm3/Nm | |
3. Enhancing Conductivity of Lubricants
A base oil, which might be synthetic or petroleum based, and numerous additives are the main components of conductive lubricants. The conductivity of the conductive lubricants can be enhanced by including conductive additives with the base lubricants. These additives offer extra lubricating properties such as anti-wear, resistance to extreme pressure, and corrosion prevention. Table 4 shows the additive list used to enhance the tribological behavior of conductive lubricants along with the base lubricants, the concentration of additives, and their mechanism. The list of additives that enhanced thermal conductivity and electrical conductivity are also tabulated in Table 5 and Table 6, respectively. The friction and wear in mechanical systems can be reduced by adding nanoparticles to lubricants. Nanotechnology is recognized as the most transformative technology of the twenty-first century due to the extraordinary tribological features of nanoparticles. Various nanoparticles have lately been researched for usage as lubricant additives [112], such as, carbon nanomaterials, metallic nanomaterials (including, Ag, Cu, Ni and Mo) and metal oxide nanomaterials.
3.1. Carbon Nanomaterials
Arrangements of carbon atoms in various structural forms at the microscopic level make up a variety of materials known as carbon nanomaterials. They include carbon nanotubes (CNTs), graphene, fullerenes, carbon nanofibers, and carbon nanoparticles. Because of their vast range of morphologies, exceptional corrosion resistance, excellent mechanical behaviors, and high thermal conductivity of carbon materials, these materials have been the subject of intensive research in the fields of optics, electrochemistry, mechanics, and tribology for many years. Additionally, carbon nanomaterials with outstanding electrochemical features include zero-dimensional (0D) fullerenes, one-dimensional (1D) carbon nanotubes (CNTs), two-dimensional (2D) graphene, and three-dimensional (3D) nanodiamonds [113,114]. This different formation of carbon nanomaterials helps to increase the tribological performance as additive in lubricant and rheological performance has improved of the lubricant [115]. As a comparison, in Table 4, the COF of 5% graphite is 0.2 and wear rate is 0.0002 mm3/Nm. According to the Lee et al. [116], with raw lubricant, when 0.1% graphite nanoparticle and 0.5% graphite nanoparticle are used, the COF is reduced from 0.038 to 0.011 and 0.009, respectively. Cu-based composite Cu-Sn-Al- Fe-h-BN graphite-SiC, which has a graphite-SiC additive has a lower COF and wear rate. Which is approximately 0.5 and 1.3 × 10−5–4.3 × 10−5 mm3 N−1 m−1, respectively. In Table 4, when 1% Carbon nano-additive is used with grease the COF becomes 0.022 and wear scar diameter becomes 0.24 mm. On the other hand, Table 3 shows that 1% alkyl-phosphonium-based IL trihexyltetradecyl-phosphonium docusate with black carbon as additives reduced its COF and wear. They become 0.55 and wear volume 0.05 mm3, respectively. Additionally, again, 1% alkyl-phosphonium-based IL trihexyltetradecyl-phosphonium docusate with carbon nanotubes as additives reduced its COF to 0.59 and wear volume to 0.059 mm3.
Another name for the lubricating material hexagonal boron nitride is white graphite. It offers lubrication through lamellar sliding along the basal plane, making it suitable for high-temperature and humid conditions due to its improved thermal stability and oxidation resistance. However, compared to graphite or molybdenum disulfide (MoS2), h-BN performs less effectively in tribological tests due to stronger van der Waals forces between its layers. Nevertheless, when added to lubricating oil or water or impregnated into porous surfaces as micro-particles, h-BN can enhance the tribological performance of composites and ceramics. The coefficient of friction for h-BN in normal air ranges from 0.2 to 0.25, which decreases to less than 0.1 in humid air. At ambient temperature, h-BN has a relatively high coefficient of friction, but it decreases to 0.15 at 600 °C. The addition of h-BN to certain materials, such as silicon nitride, stainless steel, aluminum oxide, titanium diboride, and boron carbide, improves their tribological properties at high temperatures due to h-BN’s oxidation resistance and chemical stability. Generally h-BN is used enhance the tribological performance of solid lubricants. From Table 1, we can see several uses of h-BN with solid lubricants such as B4C-h-BN. The COF of B4C-h-BN is ranging from 0.591–0.321 after using h-BN. Additionally, for Ni-P-h-BN coating, At room temperature, Ni-P-35 vol.% h-BN autocatalytic composite coating exhibits a 106 mm3/(Nm) wear rate and a 0.2 friction coefficient when applied to an AISI52100 steel ball. At 360 °C, sliding against paired 15-5PH stainless steel cylinders lowered the friction coefficient from Ti-alloys to Ti-h-BN from 0.72 to 0.35. The wear resistance of Ni-Cr-W-Mo-Al-Ti-h-BN-Ag becomes 7 × 10−4 N−1 M−1.
3.2. Metallic Nanomaterials
Metallic nanoparticles are tiny particles of metal that have dimensions in the nanometer range (typically between 1 and 100 nanometers). Metallic nanoparticles (including, Ag, Cu, Ni and Mo) have various distinctive benefits. (1) By being physically pressed and/or smeared into the contact area, they can create layers that contain nanoparticles that are softer and more compliant. This layer facilitates deformation and faster running-in with greater conformal contact, resulting in reduced asperity contact pressure and shear resistance under lower friction. In addition, they provide a cushion that absorbs some of the asperity collision force, lowering noise. (2) They can contribute to the development of tribal films in two different ways: through tribochemical processes or by serving as a source of metal cations [117].
The presence of silver nanoparticles forms a protective boundary film, reducing metal-to-metal contact and enhancing the lubrication performance. They exhibit excellent anti-friction and anti-wear properties, reducing friction and preventing wear between sliding surfaces. Because of its advantageous environmental effects, chemical stability, low shear strength, accurate embed ability, ductility, and significant potential for enhancing friction reduction and anti-wear properties, silver nanoparticles received a great deal of attention [117]. Because of this, several researchers have tried to employ silver nanoparticles as lubricant additives to enhance the lubrication performance of base fluids, notably oil-based lubricants. In Table 1, when Ag is used in NiMoAl-6Al2O3 as additives, the COF and WR becomes 0.53 and 1.47 × 10−5 mm3/Nm, respectively. Again, when Ag is used in NiMoAl as additives, the COF and WR becomes 0.3 and 4.64 × 10−5 mm3/Nm, respectively. Silver with titanium improves the lubricity of titanium. The COF and WR of Ti-Ag alloy is 0.2 and 5.2 × 10−5 mm3/Nm. When Ag is used with Ni-Cr-W-Mo-Al-Ti-h-BN, its improved the tribological property of this composite. The COF and the WR become 7 × 10−4 N−1 m−1, respectively.
For PAG, there is a variation in the tribological property of PAG when we use different metals as additives, such as Cu, Ag and Sb. According to Table 2, when we use 0.2% Cu with PAG, the COF is 0.121 and wear width is 0.33 mm. However, when we use 0.2% Ag with PAG, the COF becomes 0.119 and wear width 0.31 mm. On the other hand, when we use 0.2% Sb-doped SnO2 (AOT) with PAG, the COF becomes 0.111 and wear width 0.325 mm.
MoS2 is another metallic additive. It is also help to improve the tribological behavior of lubricant. Table 2 shows that the wear volume of PG is 4.7/10−4. However, when 3% MoS2 is used as additive, the wear volume becomes 3.75/10−4 mm3. This is how a lubricant can show different tribological properties with the change in different metal additives.
3.3. Metal Oxide Nanomaterials
Metal oxide refers to a compound that consists of a metal element bonded with oxygen atoms. It is formed when a metal reacts with oxygen, resulting in the formation of a chemical compound composed of metal cations and oxide anions. Metal oxides are widely found in nature and can also be synthesized through various chemical processes. In order to decrease friction and wear, metal oxides are often employed alone or in combination as lubricant additives in base fluids. They display lubricating processes that are comparable to those of metallic nanoparticles, such as tribo-film or adsorption film creation, the rolling effect, and the repair or sintering effect. The single and double metal oxides are covered in detail in this section [117]. In Table 1, a single metal oxide, Al2O3 is used with NiMoAl-Ag. It improved the tribological performance of NiMoAl-Ag. Without Al2O3, the COF of NiMoAl-Ag is 4.64 × 10−5 mm3/Nm. However, with Al2O3, the COF of NiMoAl-Ag becomes 1.47 × 10−5 mm3/Nm.

Table 4.
List of additives used to enhance the tribological behavior of conductive lubricants.
Table 4.
List of additives used to enhance the tribological behavior of conductive lubricants.
