Stability and Thermal Conductivity of Mono and Hybrid Nanoparticles Dispersion in Double-End Capped PAG Lubricant
Abstract
:1. Introduction
2. Experimental Methodology
2.1. The Materials Specification
2.2. Nanolubricants Characterisation and Stability Evaluation
2.3. Thermal Conductivity Measurement
3. Results and Discussion
3.1. Characterisation and Stability Evaluation
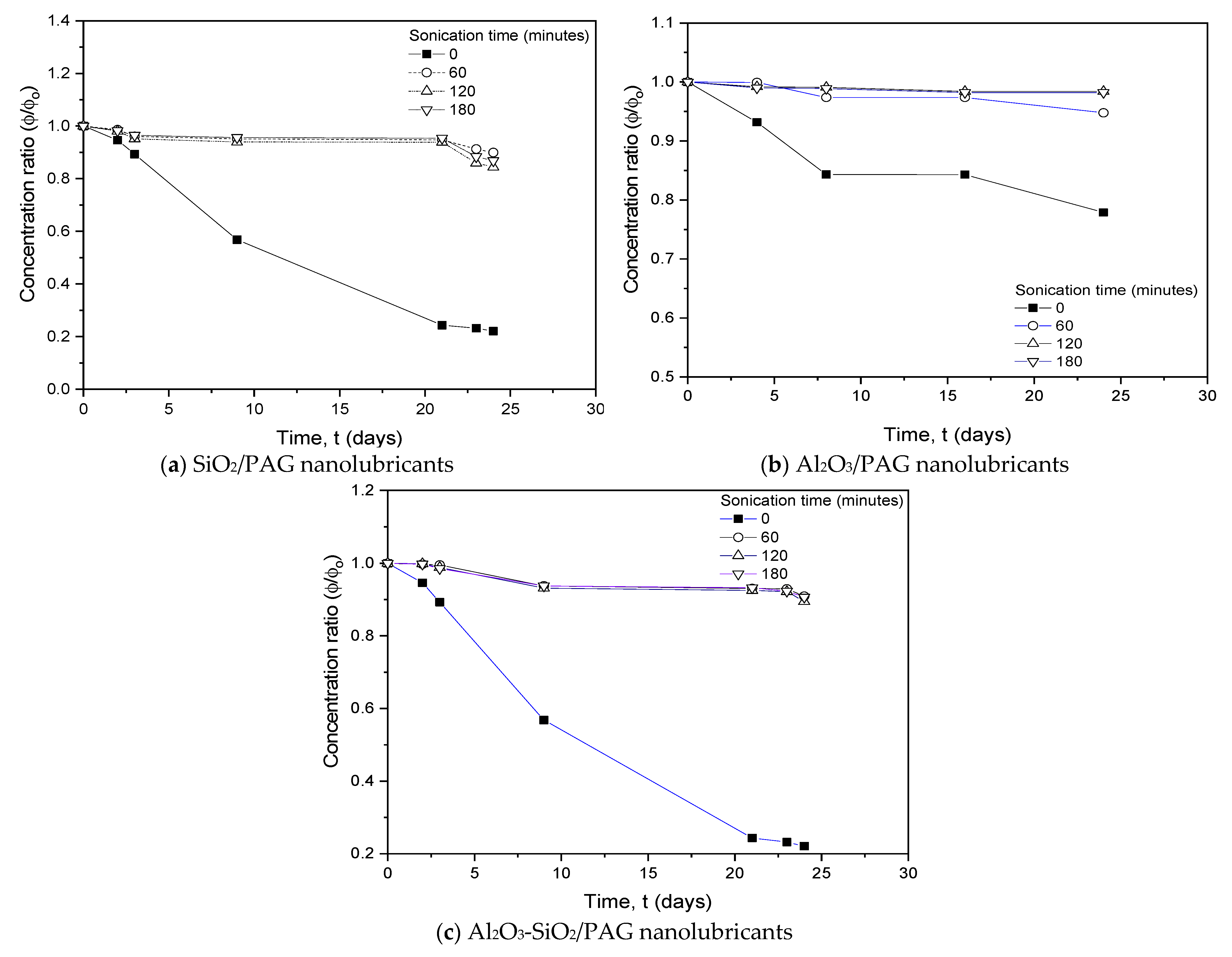
3.1.1. Sonication Time Evaluation
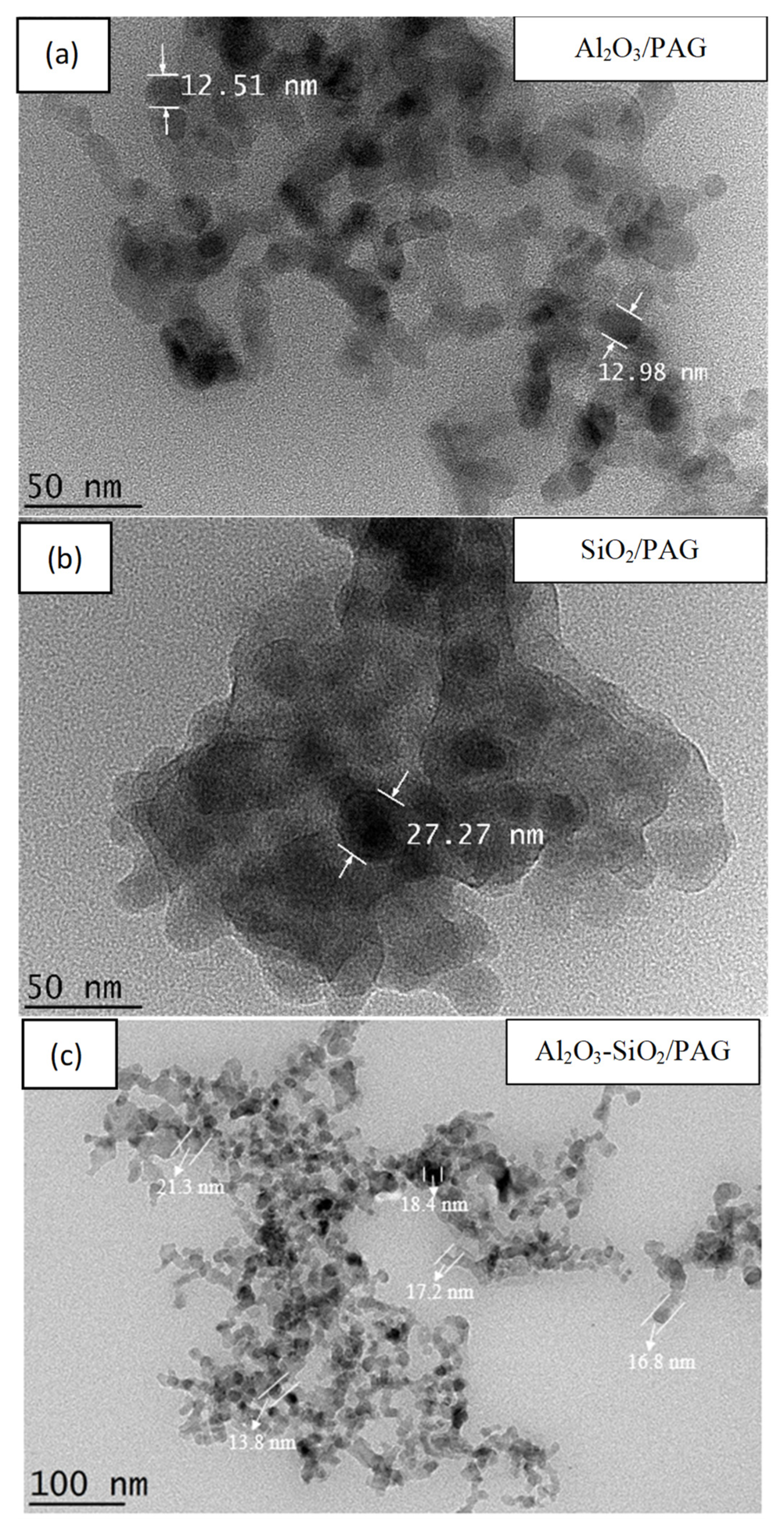
