3.1. Regulation Mechanism of Trivalent Cations with respect to the Friction Coefficient of a PVPA Superlubricity System
Our previous study showed that the surface of PVPA-modified Ti6Al4V can maintain stable superlubricity properties in monovalent salt solutions [
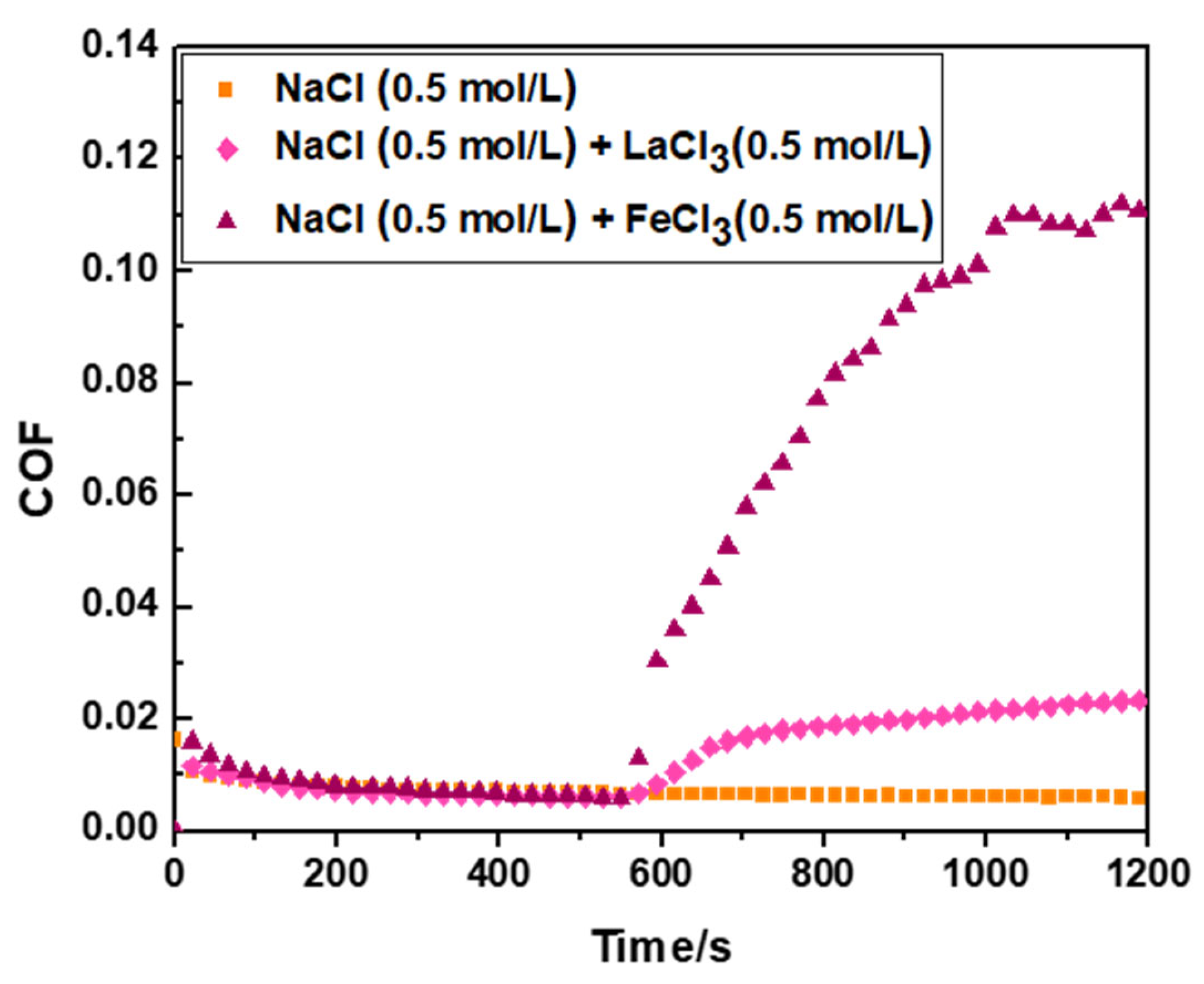
22]. In this study, 0.5 mol/L NaCl solution (6 mL) was used as the base lubricant to obtain a stable, ultra-low-friction coefficient (0.006–0.007), as shown by the square curve in
Figure 1. Following stable operation of the PVPA superlubricity system for 500–600 s, 0.5 mol/L LaCl
3 solution (2 mL) was added to the base lubricant. The friction coefficient of the system suddenly increased when LaCl
3 solution was added, as shown by the diamond curve in
Figure 1. The sudden increase in the degree of the friction coefficient was 285.24
62%. When FeCl
3 solution (2 mL) with a concentration of 0.5 mol/L was added into the basic lubricant, the friction coefficient of the PVPA superlubricity system also increased suddenly, with a degree of 1825.86
70%, as shown by the triangle curve in
Figure 1.