| Lubricants | Additives | Additives Concentration | Testing Method | Tribological Properties | Ref. | |
|---|---|---|---|---|---|---|
| Coefficient of Friction | Wear Rate | |||||
| Polyalphaolefin oil (PAO) | SWNT | 0.5 wt% | Block on ring test apparatus UMT-3 at 200 rpm, load 300 N, contact pressure 220 MPa | 0.034 | 7790 μm2 scar area | [118] |
| Polyalphaolefin oil (PAO) | MWNT | 0.5 wt% | Block on ring test apparatus UMT-3, at 200 rpm, load 300 N, contact pressure 220 Mpa | 0.013 | 7151 μm2 scar area | [118] |
| PEG200 oil | Reduced graphene oxide | 0.2 mg mL−1 | Ball-on-disc nano-tribometer. Load 500 mN | 0.06 | [30] | |
| Esterified bio-oil | Graphene/MoS2 mass ratio 3:2 | 0.5 wt% | MQ-800 four-ball tribometer test. At load 300 N, 1000 rpm | 0.017 | Wear scar diameter 0.43 mm | [119] |
| 10 W40 engine oil | (Zinc oxide) ZnO/MWCNTs (multiwalled carbon nanotubes) hybrid nanomaterial mix 3:2 | 0.25 wt% | Ball-on-disk tribotester, linear reciprocating. Load 35 N. Engine oil contains hybrid nanomaterials of zinc oxide and MWCNTs | 0.044 | Wear volume 0.09 mm3 | [120] |
| Hydraulic oil | Graphene oxide sheets include regular edges (RG) | 1 wt% | Reciprocating tribometer (UMT-3) in ball on disk mode. 2 N of load at 0.5 Hz | 0.0614 | Wear scar depth 0.151 µm | [31] |
| water | Functionalized graphene oxide (ILCAs-GO) using 1-hydroxyethyl-3-methyl imidazole tetrafluoroborate | 0.8 mg/mL | CETR UMT-3 multi-function sliding test. For 5 N Load. In water, graphene oxide was functionalized by hydroxyl-terminated ionic liquids | 0.172 | Wear volume 0.6 × 105 mm3 | [121,122] |
| Aviation Lubricant (4010 AL) | Graphene | 0.075 wt% | Four-ball tester. Ceramic Si3N4/steel GCr15 tribo-pairs. Activate 392 N and 1450 rpm. | 0.068 | Wear scar depth 0.516 µm | [123] |
| BS6500 oil | Bi 7–65 nm | 900 mg/L | Four ball tester at 1200 rpm, 392 N, 75 °C, and 30 min | 0.052 | 454 µm scar diameter | [124] |
| Teboil Ward and Chevron Taro 30 DP 40 | Cu | 3 wt% | Pin-on-disk tribometer, 0.02 mm/s, load 0.1–180 mN, 75 °C | 0.11 | 0.018 mg wear signal | [125] |
| SAE 10 mineral oil | Co | 0.5 wt% | Four ball tester at 1420 min−1, load 150 N, 75 °C, 60 min | Wear scar diameter 0.54 mm | [32] | |
| Base oil | Al2O3 | 0.1 wt% | Four ball tester at 147 N, 1450 rpm, 75 °C, 30 min | 0.057 | Wear scar diameter 348.09 µm | [126] |
| Mineral oil | Nano-sized titanium dioxide (TiO2) | 0.25 wt% | Reciprocating pin-on-disk at 0.05 m/s, load 14.7 N, 30 min | 0.09 | [25] | |
| Pure lubricant oil | ZnAl2O4—95 nm | 0.1 wt% | Four ball tribometer at 147 N, 1450 rpm, 348 K, 1800 s | 0.0643 | Wear scar diameter 257.05 µm | [127] |
| API SL/CF 10W-40 engine oil | FeS | 2 wt% | Pin-one-disc system at load 50 N, 150 rpm, 20 min | 0.018 | [128] | |
| Blend of PAO 4 and PAO 40 | IF-MoS2 | 1 wt% | High frequency reciprocating rig (HFRR) at load 10 N, 80 °C | 0.06 | Wear scar depth 1.2 µm | [129] |
| Dioctylsebacate (DOS) | MoS2 50–100 nm | 2 wt% | Reciprocating ball-on-disc tribometer at load 7.84 N, 0.1 m/s, 60 °C, 75 min | 0.095 | Wear scar diameter 220 µm | [130] |
| Paraffin oil | Nanocomposite of Ag and Graphene (with laser irradiation) 56 nm | 0.1 wt% | Four ball tribometer at 392 N, 1200 rpm, 30 min | 0.061 | Wear scar diameter 0.496 mm | [26] |
| 10w40 engine oil | Nano-Ag/MWCNTs 5–15 nm | 0.18 wt% | Four-ball machine operating at 392 N, 1200 RPM, 75 °C, and 60 min | 0.053 | Wear scar diameter 0.35 mm | [27] |
| Engine oil for Diesel engine (CD 15W-40) | 185.96 nm layered double hydroxide (LDH)-la-doped Mg/Al | 100 mL of oil with 0.5 g of LDH | Four ball tester at 392 N, 1200 rpm, 60 min | 0.093 | [33] | |

Table 5.
List of additives used to enhance the thermal conductivity of lubricant materials.
Table 5.
List of additives used to enhance the thermal conductivity of lubricant materials.
| Lubricants | Additives | Additives Concentration | Method | Thermal Conductivity (W/mK) | Ref. |
|---|---|---|---|---|---|
| Polyalphaolefin oil (PAO) | SWNT | 11.60 wt% | Hot DiskTM thermal constants analyzer, 0.0471/K TCR, kapton disk type. Temperature range between 100 °C and 177 °C. | 0.26 | [131] |
| Polyalphaolefin oil (PAO) | MWNT | 19.00 wt% | Hot DiskTM thermal constants analyzer, 0.0471/K TCR, kapton disk type. Temperature range between 100 °C and 177 °C. | 0.27 | [131] |
| SAE 20 W50 engine oil | MWCNTs | 0.1 wt% | KD2-Pro equipment for analyzing the thermal properties and transient hot wire system at temperature 20 °C. | 0.19 | [132] |
| Diesel oil (DO) | Surfactant oleic acid -MWCNT | 0.5 wt% | KD2 Pro thermal analyzer. All samples’ thermal conductivity was measured four times, with the findings being reported as an average. Temperature range between 5 °C and 100 °C. | 0.293 | [133] |
| Diesel oil (DO) | Hexylamine -MWCNT | 0.5 wt% | Thermal tester KD2 Pro. Four measurements were made to determine the thermal conductivity of each sample, and the average amount of data is presented at 80 °C. | 0.321 | |
| TIM silicone grease | MWCNTs | 2–3 µm in Strong acid/base | T-type thermocouples at 0.05 °C, 10 Kpa contact pressure, two copper plates and the heat flux, the equivalent thermal conductivity and resistance of the composites. | 4.267 | [134] |
| Krytox XHT750 | Helix MWNT | 15 wt% | Hot Disk™ thermal constants analyzer: 3.189 mm sensor radius, 6 mm measurement depth, room temperature, 0.012 W power, 10 s, 0.0471/K TCR, and Kapton disk type. temperature range between 16.85 °C and 86.85 °C. | 0.19 | [135] |
| PAO Durasyn 166 | MWNT-OH | 7.5 wt% | Hot Disk™ thermal constants analyzer: Measurement parameters: room temperature, 6 mm measurement depth, 0.012 W power, 10 s duration, 3.189 mm sensor radius, 0.0471/K TCR, and Kapton disk type, temperature range between 16.85 °C and 86.85 °C. | 0.31 | [135] |
| Durasyn 166 PAO | Pr-24-XT-HHT CNF Pyrograf | 10 wt% | Thermal constants analyzer Hot DiskTM: 6.18 mm measurement depth, room temperature, 0.012 W power, 10 s, 3.189 mm sensor radius, 0.0471/K TCR, Kapton disk type, temperature range between 16.85 °C and 86.85 °C. | 0.68 | [135] |
| Petro-Canada N650HT | IMERYS Su-per 65 Carbon Black, 0.75 weight percent, MWNT-OH | 6.8 wt% | Hot Disk™ thermal constants analyzer: 3.189 mm sensor radius, 6 mm measurement depth, room temperature, 0.012 W power, 10 s, 0.0471/K TCR, and Kapton disk type, temperature range between 16.85 °C and 86.85 °C. | 0.30 | [135] |
| ROYCO 808 | MWNT-OH | 7.5 wt% | Hot Disk™ thermal constants analyzer: 3.189 mm sensor radius, 6 mm measurement depth, room temperature, 0.012 W power, 10 s, 0.0471/K TCR, Kapton disk type, drift, temperature range between 16.85 °C and 86.85 °C. | 0.32 | [135] |
| Ethylene glycol | MWNT | 12.5 wt% | Hot Disk™ thermal constants analyzer: Measurement parameters: room temperature, 6 mm measurement depth, 0.012 W power, 10 s duration, 3.189 mm sensor radius, 0.0471/K TCR, and Kapton disk type, temperature range between 20 °C and 22 °C. | 0.38 | [135] |
| MG Chemicals Silicone Heat Transfer Compound | Pyrograf Pr-19-XT-HHT CNF | 5 wt% | Thermal constants analyzer Hot DiskTM: 6.18 mm measurement depth, room temperature, 0.012 W power, 10 s, 3.189 mm sensor radius, 0.0471/K TCR, Kapton disk type, temperature range between 20 °C and 22 °C. | 1.70 | |
| Discarded silicon oil from a water bath heater | CNF-19 | 4.9 wt% | Hot Disk™ thermal constants analyzer: 3.189 mm sensor radius, 6 mm measurement depth, room temperature, 0.012 W power, 10 s, 0.0471/K TCR, and Kapton disk type, temperature range between 20 °C and 22 °C. | 0.51 | |
| NYE 758 G grease | CNF-19 | 5 wt% | Hot Disk™ thermal constants analyzer: Measurement parameters: room temperature, 6 mm measurement depth, 0.012 W power, 10 s duration, 3.189 mm sensor radius, 0.0471/K TCR, and Kapton disk type, temperature range between 20 °C and 22 °C. | 0.48 | |
| Valvoline cerulean grease | CNF-19 | 3.7 wt% | Hot Disk™ thermal constants analyzer: 6 mm measurement depth, room temperature, 3.189 mm sensor radius, 0.0471/K TCR, Kapton disk type, drift, 0.012 W power, temperature range between 20 °C and 22 °C. | 0.35 | |
| R-134a nano-refrigerant | Al2O3 | 5 wt% | Thermal constants analyzer at 26.85 °C. temperature. | 0.0985 | [136] |
| R-134a nano-refrigerant | Spherical ZnO | 10 wt% | KD2 pro thermal analyzer (Decagon Devices) at Temperature range between 9.85 °C and 33.85 °C. | 0.095 | [137] |
| R-134a nano-refrigerant | Cube ZnO | 10 wt% | KD2 pro thermal analyzer (Decagon Devices) at Temperature range between 9.85 °C and 33.85 °C. | 0.105 | |
| Heat transfer oil | Ag—20 nm | 0.72 wt% | a Decagon Devices KD2 Pro KS-1 thermal analyzer. The maximum variance for the transient hot wire approach is 5.0%. 0.02 to 2.00 thermal conductivity is acceptable at 99.85 °C temperature. | 0.165 | [138] |
| SAE 20 W50 | CuO | 0.1 wt% | KD2 Pro equipment for analyzing the thermal properties and the transient hot wire system for measuring thermal conductivity at 20 °C temperature. | 0.172 | [139] |
| Ethylene glycol (EG) | ZnO—30 nm | 10.5 wt% | Using a transient hot-wire mechanism, the thermal conductivities of the nanofluids were determined with an accuracy of 2–3% and a measurement range of 0.001–20 W/mK, at 55 °C temperature. | 0.287 | [140] |
| SAE 20 W50 | carbon nanoballs (CNBs)—70 nm | 0.1 wt% | The KD2-Pro is used for measuring thermal conductivity of base oil and nanofluids. at 20 °C temperature. | 0.1954 | [28] |
| SAE 20 W50 | Fullerene—10 nm | 0.1 wt% | Base oils and nanofluids are measured for their thermal conductivity using the KD2-Pro. at 20 °C temperature. | 0.172 |

Table 6.
List of additives used to enhance electrical conductivity of lubricant materials.
Table 6.
