Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
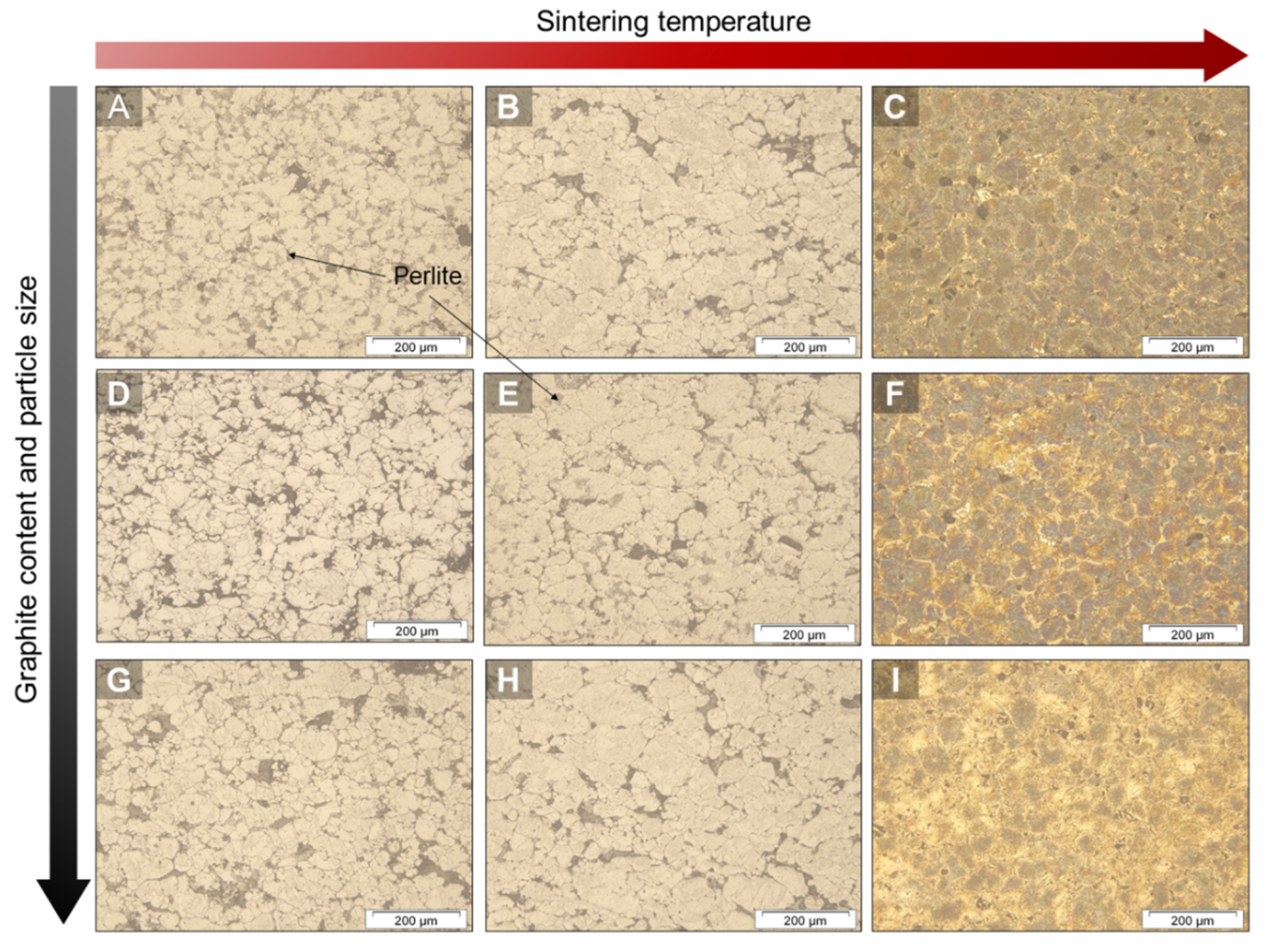
