Abstract
The lubrication properties of nanoparticles are of great interest to the manufacturing industry and led to the development of the nano-minimum quantity lubrication (NMQL) cooling strategy. To evaluate the sustainability characteristics of nano-minimum quantity lubrication, apart from analyzing the benefits of increasing machining efficiency, it is also essential to evaluate the potential detrimental effects of nanoparticles on human health and the environment. Existing literature provides substantial data on the benefits of nano-minimum quantity lubrication machining. However, the current literature does not provide researchers in the machining sector a comprehensive analysis of the toxicity of the nanoparticles used in nano-minimum quantity lubrication. This study aims to provide a comprehensive review that addresses the toxicity levels of the most frequently used nanoparticles in NMQL machining. To understand the impacts of nanoparticles on the human body and the environment, in vitro studies that evaluate the nanoparticles’ toxicity on human cells and in vitro/in vivo studies on other living organisms are considered. The results from toxicity studies on each of the chosen nanoparticles are summarized and presented in chronological order. The reviewed studies indicate transition metal dichalcogenides (MoS2 and WS2) exhibit very low toxicity when compared to other nanoparticles. The toxicity of hBN and AL2O3 nanoparticles varies depending on their lengths and crystalline structures, respectively. In conclusion, a chart that maps the toxicity levels of nanoparticles on seven different human cell lines (human lung epithelial cells (A549), human bronchial epithelial cells (Nl-20), AGS human gastric cells, human epidermal cells (HEK), human liver-derived cells (HepG2), human endothelial cells and human peripheral cells), representing exposures by inhalation, ingestion and dermal contact, was developed for easy and quick insights. This is the first attempt in open literature to combine the results of the experimental investigations of nano-minimum quantity lubrication cooling and the toxicity studies of nanoparticles, allowing researchers to make informed decisions in the selection of the most sustainable nanoparticles in the nano-minimum quantity lubrication machining process.
1. Introduction
The machining and manufacturing sectors are among the most influential markets today with over 100 billion dollars in annual expenditures in the United States alone [1]. The importance of sustainability in machining processes is shown by the sheer volume of production involved and the corresponding potential environmental hazards. The dry machining of metals involves a very high generation of heat in the cutting zone and high cutting forces, which leads to tool wear, workpiece hardening and an increased surface roughness. To minimize these effects, lubrication and cooling via flood cooling techniques are generally employed in machining processes; however, the storage, use and disposal of these conventional lubricants have resulted in an increased environmental impact. Furthermore, due to their inherent toxicity, some lubricants are harmful to machine operators. To avoid the excessive use of cutting fluids, and thereby reduce the potential environmental hazards, many researchers have studied minimum quantity lubrication (MQL) as a viable alternative [2], and literature provides positive results for MQL [3,4,5]. The MQL technique works with a flow rate of about 50 m/h to 2000 mL/h, while the conventional flood cooling flow rate is approximately 1200 × 103 mL/h [6,7]. However, the benefits of the MQL technique are limited due to the low cooling capacities of base fluids and the clogging of debris at the cutting zone when MQL is applied [8,9]. Nano-minimum quantity lubrication (NMQL) was developed to overcome the shortcomings of regular MQL.
Nanoparticles added to a base oil enhance the thermal conductivity of the resultant nanofluid. Therefore, machining with the NMQL technique reduces the cutting tool temperature due to an increased heat transfer coefficient. Further, a nanoparticle rolling effect at the cutting edge reduces contact friction and results in reduced contact forces [10,11]. Zhang et al. conducted studies to investigate the effectiveness of the NMQL grinding of grade 45 steel with MoS2 nanoparticles immersed in different vegetable oils [12]. The authors employed a 2% and a 5% MoS2 mass fraction and used four different base oils: liquid paraffin, palm oil, rapeseed oil and a combination of palm oil and soybean oil. Palm oil with MoS2 nanoparticles was observed to provide the best results. Increasing the mass fraction up to 6% produced an improved lubricating performance, while at a mass fraction exceeding 6%, a deteriorating lubrication effect was the result. Kumar et al. compared the effectiveness of different mono and hybrid nanofluids in the grinding process of silicon nitride [13] and reported positive results with NMQL. A cutting fluid with mono nanoparticles (NPs) performed subpar compared to a fluid with hybrid NPs. Among the mono NPs, MoS2 resulted in a lower grinding force, surface finish and specific grinding energy while a MoS2-WS2 hybrid performed the best overall. The authors further stated that with dry and flood cooling, the grinding forces started to increase over time; however, when a nanoparticle jet MQL (NJMQL) was employed, the grinding forces were constant. The lubrication capability was also increased with NPs, and this was supported by the formation of small, segmented chips when an NJMQL was employed. Similarly, Roshan et al. reported a superior performance with AL2O3 nanoparticles mixed with palm oil in a NJMQL setup for grinding Inconel 718 [14]. The authors reported the lowest specific grinding energy for a 0.5% wt. of NPs and a lower surface roughness for a 1% wt. of NPs. The increase in the surface quality with an increase in the NPs was attributed to better lubrication. This was facilitated by a rolling effect of the NPs at the cutting interface and a superior cooling ability of the NPs due to their high thermal conductivity.
Eltaggaz et al. investigated the influence of NMQL in the turning of austempered ductile iron (ADI) [15]. An oxide of aluminum with an α nanocrystalline structure was dispersed in the air–oil mixture. NMQL performed better than the pure MQL and reduced the flank wear. A comparative study conducted by the authors to evaluate the advantages of various cooling strategies (dry, flood, MQL and NMQL) found that NMQL machining performed better than the base MQL and was comparable to flood cooling [16]. The authors conducted further studies in the machining of titanium with AL2O3 nanoparticles and found that the seizure zone was reduced by using NMQL when compared to the base MQL cooling [17]. Further, the authors concluded that nanoparticle concentration positively impacted the tool life and quality of the surface finish. Further improvements in milling performance were reported in studies on ferritic stainless steel [18] and TiAIN-coated carbide surfaces [19] using graphene and hBN nanoparticles. Table 1 provides a summary of the studies on nano-MQL cooling and highlights the important conclusions drawn by the authors.

Table 1.
Machining processes and materials studied in the literature on NMQL cooling strategy.
The increased performance of the NMQL cooling strategy can be attributed to two factors: an increased thermal conductivity of the base oil due to the addition of highly conductive nanoparticles, which resulted in an increased heat removal rate and reduced friction induced by the rolling effect of nanoparticles at the tool–chip interface. The increased nanoparticle concentration developed a thick protective layer on the tool and work surface [16]. The reduction in friction was achieved by reducing the contact between the tool and the workpiece. An exaggerated view of the tool–chip contact interface in an NMQL cooling process is shown in Figure 1. The nanoparticles in the base oil produce a rolling effect and thereby increase the sliding action of the tool, therefore reducing the friction. The highlighted studies clearly indicate a positive effect of using the NMQL cooling strategy. From Table 1 and an existing review paper [24] on NMQL machining, the most frequently used nanoparticles are tungsten disulfide (WS2), molybdenum disulfide (MoS2), boron nitride (BNNT and hBN), carbon nanotubes (CNT, SWCNT and MWCNT) and oxides of zinc (ZnO) and aluminum (AL2O3).