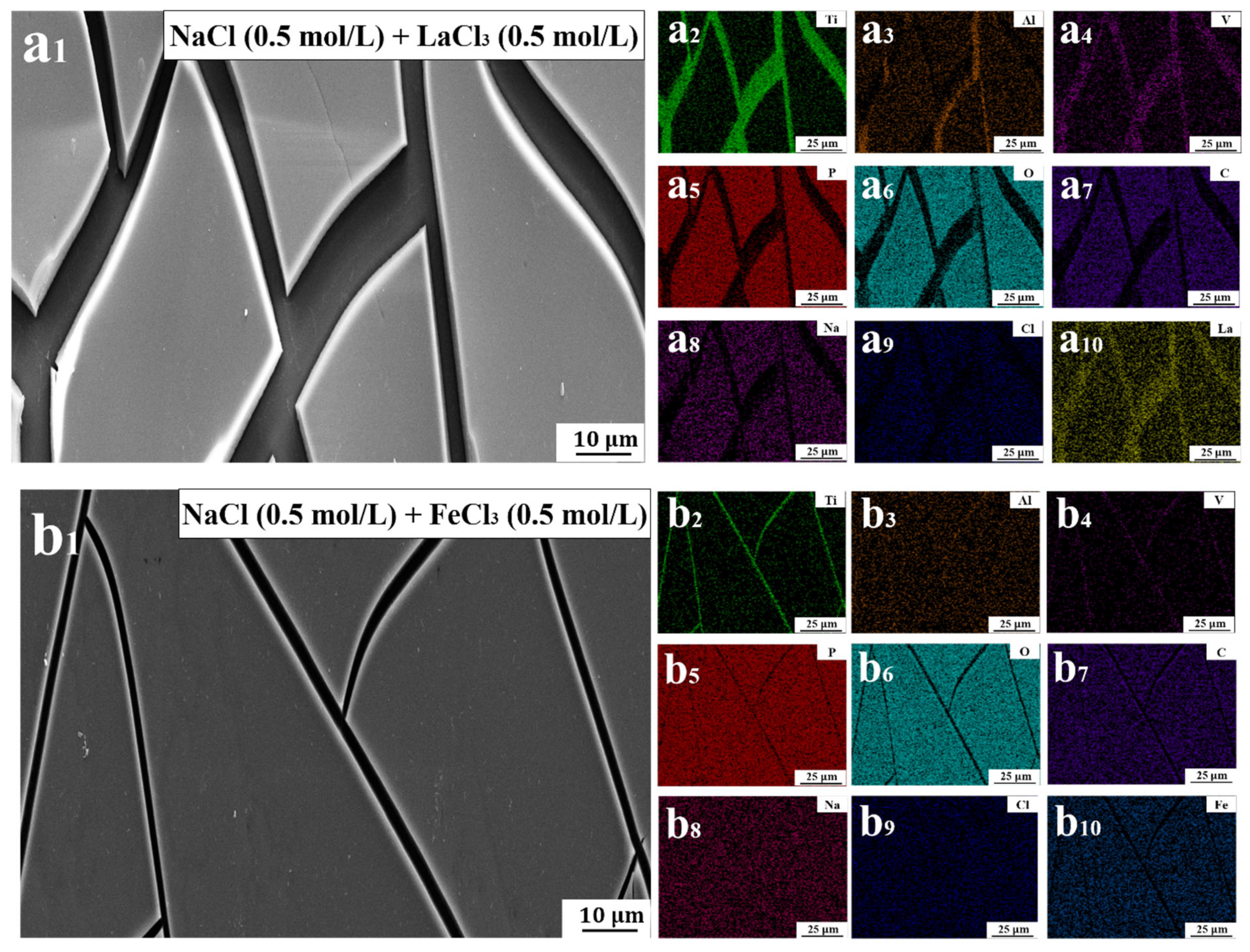
Based on the volume ratio of trivalent salt solution and basic lubricant in the addition process, a mixture solution of basic lubricant and trivalent salt solution was prepared in a ratio of 3:1 (basic lubricant: trivalent salt solution). SEM and EDS were used to detect the morphology and element distribution of PVPA-modified surfaces after soaking in NaCl/LaCl3 and NaCl/FeCl3 mixed solutions, respectively, to establish the relationship between the change in surface structure and element distribution and the change in friction coefficient.