List of additives used to enhance electrical conductivity of lubricant materials.
| Lubricants | Additives | Additives Concentration | Characterization Method | Electrical Conductivity (Ω·cm) | Ref. |
|---|---|---|---|---|---|
| Diesel oil (DO) | surfactant oleic acid -MWCNT | 0.5 wt% | Electrical property analyzer at 80 °C | 476.3 μS/ cm | [133] |
| Diesel oil (DO) | Hexylamine-MWCNT | 0.5 wt% | Electrical property analyzer at 80 °C | 409.2 μS/ cm | |
| Diesel oil (DO) | surfactant oleic acid-graphene nanoplatelets | 0.5 wt% | Electrical property analyzer at 80 °C | 492.6 μS/ cm | |
| Diesel oil (DO) | Hexylamine-graphene nanoplatelets | 0.5 wt% | Electrical property analyzer at 80 °C | 429.9 μS/ cm | |
| Diesel oil (DO) | surfactant oleic acid-graphene nanoplatelets/MWCNT | 0.5 wt% | Electrical property analyzer at 80 °C | 482.2 μS/ cm | |
| 50% Ethylene Glycol, 50% Water | MWNT-OH | 5.68 wt% | Keithley digit multimeter for electrical resistivity measurement | 11.9 | [141] |
| Epoxy with Ag flakes | diethylene glycol butyl ether (DGBE) | 2 pph | The four-point probe on a Keithley 2000 multimeter was used to gauge the bulk resistance of the ECA samples. | 3.4 × 10−3 | [142] |
| Glycerol | MWNT-OH, 3% Cu (Nano) | 4.5 wt% | Keithley digit multimeter for electrical resistivity measurement | 178 | [141] |
| Glycerol | CNF | 12 wt% | Keithley digit multimeter for electrical resistivity measurement | 175 | [141] |
| 75% Glycerol, 25% Water | MWNT-OH | 4.5 wt% | Keithley digit multimeter for electrical resistivity measurement | 10 | [141] |
| Polyalphaolefin oil (PAO) | SWNT | 11.60 wt% | Using volume resistivity test cells provided by Electro-Tech Systems, Inc. | 3000 | [131] |
| Polyalphaolefin oil (PAO) | MWNT | 19.00 wt% | Using volume resistivity test cells | 4000 | [131] |
| Polyalphaolefin oil (PAO) | Helix MWNT | 20 wt% | Keithley digit multimeter for electrical resistivity measurement | 2700 | [141] |
| Polyalphaolefin oil (PAO) | CNF-MWNT-OH | 4.48 wt% | Keithley digit multimeter for electrical resistivity measurement | 138 | |
| Petro-Canada N650HT | MWNT | 8.4 wt% | Keithley digit multimeter for electrical resistivity measurement | 7880 | |
| Petro-Canada N650HT | MWNT-OH | 7.5 wt% | Keithley digit multimeter for electrical resistivity measurement | 22.4 | |
| ROYCO 500 | MWNT-OH | 7.5 wt% | Keithley digit multimeter for electrical resistivity measurement | 80 | |
| Vaseline oil | oleic acid | 2 wt% | MTE-3 device, the loads ranged from 10 to 60 cN, with sliding velocities between 0.05 and 4.3 mm/s and currents under 1 mA | 0.52 Ωm | [143] |
4. Tribological Behavior of Different Conductive Lubricants
According to the definition, tribology is a physiological investigation of interacting surfaces in motion [144]. The tribological property is mainly configured with COF (coefficient of friction) and WR (wear rate). Tribological properties can be affected by different parameters such as concentration, weight, friction, temperature, time, and load. With the increase or decrease in these parameters, the tribological properties of different types of lubricant materials can be changed. Tribological behavior affects the conductivity of lubricant materials. The lubricants usually become more conductive with minimal tribological behavior.
4.1. Tribological Behavior of Conductive Solid Lubricants
The tribological behavior means COF and WR of conductive solid lubricants represent the base lubricants along with the additives, lubricant material types, and the application fields of the lubricants as well. Here, Table 1 summarizes the tribological behavior of different conductive solid lubricants. In Table 1, 5% graphite at 10 N load possesses COF 0.2 and wear rate 0.0002 mm3/Nm. However, with the increase in graphite content from 5% to 20% decreases the wear rate considerably [84]. Likewise, the remaining data in Table 1 exhibit the recent progress on tribological behaviors of conductive solid lubricants.
4.2. Tribological Behavior of Conductive Liquid and Semisolid Lubricants
Table 5 and Table 6 represent the tribological data of semisolid and liquid lubricants, respectively. In Table 2, polyalkylene glycol (PAG) with 0.2% Ag grease has higher COF but lower wear width than polyalkylene glycol (PAG) with 0.2% Sb grease [96]. Choline-based stearic acid ([Ch][SA]) ionic liquid has lower COF and wear volume than choline-based palmitic acid ([Ch][PA]) liquid tabulated in Table 3. Similarly, the residual data presented in Table 5 and Table 6 show evolution of tribological data of conductive semisolid and liquid lubricants.
5. Characteristics of Lubricants for Advance Application
The main component of lubricating fluid is base oil (BO) [41]. Most of the lubricants first worked as BOs and different additives are included to improve their performance and conserve energy. BOs and their viscosities are responsible for cooling performance and additives play crucial roles to enhance the properties of the lubricants for using it in different dynamic fields [29]. The enhanced properties of the lubricants enable these to be used in different advanced fields of application and examples include electric vehicles (EV), space, and electromagnetic interference (EMI).
Lubricants in EVs need to have a higher level of electrical insulation to prevent arcing since they direct contact with e-motor and other electrical components of the vehicle [41]. The operating environment for EVs can be tough and include high temperatures, increased oxidation, and particle abrasion. The lubricants must have consistent, steady dielectric characteristics to function under these circumstances. Additionally, the lubricant comes into contact with various materials, which could cause the components to break, swell, crack, etc. [145]. Due to its excellent electrical conductivity, copper is used for the majority of these components. Therefore, the lubricant must have high compatibility with copper. For efficient and durable utilization, there is a specific range for operating temperature for the electric engine and other electronic components. The lubricants must have a high heat dissipation rate of up to 180 °C [146].
To ensure the dependability, accuracy, and long-term life of numerous movable mechanical components, space lubrication design for the aerospace sector is extremely significant [147,148]. Certain characteristics are especially crucial when choosing lubricants for use in the space area because of the inherent difficulties and conditions of space [149]. Particularly conductive lubricants offer distinctive properties that make them appropriate for some space applications. Here are some characteristics of conductive lubricants for use in the space field including thermal conductivity [150], electrical conductivity [151], compatibility with materials [152], vacuum stability [153,154], lubricity and wear resistance [155], nonflammability [156], and longevity [157].
Conductive lubricants are useful in EMI shielding applications because of their unique properties, which enable EMI interference management and reduction possible [158]. Some vital characteristics [158,159,160] of conductive lubricants for EMI shielding are including electrical conductivity, shielding effectiveness, frequency range, stability, compatibility, ease of application, and durability. These characteristics make conductive lubricants useful for shielding EMI, lowering electromagnetic interference, and preserving the integrity and functionality of sensitive electronic equipment.
6. Lubricating Mechanism of Conductive Lubricants
Lubrication and thermal or electrical conduction are two essential components of the lubrication process of conductive lubricants. Conductive lubricants work similarly to traditional lubricants by minimizing wear and friction on moving surfaces. In order to facilitate motion and avoid metal-to-metal contact, they create a thin, protective coating between the touching surfaces. This lubricating coating assists in reducing wear, heat generation, and frictional losses within the system. Thermal conduction enables the lubricant to dissipate heat from the hot region to the cold region therefore the mating surfaces can be within controlled temperature. The incorporation of conductive additives into the lubricant formulation allows conductive lubricants to attain electrical conductivity. The lubricant’s electrical conductivity creates a conductive route that facilitates the movement of electrical current between the contacting surfaces. Lubricating mechanism of conductive solid lubricants and conductive liquid lubricants are explained below.
6.1. Lubricating Mechanism of Conductive Solid Lubricants
Industries have used solid lubricating oils for a long time to minimize wear and friction under various conditions [161]. Molybdenum disulfide (MoS2) [162], tungsten disulfide (WS2) [163], calcium sulfonate [164], and graphite [165] are the most commonly used conductive solid lubricants. Though these have unique structures and physicochemical properties, these are utilized as strongly bonded protective coatings [166]. These substances create a shield that prevents friction pairs from coming into direct contact, which improves the effectiveness of friction reduction and wear resistance [167]. The scientific and industrial sectors hold solid lubricating coatings in high respect for their exceptional chemical inertness, great mechanical strength, and hardness, as well as their exceptional capabilities for minimizing friction and resisting wear [168]. Since they reduce abrasion, shear, and adhesion, the least amount of friction and wear is normally provided by their strong protective coatings.
In contrast, liquid lubricants have several special benefits, such as the simplicity of wear replenishment, the lack of mechanical noise, and the capacity to eliminate wear debris. Therefore, solid–liquid duplex lubricating coatings by mixing liquid lubricants with solid lubricants are necessary for advanced applications [169]. Carbon has become the most attention compared to other solid lubricants because of its outstanding properties. Carbon exists in a variety of forms, and each form’s features are determined by its unique structure [170]. Diamond-like carbon (DLC) is one of the most capable carbon-based protective coatings due to its exceptional physical, and tribological properties [171].
Given the substantial progress in solid films and liquid lubricants over the past few decades, it is hoped that solid and liquid lubricating systems will lessen individual drawbacks through synergistic effects. As a result, solid–liquid systems based on carbon were created. Xiaoqiang FAN et al. [169] concentrated on creating solid–liquid duplex lubricating coatings by mixing liquid lubricants with diamond-like carbon sheets. They formed and transferred a solid lubricant coating on a steel ball. Figure 4 represents the potential lubricating mechanism for DLC-based solid–liquid composite systems. According to the study of Wang’s group [172] the potential lubricating mechanism of carbon-based solid–liquid duplex coatings can be explained as follows.
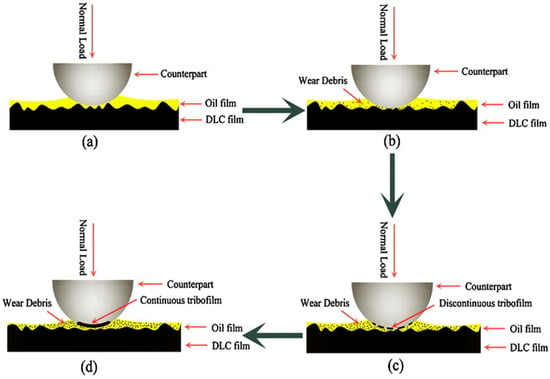
Figure 4.
The proposed lubricating mechanism for solid–liquid duplex coatings is shown schematically in the diagram. A thorough illustration of the friction process’ transfer layer creation was provided. (a) shows the distribution of the DLC film along with the surface asperities, (b) shows some wear debris that are scattered across the contact area, (c) shows the transfer of the film from the disc to the steel ball, and (d) shows the development of a continuous tribofilm on the steel ball. Reprinted with permission from [172].