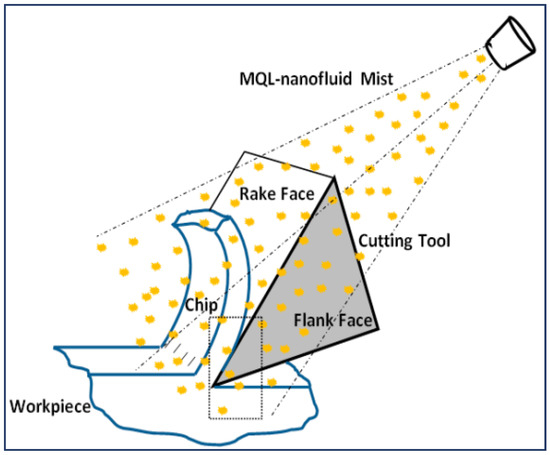
Figure 1.
NMQL cooling mechanism [19].
The science of sustainability addresses all the different aspects involved in the development and the use of any technology. To evaluate the sustainability characteristics of nano-minimum quantity lubrication, apart from analyzing the benefits of increasing machining efficiency, it is also essential to evaluate the potential detrimental effects of nanoparticles on human health and the environment. As was highlighted, the existing literature provides substantial data on the benefits of the NMQL machining process when compared to pure MQL and other conventional cooling strategies [3,25,26]. However, the current literature does not provide researchers in the machining sector a comprehensive analysis of the toxicity of the nanoparticles used in nano-minimum quantity lubrication. Pereira et al. conducted a lifecycle assessment to study the environmental effects of the biodegradable oils used in minimum quantity lubrication [27], but similar studies are lacking in the field of nano-MQL lubrication. The current study aims to provide a comprehensive review that addresses the toxicity levels of the most frequently used nanoparticles in NMQL machining. To understand the impacts of nanoparticles on the human body and the environment, in vitro studies that evaluate the nanoparticle toxicity on human cells and in vitro/in vivo studies on other living organisms are considered. Further, the machining efficiency of the chosen nanoparticles in the machining industry is highlighted with relevant studies from the literature. The culmination of the results on both the effectiveness of nanofluids in machining and their corresponding toxicity is expected to reveal a better understanding of the sustainability of machining with nanofluids.
2. Research Motivation and Methodology
The literature provides adequate justification towards the positive impact of NMQL cooling strategy in reducing the cutting forces and cutting temperatures in machining. However, the existing research on NMQL machining does not address the toxicity of nanoparticles. This is attributed to a lack of understanding and unavailability of toxicity-related information on nanoparticles. In most cases, research involving nanoparticle toxicity employs very different measurement parameters and therefore makes it very difficult for non-experts to interpret the results. Hence, to completely address the sustainability aspect of NMQL machining, it is important to analyze the toxicity of the nanoparticles before their selection and implementation. Understanding the possible impacts of nanoparticles on human body and other living organisms will motivate researchers to exercise caution in their use. Further, understanding the effect of nanoparticles on the environment (soil and water bodies) will help researchers develop the required disposal plan. The current research aims to address these concerns and develop a review paper on toxicity of nanoparticles used in the machining studies. The results from the available toxicity studies are presented in easily understandable terms and portray the impacts of the nanoparticles on humans, animals, other living organisms and the environment. The review was developed with a focus on clarity of information and ease of understanding. As stated earlier, since the review paper is aimed at aiding researchers in the manufacturing industry, it was important to present the results from toxicity studies in a simple manner but without tarnishing the significance of the data. Following methodology was used in developing this review:
- Review development phase—The review was developed in two stages. In stage one, the effectiveness of NMQL cooling strategy in machining was established from studies available in literature, and six nanoparticles (some nanoparticle families) were chosen for toxicity analysis in stage two of the review. Due to the presence of detailed review papers on NMQL machining’s performance, this section of the review was kept brief, and it highlights only some studies in each of the machining categories. Further, in this section, reference is made to available review papers in current literature on NMQL machining’s performance. Stage two focused on building the toxicity review for each of the nanoparticles selected in stage one, and it is the main contribution of this review paper. The following methodology was used for stage two:
- In vivo and in vitro studies on both human cells and other living organisms were showcased for all nanoparticles. Studies on aquatic life and bacteria helped with estimating the impacts of the nanoparticles on the environment and may help create a safe disposal procedure. Further, in vitro studies on human cells provided information on the possible impacts of working with nanoparticles during the machining process.
- The following nanoparticle and toxicity test characteristics were made available from each study: nanoparticle size, nanoparticle concentration in the test medium and duration of exposure to the nanoparticle.
- For the development of the toxicity chart, only studies measuring cell viability for seven different human cell lines (human lung epithelial cells (A549), human bronchial epithelial cells (Nl-20), AGS human gastric cells, human epidermal cell (HEK), human liver-derived cells (HepG2), human endothelial cells and human peripheral cells) were considered.
- Results communication and dissemination phase—The following considerations were made in presenting the results in this review:
- The results from the toxicity studies were presented in table format for each of the selected nanoparticles and in understandable terms.
- All studies on each investigated nanoparticle were presented in a chronological order. This was to account for the developing technologies as well as to provide a better insight into some contradictory toxicity results available in the literature.
- For the development of the toxicity chart, only studies measuring cell viability for seven different human cell lines (human lung epithelial cells (A549), human bronchial epithelial cells (Nl-20), AGS human gastric cells, human epidermal cell (HEK), human liver-derived cells (HepG2), human endothelial cells and human peripheral cells) were considered.
The following sections present the toxicity studies and their results for these selected nanoparticles.
3. Toxicity Studies of Nanoparticles
The significance of reducing the amount of lubricant used gave rise to the implementation of MQL techniques, which were then further developed into nano-minimum quantity lubrication. As shown in Table 1, many studies in the literature reflect the positive impacts of introducing nanoparticles in the base oil of a minimum quantity lubrication system. However, the toxicology of the nanoparticles must be understood in order to completely address the sustainability aspect of the machining process with NMQL. Nanoparticles are used in various applications and depending on their usage; nanoparticles may enter the human system by inhalation, oral intake or dermal contact [28,29], as shown in Figure 2.
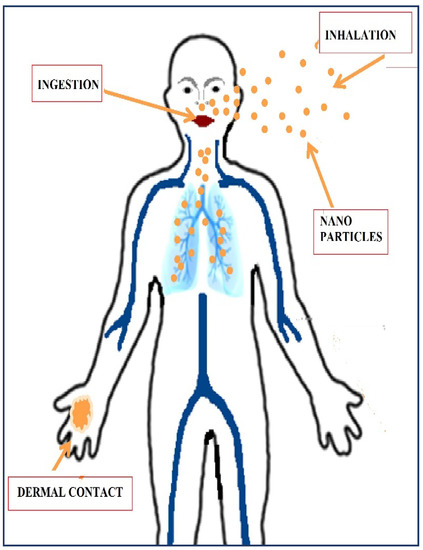
Figure 2.