The morphologies of PVPA-modified surfaces after soaking in NaCl/LaCl
3 and NaCl/FeCl
3 mixed solutions are shown in
Figure 2(a
1) and (b
1), respectively. PVPA coatings soaked in the two mixed solutions both exhibited cracking phenomena, with sharp edges appearing on the coated surface. Such sharp edges aggravate the wear of PTFE [
23], as shown in
Figure S1 in the Supplementary Materials Section S1. This could be the main reason for the sudden increase in friction coefficient. The distributions of elements on the PVPA-modified surfaces after soaking in NaCl/LaCl
3 and NaCl/FeCl
3 mixed solutions are shown in
Figure 2(a
2–a
10) and (b
2–b
10), respectively. The atomic percentages of elements on the two sample surfaces are shown in
Table 1.
The atomic percentages of Ti, Al and V on the PVPA-modified surface after soaking in NaCl/LaCl
3 mixed solution were significantly higher than those after soaking in an NaCl/FeCl
3 mixed solution. The degree of damage of trivalent lanthanum ions on the PVPA coating was greater than that of trivalent iron ions on the PVPA coating, as also proven by the difference in P element on the surface of the two samples. Compared to the PVPA coating surface, the trivalent lanthanum ion tends to adsorb on the exposed Ti6Al4V surface after the coating cracks, as shown in
Figure 2(a
10). However, the trivalent iron tends to adsorb on PVPA surface, as shown in
Figure 2(b
10).
The presence of salt ions in solutions may be the main reason for the adsorption difference. Sung et al. reported that after LaCl
3 was dissolved in neutral water (pH 5.7–7), the trivalent lanthanum ion mainly existed in the form of La
3+. However, when FeCl
3 was dissolved in neutral water, the trivalent iron mainly existed in the form of
, and the pH value of the water reduced as a result of the subsequent reaction [
24]:
The pH values of deionized water, basic lubricant (0.5 mol/L NaCl solution) and mixed solutions were measured. NaCl solution and trivalent salt solution were mixed in a volume ratio of 3:1 (NaCl solution: trivalent salt solution). The results are presented in
Table 2.
As shown by detection results in
Table 2, compared to deionized water, the pH value of NaCl solution does not change. After mixing the NaCl solution and the LaCl
3 solution, the pH value of the mixed solution did not obviously change. When he NaCl solution was mixed with FeCl
3 solution, the pH value of the solution decreased to 2.2. We speculate that the change shown in Equation (2) took place in the NaCl/FeCl
3 mixed solution, and the value of
n is generally identified as 3 [
24].
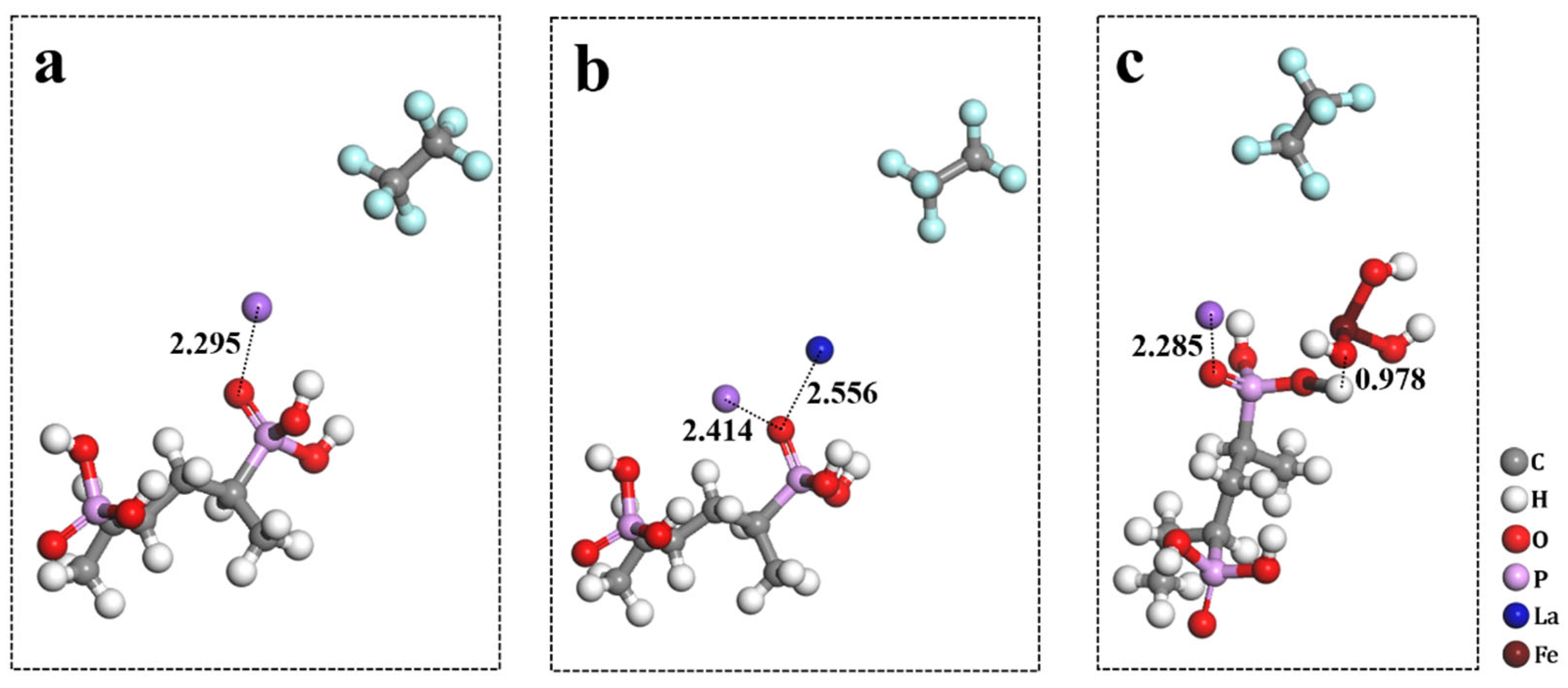
Molecular dynamics simulation was used to study the interface change of PVPA induced by La
3+ and Fe(OH)
3. The effect of interface change on the sudden change in friction coefficient was analyzed. The interaction energy between PVPA chains and ions can effectively reflect the change in the PVPA interface. When studying the role of introduced cations, the role of anions can be ignored. To more clearly reflect the interactions between the introduced cations and PVPA chains, as well as those between Na
+ and the PVPA chains before and after the introduction, Cl
− and water molecules were removed from the new system, as shown in