3.1.2. Micrograph Evaluation
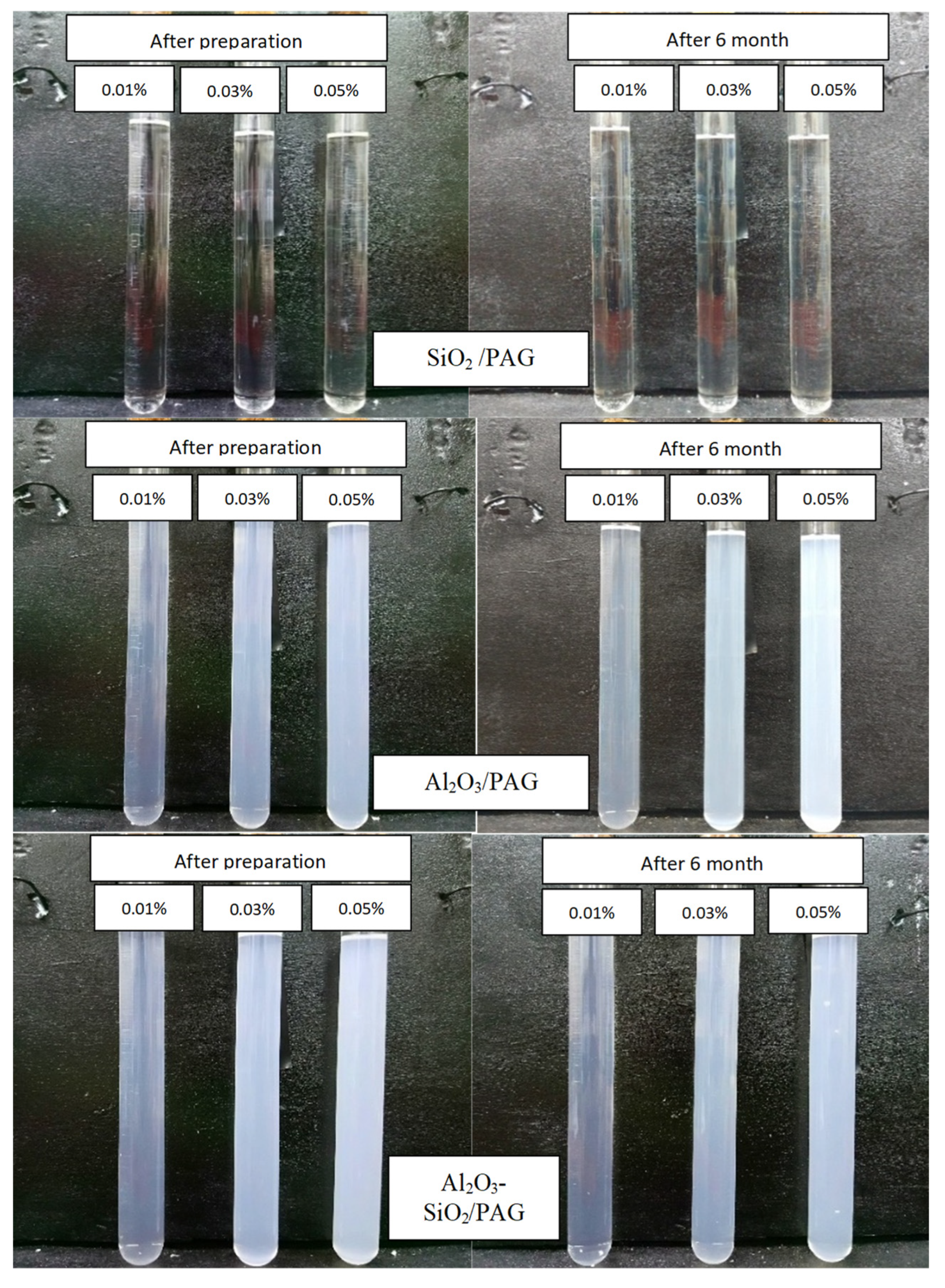
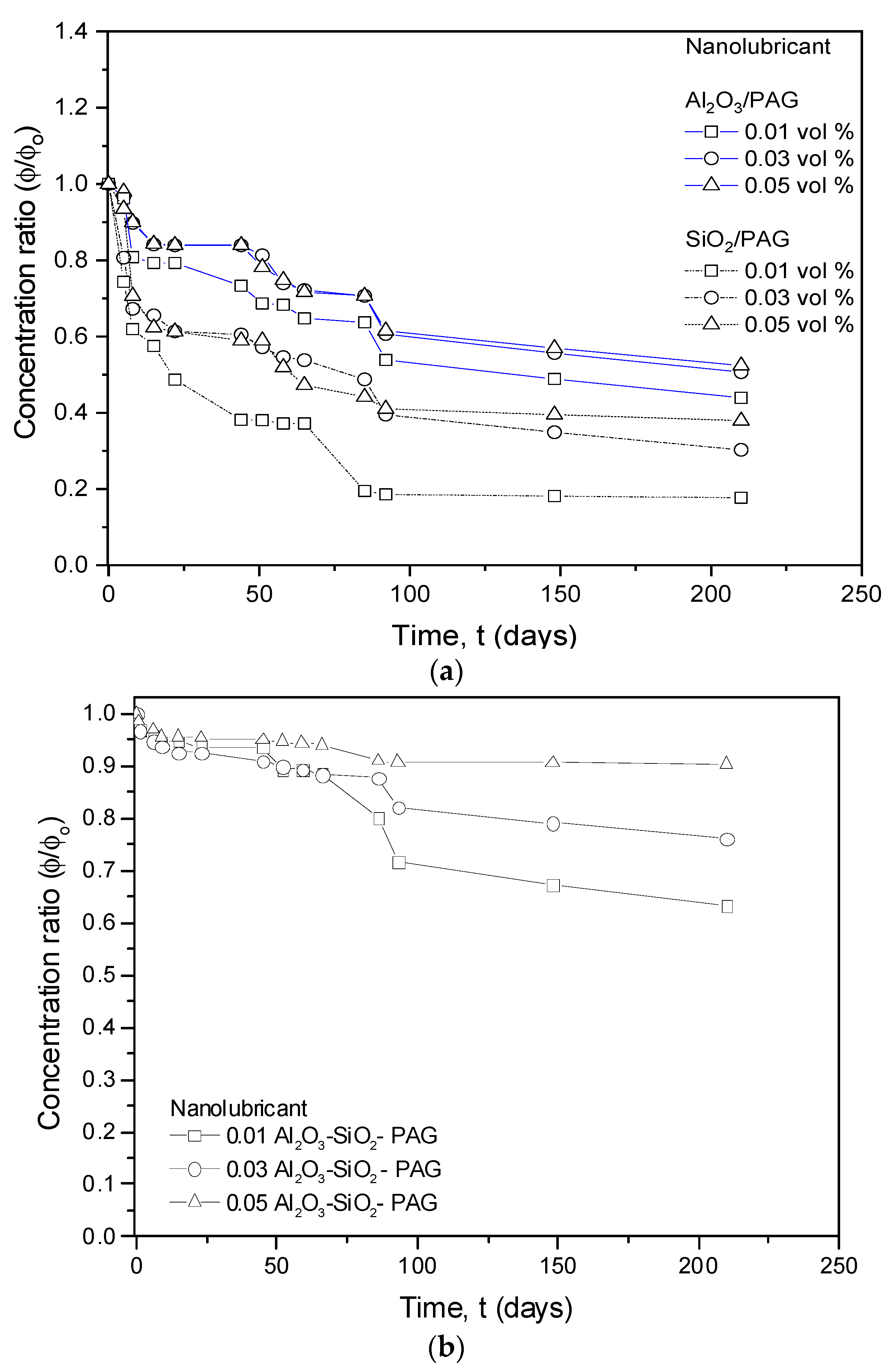
3.1.3. Visual Sedimentation Observation
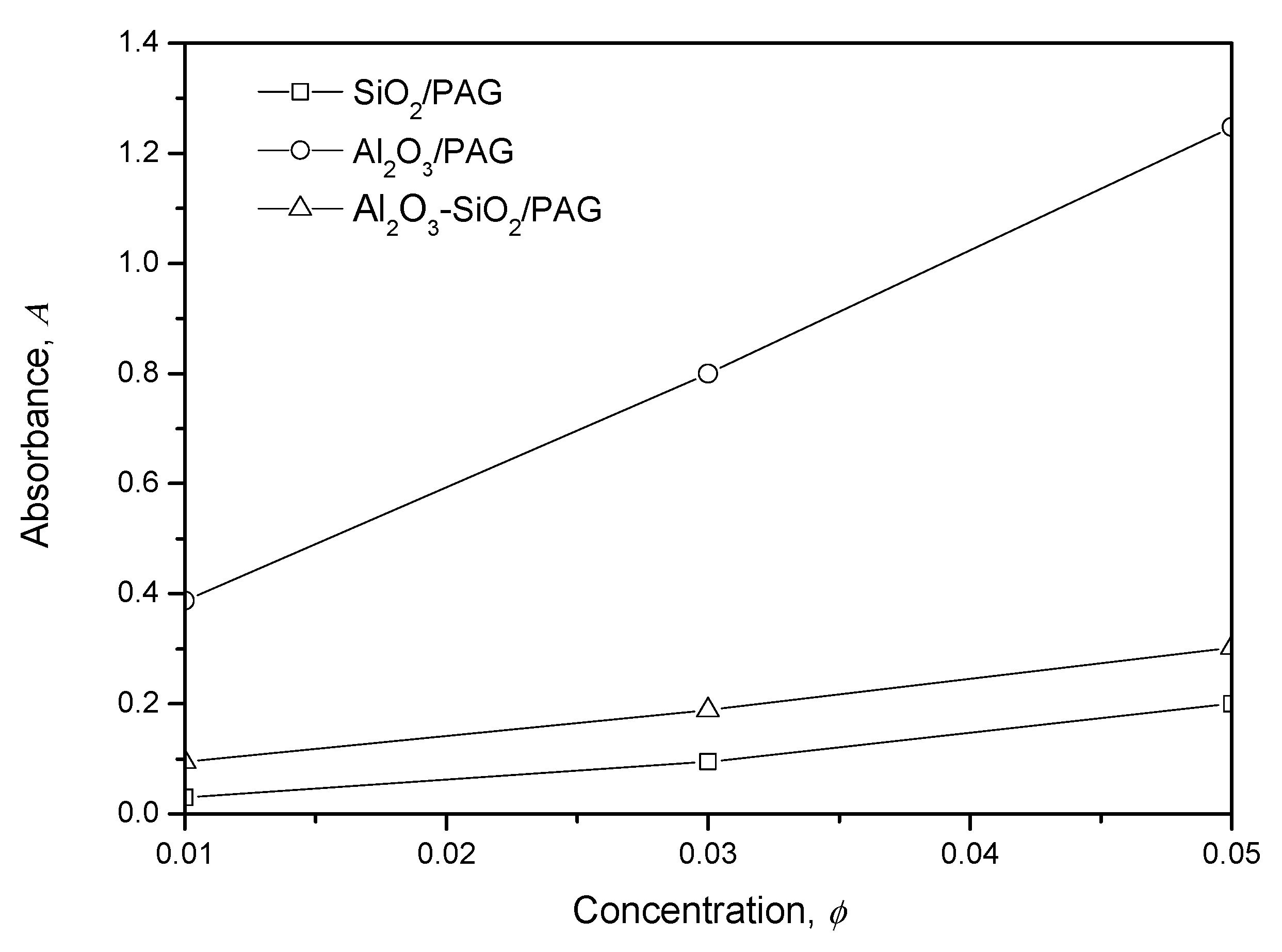
3.1.4. UV-Vis Spectrophotometer Evaluation
3.1.5. Zeta Potential Evaluation
3.2. Thermal Properties Evaluation
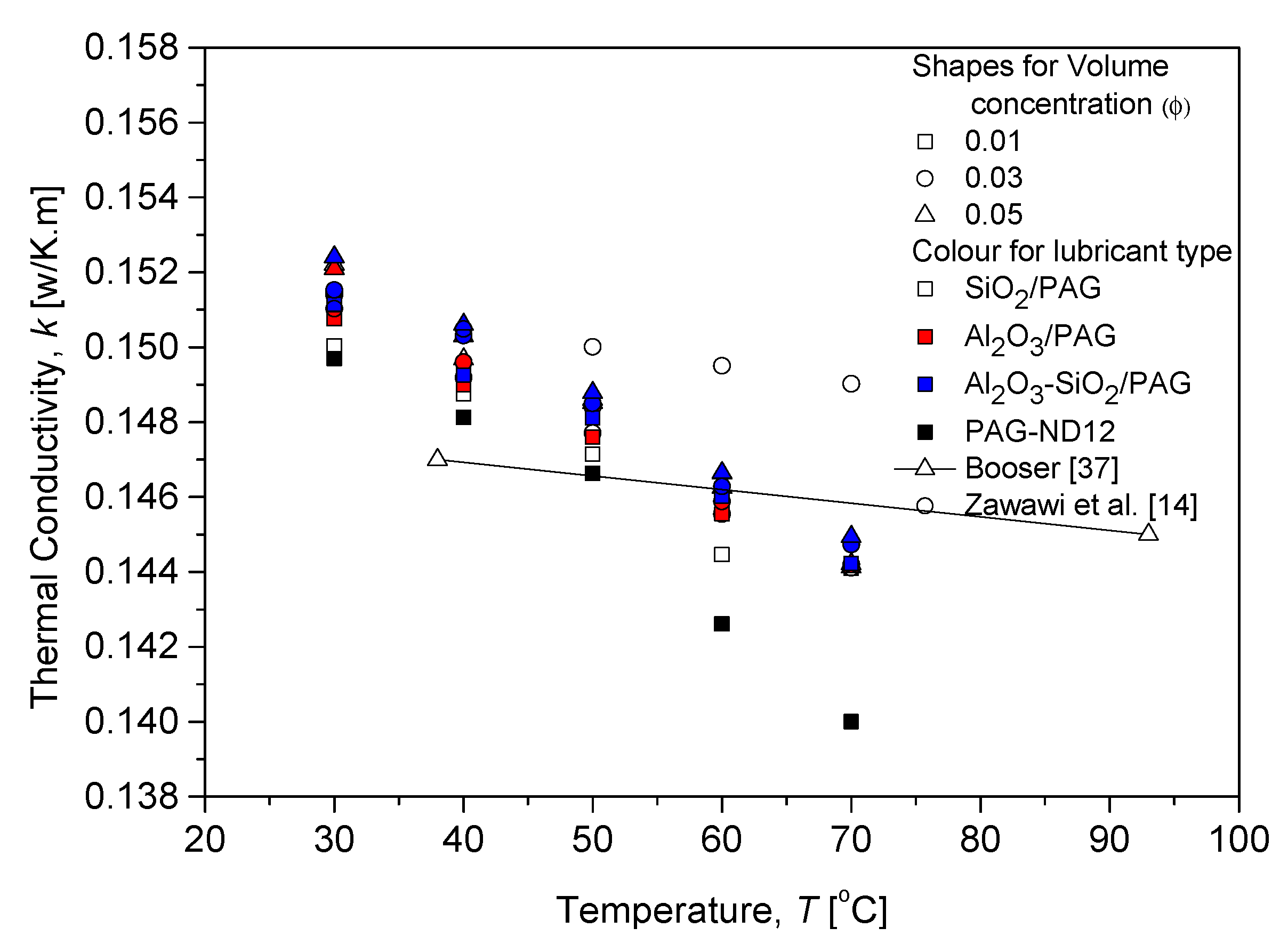
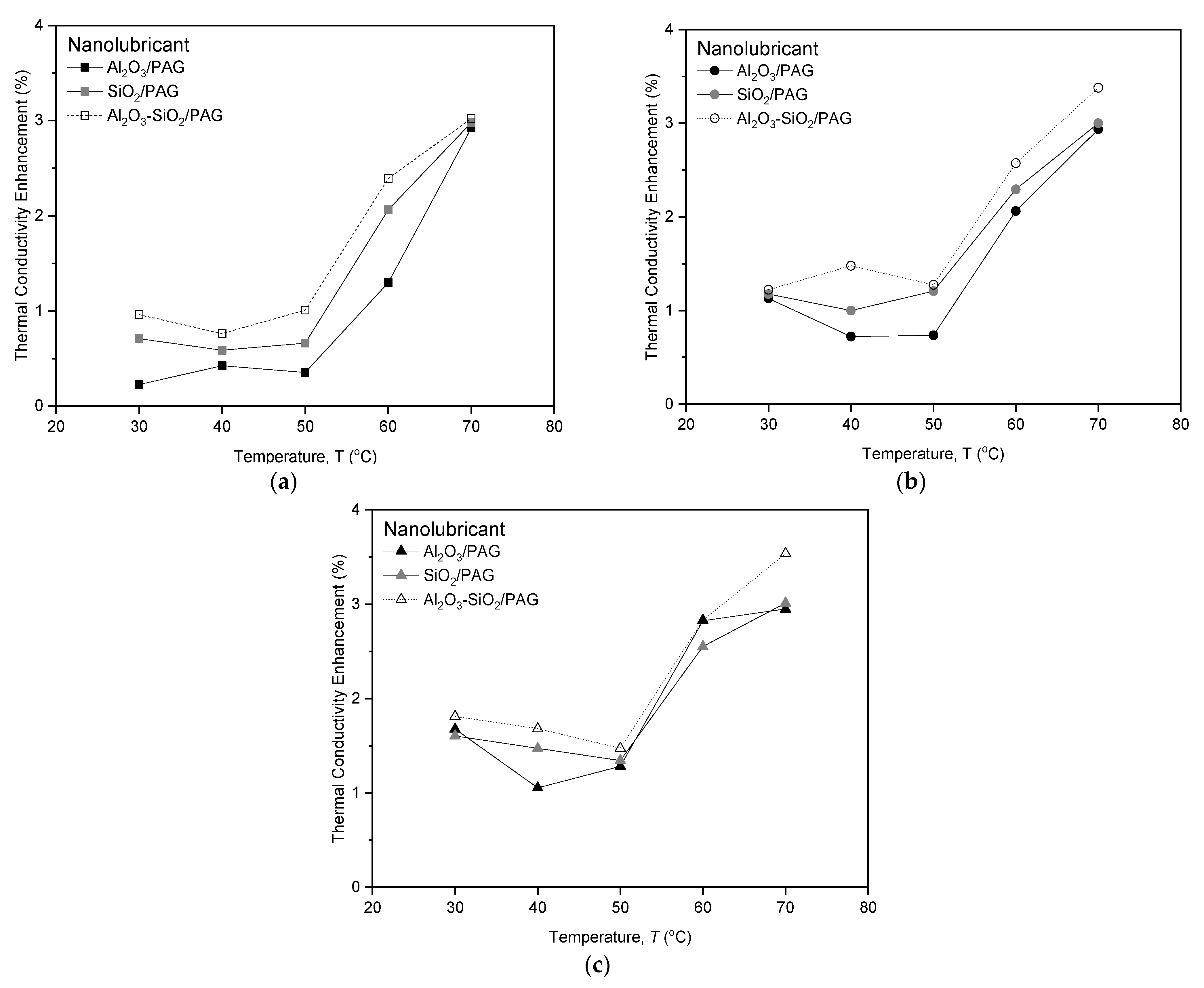
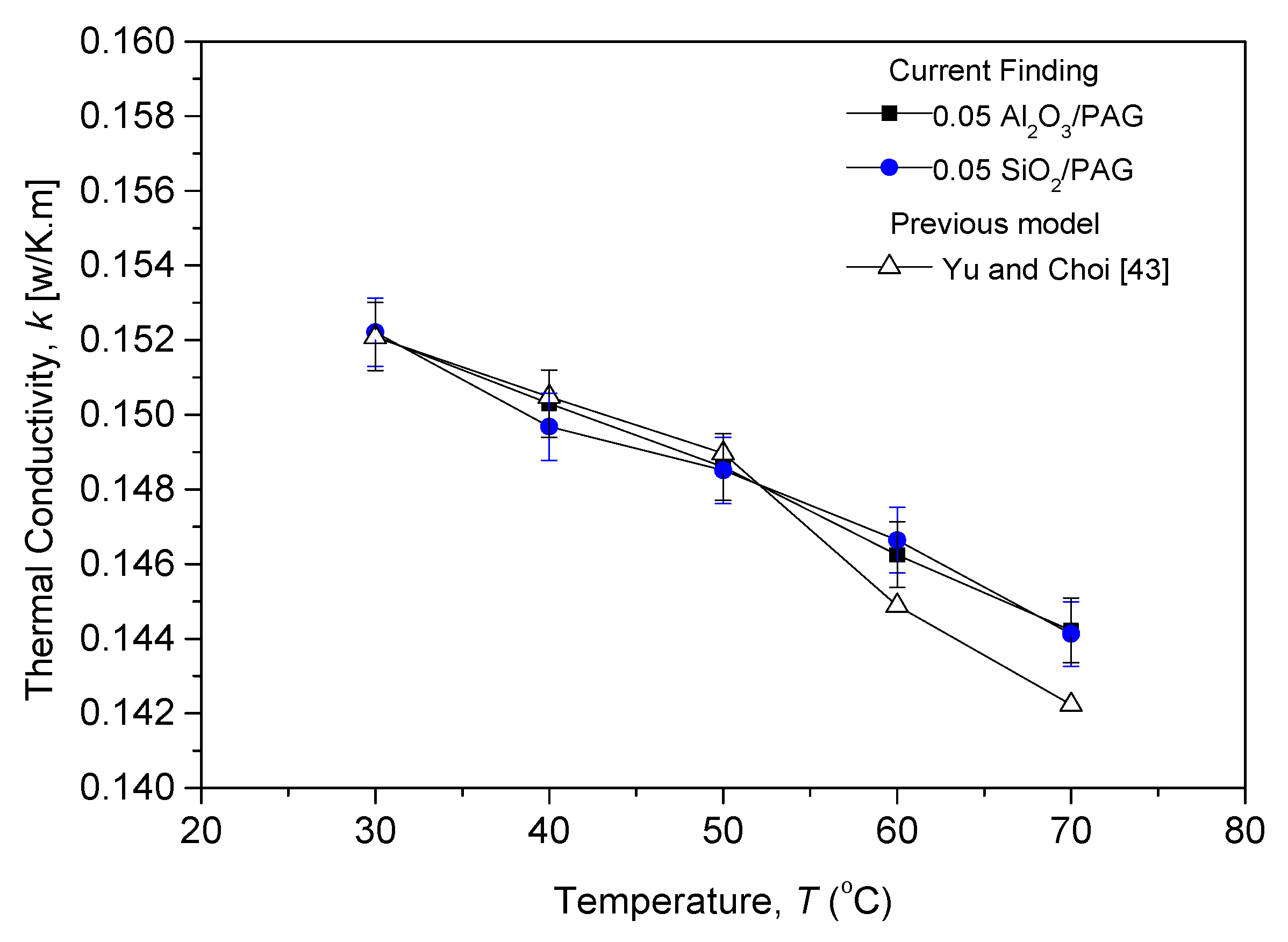
3.2.1. Thermal Conductivity Evaluation