In Figure 4, the upper oil film is represented as a yellow layer, and the DLC film layer is displayed as a black layer. The loaded duplex lubricating layer is continually approached by the counter face ball at the beginning of the test. The DLC film in Figure 4a was equally dispersed across the surface and had several asperities. Early on, some wear debris was scattered across the contact area (Figure 4b). A layer of coating is transferred from the disc to the steel ball as a result of repeated sliding in the oil. As can be seen in Figure 4c, the transfer film was first discontinuous and very thin. The transfer layer developed a continuous tribofilm because of the intense contact between the oil and DLC film debris (Figure 4d).
6.2. Lubricating Mechanism of Conductive Liquid Lubricants
The need for mechanical components to operate in a reliable and precise manner over an extended period drives researchers to investigate high-performance lubricants better suited for advanced uses, i.e., space technology, and electrical vehicles. Liquid lubricants have proven to have certain particular advantages for handling lubrication problems, such as low friction and wear, low mechanical noise, ease of replenishment, ability to remove worn debris, and relative environmental adaptability [169,173]. The materials including silicone oils [174], mineral oils [175], perfluoropolyethers (PFPE) [176], polyalphaolephines (PAO) [177], multiply-alkylated cyclopentanes (MACs) [178], silahydrocarbons (SiHC) [179] are mainly ionic liquids (ILs) [179].
ILs are ionic salts having melting points lower than room temperature. They are composed of a relatively large organic cation and an inorganic anion with weak coordination [180]. Due to their unique qualities, ILs have gained a lot of attention in a variety of applications. ILs have thus been researched for use with steel, aluminum, copper, and steel with changed surface coatings as flexible lubricants and lubricant additives [93,181,182,183]. The possible mechanism of ILs as conductive lubricants can be described below:
Figure 5 illustrates the lubricating mechanism of ILs as lubricant additive for steel-to-steel contacts [184]. The creation of stable lubrication films on sliding surfaces for blank base oil is exceedingly challenging at zero potential and low load due to the absence of polar functional groups, which interact chemically and physically with substrate surfaces. The long linear alkyl chain of the ions created a dense inner layer in the mixtures fortified with ILs. Cations and anions will absorb onto the surfaces of pairs by intermolecular and coulomb forces. Mixtures become more lubricious at negative potential. On the other hand, with positive potential, the lubricity of IL mixes decreases.
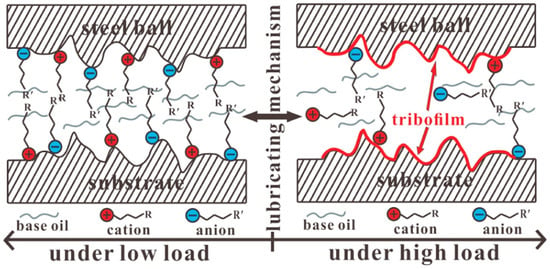
Figure 5.
The lubricity mechanism of ILs lubricant for steel-to-steel contacts. Reprinted with permission from [184].
However, to protect the sliding pairs under high-stress circumstances, the ILs engage in chemical reaction with the substrate surface and create reaction films. At this stage, the tribofilm and IL absorption combine to improve the basic oils’ ability to lubricate [185].
7. Superlubricity of Conductive Lubricants
The word “superlubricity” refers to a lubrication state with a very low coefficient of friction-below 0.001-in the fluid [186,187,188]. Researchers have concentrated on enhancing the performance of lubrication to decrease friction and wear and to increase the effectiveness and durability of two sliding surfaces [187]. In this regard, the concept of superlubricity was introduced in 1990 [189,190]. The concept of superlubricity has gained significant attention due to its potential applications in various fields, including nanotechnology, engineering, and even in the development of advanced lubricants [191]. The impact of superlubricity can be particularly fascinating in the context of conductive lubricants. Conductive lubricants are specially formulated lubricating materials that possess electrical and thermal conductivity properties [192]. The application of superlubricity to conductive lubricants can offer several advantages including reduction in friction and wear. Superlubricity considerably minimizes surface-to-surface friction [193]. As a result, there is significantly less wear and tear on the lubricated parts. This is especially advantageous for conductive lubricants because it maintains the reliability and lifetime of electrical contacts and connectors by protecting their integrity. Increased energy efficiency is another advantage of superlubricants having conductivity [194]. The ability of superlubricity to reduce frictional losses can help systems that use conductive lubricants operate more efficiently in terms of energy use [195]. The efficiency of the entire system is increased by reducing the energy losses brought on by friction. This reduces the amount of electricity that is lost as heat. Table 7 summarizes the superlubricity of conductive lubricants.

Table 7.
Superlubricity of conductive lubricants.
8. Conclusions and Outlook
This review provides a recent summary of the research on thermally and electrically conductive lubricants. A full study of recent advancements in the disciplines of conductivity-based lubricants, methods for improving their conductivity, tribological characteristics, and lubrication mechanisms are also provided in this article. Since conventional lubricants have a low lubrication efficiency, it is further difficult to minimize friction between tribosurfaces, which has been published in the literature. Conductive lubricants are an ideal choice for tribological applications due to their superior mechanical, thermal, electrical, and biological properties. This review provides a more structured tabulated summary of the methodologies, experimental techniques, environmental factors, applications, and tribological characteristics of conductive lubricants and lubricant additives.
A significant thermal feature that differs from lubricant to lubricant is thermal conductivity. Lubricants often have lower thermal conductivity than other materials, which results in less heat being transferred between two moving parts while decreasing friction. To ensure that the heat generated by friction can be efficiently transported from the moving surface contact to the surrounding environment, a lubricant’s superior thermal conductivity is essential. Different lubricants’ thermal conductivities are not necessarily the same, but this can be managed with the correct additives. Compressor oil, for instance, is a mineral oil-based lubricant with a lower thermal conductivity than synthetic lubricants such as polyol esters and polyalphaolefins (PAOs). Nowadays, several nanoparticles including CuO, Al2O3, Cu, multi-walled carbon nanotubes (MWCNTs), fullerene (C60), and others are used to improve the thermal conductivity of lubricants. Table 5 makes it clear that the addition of MWNT or SWNT as an additive improves the thermal characteristics of polyalphaolefin oil (PAO). With MWNT, thermal conductivity can reach 0.27 W/mK. With the addition of MWNT-OH, PAO Durasyn 166 has a thermal conductivity of up to 0.31 W/mK (Table 5). Additionally, Table 5 shows that PAO Durasyn 166 with Pyrograf Pr-24-XT-HHT CNF additive has a thermal conductivity of 0.68 W/mK.
The ability of lubricants to carry electric current is referred to as electrical conductivity. To reduce the risk of electrical current flow and potential damage in applications involving electrical components or circuits, lubricants are often manufactured to have low electrical conductivity. A lubricant’s composition and the kind of additives it contains determine its electrical conductivity. The base oils used in lubricants might be mineral, synthetic, or vegetable oils, and they may also contain a variety of additives to improve their performance. Petroleum-derived mineral oils often have a low electrical conductivity. However, some additives, including polar additives or conductive metal powders, can be added to increase the lubricant’s electrical conductivity. Table 6 shows, when we use surfactant oleic acid–MWCNT as additive with diesel oil (DO) the electrical conductivity becomes 476.3 μS/cm. When hexylamine–MWCNT is used as additive with DO, the electrical conductivity becomes 409.2 μS/cm. Again, with surfactant oleic acid-graphene nanoplatelets additive the electrical conductivity of DO becomes 492.6 μS/cm. Here, the addition of new additives causes diesel oil to change in conductivity.
Synthetic oils, on the other hand, can have varying levels of electrical conductivity depending on their composition. Some synthetic oils may have higher electrical conductivity compared to mineral oils, but they still generally formulated to have low conductivity to prevent electrical issues. Vegetable oils, often used in environmentally friendly or bio-based lubricants, usually have higher electrical conductivity compared to mineral or synthetic oils. However, their electrical conductivity is still relatively low in comparison to conductive materials. Table 6 has some examples of it. The conductivity of glycerol with MWNT-OH, 3% Cu additive is 178 Ω·cm. Again, the conductivity of glycerol with CTN additive is 175 Ω·cm. The electrical conductivity of 75% glycerol and 25% water with MWNT-OH additive is 10 Ω·cm. Some lubricants are designed to have low electrical conductivity but there are specialized lubricants available with higher electrical conductivity for specific applications where electrical grounding or conductivity desired. These lubricants typically used in applications such as electrical contacts or as conductive greases for grounding purposes.
Lubricants that transfer heat and electricity are essential for a variety of cutting-edge applications, including EMI, EVs, and marine. There are many articles on lubrication performance in the literature, but relatively few assess thermal and electrical properties or thoroughly explore the relationship between conductivity and lubrication performance. Therefore, for more advanced applications, researchers should investigate into the structural and nanoscale characteristics of conductive lubricants. Tribological properties of lubricating oils change with the increase in temperature, which degrades the performances. Numerous studies have been performed to enhance the tribological performance of lubricants along with different additives. However, very little or no literature has yet examined thoroughly the impacts of lubricant conductivity on tribological characteristics. Further, very few studies have combined thermally and electrically conductive lubricants and the researcher should investigate the connections between conductivity and tribological features for different fabrication methods. Additionally, structural research or nanoscale research can aid in understanding the lubricating mechanism. To choose the right lubricants, a detailed understanding of the lubrication process is essential. To commercialize, we must explore less expensive alternatives. Therefore, it is necessary to produce cheaper and more accessible lubricants with improved thermal and electrical conductivity for prospective future advanced applications such as aerospace, EMI, EVs, and marine.