Figure 3. Notably, the removal behavior did not affect the specific value of the interaction energy.
The minimum distances of La
3+ and Fe(OH)
3 from the PVPA molecular chain were 2.556 Å and 0.978 Å, respectively, as shown in
Figure 3b,c. The interaction energies of La
3+ and Fe(OH)
3 with the PVPA molecular chain were −30.89 Kcal/mol and −155.3 Kcal/mol, respectively, as shown in
Table 3. The calculation method is described in the
Supplementary Materials Section S2. Fe(OH)
3 exhibited a greater degree of adsorption on the PVPA surface than La
3+. It is believed that La
3+ binds to the oxygen atoms in the phosphate radical group on the PVPA molecular chain through electrostatic interaction. Furthermore, oxygen atoms in Fe(OH)
3 can form hydrogen bonds with the hydrogen atoms in the phosphate radical groups on the PVPA molecular chain. Bonding to PVPA chains by hydrogen bonding is stronger than that of by electrostatic effect. Both La
3+ and Fe(OH)
3 can bind to one to three phosphate groups, although with differences in binding methods. These phosphate groups may come from the same PVPA molecular chain or from adjacent PVPA molecular chains. Such a bridging effect can directly damage the stability of the PVPA coating and even cause cracking of the coating, as shown in
Figure 2(a
1,b
1). This may be the main reason for the increase in friction coefficient.
The friction coefficients of the PVPA superlubricity system both increased suddenly due to the bridging effect of La3+ and Fe(OH)3 with the PVPA molecular chain, but the degree of the sudden increase in friction coefficient varied. We speculate that the degree of sudden increase in friction coefficient is closely related to the degree of hydration of introduced ions. Highly hydrated ions adsorbed on the surface can contribute a certain degree of lubrication and reduce the degree of sudden increase in friction coefficient.
In this study, the degree of hydration of introduced ions in the PVPA superlubricity system are reflected by the interaction energy between the introduced ion and surrounding water molecules. The greater the interaction energy between the introduced ion and surrounding water molecules, the stronger the degree of attraction of the introduced ion to surrounding water molecules and the higher the degree of hydration of the introduced ion. The interaction energies of La
3+ and Fe(OH)
3 with surrounding water molecules are −61.44 Kcal/mol and −24.78 kcal/mol, respectively, as shown in
Table 3. Compared with Fe(OH)
3, La
3+ adsorbed on the surface has a stronger attraction to surrounding water molecules. In other words, La
3+ adsorbed on the surface can result in an improved lubrication effect. In addition, EDS results showed that part of the La
3+ can adsorb on the Ti6Al4V surface exposed after PVPA coating cracking. These La
3+ ions can play the role of avoiding direct contact between the two friction pairs to a certain extent after attracting water molecules. Although the degree of damage of La
3+ to the PVPA coating is greater than that of Fe(OH)
3, combination of the stronger attraction of La
3+ to surrounding water molecules and the stable adsorption of La
3+ on exposed the Ti6AlV surface lead to a sudden increase in the degree of friction coefficient induced by La
3+ less than that of Fe(OH)
3.