Nanoparticle routes of exposure [19].
Nanoparticles’ sizes and surface charges determine their reactions with biological fluids, and hence, it is essential to understand the capacity of the body’s biological barriers in identifying these contaminants [30]. Studies have shown that smaller nanoparticles travel to the alveolar region and then to the secondary organs, resulting in increased toxicity and causing systemic effects. In contrast, larger particles are likely to be deposited in the upper airways [31,32]. Further, the impacts of nanoparticles on the environment and other living organisms are also important to realize a safe disposal procedure for the nanofluids. Some terminologies relevant to the discussion ahead are detailed below:
- In vitro studies—Toxicity studies conducted outside a living organism. Usually, a cell culture is developed and the nanoparticles are then added to the cell culture for certain durations of time to examine their effects.
- In vivo studies—Toxicity studies conducted by injecting a living organism/animal with a certain dose of nanoparticles. The impacts on the organs/functions of the animal are then studied.
- Cell viability—Cell viability is defined as the number of healthy cells in a sample and can be expressed in percentage. Many studies focus on evaluating concentrations of nanoparticles that reduce the cell viability of a given sample to 50%.
- Cell morphology—Describes the shape, structure, form and size of cells. Changes in cells’ morphology might indicate negative impacts on cell function.
The following sections present both in vivo and in vitro research on toxicity levels for the selected frequently used nanoparticles.
3.1. Molybdenum Disulfide (MoS2)
MoS2 is one of the important transition-metal dichalcogenides (TMD) with growing applications in the industry. The low friction coefficient of MoS2 particles (µ = 0.003) has guaranteed this substance a place as a general application lubricant [33]. In vitro analyses to study the toxicity of MoS2, WS2 and WSe2 particles on human lung epithelial cells were conducted by Teo et al. [34]. The study highlighted the relative inertness of transition-metal dichalcogenides when compared to their organic analogs, such as graphene oxide. The viability of the cells was not significantly altered by the addition of MoS2 and WS2 particles. Furthermore, among the TMDs tested, WSe2 resulted in the least cell viability while WS2 and MoS2 had very low toxicological effects on cells. Table 2 presents the toxicity studies on MoS2 particles in the chronological order of publication. The following important observations were made:

Table 2.
MoS2 toxicity studies in chronological order.
- The in vitro studies on human cell lines generally provide a very low toxicity when MoS2 nanoparticles are added to cell cultures.
- The method of nanoparticle exfoliation is highlighted as critical in determining the toxicity levels of MoS2 nanoparticles.
- A study on Escherichia coli to study the effects of MoS2 in natural water provided a high mortality rate. Thus, it indicates a need to be careful in the disposal of the nanoparticles. Further, a lack of in vivo studies on MoS2 has been noted. This is attributed to the relative newness of MoS2 nanoparticles when compared to CNT and metal oxides.
3.2. Tungsten Disulfide (WS2)
Similar to MoS2 nanoparticles, tungsten disulfide (WS2) particles are part of the TMD family of 2D nanomaterials and exhibit excellent lubrication properties with a dynamic friction coefficient of 0.03 and a static friction coefficient of 0.07 [42]. Table 3 presents the toxicity studies on WS2 particles in the chronological order of publication. The following important observations were made:

Table 3.
WS2 toxicity studies in chronological order.
- Similar to MoS2 nanoparticles, in vitro studies on human cell lines generally provide a very low toxicity for WS2 nanoparticles.
- A study on Escherichia coli to study the effect of WS2 in natural water provided a high mortality rate. Additionality, a study on the effects of WS2 nanoparticles on a fungus also resulted in high levels of toxicity. Therefore, it is essential to develop a safe disposal mechanism to protect the environment from exposure to these nanoparticles.
- Further, both WS2 and MoS2 have limited number of in-vivo investigation of their toxicity. This is attributed to the relative newness of TMDs.
3.3. Hexagonal Boron Nitride (hBN)
The chemical composition of hBN consists of equal amounts of boron and nitrogen atoms, synthetically manufactured from boric acid (H3BO3) or boron trioxide (B2O3). It has very good lubricating properties [46]. Horvath et al. studied the effects of boron nitride nanotubes (BNNT) on human lung alveoli and embryonic kidney cells in an in vitro analysis [47]. It was concluded that boron nitride nanotubes are cytotoxic and their toxicity is greater than that of carbon nanotubes. The authors hypothesized that the increased toxicity of BNNT could be due to their rod-like structure and highlighted the need for in vivo studies before larger implementations in medical fields. In contrast to Horvath’s work, Turco et al. studied the cell viability, cytoskeleton integrity and DNA damage in human vein endothelial cells and reported that BNNT had a non-significant effect on the cells [48]. It was reported that a modest reduction in cell viability occurred at only the highest concentrations (100 µg mL−1). Further, Campatelli et al. [49,50], in a direct response to the work by Horvath et al. [47], conducted a further investigation on the toxicity of hBN nanoparticles. The research highlighted that hBN nanoparticles with lengths greater than 10 µm (similar to the ones used in the works of Horvath et al.) produce toxic effects in the cell, while shorter hBN nanoparticles do not exhibit toxic effects on the same cell lines. Table 4 presents the toxicity studies on BN-NT/hBN nanoparticles in the chronological order of publication. The following important observations were made:
- The early literature on hBN provided contradictory results on toxicity. However, further research has highlighted the cause for the discrepancy in results. The lengths of nanoparticles are crucial in determining the particles’ toxicity levels.
- In vivo studies on mice showcased a dose-dependent increase in toxicity. Therefore, it is very important to understand the toxicity of hBN nanoparticles relative to their concentration levels.
- Soil worms (C. elegans) were impacted by the presence of nanoparticles in their systems. This highlights the need to be cautious in the disposal of the nanoparticles in the environment.
- Study by Xin et al. [51] estimated 40 µg as equal to almost approximately 2–3 decades of work exposure to humans, and 4 µg was estimated to be about 2–7 years of work exposure. Such estimates are important in understanding the safety criteria for the use and implementation of these nanoparticles.

Table 4.
hBN toxicity studies in chronological order.