3.2.2. Regression Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pabon, J.J.; Khosravi, A.; Belman-Flores, J.; Machado, L.; Revellin, R. Applications of refrigerant R1234yf in heating, air conditioning and refrigeration systems: A decade of researches. Int. J. Refrig. 2020, 118, 104–113. [Google Scholar] [CrossRef]
- Nair, V. HFO refrigerants: A review of present status and future prospects. Int. J. Refrig. 2021, 122, 156–170. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K.; Hishinuma, N. Alteration of Thermal Conductivity and Viscosity of Liquid by Dispersing Ultra-Fine Particles. Dispersion of Al2O3, SiO2 and TiO2 Ultra-Fine Particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab. (ANL): Argonne, IL, USA, 1995.
- Zawawi, N.N.M.; Azmi, W.H.; Sharif, M.Z.; Shaiful, A. Composite nanolubricants in automotive air conditioning system: An investigation on its performance. IOP Conf. Series Mater. Sci. Eng. 2019, 469, 012078. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C.; Beriache, M. Applications of nanorefrigerant and nanolubricants in refrigeration, air-conditioning and heat pump systems: A review. Int. Commun. Heat Mass Transf. 2015, 68, 91–97. [Google Scholar] [CrossRef]
- Booser, E.R. CRC Handbook of Lubrication and Tribology, Volume III: Monitoring, Materials, Synthetic Lubricants, and Applications; CRC Press: Boca Raton, FL, USA, 1993; Volume 3. [Google Scholar]
- Kawaguchi, Y.; Kaneko, M.; Takagi, M. The performance of end capped PAG as a refrigeration oil for HFC134a. In Proceedings of the International Compressor Engineering Conference, West Lafayette, IN, USA, 14–17 July 1998; Purdue University: West Lafayette, IN, USA, 2010; pp. 267–273. [Google Scholar]
- Denso-Europe-B.V. What You Need to Know about DENSO Compressor Oils! Available online: https://www.denso-technic.com/images/document/a-c-and-thermal/en/denso-what-you-need-to-know-about-denso-compressor-oil-nd-8-and-nd-12-part1-en.pdf (accessed on 31 January 2022).