In our previous study [
16], we found that the introduction of new monovalent cations during the friction process disturbed the state of the monovalent cations in the base lubricant to a certain extent and that this disturbance could affect the change in the friction coefficient. In this study, the introduction of La
3+ and Fe(OH)
3 also disturbed the state of Na
+ to a certain extent. In the original system, the minimum distance of Na
+ to the PVPA molecular chain was 2.295 Å, as shown in
Figure 3a. With the introduction of La
3+ and Fe(OH)
3, the minimum distance of Na
+ to the PVPA chain changed to 2.414 Å and 2.285 Å, respectively, as shown in
Figure 3b,c. This change was influenced by both the adsorption degree of Na
+ on PVPA and the repulsion of Na
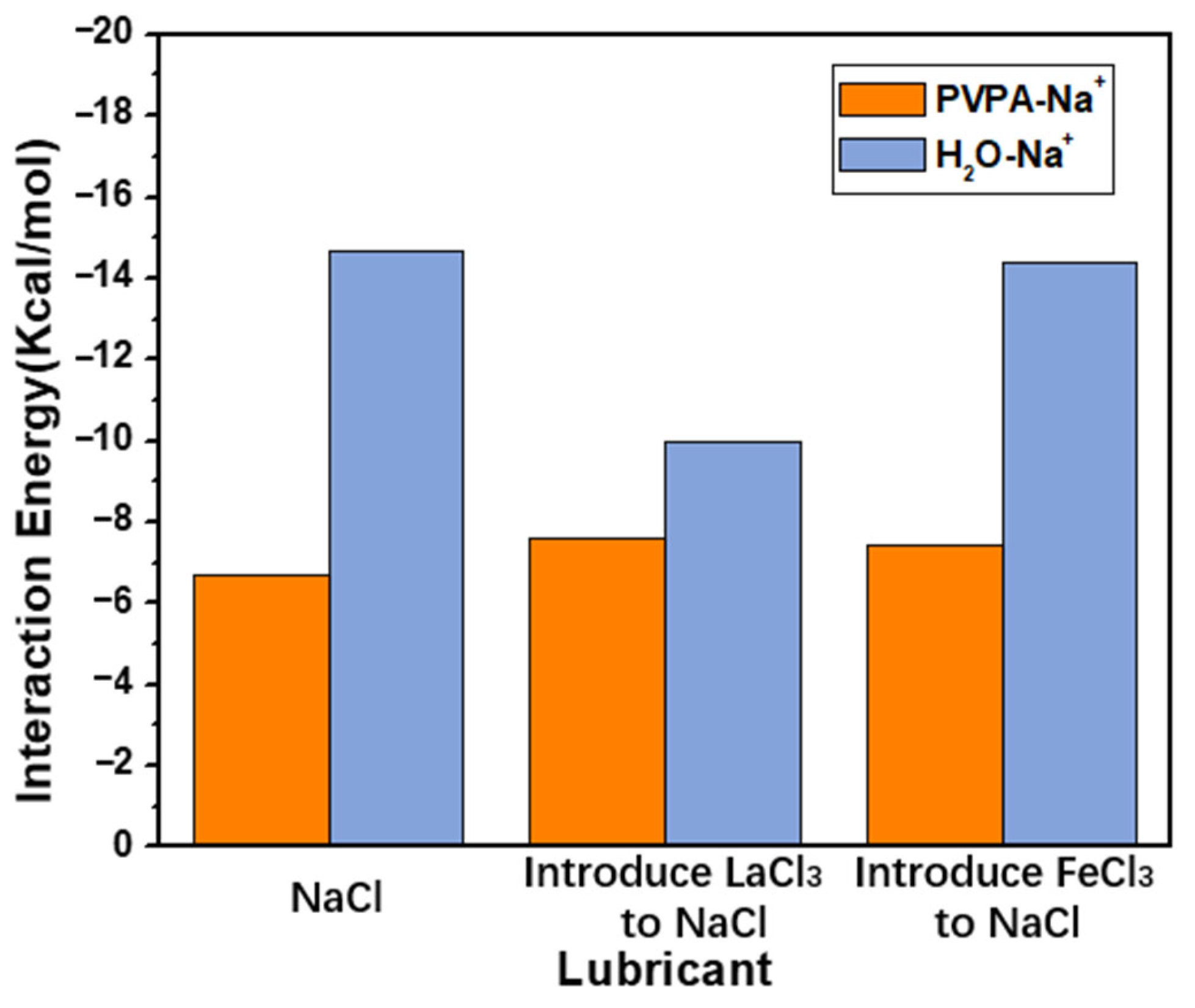
+ by introduced ions. Before the introduction of new ions, the interaction energy between the PVPA molecular chain and Na
+ was −6.68 Kcal/mol. After the introduction of La
3+ and Fe(OH)
3, the interaction energies between the PVPA molecular chain and Na
+ were −7.59 Kcal/mol and −7.45 Kcal/mol, respectively, as shown in
Figure 4. Both La
3+ and Fe(OH)
3 can enhance the adsorption degree of Na
+ on the PVPA molecular chain. The interaction energies of Na
+ with La
3+ and Fe(OH)
3 were 26.38 Kcal/mol and 2.37 Kcal/mol, respectively. Compared with La
3+, the repulsion effect of Fe(OH)
3 on Na
+ can be ignored. Therefore, after the introduction of Fe(OH)
3, the adsorption degree of Na
+ on the PVPA molecular chain was enhanced, resulting in a decrease in the minimum distance between Na
+ and the PVPA molecular chain. The introduced La
3+ had a significant repulsion effect on Na
+. The minimum distance from Na
+ to the PVPA increased under the combined effect of the attraction effect of PVPA on Na
+ and Na
+ repulsion by La
3+.
The introduction of new ions also interfered with the attraction of Na
+ to surrounding water molecules. Before the introduction of new ions, the interaction energy between Na
+ and surrounding water molecules was −14.67 Kcal/mol. After the introduction of La
3+ and Fe(OH)
3, the interaction energies of Na
+ and surrounding water molecules were −10 Kcal/mol and −14.37 Kcal/mol, respectively, as shown in
Figure 4. Compared to Fe(OH)
3, the introduction of La
3+ led to a greater decrease in the attraction of Na
+ to surrounding water molecules. The state change of Na