Author Contributions
The first three authors contributed equally. Conceptualization, B.B., H.Y., A.S.M.N. and M.M.R.; methodology, B.B., M.I., M.J.S., S.H., M.H.R. and M.M.R.; writing—original draft preparation, B.B., M.J.S. and M.I.; writing—review and editing, M.M.R., M.I., M.H.R. and S.H.; visualization, A.S.M.N. and H.Y.; supervision, M.M.R.; project administration, M.M.R.; funding acquisition, M.M.R. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the Department of Industrial and Production Engineering for its support and motivation.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| COF = Coefficient of Friction | WR = Wear Rate |
| EVs = Electric Vehicles | EHV = Electric Hybrid Vehicles |
| ICEVs = Internal Combustion Engine Vehicles | EHL = Elastohydrodynamic Lubrication |
| PAO = Polyalphaolefin Oil | IL = Ionic Liquid |
| DO = Diesel Oil | PAG = Polyalkylene Glycol |
| EMI = Electromagnetic Interference | PFPE = Perfluoropolyethers |
| MACs = Multiply-Alkylated Cyclopentanes | SiHC = Silahydrocarbons |
| MoS2 = Molybdenum Disulfide | MoSe2 = Molybdenum Diselenide |
References
- Chinnachamy, R.; Durairaj, V.; Saravanamuthu, M.; Rajagopal, V. Evaluation of the effect of silver nanoparticles on the tribological and thermophysical properties of bio-lubricants. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2023, 237, 410–417. [Google Scholar]
- Woydt, M. The importance of tribology for reducing CO2 emissions and for sustainability. Wear 2021, 474, 203768. [Google Scholar]
- Sawyer, W.G. Leonardo da Vinci on Wear. Biotribology 2021, 26, 100160. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, P. Experimental study of Carbon Nanotubes to enhance Tribological Characteristics of Lubricating Engine Oil SAE10W40. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1225, 012052. [Google Scholar]
- Ahmed, N.S.; Nassar, A.M. Lubrication and lubricants. In Tribology: Fundamentals and Advancements; Intech Open: Rijeka, Croatia, 2013; pp. 55–76. [Google Scholar]
- Kaneta, M.; Matsuda, K.; Nishikawa, H. Effects of thermal properties of contact materials and slide-roll ratio in elastohydrodynamic lubrication. J. Tribol. 2022, 144, 061603. [Google Scholar]
- Gatti, S.F.; Gatti, F.; Amann, T.; Kailer, A.; Moser, K.; Weiss, P.; Seidel, C.; Rühe, J. Tribological performance of electrically conductive and self-lubricating polypropylene–ionic-liquid composites. RSC Adv. 2023, 13, 8000–8014. [Google Scholar]
- Mustafa, W.A.A.; Dassenoy, F.; Sarno, M.; Senatore, A. A review on potentials and challenges of nanolubricants as promising lubricants for electric vehicles. Lubr. Sci. 2022, 34, 1–29. [Google Scholar]
- Parenago, O.P.; Lyadov, A.S.; Maksimov, A.L. Development of Lubricant Formulations for Modern Electric Vehicles. Russ. J. Appl. Chem. 2022, 95, 765–774. [Google Scholar] [CrossRef]
- Wu, L.; Yan, J.; Cao, Z.; Xia, Y.; Wu, H. Investigation on the electrical conductivity and tribological properties of NbSe2-doped lubricating grease. Mater. Res. Express 2022, 9, 085201. [Google Scholar] [CrossRef]
- Hilton, M.R.; Fleischauer, P.D. Applications of solid lubricant films in spacecraft. Surf. Coat. Technol. 1992, 54, 435–441. [Google Scholar]
- Voevodin, A.; Muratore, C.; Aouadi, S. Hard coatings with high temperature adaptive lubrication and contact thermal management: Review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C.J.S.; Technology, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Xiao, S.; He, X.; Zhao, Z.; Huang, G.; Yan, Z.; He, Z.; Zhao, Z.; Chen, F.; Yang, J. Strong anti-polyelectrolyte zwitterionic hydrogels with superior self-recovery, tunable surface friction, conductivity, and antifreezing properties. Eur. Polym. J. 2021, 148, 110350. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Su, Y.; Li, J. Real time numerical simulation of thermal conductivity of marine gas turbine lubricating oil under complex sea conditions. Therm. Sci. 2021, 25, 4075–4081. [Google Scholar] [CrossRef]
- Song, J. Research progress of ionic liquids as lubricants. ACS Omega 2021, 6, 29345–29349. [Google Scholar] [PubMed]
- Zaharin, H.; Ghazali, M.; Rasheed, A.; Khalid, M.; Otsuka, Y. Tribological Performance of Hybrid Ti3C2/Graphene Additive on Outboard Engine Oil. In Proceedings of the 3rd Malaysian International Tribology Conference, Langkawi, Malaysia, 28–30 September 2020; pp. 146–153. [Google Scholar]
- Li, P.; Zhang, Z.; Yang, M.; Yuan, J.; Jiang, W. Synchronously improved thermal conductivity and tribological performance of self-lubricating fabric liner composites via integrated design method with copper yarn. Tribol. Int. 2021, 164, 107204. [Google Scholar] [CrossRef]
- Gonda, A.; Capan, R.; Bechev, D.; Sauer, B. The Influence of Lubricant Conductivity on Bearing Currents in the Case of Rolling Bearing Greases. Lubricants 2019, 7, 108. [Google Scholar] [CrossRef]
- Kudelina, K.; Asad, B.; Vaimann, T.; Rassõlkin, A.; Kallaste, A.; Van Khang, H. Methods of Condition Monitoring and Fault Detection for Electrical Machines. Energies 2021, 14, 7459. [Google Scholar] [CrossRef]
- Abdollahzadeh Jamalabadi, M.Y.; Alamian, R.; Yan, W.-M.; Li, L.K.B.; Leveneur, S.; Safdari Shadloo, M. Effects of Nanoparticle Enhanced Lubricant Films in Thermal Design of Plain Journal Bearings at High Reynolds Numbers. Symmetry 2019, 11, 1353. [Google Scholar] [CrossRef]
- Narita, K.; Takekawa, D. Lubricants Technology Applied to Transmissions in Hybrid Electric Vehicles and Electric Vehicles; 0148-7191; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2019. [Google Scholar]
- Sampathkumar, S. Effect of diamond like carbon coatings on reducing sliding fit joint interfacial wear damage on Al5083 alloy. Mater. Today Proc. 2023. [CrossRef]
- Ngaile, G.; Botz, F. Performance of Graphite and Boron-Nitride-Silicone Based Lubricants and Associated Lubrication Mechanisms in Warm Forging of Aluminum. J. Tribol. 2008, 130, 021801. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO2 as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-dispersed Ag/Graphene nanocomposite as lubricant additive to reduce friction and wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Effective lubricant additive of nano-Ag/MWCNTs nanocomposite produced by supercritical CO2 synthesis. Tribol. Int. 2018, 118, 180–188. [Google Scholar] [CrossRef]
- Ettefaghi, E.-o.-l.; Rashidi, A.; Ahmadi, H.; Mohtasebi, S.S.; Pourkhalil, M. Thermal and rheological properties of oil-based nanofluids from different carbon nanostructures. Int. Commun. Heat Mass Transf. 2013, 48, 178–182. [Google Scholar] [CrossRef]
- Kwak, Y.; Cleveland, C.; Adhvaryu, A.; Fang, X.; Hurley, S.; Adachi, T. Understanding Base Oils and Lubricants for Electric Drivetrain Applications; 0148-7191; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2019. [Google Scholar]
- Gupta, B.; Kumar, N.; Panda, K.; Dash, S.; Tyagi, A.K. Energy efficient reduced graphene oxide additives: Mechanism of effective lubrication and antiwear properties. Sci. Rep. 2016, 6, 18372. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, J.; Wang, W.; He, Y.; Luo, J. Influence of the micromorphology of reduced graphene oxide sheets on lubrication properties as a lubrication additive. Tribol. Int. 2017, 119, 614–621. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiza, R.; Prosyčevas, I.; Kreivaitis, R. Tribological properties of lubricant additives of Fe, Cu and Co nanoparticles. Tribol. Int. 2013, 60, 224–232. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 31246. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, P.; Mamatha, T. A review on tribological performance of lubricants with nanoparticles additives. Mater. Today Proc. 2020, 25, 586–591. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Abd Hamid, S.B. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrogen Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Tung, S.C.; Woydt, M.; Shah, R. Global insights on future trends of hybrid/EV driveline lubrication and thermal management. Front. Mech. Eng. 2020, 6, 571786. [Google Scholar] [CrossRef]
- Shah, R.; Tung, S.; Chen, R.; Miller, R. Grease performance requirements and future perspectives for electric and hybrid vehicle applications. Lubricants 2021, 9, 40. [Google Scholar] [CrossRef]
- Koch, J.; Schuettler, M.; Pasluosta, C.; Stieglitz, T. Electrical connectors for neural implants: Design, state of the art and future challenges of an underestimated component. J. Neural Eng. 2019, 16, 061002. [Google Scholar] [CrossRef] [PubMed]
- Angadi, S.V.; Jackson, R.L.; Pujar, V.; Tushar, M.R. A comprehensive review of the finite element modeling of electrical connectors including their contacts. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 836–844. [Google Scholar] [CrossRef]
- Chen, Y.; Jha, S.; Raut, A.; Zhang, W.; Liang, H. Performance characteristics of lubricants in electric and hybrid vehicles: A review of current and future needs. Front. Mech. Eng. 2020, 6, 571464. [Google Scholar] [CrossRef]
- Marine World. Marine Engineering Study Material. Available online: https://marineengineeringstudymaterial.wordpress.com/2020/10/02/lubrication-system-2/ (accessed on 21 May 2023).
- Aurolube. What Are the Different Types of Lubricants and What Are Their Applications? Available online: https://aurolube.com/blog/different-types-of-lubricants-and-their-applications/ (accessed on 21 May 2023).