- Sharif, M.; Azmi, W.; Redhwan, A.; Mamat, R. Investigation of thermal conductivity and viscosity of Al2O3/PAG nanolubricant for application in automotive air conditioning system. Int. J. Refrig. 2016, 70, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Sanukrishna, S.; Prakash, M.J. Experimental studies on thermal and rheological behaviour of TiO2-PAG nanolubricant for refrigeration system. Int. J. Refrig. 2018, 86, 356–372. [Google Scholar] [CrossRef]
- Sanukrishna, S.; Vishnu, S.; Prakash, M.J. Experimental investigation on thermal and rheological behaviour of PAG lubricant modified with SiO2 nanoparticles. J. Mol. Liq. 2018, 261, 411–422. [Google Scholar] [CrossRef]
- Makarova, V.V.; Gorbacheva, S.N.; Antonov, S.V.; Ilyin, S.O. On the Possibility of a Radical Increase in Thermal Conductivity by Dispersed Particles. Russ. J. Appl. Chem. 2020, 93, 1796–1814. [Google Scholar] [CrossRef]
- Zawawi, N.; Azmi, W.; Redhwan, A.; Sharif, M.; Samykano, M. Experimental investigation on thermo-physical properties of metal oxide composite nanolubricants. Int. J. Refrig. 2018, 89, 11–21. [Google Scholar] [CrossRef]
- Zawawi, N.; Azmi, W.; Redhwan, A.; Sharif, M.; Sharma, K. Thermo-physical properties of Al2O3-SiO2/PAG composite nanolubricant for refrigeration system. Int. J. Refrig. 2017, 80, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Geng, Y.; Al-Rashed, A.A.A.A.; Mahmoudi, B.; Alsagri, A.S.; Shahsavar, A.; Talebizadehsardari, P. Characterization of the nanoparticles, the stability analysis and the evaluation of a new hybrid nano-oil thermal conductivity. J. Therm. Anal. 2020, 139, 1553–1564. [Google Scholar] [CrossRef]
- Sharif, M.Z.; Azmi, W.H.; Redhwan, A.A.M.; Zawawi, N.N.M.; Mamat, R. Improvement of nanofluid stability using 4-step UV-vis spectral absorbency analysis. J. Mech. Eng. 2017, SI 4, 233–247. [Google Scholar]
- Azmi, W.; Sharma, K.; Mamat, R.; Najafi, G.; Mohamad, M. The enhancement of effective thermal conductivity and effective dynamic viscosity of nanofluids–A review. Renew. Sustain. Energy Rev. 2016, 53, 1046–1058. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Aluminum Oxide (13 nm) Produced by Sigma Aldrich. Available online: https://www.sigmaaldrich.com/MY/en/product/aldrich/718475 (accessed on 15 December 2022).
- Xu, J.; Bandyopadhyay, K.; Jung, D. Experimental investigation on the correlation between nano-fluid characteristics and thermal properties of Al2O3 nano-particles dispersed in ethylene glycol–water mixture. Int. J. Heat Mass Transf. 2016, 94, 262–268. [Google Scholar] [CrossRef]
- Singh, D.J.; Timofeeva, E.V.; Yu, W.; Routbort, J.L.; France, D.M.; Smith, D.Y.; Lopez-Cepero, J.M. An investigation of silicon carbide-water nanofluid for heat transfer applications. J. Appl. Phys. 2009, 105, 064306. [Google Scholar] [CrossRef]
- Li, X.; Cao, Z.; Zhang, Z.; Dang, H. Surface-modification in situ of nano-SiO2 and its structure and tribological properties. Appl. Surf. Sci. 2006, 252, 7856–7861. [Google Scholar] [CrossRef]
- Zawawi, N.; Azmi, W.; Ghazali, M. Tribological performance of Al2O3–SiO2/PAG composite nanolubricants for application in air-conditioning compressor. Wear 2022, 492–493, 204238. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Qingping, C.; Turkson, R.F.; Bicheng, C. Improving the tribological characteristics of piston ring assembly in automotive engines using Al2O3 and TiO2 nanomaterials as nano-lubricant additives. Tribol. Int. 2016, 103, 540–554. [Google Scholar] [CrossRef]
- Zawawi, N.N.M.; Azmi, W.H.; Ghazali, M.F.; Ramadhan, A.I. Performance optimization of automotive air-conditioning system operating with Al2O3-SiO2/PAG composite nanolubricants using Taguchi Method. Automot. Exp. 2022, 5, 121–136. [Google Scholar] [CrossRef]
- Zawawi, N.N.M.; Azmi, W.H.; Redhwan, A.A.M.; Sharif, M.Z. Thermo-physical properties of metal oxides composite nanolubricants. J. Mech. Eng. 2018, 5, 28–38. [Google Scholar]
- Beer, A. Bestimmung der absorption des rothen lichts in farbigen flussigkeiten. Ann. Physik 1852, 162, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Leonard, J.; Nettleship, I.; Lee, J.; Soong, Y.; Martello, D.; Chyu, M. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic nanosilica-stabilized graphite particles for improving thermal conductivity of paraffin wax-based phase-change materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Witharana, S.; Palabiyik, I.; Musina, Z.; Ding, Y. Stability of glycol nanofluids—The theory and experiment. Powder Technol. 2013, 239, 72–77. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef] [Green Version]
- Redhwan, A.A.M.; Azmi, W.H.; Sharif, M.Z.; Mamat, R.; Samykano, M.; Najafi, G. Performance improvement in mobile air conditioning system using Al2O3/PAG nanolubricant. J. Therm. Anal. Calorim. 2019, 135, 1299–1310. [Google Scholar] [CrossRef]
- Zawawi, N.N.M.; Azmi, W.H.; Sharif, M.Z.; Najafi, G. Experimental investigation on stability and thermo-physical properties of Al2O3–SiO2/PAG nanolubricants with different nanoparticle ratios. J. Therm. Anal. Calorim. 2019, 135, 1243–1255. [Google Scholar] [CrossRef]
- Powell, R.W.; Ho, C.Y.; Liley, P.E. Thermal Conductivity of Selected Materials; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1966; Volume 8.