+ can be ignored compared with the change in the interface state caused by the introduction of ions. In other words, the perturbation of the cations in the base lubricant resulting from the introduction of multivalent cations had a negligible influence on the change in friction coefficient.
The above study shows that the friction coefficient of a PVPA superlubricity system can increase suddenly when adding LaCl3 or FeCl3 solution into the basic lubricant during the friction process. The increase in friction coefficient could be caused by the tearing off of microbands of PTFE from the ball surface, as a sharpened profile of the PVPA coating was generated by the bridge effect between introduced ions and the PVPA molecular chains. Trivalent lanthanum ions in the solution mainly exist in the form of La3+, which is adsorbed on the surface through an electrostatic effect. These trivalent cations can enhance the hydration degree of the surface by attracting surrounding water molecules, playing a lubrication role. However, trivalent iron ions mainly exist in the form of Fe(OH)3, which can be connected to PVPA surface by hydrogen bonds. However, their attraction to surrounding water molecules is less than that of La3+, resulting in a sudden increase in friction coefficient greater than that of La3+.
3.2. Regulation of Ion Concentration with respect to the Friction Coefficient of PVPA Superlubricity Systems
A related study showed that the bridging effect between salt ions and polymers is concentration-dependent [
25], which may lead to changes in friction results. Concentrations of 0.01 mol/L, 0.1 mol/L and 1 mol/L were selected for LaCl
3 and FeCl
3 to add to basic lubricant during the friction process in this study. Combined with the result obtained with a concentration of 0.5 mol/L reported in
Section 3.1, the influence of ion concentration on the sudden change in friction coefficient was investigated.
The pH values 0.5 mol/L NaCl solution mixed with different concentrations of trivalent salt solution were detected. The volume ratio of NaCl solution and trivalent salt solution in the mixed solution was 3:1. The results are presented in
Table 4. The concentration of LaCl
3 in the mixed solution had no obvious effect on the pH value of the mixed solution. Trivalent lanthanum ions in the mixed solution are always presented as La
3+. According to Equation (2), the more Fe
3+ reacts with water molecules, the lower the pH value of the solution. With increased FeCl
3 concentration, the pH value of the NaCl/FeCl
3 mixed solution decreased continuously. Almost all the trivalent iron ions in mixed solutions with varying FeCl
3 concentrations appear in the form of Fe(OH)
3 [
24].