Table 4.
hBN toxicity studies in chronological order.
| Type of Study | Concentration | Diameter (nm) | Time of Exposure | Cell Line/Organism | Major Outcomes |
|---|---|---|---|---|---|
| In vitro | 5 µg/mL of PEI-BNNT (1:10) | 72 h | Human neuroblastoma cell line (SH-SY5Y) | No adverse effects on metabolism, viability or cellular replication were reported. A good cell viability was maintained throughout the test period [49]. | |
| In vitro | 2 µg/mL | <80 | 48 h | Human lung epithelial cells (A549) alveolar macrophages (RAW 264.7) fibroblast cells (3T3-L1) Human embryonic kidney cells (HEK293) | Shape and geometry are crucial parameters that dictate the toxicity of nanomaterials. BNNT was found to exhbit toxicity to cell lines at low concentrations [47]. |
| In vitro | 0–100 µg/mL | 75–220 | 72 h | Human vein endothelial cells (HUVECs) | BNNT had a non-significant effect on the cells. It was reported that a modest reduction in cell viability occurred at only the highest concentrations (100 µg mL−1) [48]. |
| In vitro | 0–100 µg/mL | 10–80 | 24–48–72 h | Human neuroblastoma SH-SY5Y cells Human umbilical vein endothelial cells (HUVECs) | Both cell lines exhibited a high viability even at high concentrations of 20 µg/mL. A shorter BNNT was observed to have a low cytotoxicity when compared to longer nanotubes. The same BNNTs with longer lengths (10 nm) were found to be toxic at concentrations as low as 2 µm [52]. |
| In vitro | 25 µg/mL | 24 h | Human cells | Cell stiffness was calculated using atomic forced microscopy. It was seen that there was no significant change in the cell stiffness before and after hBN uptakes. Therefore, the authors posed it as safe for biomedical use. Further in vivo studies are encouraged [53]. | |
| In vitro/in vivo | 0–100 µg/mL and 40 µg | 49 | 24 h | NLRP3-deficient human monocytic cells C57BL/6 J male mice | Both in vitro and in vivo studies resulted in acute inflammation and toxicity due to BNNT contaminations [54]. |
| In vitro | 0–20 µg/mL | <50 | Human hepatoma HepG2 | At 30 μg/mL, MoS2 and BN nanoparticles reduced cell viability [38]. | |
| In vivo | 1–500 µg/mL | 150 | 0–30 days | Caenorhabditis elegans (C. elegans) | It was seen that up to a concentration of 100 µg mL−1, BNNTs did not cause any significant alteration to the growth, locomotion, lifespan or progeny of the C. elegans nematodes. However, at concentrations over 100 µg mL−1, BNNTs significantly reduced growth and locomotion and affected other characteristics [55]. |
| In vitro | 0.025–0.4 mg/mL | 50–190 | 24 and 48 h | Human normal skin fibroblast (CCD-1094Sk and ATCC® CRL 2120™) Madin–Darby canine kidney (MDCK) cells | At a low concentration of 0.025–0.1 mg/mL, no cytotoxcity was observed. However, at concentrations over 0.2 mg/mL, a mild cytotoxicity was noted on CRL-2120 cells. The authors concluded that at concentrations below 0.1 mg/mL, hBN can be a safe oral care product [56]. |
| In vivo | 4 and 40 µg | 13–23 | 4 h 1–7 days 1–2 months | Male C57BL/6 J mice | A concentration of 40 µg caused the greatest amount of damage to the lungs. 40 µg was estimated as equal to almost approximately 2–3 decades of work exposure to humans. 4µg was estimated to be about 2–7 years of work exposure, but resulted in no toxicity [51]. |
| In vivo | 50–3200 µg/kg | 50–200 | 24 h | Wistar albino rats | At concentrations below 1600 µg/kg, no toxicity was observed. Concentrations of 1600 µg/kg and 3200 µg/kg caused significant damage to the liver [57]. |
3.4. Aluminum Oxide (AL2O3)
Metallic oxide nanoparticles have various applications within the industry due to their physical and chemical properties, such as transparency, high isoelectric effects and photocatalytic efficiency [58]. AL2O3 also exhibits a resistance to chemical corrosion [59]. Weisheng et al. compared the cytotoxicity of AL2O3 nanoparticles on human bronchioloalveolar carcinoma cells (A549) with that of titanium dioxide (TiO2) and cerium oxide (CeO2) [60]. The A549 cell viability was unaffected up to a concentration of 5 µg mL−1; however, at dosages of 10 µg mL−1 and 25 µg mL−1, the cell viability was reduced to 86% and 82.8%, respectively. It was determined that CeO2 caused the cell viability to be reduced to 68.3% at 25 µg mL−1, while TiO2 resulted in a cell viability of 89.3% at the maximum concentration of 25 µg mL−1. The level of toxicity was determined to decrease in the following order: CeO2 > AL2O3 > TiO2. Noguiera et al. experimentally analyzed the effects of different crystalline forms of AL2O3 on mouse neuroblastoma cells (N2A) and human bronchial epithelial cells (BEAS-2B) [61]. The two crystalline forms that were studied—alpha AL2O3 (α-AL2O3), which has a hexagonal structure, and eta AL2O3 (η-AL2O3)—revealed different toxicology results. This gives strength to the hypothesis that the crystalline forms influence the nanoparticle toxicity. Both α and η-AL2O3 resulted in a decreased cell viability in both N2A and BEAS-2B cells with the latter crystalline form showing a greater toxicity. The decrease in cell viability was dependent on both the concentrations and the durations of cell exposure to these NPs. Table 5 represents the toxicity studies on AL2O3 nanoparticles in the chronological order of publication. The following important observations were made:

Table 5.
AL2O3 toxicity studies in chronological order.
- AL2O3 nanoparticles are relatively less toxic when compared to other metal oxides, such ZnO and SiO2.
- Dose-dependent increases in toxicity were observed. Low concentrations of AL2O3 nanoparticles of up to 100 µg/mL−1 resulted in low toxicity levels in human cell lines. However, in fish cells, higher toxicity levels were observed for the same levels of nanoparticle concentration.
- In vivo studies on mice also showcased inflammation and damage to the liver.
3.5. Zinc Oxide (ZnO)
The metallic oxide of zinc is used in many applications, including cosmetics for protection against UV rays [62]. Weisheng et al. investigated the toxicity of ZnO nanoparticles on human lung epithelial cells. This study found that there was a 75–85% decrease in cell viability between concentrations of 18 and 25 µg mL−1, respectively [80]. The authors reported a steep decline in cell viability for ZnO compared to the NPSs of other metallic oxides. This observation was supported by Qiang et al. in a comparative study of different metallic oxides [59]. ZnO resulted in the greatest decrease in cell viability when compared to metallic oxides of aluminum, silica and titanium. Sliwinska et al. further confirmed the non-biocompatibility of ZnO nanoparticles with human peripheral blood lymphocytes [58]. Table 6 represents the toxicity studies on ZnO nanoparticles in the chronological order of publication. The following important observations were made:

Table 6.
ZnO toxicity studies in chronological order.
- High levels of toxicity were observed at even low concentrations within in vitro cytotoxicity studies on human cell lines.
- In vivo studies also showcased high levels of toxicity and damage to the liver.
3.6. Carbon Nanotubes (CNT, SWCNT and MWCNT)
Carbon nanotubes, due to their excellent structural, mechanical, electrical and optical properties, are used in many industrial applications. A good amount of literature exists on the toxicity levels of CNTs due to their longer presence in the industry. An in vivo toxicity study on mice comparing CNTs to asbestos observed that CNTs result in the formation of a scar-like structure (lesion) similar to asbestos that is a carcinogenic. Table 7 represents the toxicity studies on CNT nanoparticles in the chronological order of publication. The following important observations were made:

Table 7.
CNT toxicity studies in chronological order.
- High levels of toxicity were observed at even low concentrations within in vitro cytotoxicity studies on human cell lines. Some contradictory results are also available in the literature.
- In vivo studies highlighted short-term impairments of fear, memory and morphological changes and an increased heartbeat.