- Singh, R.; Dureja, J.S.; Dogra, M.; Gupta, M.K.; Mia, M.; Song, Q. Wear behavior of textured tools under graphene-assisted minimum quantity lubrication system in machining Ti-6Al-4V alloy. Tribol. Int. 2020, 145, 106183. [Google Scholar] [CrossRef]
- Du, C.; Sheng, C.; Liang, X.; Rao, X.; Guo, Z. Effects of temperature on the tribological properties of cylinder-liner piston ring lubricated with different oils. Lubricants 2023, 11, 115. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Zheng, Z.; Qiao, D.; Feng, D.; Gong, Z.; Dong, G. Effect of functional groups on tribological properties of lubricants and mechanism investigation. Friction 2023, 11, 911–926. [Google Scholar] [CrossRef]
- Birleanu, C.; Pustan, M.; Cioaza, M.; Molea, A.; Popa, F.; Contiu, G. Effect of TiO2 nanoparticles on the tribological properties of lubricating oil: An experimental investigation. Sci. Rep. 2022, 12, 5201. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.A.A.; Takhakh, A.M.; Al-Waily, M. A review of use of nanoparticle additives in lubricants to improve its tribological properties. Mater. Today Proc. 2022, 52, 1442–1450. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, Y.; Liu, L.; Feng, X. Study on the conductive and tribological properties of copper sliding electrical contacts lubricated by ionic liquids. Tribol. Int. 2019, 130, 27–35. [Google Scholar] [CrossRef]
- Lin, F.; Xia, Y.; Feng, X. Conductive and tribological properties of TiN-Ag composite coatings under grease lubrication. Friction 2021, 9, 774–788. [Google Scholar] [CrossRef]
- Feng, X.; Hu, C.; Xia, Y.; Wang, Y. Study on conductivity and tribological properties of polyaniline/molybdenum disulfide composites in lithium complex grease. Lubr. Sci. 2022, 34, 182–195. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Y.; Hu, C.; Feng, X. Conductivity and tribological properties of IL-PANI/WS2 composite material in lithium complex grease. Friction 2023, 11, 977–991. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L. Tribological properties of conductive lubricating greases. Friction 2014, 2, 343–353. [Google Scholar] [CrossRef]
- Kaneta, M.; Sperka, P.; Yang, P.; Krupka, I.; Yang, P.; Hartl, M. Thermal elastohydrodynamic lubrication of ceramic materials. Tribol. Trans. 2018, 61, 869–879. [Google Scholar] [CrossRef]
- Rasul, M.G.; Kiziltas, A.; Bin Hoque, M.S.; Banik, A.; Hopkins, P.E.; Tan, K.-T.; Arfaei, B.; Shahbazian-Yassar, R. Improvement of the thermal conductivity and tribological properties of polyethylene by incorporating functionalized boron nitride nanosheets. Tribol. Int. 2022, 165, 107277. [Google Scholar] [CrossRef]
- Czel, G.; Sycheva, A.; Janovszky, D. Effect of different fillers on thermal conductivity, tribological properties of Polyamide 6. Sci. Rep. 2023, 13, 845. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Liu, P.; Cui, H.; Zhou, H.; Ma, F.; Li, W. Tribological Properties and Electrical Conductivity of Carbon Nanotube-Reinforced Copper Matrix Composites. J. Mater. Eng. Perform. 2022, 31, 4955–4962. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, H.; Xu, X.; Zhang, X.; Guo, Z. The effect of surface texture and conductive grease filling on the tribological properties and electrical conductivity of carbon brushes. Tribol. Int. 2021, 153, 106637. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, P.; Ni, J.; Shao, X.; Zhao, L.; Huang, B.; Ge, B.; Ban, C. The Electrical conductivities and Tribological properties of Vacuum Hot-Pressed Cu/Reduced Graphene Oxide Composite. J. Mater. Eng. Perform. 2017, 26, 4434–4441. [Google Scholar] [CrossRef]
- Chen, T.; Song, C.; Liu, Z.; Wang, L.; Hou, X.; Lu, H.; Zhang, Y. Current-carrying tribological properties of an elastic roll ring under different currents. Wear 2023, 514–515, 204590. [Google Scholar] [CrossRef]
- Sarkar, M.; Mandal, N. Solid lubricant materials for high temperature application: A review. Mater. Today Proc. 2022, 66, 3762–3768. [Google Scholar] [CrossRef]
- Mittal, D.; Singh, D.; Sharma, S.K. Thermal Characteristics and Tribological Performances of Solid Lubricants: A Mini Review. In Advances in Rheology of Materials; Dutta, A., Ali, H.M., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Schmidt, F.; Hebart, M.N.; Fleming, R.W. Core dimensions of human material perception. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Rawat, S.S.; Harsha, A.P. Current and future trends in grease lubrication. In Automotive Tribology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 147–182. [Google Scholar]
- Modigell, M.; Pola, A.; Tocci, M. Rheological characterization of semi-solid metals: A review. Metals 2018, 8, 245. [Google Scholar] [CrossRef]
- Cai, M.; Yu, Q.; Liu, W.; Zhou, F. Ionic liquid lubricants: When chemistry meets tribology. Chem. Soc. Rev. 2020, 49, 7753–7818. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, N.W.M.; Kalam, M.A.; Masjuki, H.H.; Al Mahmud, K.A.H.; Yunus, R. The effect of temperature on tribological properties of chemically modified bio-based lubricant. Tribol. Trans. 2014, 57, 408–415. [Google Scholar] [CrossRef]
- Bobzin, K.; Bartels, T.; Mang, T. Industrial Tribology: Tribosystems, Friction, Wear and Surface Engineering, Lubrication; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bay, N. The state of the art in cold forging lubrication. J. Mater. Process. Technol. 1994, 46, 19–40. [Google Scholar] [CrossRef]
- Sultana, M.N.; Dhar, N.R.; Zaman, P.B. A review on different cooling/lubrication techniques in metal cutting. Am. J. Mech. Appl. 2019, 7, 71–87. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Y. Boundary lubrication by adsorption film. Friction 2015, 3, 115–147. [Google Scholar] [CrossRef]
- Etsion, I. Modeling of surface texturing in hydrodynamic lubrication. Friction 2013, 1, 195–209. [Google Scholar] [CrossRef]
- Lugt, P.M.; Morales-Espejel, G.E. A review of elasto-hydrodynamic lubrication theory. Tribol. Trans. 2011, 54, 470–496. [Google Scholar] [CrossRef]
- Jackson, R.L.; Angadi, S. Modelling of lubricated electrical contacts. Lubricants 2022, 10, 32. [Google Scholar] [CrossRef]
- Zin, V.; Barison, S.; Agresti, F.; Colla, L.; Pagura, C.; Fabrizio, M. Improved tribological and thermal properties of lubricants by graphene based nano-additives. RSC Adv. 2016, 6, 59477–59486. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, H.S.; Lee, J.K.; Jung, W.H. Thermal conductivity and lubrication characteristics of nanofluids. Curr. Appl. Phys. 2006, 6, e67–e71. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Li, S.; Guo, L.; Yang, Z.; Zhang, X.; Wang, T.; Wang, Q. 3D structurally advanced graphene oxide/h-BN hybrid for solid self-lubrication with enhanced thermal conductivity. Tribol. Int. 2022, 176, 107918. [Google Scholar] [CrossRef]
- Shah, R.; Gashi, B.; Rosenkranz, A. Latest developments in designing advanced lubricants and greases for electric vehicles—An overview. Lubr. Sci. 2022, 34, 515–526. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- McCoy, B. Next generation driveline lubricants for electrified vehicles. Tribol. Lubr. Technol. 2021, 77, 38–40. [Google Scholar]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Baiming, C.; Qinling, B.; Jun, Y.; Yanqiu, X.; Jingcheng, H. Tribological properties of solid lubricants (graphite, h-BN) for Cu-based P/M friction composites. Tribol. Int. 2008, 41, 1145–1152. [Google Scholar]
- Du, L.; Huang, C.; Zhang, W.; Li, T.; Liu, W. Preparation and wear performance of NiCr/Cr3C2–NiCr/hBN plasma sprayed composite coating. Surf. Coat. Technol. 2011, 205, 3722–3728. [Google Scholar] [CrossRef]
- Baradeswaran, A.; Perumal, A.E. Wear and mechanical characteristics of Al 7075/graphite composites. Compos. Part B Eng. 2014, 56, 472–476. [Google Scholar] [CrossRef]
- Hudec, T.; Izai, V.; Satrapinskyy, L.; Huminiuc, T.; Roch, T.; Gregor, M.; Grančič, B.; Mikula, M.; Polcar, T. Structure, mechanical and tribological properties of MoSe2 and Mo-Se-N solid lubricant coatings. Surf. Coat. Technol. 2021, 405, 126536. [Google Scholar] [CrossRef]
- Efeoglu, I.; Baran, Ö.; Yetim, F.; Altıntaş, S. Tribological characteristics of MoS2–Nb solid lubricant film in different tribo-test conditions. Surf. Coat. Technol. 2008, 203, 766–770. [Google Scholar] [CrossRef]
- Zhong, H.; Feng, X.; Jia, J.; Yi, G. Tribological characteristics and wear mechanisms of NiMoAl composite coatings in reversible temperature cycles from RT to 900 °C. Tribol. Int. 2017, 114, 48–56. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Zhou, H.; Chen, J.; An, Y.; Yan, F. HVOF-sprayed adaptive low friction NiMoAl–Ag coating for tribological application from 20 to 800 °C. Tribol. Lett. 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Li, D.-X.; You, Y.-L.; Deng, X.; Li, W.-J.; Xie, Y. Tribological properties of solid lubricants filled glass fiber reinforced polyamide 6 composites. Mater. Des. 2013, 46, 809–815. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Y.; Liu, Z. Comparative study of the tribological properties of ionic liquids as additives of the attapulgite and bentone greases. Lubr. Sci. 2012, 24, 174–187. [Google Scholar] [CrossRef]
- Anand, G.; Saxena, P. A review on graphite and hybrid nano-materials as lubricant additives. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012201. [Google Scholar] [CrossRef]
- Wu, L.; Xia, Y.; Xiong, S.; Wu, H.; Chen, Z. Effect of ionic liquids modified nano-TiO2 as additive on tribological properties of silicone grease. Mater. Res. Express 2021, 8, 105011. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L.; Pu, J.; Chen, T.; Zhang, H. Study of the Conductivity and Tribological Performance of Ionic Liquid and Lithium Greases. Tribol. Lett. 2014, 53, 281–291. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Q.; Zhao, G.; Liu, J.; Wang, X. Tribological properties of alkylphenyl diphosphates as high-performance antiwear additive in lithium complex grease and polyurea grease for steel/steel contacts at elevated temperature. Ind. Eng. Chem. Res. 2014, 53, 5660–5667. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, Y.; Ge, X. Conductive capacity and tribological properties of several carbon materials in conductive greases. Ind. Lubr. Tribol. 2016, 68, 577–585. [Google Scholar] [CrossRef]