The introduction of LaCl
3 solutions with different concentrations in the friction process can cause the friction coefficient to suddenly increase, as shown in
Figure 5. For concentrations of 0.01 mol/L, 0.1 mol/L and 1 mol/L, the friction coefficient of the PVPA superlubricity system increased by 518.46 ± 92%, 574.24 ± 88% and 360.90 ± 65%, respectively. Combined with the increase in the friction coefficient at a concentration of 0.5 mol/L in reported
Section 3.1 (285.24 ± 62%), it can be concluded that a higher concentration of LaCl
3 does not result in a greater degree of sudden increase in friction coefficient. Instead, there is an extreme value of concentration at which the maximum of the sudden increase degree of friction coefficient can be obtained.
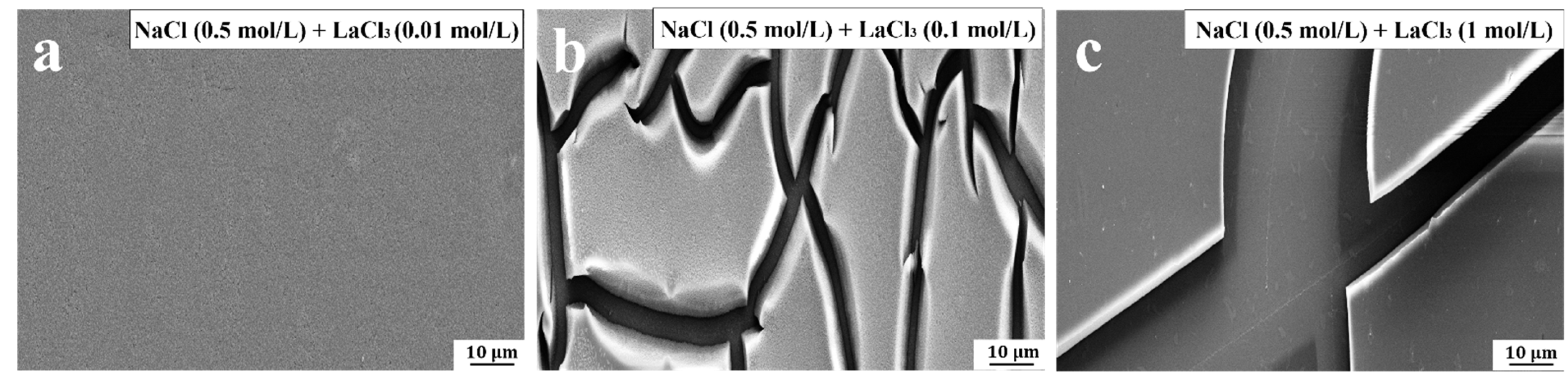
SEM was used to observe the surface microstructures of PVPA-modified surfaces soaked in NaCl/LaCl
3 mixed solutions with varying concentrations of LaCl
3. The influence of the concentration of La
3+ on the PVPA surface morphology was investigated. After soaking the PVPA coating in a mixed solution containing 0.01 mol/L LaCl
3, no cracks appeared on the coating surface, as shown in
Figure 6a. When the concentration of LaCl
3 was increased to 0.1 mol/L, obvious cracks appeared on the coating surface, as shown in
Figure 6b. When the concentration of LaCl
3 was 0.5 mol/L and 1 mol/L, obvious exposed Ti6Al4V appeared on the surface, as shown in
Figure 2(a1) and
Figure 6c, respectively.
The bridge effect between La3+ and the PVPA molecular chain is the main reason for the sudden increase in the friction coefficient. The degree of bridging effect can be reflected by the degree of PVPA cracking, which is related to the concentration of LaCl3 in the mixed solution. The higher the concentration of LaCl3 in the mixed solution, the more obvious the cracks on the PVPA coating. However, the coating did not peel off completely. We speculate that the main role of La3+ is to bridge the PVPA molecular chain and destroy the coating structure before the appearance of surface exposure of Ti6Al4V due to coating cracks. In this process, the higher the concentration of LaCl3, the more La3+ is involved in the bridging effect and the greater the degree of sudden increase in friction coefficient. La3+ tends to adsorb on exposed Ti6Al4V surfaces when the surface exposure of Ti6Al4V occurs due to coating cracks. At this point, the main role of La3+ is to play a lubrication role by attracting surrounding water molecules, which can inhibit the sudden increase in friction coefficient to a certain extent. Therefore, the sudden increase in friction coefficient at concentrations of 0.5 mol/L and 1 mol/L is less than that at 0.01 mol/L and 0.1 mol/L, respectively. When surface exposure of Ti6Al4V occurs due to coating cracks, as the concentration of LaCl3 continues to increase, it can continue to damage the coating structure. Therefore, the sudden increase in friction coefficient caused by the introduction of LaCl3 solution with a concentration of 1 mol/L is greater than that of 0.5 mol/L.