- Naumann, R.J.; Lehoczky, S.L. Effect of variable thermal conductivity on isotherms in Bridgman growth. J. Cryst. Growth 1983, 61, 707–710. [Google Scholar] [CrossRef]
- Booser, E.R. Tribology Data Handbook: An Excellent Friction, Lubrication, and Wear Resource; CRC press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Sundar, L.S.; Farooky, H.; Sarada, S.N.; Singh, M. Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int. Commun. Heat Mass Transf. 2013, 41, 41–46. [Google Scholar] [CrossRef]
- Xing, M.; Yu, J.; Wang, R. Experimental study on the thermal conductivity enhancement of water based nanofluids using different types of carbon nanotubes. Int. J. Heat Mass Transf. 2015, 88, 609–616. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xu, J.; Du, K.; Zhang, X. Recent developments on viscosity and thermal conductivity of nanofluids. Powder Technol. 2017, 317, 348–369. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Maxwell Model. J. Nanoparticle Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Sharma, K.V.; Sarma, P.K.; Azmi, W.H.; Mamat, R.; Kadirgama, K. Correlations to predict friction and forced convection heat transfer coefficients of water based nanofluids for turbulent flow in a tube. Int. J. Microscale Nanoscale Therm. Fluid Transp. Phenom. 2012, 3, 283. [Google Scholar]


| Property | Unit | Al2O3 | SiO2 |
|---|---|---|---|
| Average particle diameter | nm | 13 | 30 |
| Density | kg·m−3 | 4000 | 2220 |
| Purity | % | 99.9 | 99.9 |
| Molecular mass | g(mol)−1 | 101.96 | 60.08 |
| Specific heat | J(kg·K)−1 | 773 | 745 |
| Thermal Conductivity | W(m·K)−1 | 36 | 1.4 |
| No. | Properties | Value |
|---|---|---|
| 1 | ISO viscosity grade | 46 |
| 2 | Kinematic viscosity at 40 °C | 39.3 cP |
| 3 | Kinematic viscosity at 100 °C | 8.45 cP |
| 4 | Viscosity index | 216.157 |
| 5 | Density at 40 °C | 1.021 g(cm)−3 |
| 6 | Density at 100 °C | 0.976 g(cm)−3 |
| 7 | Flash point | −36 °C |
| No. | Nanolubricants | Average Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 1 | Al2O3/PAG | 142 | 82.6 |
| 2 | SiO2/PAG | 152 | 80.6 |
| 3 | Al2O3-SiO2/PAG | 135 | 140.0 |
| Nanolubricants | A | B | Average Deviation | Standard Deviation |
|---|---|---|---|---|
| Al2O3 | 28.80 | 0.015 | 0.61 | 0.38 |
| SiO2 | 29.26 | 0.012 | 0.64 | 0.38 |
| Al2O3-SiO2/PAG | 32.90 | 0.017 | 0.66 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, M.Z.; Azmi, W.H.; Ghazali, M.F.; Zawawi, N.N.M.; Hendrawati, T.Y. Stability and Thermal Conductivity of Mono and Hybrid Nanoparticles Dispersion in Double-End Capped PAG Lubricant. Lubricants 2023, 11, 1. https://doi.org/10.3390/lubricants11010001
Sharif MZ, Azmi WH, Ghazali MF, Zawawi NNM, Hendrawati TY. Stability and Thermal Conductivity of Mono and Hybrid Nanoparticles Dispersion in Double-End Capped PAG Lubricant. Lubricants. 2023; 11(1):1. https://doi.org/10.3390/lubricants11010001
Chicago/Turabian StyleSharif, Mohd Zaki, Wan Hamzah Azmi, Mohd Fairusham Ghazali, Nurul Nadia Mohd Zawawi, and Tri Yuni Hendrawati. 2023. "Stability and Thermal Conductivity of Mono and Hybrid Nanoparticles Dispersion in Double-End Capped PAG Lubricant" Lubricants 11, no. 1: 1. https://doi.org/10.3390/lubricants11010001
APA StyleSharif, M. Z., Azmi, W. H., Ghazali, M. F., Zawawi, N. N. M., & Hendrawati, T. Y. (2023). Stability and Thermal Conductivity of Mono and Hybrid Nanoparticles Dispersion in Double-End Capped PAG Lubricant. Lubricants, 11(1), 1. https://doi.org/10.3390/lubricants11010001