4. Discussion
MQL is a mist lubrication strategy, and hence, it has a high probability of resulting in airborne impurities, such as nanoparticles. This review details the toxicity of six nanoparticles frequently used in nano-MQL machining. From the available literature, it is evident that nanoparticles improve machining performance. However, they also impact human health and the environment. Therefore, it is critical for researchers and workers experimenting in the use of NPs in the machining sector to be cautious regarding the handling, preparation and operation of nanofluids. The most frequently used nanoparticles in the machining studies available in the literature are identified as tungsten disulfide (WS2), molybdenum disulfide (MoS2), boron nitride (BNNT and hBN), carbon nanotubes (CNT, SWCNT and MWCNT) and oxides of zinc (ZnO) and aluminum (AL2O3). MoS2 nanoparticle additives improve the machining performance in minimum quantity lubrication machining. In a comparative study with different nanoparticles, MoS2 performed the best with a low surface roughness and lower cutting forces [13]. The machining performance of WS2 was also comparable to that of MoS2. The toxicity studies for MoS2 and WS2 nanoparticles presented in Table 2 and Table 3, respectively, attest the very low toxicity of TMDs (MoS2 and WS2). Therefore, the high machining performance and low toxicity present TMDs as an ideal choice for nano-minimum quantity lubrication. However, important observations from the toxicity studies on MoS2 indicate that the method of manufacturing affects their toxicity. Further, WS2 nanoparticles exhibit toxicity in natural water and affect fungi growth. The experimental investigations for MoS2 and WS2 listed in Table 1 do not provide details related to their methods of manufacturing or the disposal mechanisms employed. This review highlights the need for researchers to clearly indicate these parameters to achieve a proper sustainability analysis.
The toxicity of hBN nanoparticles varies depending on the length. A high toxicity is observed at larger lengths, while very low toxicity levels are seen at shorter lengths. Additionally, the chronological presentation of toxicity studies on hBN nanoparticles show the development of a consensus regarding how the lengths of nanotubes effect their toxicity. However, in vivo studies presented some concerning results on the impacts of hBN nanoparticles over certain concentrations. The in vivo study conducted on C. elegans showcased the detrimental effects on the growth, the locomotion and the progeny of these nematodes [55]. Although the results are not analogous to humans, it does raise important questions regarding possible similarities in its toxicity on the human body. The NMQL machining studies with hBN additives provided a good machining performance. However, due to few alarming toxicity results present in the literature, care must be taken in the selection of hBN nanoparticles. If the use of hBN nanoparticles cannot be avoided, particles with short lengths must be used. Similar to hBN nanoparticles, the toxicity of AL2O3 is affected by its crystalline structures. γ-AL2O3 NPs were more toxic than α-AL2O3 NPs at all concentrations. Therefore, researchers need to avoid the use γ-AL2O3 nanoparticles. ZnO exhibits a very strong toxicity and must be avoided in machining studies. Similarly, carbon nanotubes have been seen to form lesions similar to the effects of asbestos.
For a quick and easy understanding of the toxicity levels of the chosen nanoparticles, an attempt was made to develop a chart that maps the toxicity levels of the nanoparticles on seven different human cell lines representing the possible exposure routes of nanoparticles. Many researchers have used human lung epithelial cells (A549) for toxicity studies representing exposure to inhaled nanoparticles. Bronchial epithelial cells (NL-20) have also been used. The effects of nanoparticle ingestion have been represented by using in vitro toxicity studies on human gastric cell lines (AGS), human epidermal keratinocytes (HEKs) and human liver-derived cells (HepG2), while blood-related toxicity has been represented by using in vitro studies on human peripheral blood cells and human endothelial cells. Cell viability is defined as the number of healthy cells in a sample [95] and was used as a marker to establish the comparison chart seen in Table 8. It must be noted that all studies seen in the toxicity studies of individual nanoparticles were not included in the chart. In order to facilitate a comparison, only in vitro studies on select human cell lines were included in the chart. The cell viability marker is represented as a percentage and provides an easy understanding of the toxicity levels for each nanoparticle.

Table 8.
Comparison of nanoparticle toxicities on human cells.
5. Conclusions
The literature on machining with NMQL does not explicitly address the safety aspect of dealing with nanoparticles. This study is an attempt to bridge that gap in the literature by providing the toxicity details regarding the most-used nanoparticles in machining. The review includes both in vivo and in vitro studies assessing the toxicity of the nanoparticles on human cells and other organisms. This will allow researchers to understand the impacts of nanoparticles on human health as well as on the environment and thereby devise appropriate methodologies for the safe handling and disposal of these nanoparticles. Further, for the easy assessment of toxicity levels, a toxicity chart for nanoparticles was developed. Cell viability measured from in vitro toxicity studies of nanoparticles on seven different cell lines representing three possible exposure routes (inhalation, ingestion and dermal contact) was used as a marker to establish the visual representation found in Table 8. This table compares the toxicity of six different nanoparticles used in NMQL machining processes. The following conclusions have been drawn from this review:
- Transition metal dichalcogenides (MoS2 and WS2) exhibit a very low toxicity when compared to other nanoparticles and provide a very good machining performance with a good surface finish and lower cutting forces. Among the MoS2 and WS2 nanoparticles, MoS2 provides a better surface finish and exhibits a lower toxicity. However, a lack of in vivo studies and the relative infancy of the toxicity research on these nanoparticles must be considered.
- The toxicity of hBN nanoparticles varies depending on the length. A high toxicity was observed at larger lengths, while very low toxicity levels are seen at shorter lengths. Hence, machining with hBN nanoparticles must be done with only short hBN nanoparticles. Similarly, care must be taken in the selection of AL2O3 nanoparticles. The toxicity of AL2O3 nanoparticles varies depending on their crystalline structures but generally exhibits a low toxicity on human cells. However, results from in vivo studies of both hBN and AL2O3 highlight the concern and the need to accurately understand the importance of nanoparticle concentrations.
- ZnO exhibited very high levels of toxicity in both in vitro and in vivo studies, and therefore, irrespective of machining performance, researchers must avoid their use in machining operations. In vivo studies for carbon nanotube toxicity predicted a high toxicity, while in vitro toxicity studies provided contradictory results.
- Some nanoparticles for the same concentration exhibited a higher toxicity in non-human species. This provides researchers information to develop disposal guidelines and highlights the need for the proper disposal of nanofluids after machining.
- The comparisons developed in Table 8 provide an easy interpretation of the toxicity levels of the six nanoparticles that were considered. Cell viability is an important marker for toxicity studies and provides easy interpretations. However, a lack of uniformity in nanoparticle concentrations and the methods employed are limitations. Future research can aim to develop the chart with consistent nanoparticle concentrations and same methods of toxicity analyses.
This is the first attempt to combine the results of the experimental investigations of nano-MQL cooling and the toxicity studies of nanoparticles, allowing researchers to make informed decisions in the selection of the most sustainable nanoparticles in the nano-MQL machining process. Simulation studies enable researchers to understand key parameters, such as temperature changes, heat transfer coefficients, cutting forces and the effects of jet radius and location [96]. The relative difficulty of establishing a realistic model to simulate the effects of nano-minimum quantity lubrication is another reason experimental investigations are unavoidable. The development of CFD and FEM models to simulate nanofluid lubrication to minimize experimentation must be considered and is a topic of future study.