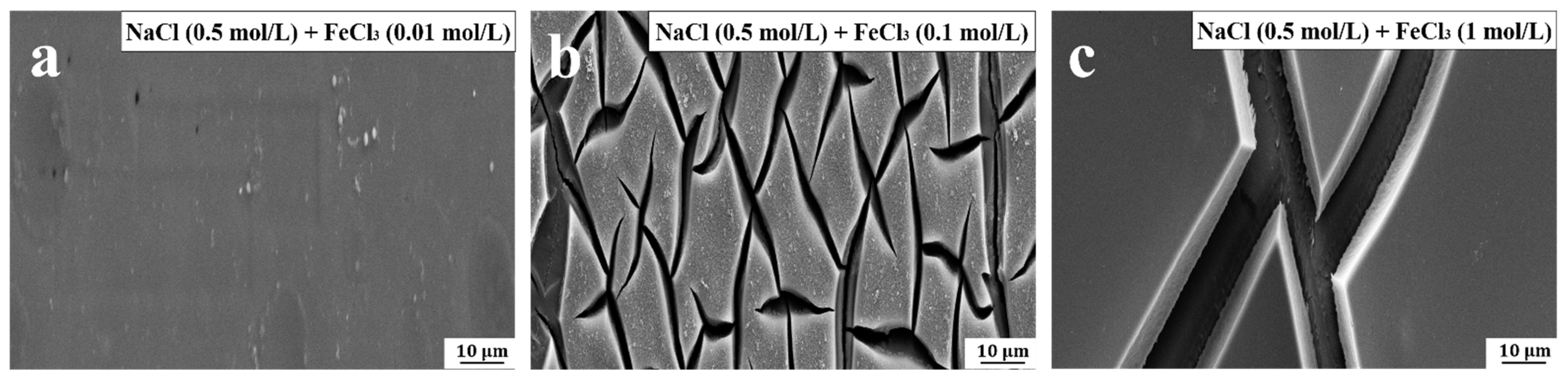
The introduction of FeCl
3 solution with varying concentrations in the friction process can cause the friction coefficient of the PVPA superlubricity system increase suddenly, as shown in
Figure 7. When the concentrations of FeCl
3 are 0.01 mol/L, 0.1 mol/L and 1 mol/L, the degree of sudden increase in the friction coefficient are 825.37 ± 95%, 2648.14 ± 102% and 2000 ± 77%, respectively. Compared to trivalent lanthanum ions, trivalent iron ions almost always exist in the form of Fe(OH)
3 in mixed solutions with varying concentrations of FeCl
3. Combined with the degree of sudden increase in the friction coefficient (1825.86 ± 70%) with a concentration of 0.5 mol/L reported in
Section 3.1, it can be concluded that the dependence of Fe(OH)
3 on the degree of induction of the sudden increase in friction coefficient with respect to concentration is similar to that of La
3+. There is an extreme concentration value at which the maximum degree of sudden increase in friction coefficient can be obtained.
Figure 8 shows the microstructures of PVPA-modified surfaces soaked in NaCl/FeCl
3 mixed solutions with varying concentrations of FeCl
3. Fe(OH)
3 binds to more than one hydroxyl group from the same or an adjacent molecular chain by hydrogen bonding, and the degree of this special bridging effect is also dependent on the concentration of FeCl
3. When the concentration of FeCl
3 in the mixed solution increases from 0.01 M to 0.1 M, cracks begin to appear on the surface of the PVPA coating, as shown in
Figure 8a,b. When the concentration of FeCl
3 is 1M, an obvious Ti6Al4V bare part appears on the surface, as shown in
Figure 8c. We speculate that when the concentration of FeCl
3 is low, one Fe(OH)
3 molecule will be hydrogen-bonded to three hydroxyl groups. When the concentration increases, some Fe(OH)
3 molecules can only bind to one hydroxyl group, so the Fe(OH)
3 will not cause further damage to the coating and can attract the surrounding water molecules for lubrication. This may be an important reason for the existence of extreme degree of the sudden increase in friction coefficient.
The above analysis indicates that a PVPA coating can respond to the presence of trivalent ions, even at low concentrations. In addition, the degree of the sudden increase in friction coefficient caused by the introduction of trivalent ions in the friction process is concentration-dependent. There is an extreme concentration at which the maximum degree of increase in the friction coefficient can be obtained.