- Ge, X.; Xia, Y.; Shu, Z.; Zhao, X. Conductive grease synthesized using nanometer ATO as an additive. Friction 2015, 3, 56–64. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Hu, Y.; Hou, B. Tribological performance and conductive capacity of Ag coating under boundary lubrication. Tribol. Int. 2017, 110, 161–172. [Google Scholar] [CrossRef]
- Liu, W.; Ye, C.; Gong, Q.; Wang, H.; Wang, P. Tribological performance of room-temperature ionic liquids as lubricant. Tribol. Lett. 2002, 13, 81–85. [Google Scholar] [CrossRef]
- Wang, R.; Sun, C.; Yan, X.; Guo, T.; Xiang, W.; Yang, Z.; Yu, Q.; Yu, B.; Cai, M.; Zhou, F. Influence of the molecular structure on the tribological properties of choline-based ionic liquids as water-based additives under current-carrying lubrication. J. Mol. Liq. 2023, 369, 120868. [Google Scholar] [CrossRef]
- Tuero, A.G.; Sanjurjo, C.; Rivera, N.; Viesca, J.L.; González, R.; Battez, A.H. Electrical conductivity and tribological behavior of an automatic transmission fluid additised with a phosphonium-based ionic liquid. J. Mol. Liq. 2022, 367, 120581. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G.D. Thermal conductivity enhancement of stearic acid using expanded graphite for low temperature thermal storage. Int. J. Eng. Sci. Innov. Technol. 2014, 3, 531–536. [Google Scholar]
- Gadilohar, B.L.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z.; Li, Y.; Chen, L. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol. Energy 2010, 84, 339–344. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Kelkar, M.S.; Maginn, E.J. Effect of temperature and water content on the shear viscosity of the ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide as studied by atomistic simulations. J. Phys. Chem. B 2007, 111, 4867–4876. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, X.; Zhang, S.; Liang, Y.; Bao, M.; Liu, W. Investigation of tribological behavior of Al–Si alloy against steel lubricated with ionic liquids of 1-diethylphosphonyl-n-propyl-3-alkylimidazolium tetrafluoroborate. J. Tribol. 2008, 130, 034501. [Google Scholar] [CrossRef]
- Suzuki, A.; Shinka, Y.; Masuko, M. Tribological characteristics of imidazolium-based room temperature ionic liquids under high vacuum. Tribol. Lett. 2007, 27, 307–313. [Google Scholar] [CrossRef]
- Tarasov, S.; Kolubaev, A.; Belyaev, S.; Lerner, M.; Tepper, F. Study of friction reduction by nanocopper additives to motor oil. Wear 2002, 252, 63–69. [Google Scholar] [CrossRef]
- Brouwer, M.D.; Gupta, L.A.; Sadeghi, F.; Peroulis, D.; Adams, D. High temperature dynamic viscosity sensor for engine oil applications. Sens. Actuators A Phys. 2012, 173, 102–107. [Google Scholar] [CrossRef]
- Jimenez, A.-E.; Bermúdez, M.-D. Ionic liquids as lubricants for steel–aluminum contacts at low and elevated temperatures. Tribol. Lett. 2007, 26, 53–60. [Google Scholar] [CrossRef]
- Boldoo, T.; Lee, M.; Cho, H. Enhancing the solar energy conversion and harvesting characteristics of multiwalled carbon nanotubes-modified 1-hexyl-3-methyl-imidazolium cation ionic liquids. Int. J. Energy Res. 2022, 46, 8891–8907. [Google Scholar] [CrossRef]
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Shah, S.; Chiou, Y.-C.; Lai, C.Y.; Apostoleris, H.; Rahman, M.M.; Younes, H.; Almansouri, I.; Al Ghaferi, A.; Chiesa, M.J.C. Impact of short duration, high-flow H2 annealing on graphene synthesis and surface morphology with high spatial resolution assessment of coverage. Carbon 2017, 125, 318–326. [Google Scholar] [CrossRef]
- Mohamed, A.; Tirth, V.; Kamel, B.M. Tribological characterization and rheology of hybrid calcium grease with graphene nanosheets and multi-walled carbon nanotubes as additives. J. Mater. Res. Technol. 2020, 9, 6178–6185. [Google Scholar] [CrossRef]
- Lee, C.-G.; Hwang, Y.-J.; Choi, Y.-M.; Lee, J.-K.; Choi, C.; Oh, J.-M. A study on the tribological characteristics of graphite nano lubricants. Int. J. Precis. Eng. Manuf. 2009, 10, 85–90. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, W.; Liang, W.; Yan, T.; Li, T.; Zhang, L.; Li, S. Inorganic nanomaterial lubricant additives for base fluids, to improve tribological performance: Recent developments. Friction 2022, 10, 645–676. [Google Scholar] [CrossRef]
- Nunn, N.; Mahbooba, Z.; Ivanov, M.G.; Ivanov, D.M.; Brenner, D.W.; Shenderova, O. Tribological properties of polyalphaolefin oil modified with nanocarbon additives. Diam. Relat. Mater. 2015, 54, 97–102. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, Y.; Dearn, K.D.; Zheng, X.; Yao, L.; Hu, X. Synergistic lubricating behaviors of graphene and MoS2 dispersed in esterified bio-oil for steel/steel contact. Wear 2015, 342–343, 297–309. [Google Scholar] [CrossRef]
- Vardhaman, B.S.A.; Amarnath, M.; Ramkumar, J.; Mondal, K. Enhanced tribological performances of zinc oxide/MWCNTs hybrid nanomaterials as the effective lubricant additive in engine oil. Mater. Chem. Phys. 2020, 253, 123447. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.; Roy, R.; Younis, H.; AlNahyan, M.; Younes, H. Carbon Nanomaterial-Based Lubricants: Review of Recent Developments. Lubricants 2022, 10, 281. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Li, X.; Li, D.; Zhu, M. Hydroxyl-terminated ionic liquids functionalized graphene oxide with good dispersion and lubrication function. Tribol. Int. 2020, 148, 106350. [Google Scholar] [CrossRef]
- Wu, L.; Gu, L.; Jian, R. Lubrication mechanism of graphene nanoplates as oil additives for ceramics/steel sliding components. Ceram. Int. 2021, 47, 16935–16942. [Google Scholar] [CrossRef]
- Flores-Castañeda, M.; Camps, E.; Camacho-López, M.; Muhl, S.; García, E.; Figueroa, M. Bismuth nanoparticles synthesized by laser ablation in lubricant oils for tribological tests. J. Alloys Compd. 2015, 643, S67–S70. [Google Scholar] [CrossRef]
- Scherge, M.; Böttcher, R.; Kürten, D.; Linsler, D. Multi-Phase Friction and Wear Reduction by Copper Nanopartices. Lubricants 2016, 4, 36. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Huang, X.; Huang, L.; Yang, F. Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceram. Int. 2014, 40, 7143–7149. [Google Scholar] [CrossRef]
- Song, X.; Zheng, S.; Zhang, J.; Li, W.; Chen, Q.; Cao, B. Synthesis of monodispersed ZnAl2O4 nanoparticles and their tribology properties as lubricant additives. Mater. Res. Bull. 2012, 47, 4305–4310. [Google Scholar] [CrossRef]
- Zhou, L.H.; Wei, X.C.; Ma, Z.J.; Mei, B. Anti-friction performance of FeS nanoparticle synthesized by biological method. Appl. Surf. Sci. 2017, 407, 21–28. [Google Scholar] [CrossRef]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Hu, K.; Xu, Y.; Hu, X. Formation of an adsorption film of MoS2 nanoparticles and dioctyl sebacate on a steel surface for alleviating friction and wear. Tribol. Int. 2015, 92, 172–183. [Google Scholar] [CrossRef]
- Hong, H.; Thomas, D.; Waynick, A.; Yu, W.; Smith, P.; Roy, W. Carbon nanotube grease with enhanced thermal and electrical conductivities. J. Nanopart. Res. 2010, 12, 529–535. [Google Scholar] [CrossRef]
- Ettefaghi, E.-o.-I.; Ahmadi, H.; Rashidi, A.; Nouralishahi, A.; Mohtasebi, S.S. Preparation and thermal properties of oil-based nanofluid from multi-walled carbon nanotubes and engine oil as nano-lubricant. Int. Commun. Heat Mass Transf. 2013, 46, 142–147. [Google Scholar] [CrossRef]
- Naddaf, A.; Zeinali Heris, S. Experimental study on thermal conductivity and electrical conductivity of diesel oil-based nanofluids of graphene nanoplatelets and carbon nanotubes. Int. Commun. Heat Mass Transf. 2018, 95, 116–122. [Google Scholar] [CrossRef]
- Yujun, G.; Zhongliang, L.; Guangmeng, Z.; Yanxia, L. Effects of multi-walled carbon nanotubes addition on thermal properties of thermal grease. Int. J. Heat Mass Transf. 2014, 74, 358–367. [Google Scholar] [CrossRef]
- Christensen, G.; Younes, H.; Hong, G.; Lou, D.; Hong, H.; Widener, C.; Bailey, C.; Hrabe, R. Hydrogen bonding enhanced thermally conductive carbon nano grease. Synth. Met. 2020, 259, 116213. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Saadah, A.; Saidur, R.; Khairul, M.A.; Kamyar, A. Thermal performance analysis of Al2O3/R-134a nanorefrigerant. Int. J. Heat Mass Transf. 2015, 85, 1034–1040. [Google Scholar] [CrossRef]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. Effect of Shape on Thermophysical and Heat Transfer Properties of ZnO/R-134a Nanorefrigerant. Mater. Today Proc. 2018, 5, 1635–1639. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Ettefaghi, E.-o.-l.; Ahmadi, H.; Rashidi, A.; Mohtasebi, S.S.; Alaei, M. Experimental evaluation of engine oil properties containing copper oxide nanoparticles as a nanoadditive. Int. J. Ind. Chem. 2013, 4, 28. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2015, 88, 363–368. [Google Scholar] [CrossRef]
- Christensen, G.; Yang, J.; Lou, D.; Hong, G.; Hong, H.; Tolle, C.; Widener, C.; Bailey, C.; Hrabe, R.; Younes, H. Carbon nanotubes grease with high electrical conductivity. Synth. Met. 2020, 268, 116496. [Google Scholar] [CrossRef]
- Daoqiang, L.; Tong, Q.K.; Wong, C.P. A study of lubricants on silver flakes for microelectronics conductive adhesives. IEEE Trans. Compon. Packag. Technol. 1999, 22, 365–371. [Google Scholar] [CrossRef]
- Myshkin, N.K.; Konchits, V.V. Evaluation of the interface at boundary lubrication using the measurement of electric conductivity. Wear 1994, 172, 29–40. [Google Scholar] [CrossRef]
- Yan, Y. 7—Tribology and tribo-corrosion testing and analysis of metallic biomaterials. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 178–201. [Google Scholar] [CrossRef]
- Rivera, N.; Viesca, J.L.; García, A.; Prado, J.I.; Lugo, L.; Battez, A.H. Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles. Appl. Sci. 2022, 12, 8911. [Google Scholar] [CrossRef]
- Bouvy, C.; Baltzer, S.; Jeck, P.; Gißing, J.; Lichius, T.; Eckstein, L. Holistic vehicle simulation using modelica—An application on thermal management and operation strategy for electrified vehicles. In Proceedings of the 9th International MODELICA Conference, Munich, Germany, 3–5 September 2012; pp. 264–270. [Google Scholar]