Author Contributions
Literature, I.N. and A.E.; writing—original draft preparation, I.N. and A.E.; writing—review and editing, S.P.; visualization, I.N.; supervision, I.D.; funding acquisition, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support provided by the NSERC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Black, J.T.; Kohser, R.A. DeGarmo’s Materials and Processes in Manufacturing, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Dixit, U.S.; Sarma, D.K.; Davim, J.P. Environmentally Friendly Machining; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Boswell, B.; Islam, M.N.; Davies, I.J.; Ginting, Y.R.; Ong, A.K. A review identifying the effectiveness of minimum quantity lubrication (MQL) during conventional machining. Int. J. Adv. Manuf. Technol. 2017, 92, 321–340. [Google Scholar] [CrossRef]
- Dureja, J.S.; Singh, R.; Singh, T.; Singh, P.; Dogra, M.; Bhatti, M.S. Performance evaluation of coated carbide tool in machining of stainless steel (AISI 202) under minimum quantity lubrication (MQL). Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Carou, D.; Rubio, E.M.; Davim, J.P. A note on the use of the minimum quantity lubrication (MQL) system in turning. Ind. Lubr. Tribol. 2015, 67, 256–261. [Google Scholar] [CrossRef]
- Klocke, F.; Eisenblätter, G. Dry cutting—State of research. VDI Ber. 1998, 46, 159–188. [Google Scholar]
- Tschätsch, H. Applied Machining Technology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–23. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Yılmaz, V.; Güllü, A. Analysis of cutting parameters and cooling/lubrication methods for sustainable machining in turning of Haynes 25 superalloy. J. Clean. Prod. 2016, 133, 172–181. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Effects of Minimum Quantity Lubrication (MQL) in machining processes using conventional and nanofluid based cutting fluids: A comprehensive review. J. Clean. Prod. 2016, 127, 1–18. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammad, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Progress of Nanofluid Application in Machining: A Review. Mater. Manuf. Process. 2015, 30, 813–828. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of MoS2 nanoparticles in jet MQL grinding with different types of vegetable oil as base oil. J. Clean. Prod. 2015, 87, 930–940. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, S.; Aravindan, S. Experimental investigations on surface grinding of silicon nitride subjected to mono and hybrid nanofluids. Ceram. Int. 2019, 45, 17447–17466. [Google Scholar] [CrossRef]
- Virdi, R.L.; Chatha, S.S.; Singh, H. Experiment evaluation of grinding properties under Al2O3 nanofluids in minimum quantity lubrication. Mater. Res. Express 2019, 6, 096574. [Google Scholar] [CrossRef]
- Eltaggaz, A.; Hegab, H.; Deiab, I.; Kishawy, H.A. Hybrid nano-fluid-minimum quantity lubrication strategy for machining austempered ductile iron (ADI). Int. J. Interact. Des. Manuf. 2018, 12, 1273–1281. [Google Scholar] [CrossRef]
- Eltaggaz, A.; Zawada, P.; Hegab, H.A.; Deiab, I.; Kishawy, H.A. Coolant strategy influence on tool life and surface roughness when machining ADI. Int. J. Adv. Manuf. Technol. 2018, 94, 3875–3887. [Google Scholar] [CrossRef]
- Eltaggaz, A.; Nouzil, I.; Deiab, I. Machining ti-6al-4v alloy using nano-cutting fluids: Investigation and analysis. J. Manuf. Mater. Process. 2021, 5, 42. [Google Scholar] [CrossRef]
- Uysal, A. Investigation of flank wear in mql milling of ferritic stainless steel by using nano graphene reinforced vegetable cutting fluid. Ind. Lubr. Tribol. 2016, 68, 446–451. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Do, I.; Kwon, P. A tribological study of vegetable oil enhanced by nano-platelets and implication in MQL machining. Int. J. Precis. Eng. Manuf. 2012, 13, 1077–1083. [Google Scholar] [CrossRef]
- Yıldırım, Ç.V.; Sarıkaya, M.; Kıvak, T.; Şirin, Ş. The effect of addition of hBN nanoparticles to nanofluid-MQL on tool wear patterns, tool life, roughness and temperature in turning of Ni-based Inconel 625. Tribol. Int. 2019, 134, 443–456. [Google Scholar] [CrossRef]
- Yıldırım, Ç.V. Experimental comparison of the performance of nanofluids, cryogenic and hybrid cooling in turning of Inconel 625. Tribol. Int. 2019, 137, 366–378. [Google Scholar] [CrossRef]
- Chetan; Ghosh, S.; Rao, P.V. Comparison between sustainable cryogenic techniques and nano-MQL cooling mode in turning of nickel-based alloy. J. Clean. Prod. 2019, 231, 1036–1049. [Google Scholar] [CrossRef]
- Şirin, Ş.; Kıvak, T. Performances of different eco-friendly nanofluid lubricants in the milling of Inconel X-750 superalloy. Tribol. Int. 2019, 137, 180–192. [Google Scholar] [CrossRef]
- Said, Z.; Gupta, M.; Hegab, H.; Arora, N.; Khan, A.M.; Jamil, M.; Bellos, E. A comprehensive review on minimum quantity lubrication (MQL) in machining processes using nano-cutting fluids. Int. J. Adv. Manuf. Technol. 2019, 105, 2057–2086. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.; Ding, W.; Chen, Y.; Mao, C.; Xu, X.; Liu, B.; Wang, D.; Li, H.N.; Zhang, Y.; et al. Minimum quantity lubrication machining of aeronautical materials using carbon group nanolubricant: From mechanisms to application. Chin. J. Aeronaut. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.N.; Li, C.; Huang, C.; Hafiz Muhammad, A.; Xu, X.; Mao, C.; Ding, W.; Cui, X.; Yang, M.; et al. Nano-enhanced biolubricant in sustainable manufacturing: From processability to mechanisms. Friction 2022, 10, 803–841. [Google Scholar] [CrossRef]
- Pereira, O.; Martin-Alfonso, J.E.; Rodríguez, A.; Calleja-Ochoa, A.; Fernández-Valdivielso, A.; Lopez de Lacalle, L.N. Sustainability analysis of lubricant oils for minimum quantity lubrication based on their tribo-rheological performance. J. Clean. Prod. 2017, 164, 1419–1429. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Moore, C.; Movia, D.; Smith, R.J.; Hanlon, D.; Lebre, F.; Lavelle, E.C.; Byrne, H.J.; Coleman, J.N.; Volkov, Y.; McIntyre, J. Industrial grade 2D molybdenum disulphide (MoS2): An in vitro exploration of the impact on cellular uptake, cytotoxicity, and inflammation. 2D Mater. 2017, 4, 025065. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Gosens, I.; Krystek, P.; Boere, J.A.F.; Cassee, F.R.; Fokkens, P.H.B.; Post, J.A.; Van Loveren, H.; Park, M.V.D.Z. Particle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticles. Part. Fibre Toxicol. 2014, 11, 49. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Chng, E.L.K.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem. A Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef]
- Chng, E.L.K.; Sofer, Z.; Pumera, M. MoS2 exhibits stronger toxicity with increased exfoliation. Nanoscale 2014, 6, 14412–14418. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low cytotoxicity of inorganic nanotubes and fullerene-like nanostructures in human bronchial epithelial cells: Relation to inflammatory gene induction and antioxidant response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.A.; Wang, Q.H.; Chae, J. Low Cytotoxicity and Genotoxicity of Two-Dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Z.; Wu, B.; Yu, Y.; Hou, H.; Zhang, X.X.; Ren, H.Q. Cytotoxicity and Efflux Pump Inhibition Induced by Molybdenum Disulfide and Boron Nitride Nanomaterials with Sheetlike Structure. Environ. Sci. Technol. 2017, 51, 10834–10842. [Google Scholar] [CrossRef]
- Shang, E.; Niu, J.; Li, Y.; Zhou, Y.; Crittenden, J.C. Comparative toxicity of Cd, Mo, and W sulphide nanomaterials toward E. coli under UV irradiation. Environ. Pollut. 2017, 224, 606–614. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, X.; Zhao, M.; Zhou, Q.; Hu, X. Cellular proliferation and differentiation induced by single-layer molybdenum disulfide and mediation mechanisms of proteins via the Akt-mTOR-p70S6K signaling pathway. Nanotoxicology 2017, 11, 781–793. [Google Scholar] [CrossRef]
- Zapór, L. Cytotoxicity Elicited by Molybdenum Disulphide in Different Size of Particles in Human Airway Cells. Rocz. Ochr. Sr. 2019, 21, 794–809. [Google Scholar]
- Roy, D.; Das, A.K.; Saini, R.; Singh, P.K.; Kumar, P.; Hussain, M.; Mandal, A.; Dixit, A.R. Pulse current co-deposition of Ni–WS2 nano-composite film for solid lubrication. Mater. Manuf. Processes 2017, 32, 365–372. [Google Scholar] [CrossRef]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.-H.; Aframian, D.J. Biocompatibility of tungsten disulfide inorganic nanotubes and fullerene-like nanoparticles with salivary gland cells. Tissue Eng. Part A 2015, 21, 1013–1023. [Google Scholar] [CrossRef]
- Garcia-Hevia, L.; Roehrer, I.; Mazzocchi, T.; Menciassi, A.; Ricotti, L. Cytotoxicity of pristine and functionalized tungsten disulfide particles in the urinary system. J. Nanopart. Res. 2020, 22, 273. [Google Scholar] [CrossRef]
- Domi, B.; Bhorkar, K.; Rumbo, C.; Sygellou, L.; Martin, S.M.; Quesada, R.; Yannopoulos, S.N.; Tamayo-Ramos, J.A. Toxicological assessment of commercial monolayer tungsten disulfide nanomaterials aqueous suspensions using human A549 cells and the model fungus Saccharomyces cerevisiae. Chemosphere 2021, 272, 129603. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Nano boron nitride flatland. Chem. Soc. Rev. 2014, 43, 934–959. [Google Scholar] [CrossRef]
- Horváth, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horváth, E.; Forró, L.; Schwaller, B. In vitro investigation of the cellular toxicity of boron nitride nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef]
- Del Turco, S.; Ciofani, G.; Cappello, V.; Gemmi, M.; Cervelli, T.; Saponaro, C.; Nitti, S.; Mazzolai, B.; Basta, G.; Mattoli, V. Cytocompatibility evaluation of glycol-chitosan coated boron nitride nanotubes in human endothelial cells. Colloids Surf. B Biointerfaces 2013, 111, 142–149. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Cytocompatibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: Confirmation of their potential for biomedical applications. Biotechnol. Bioeng. 2008, 101, 850–858. [Google Scholar] [CrossRef]
- Ciofani, G.; del Turco, S.; Rocca, A.; de Vito, G.; Cappello, V.; Yamaguchi, M.; Li, X.; Mazzolai, B.; Basta, G.; Gemmi, M.; et al. Cytocompatibility evaluation of gum Arabic-coated ultra-pure boron nitride nanotubes on human cells. Nanomedicine 2014, 9, 773–788. [Google Scholar] [CrossRef]
- Xin, X.; Barger, M.; Roach, K.A.; Bowers, L.; Stefaniak, A.B.; Kodali, V.; Glassford, E.; Dunn, K.H.L.; Dunn, K.H.L.; Wolfarth, M.; et al. Toxicity evaluation following pulmonary exposure to an as-manufactured dispersed boron nitride nanotube (BNNT) material in vivo. NanoImpact 2020, 19, 100235. [Google Scholar] [CrossRef]
- Campatelli, G.; Lorenzini, L.; Scippa, A. Optimization of process parameters using a Response Surface Method for minimizing power consumption in the milling of carbon steel. J. Clean. Prod. 2014, 66, 309–316. [Google Scholar] [CrossRef]
- Rasel, M.A.I.; Li, T.; Nguyen, T.D.; Gu, Y.T. The assessment of toxicity of boron nitride nanoparticle using atomic forced microscopy. IFMBE Proc. 2015, 52, 31–34. [Google Scholar] [CrossRef]
- Kodali, V.K.; Roberts, J.R.; Shoeb, M.; Wolfarth, M.G.; Bishop, L.; Eye, T.; Barger, M.; Roach, K.A.; Friend, S.; Schwegler-Berry, D.; et al. Acute in vitro and in vivo toxicity of a commercial grade boron nitride nanotube mixture. Nanotoxicology 2017, 11, 1040–1058. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Tang, C.; Lei, S.; Shen, W.; Wang, C.; Wang, G.; Wang, Z.; Wang, L. Toxicity evaluation of boron nitride nanospheres and water-soluble boron nitride in caenorhabditis elegans. Int. J. Nanomed. 2017, 12, 5941–5957. [Google Scholar] [CrossRef]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef]
- Kar, F.; Hacıoğlu, C.; Göncü, Y.; Söğüt, İ.; Şenturk, H.; Dönmez, D.B.; Kanbak, G.; Ay, N. In Vivo Assessment of the Effect of Hexagonal Boron Nitride Nanoparticles on Biochemical, Histopathological, Oxidant and Antioxidant Status. J. Clust. Sci. 2021, 32, 517–529. [Google Scholar] [CrossRef]
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Milczarek, J.; Toma, M.; Korycinska, A.; Szemraj, J.; Sliwinski, T. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol. Mech. Methods 2015, 25, 176–183. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yin, L.H.; Tang, M.; Pu, Y.P. ZnO, TiO2, SiO2, and Al2O3 nanoparticles-induced toxic effects on human fetal lung fibroblasts. Biomed. Environ. Sci. 2011, 24, 661–669. [Google Scholar] [CrossRef]