- Zhuang, W.; Fan, X.; Li, W.; Li, H.; Zhang, L.; Peng, J.; Cai, Z.; Mo, J.; Zhang, G.; Zhu, M. Comparing space adaptability of diamond-like carbon and molybdenum disulfide films toward synergistic lubrication. Carbon 2018, 134, 163–173. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Zabinski, J.S. Nanocomposite and nanostructured tribological materials for space applications. Compos. Sci. Technol. 2005, 65, 741–748. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L. Highly conductive ionic liquids toward high-performance space-lubricating greases. ACS Appl. Mater. Interfaces 2014, 6, 14660–14671. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Yin, L.; Zhao, J.; Xia, L.; Chen, L. Exceptionally high thermal conductivity of thermal grease: Synergistic effects of graphene and alumina. Int. J. Therm. Sci. 2015, 91, 76–82. [Google Scholar] [CrossRef]
- Nancarrow, P.; Mohammed, H. Ionic liquids in space technology—Current and future trends. ChemBioEng Rev. 2017, 4, 106–119. [Google Scholar] [CrossRef]
- Serles, P.; Nicholson, E.; Tam, J.; Barri, N.; Chemin, J.B.; Wang, G.; Michel, Y.; Singh, C.V.; Choquet, P.; Saulot, A. High performance space lubrication of MoS2 with tantalum. Adv. Funct. Mater. 2022, 32, 2110429. [Google Scholar] [CrossRef]
- Nyberg, E.; Schneidhofer, C.; Pisarova, L.; Dörr, N.; Minami, I. Ionic liquids as performance ingredients in space lubricants. Molecules 2021, 26, 1013. [Google Scholar] [CrossRef]
- Liu, X.; Pu, J.; Wang, L.; Xue, Q. Novel DLC/ionic liquid/graphene nanocomposite coatings towards high-vacuum related space applications. J. Mater. Chem. A 2013, 1, 3797–3809. [Google Scholar] [CrossRef]
- Gao, X.; Hu, M.; Fu, Y.; Weng, L.; Liu, W.; Sun, J. MoS2-Au/Au multilayer lubrication film with better resistance to space environment. J. Alloys Compd. 2020, 815, 152483. [Google Scholar] [CrossRef]
- Dörr, N.; Merstallinger, A.; Holzbauer, R.; Pejaković, V.; Brenner, J.; Pisarova, L.; Stelzl, J.; Frauscher, M. Five-Stage Selection Procedure of Ionic Liquids for Lubrication of Steel-Steel Contacts in Space Mechanisms. Tribol. Lett. 2019, 67, 73. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Z.; Hao, J.; Liu, W. Two strategies to improve the lubricating performance of WS2 film for space application. Tribol. Int. 2022, 175, 107825. [Google Scholar] [CrossRef]
- Na, R.; Liu, J.; Wang, G.; Zhang, S. Light weight and flexible poly (ether ether ketone) based composite film with excellent thermal stability and mechanical properties for wide-band electromagnetic interference shielding. RSC Adv. 2018, 8, 3296–3303. [Google Scholar] [CrossRef]
- Sun, L.; Huang, H.; Guan, Q.; Yang, L.; Zhang, L.; Hu, B.; Neisiany, R.E.; You, Z.; Zhu, M. Cooperative chemical coupling and physical lubrication effects construct highly dynamic ionic covalent adaptable network for high-performance wearable electronics. CCS Chem. 2023, 5, 1096–1107. [Google Scholar] [CrossRef]
- Soares, B.G.; Cordeiro, E.; Maia, J.; Pereira, E.C.L.; Silva, A.A. The effect of the noncovalent functionalization of CNT by ionic liquid on electrical conductivity and electromagnetic interference shielding effectiveness of semi-biodegradable polypropylene/poly (lactic acid) composites. Polym. Compos. 2020, 41, 82–93. [Google Scholar] [CrossRef]
- Hisakado, T.; Tsukizoe, T.; Yoshikawa, H. Lubrication mechanism of solid lubricants in oils. J. Lubr. Tech. 1983, 105, 245–252. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Source 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Nayak, A.P.; Yuan, Z.; Cao, B.; Liu, J.; Wu, J.; Moran, S.T.; Li, T.; Akinwande, D.; Jin, C.; Lin, J.-F. Pressure-modulated conductivity, carrier density, and mobility of multilayered tungsten disulfide. ACS Nano 2015, 9, 9117–9123. [Google Scholar] [CrossRef]
- Fugallo, G.; Cepellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal conductivity of graphene and graphite: Collective excitations and mean free paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, Z.; Liu, S.; Zhan, T.; Li, W.; Wang, J. Layered double hydroxides for tribological application: Recent advances and future prospective. Appl. Clay Sci. 2022, 221, 106466. [Google Scholar] [CrossRef]
- Torres, H.; Rodríguez Ripoll, M.; Prakash, B. Tribological behaviour of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Hu, Z.S.; Lai, R.; Lou, F.; Wang, L.G.; Chen, Z.L.; Chen, G.X.; Dong, J.X. Preparation and tribological properties of nanometer magnesium borate as lubricating oil additive. Wear 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Yang, H.-K.; Kim, D.-E. Tribology of multilayer coatings for wear reduction: A review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Fan, X.; Xue, Q.; Wang, L. Carbon-based solid-liquid lubricating coatings for space applications—A review. Friction 2015, 3, 191–207. [Google Scholar] [CrossRef]
- Lettington, A.H. Applications of diamond-like carbon thin films. Carbon 1998, 36, 555–560. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Xue, Q. A novel carbon-based solid-liquid duplex lubricating coating with super-high tribological performance for space applications. Surf. Coat. Technol. 2011, 205, 2738–2746. [Google Scholar] [CrossRef]
- Zaretsky, E.V. Liquid lubrication in space. Tribol. Int. 1990, 23, 75–93. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, T.; Okazaki, K.i.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, S.M.; Coelho, A.Z.; Martins, M.A.R.; Coutinho, J.A.P.; Ferreira, O.; Pinho, S.P. Evaluation of Ionic Liquids for the Sustainable Fractionation of Essential Oils. Ind. Eng. Chem. Res. 2023, 62, 6749–6758. [Google Scholar] [CrossRef]
- Yu, G.; Yan, S.; Zhou, F.; Liu, X.; Liu, W.; Liang, Y. Synthesis of dicationic symmetrical and asymmetrical ionic liquids and their tribological properties as ultrathin films. Tribol. Lett. 2007, 25, 197–205. [Google Scholar] [CrossRef]
- Kaldonski, T.J.; Cudzilo, S. Study on the Thermal Stability of Selected Ionic Liquids. Solid State Phenom. 2015, 220–221, 218–223. [Google Scholar] [CrossRef]
- Song, Z.; Liang, Y.; Fan, M.; Zhou, F.; Liu, W. Lithium-based ionic liquids as novel lubricant additives for multiply alkylated cyclopentanes (MACs). Friction 2013, 1, 222–231. [Google Scholar] [CrossRef]
- Kałdoński, T.; Wojdyna, P.P. Liquid lubricants for space engineering and methods for their testing. J. KONES 2011, 18, 163–184. [Google Scholar]
- Bonhote, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Yao, M.; Fan, M.; Liang, Y.; Zhou, F.; Xia, Y. Imidazolium hexafluorophosphate ionic liquids as high temperature lubricants for steel-steel contacts. Wear 2010, 268, 67–71. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Y.; Liu, Z.; Wen, Z. Conductive lubricating grease synthesized using the ionic liquid. Tribol. Lett. 2012, 46, 33–42. [Google Scholar] [CrossRef]
- Feng, X.; Xia, Y. Tribological properties of Ti-doped DLC coatings under ionic liquids lubricated conditions. Appl. Surf. Sci. 2012, 258, 2433–2438. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Ma, Z.; Cai, M.; Liu, W. Probing the lubricating mechanism of oil-soluble ionic liquids additives. Tribol. Int. 2017, 107, 152–162. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.-D.; Saurín, N.; Espinosa, T.; Carrión, F.-J.; Bermúdez, M.-D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Li, Y.; Cao, H.; Li, S.; Wang, B.; Fang, J.; Meng, Y.; Ma, L.; Tian, Y. Effects of abrasive particles on liquid superlubricity and mechanisms for their removal. Langmuir 2021, 37, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Ge, X.; Yi, S.; Wang, H.; Liu, Y.; Luo, J. Macroscale superlubricity achieved on the hydrophobic graphene coating with glycerol. ACS Appl. Mater. Interfaces 2020, 12, 18859–18869. [Google Scholar] [CrossRef]
- Du, C.; Yu, T.; Zhang, L.; Deng, H.; Shen, R.; Li, X.; Feng, Y.; Wang, D. Macroscale Superlubricity with Ultralow Wear and Ultrashort Running-In Period (~1 s) through Phytic Acid-Based Complex Green Liquid Lubricants. ACS Appl. Mater. Interfaces 2023, 15, 10302–10314. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.; Duan, Z.; Huang, S.; Tao, Y.; Lu, X.; Zhang, Y.; Kan, Y.; Wei, Z.; Li, D. Phononic origin of structural lubrication. Friction 2023, 11, 966–976. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xu, D. Anisotropic mechanical properties of quartz mica schist subjected to true triaxial compression. Environ. Earth Sci. 2023, 82, 204. [Google Scholar] [CrossRef]
- Sun, S.; Ru, G.; Qi, W.; Liu, W. Molecular dynamics study of the robust superlubricity in penta-graphene van der Waals layered structures. Tribol. Int. 2023, 177, 107988. [Google Scholar] [CrossRef]
- Zeng, Q. High-Temperature Superlubricity Performance of h-BN Coating on the Textured Inconel X750 Alloy. Lubricants 2023, 11, 258. [Google Scholar] [CrossRef]
- Kayode, J.F.; Afolalu, S.A.; Emetere, M.E.; Monye, S.I.; Lawal, S.L. Application and impact of tribology in energy—An overview. E3S Web Conf. 2023, 391, 01081. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Qiao, Y.; Tan, S.; Feng, S.; Ma, J.; Liu, Y.; Luo, J. 2D metal-organic frameworks with square grid structure: A promising new-generation superlubricating material. Nano Today 2021, 40, 101262. [Google Scholar] [CrossRef]
- Ayyagari, A.; Alam, K.I.; Berman, D.; Erdemir, A. Progress in superlubricity across different media and material systems—A review. Front. Mech. Eng. 2022, 8, 908497. [Google Scholar] [CrossRef]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem. Commun. 2014, 50, 4368–4370. [Google Scholar] [CrossRef] [PubMed]
- Björling, M.; Shi, Y. DLC and glycerol: Superlubricity in rolling/sliding elastohydrodynamic lubrication. Tribol. Lett. 2019, 67, 23. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Zhang, C.; Liu, Y.; Luo, J. Superlubricity and antiwear properties of in situ-formed ionic liquids at ceramic interfaces induced by tribochemical reactions. ACS Appl. Mater. Interfaces 2019, 11, 6568–6574. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Miao, K.; Zhang, K.; Zheng, W.; Chen, C. Macroscale robust superlubricity on metallic NbB2. Adv. Sci. 2022, 9, 2103815. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).