- Lin, W.; Stayton, I.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxicol. Environ. Chem. 2008, 90, 983–996. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Arl, M.; Köerich, J.S.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Comparison of cytotoxicity of α-Al2O3 and η-Al2O3 nanoparticles toward neuronal and bronchial cells. Toxicol. Vitr. 2019, 61, 104596. [Google Scholar] [CrossRef]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health Part A 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health Part A 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Chen, L.; Yokel, R.A.; Hennig, B.; Toborek, M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J. Neuroimmune Pharmacol. 2008, 3, 286–295. [Google Scholar] [CrossRef]
- Oesterling, E.; Chopra, N.; Gavalas, V.; Arzuaga, X.; Lim, E.J.; Sultana, R.; Butterfield, D.A.; Bachas, L.; Hennig, B. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol. Lett. 2008, 178, 160–166. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Ates, M.; Demir, V.; Arslan, Z.; Daniels, J.; Farah, I.O.; Bogatu, C. Evaluation of alpha and gamma aluminum oxide nanoparticle accumulation, toxicity, and depuration in Artemia salina larvae. Environ. Toxicol. 2015, 30, 109–118. [Google Scholar] [CrossRef]
- Shirazi, A.; Shariati, F.; Keshavarz, A.K.; Ramezanpour, Z. Toxic Effect of Aluminum Oxide Nanoparticles on Green Micro-Algae dunaliella salina. Int. J. Environ. Res. 2015, 9, 585–594. [Google Scholar]
- Srikanth, K.; Mahajan, A.; Pereira, E.; Duarte, A.C.; Rao, J.V. Aluminium oxide nanoparticles induced morphological changes, cytotoxicity and oxidative stress in Chinook salmon (CHSE-214) cells. J. Appl. Toxicol. 2015, 35, 1133–1140. [Google Scholar] [CrossRef]
- Park, E.J.; Sim, J.; Kim, Y.; Han, B.S.; Yoon, C.; Lee, S.; Cho, M.H.; Lee, B.S.; Kim, J.H. A 13-week repeated-dose oral toxicity and bioaccumulation of aluminum oxide nanoparticles in mice. Arch. Toxicol. 2015, 89, 371–379. [Google Scholar] [CrossRef]
- Rajiv, S.; Jerobin, J.; Saranya, V.; Nainawat, M.; Sharma, A.; Makwana, P.; Gayathri, C.; Bharath, L.; Singh, M.; Kumar, M.; et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum. Exp. Toxicol. 2016, 35, 170–183. [Google Scholar] [CrossRef]
- Benavides, M.; Fernández-Lodeiro, J.; Coelho, P.; Lodeiro, C.; Diniz, M.S. Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassius auratus. Environ. Sci. Pollut. Res. 2016, 23, 24578–24591. [Google Scholar] [CrossRef]
- Murali, M.; Suganthi, P.; Athif, P.; Bukhari, A.S.; Mohamed, H.S.; Basu, H.; Singhal, R. Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. J. Trace Elem. Med. Biol. 2017, 44, 125–131. [Google Scholar] [CrossRef]
- Akbaba, G.B.; Türkez, H. Investigation of the Genotoxicity of Aluminum Oxide, β-Tricalcium Phosphate, and Zinc Oxide Nanoparticles In Vitro. Int. J. Toxicol. 2018, 37, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chung, Y.H.; Seo, D.S.; Choi, H.S.; Lim, C.H. Twenty-eight-day repeated inhalation toxicity study of aluminum oxide nanoparticles in male Sprague-Dawley rats. Toxicol. Res. 2018, 34, 343–354. [Google Scholar] [CrossRef]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E.N. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Al-Hamadani, M.Y.I.; Kamel, M.A. Reproductive Toxicity of Aluminum Oxide Nanoparticles and Zinc Oxide Nanoparticles in Male Rats. Nano Part. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Anand, A.S.; Gahlot, U.; Prasad, D.N.; Kohli, E. Aluminum oxide nanoparticles mediated toxicity, loss of appendages in progeny of Drosophila melanogaster on chronic exposure. Nanotoxicology 2019, 13, 977–989. [Google Scholar] [CrossRef]
- Boran, H.; Şaffak, S. Transcriptome alterations and genotoxic influences in zebrafish larvae after exposure to dissolved aluminum and aluminum oxide nanoparticles. Toxicol. Mech. Methods 2020, 30, 546–554. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Huang, C.-C.; Ma, Y.; Shannon, K.B.; Chen, D.-R.; Huang, Y.-W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Guan, R.; Kang, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, S.; Alapati, S.; Ganapathy, S.; Anumolu, G.; Pully, N.R.; Prakhya, B.M. Toxicity of zinc oxide nanoparticles through oral route. Toxicol. Ind. Health 2012, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Kim, S.-J.; Lee, T.-J.; Kim, G.-Y.; Meang, E.; Hong, J.-S.; Kim, Y.-H.; Koh, S.-B.; Hong, S.-G.; Sun, Y.-S.; et al. A 90-day study of sub-chronic oral toxicity of 20nm positively charged zinc oxide nanoparticles in Sprague Dawley rats. Intern. J. Nanomed. 2014, 9, 93–107. [Google Scholar]
- Davoren, M.; Herzog, E.; Casey, A.; Cottineau, B.; Chambers, D.; Byrne, H.J.; Lyng, F.M. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol. Vitr. 2007, 21, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Zeng, L.; Barron, A.R.; Monteiro-Riviere, N.A. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int. J. Toxicol. 2007, 26, 103–113. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Clichici, S.; Biris, A.R.; Catoi, C.; Filip, A.; Tabaran, F. Short-term splenic impact of single-strand DNA functionalized multi-walled carbon nanotubes intraperitoneally injected in rats. J. Appl. Toxicol. 2014, 34, 332–344. [Google Scholar] [CrossRef]
- Reddy, A.R.N.; Krishna, D.R.; Himabindu, V.; Reddy, Y.N. Single walled carbon nanotubes induce cytotoxicity and oxidative stress in HEK293 cells. Toxicol. Environ. Chem. 2014, 96, 931–940. [Google Scholar] [CrossRef]
- Dal Bosco, L.; Weber, G.E.; Parfitt, G.M.; Paese, K.; Gonçalves, C.O.; Serodre, T.M.; Furtado, C.A.; Santos, A.P.; Monserrat, J.M.; Barros, D.M. PEGylated carbon nanotubes impair retrieval of contextual fear memory and alter oxidative stress parameters in the rat hippocampus. BioMed Res. Int. 2015, 2015, 104135. [Google Scholar] [CrossRef]
- Shang, S.; Yang, S.Y.; Liu, Z.M.; Yang, X. Oxidative damage in the kidney and brain of mice induced by different nano-materials. Front. Biol. 2015, 10, 91–96. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Azimirad, V.; Alimohammadi, M.; Shahabi, P.; Sadighi, M.; Nejad, G.G. The cardiac effects of carbon nanotubes in rat. BioImpacts 2016, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. Cell Viability Assays: Introduction; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Nouzil, I.; Pervaiz, S.; Kannan, S. Role of jet radius and jet location in cryogenic machining of Inconel 718: A finite element method based approach. Int. J. Interact. Des. Manuf. 2021, 15, 1–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).