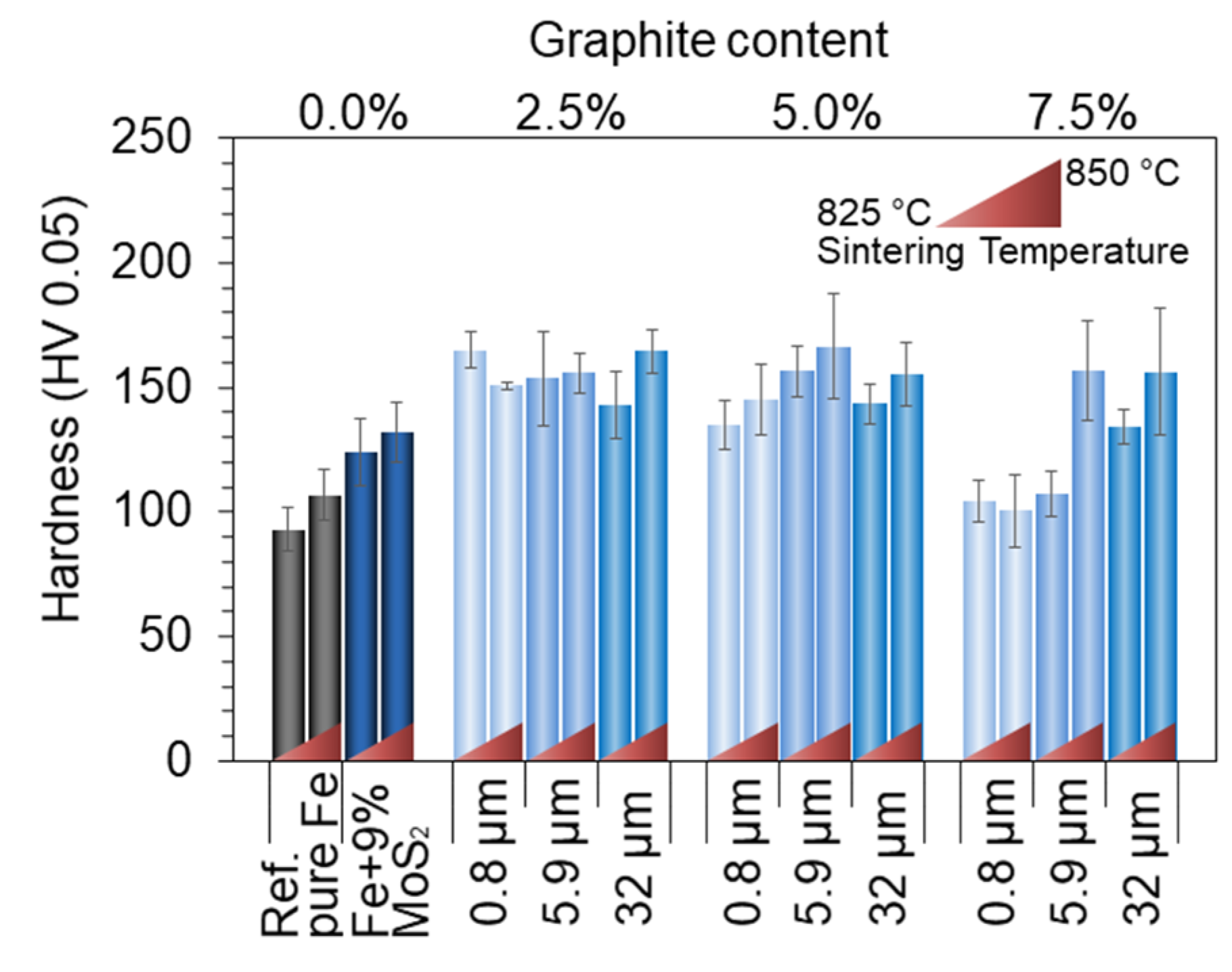
3.1. Microstructural Analysis
3.2. Phase Transition and Thermal Stability
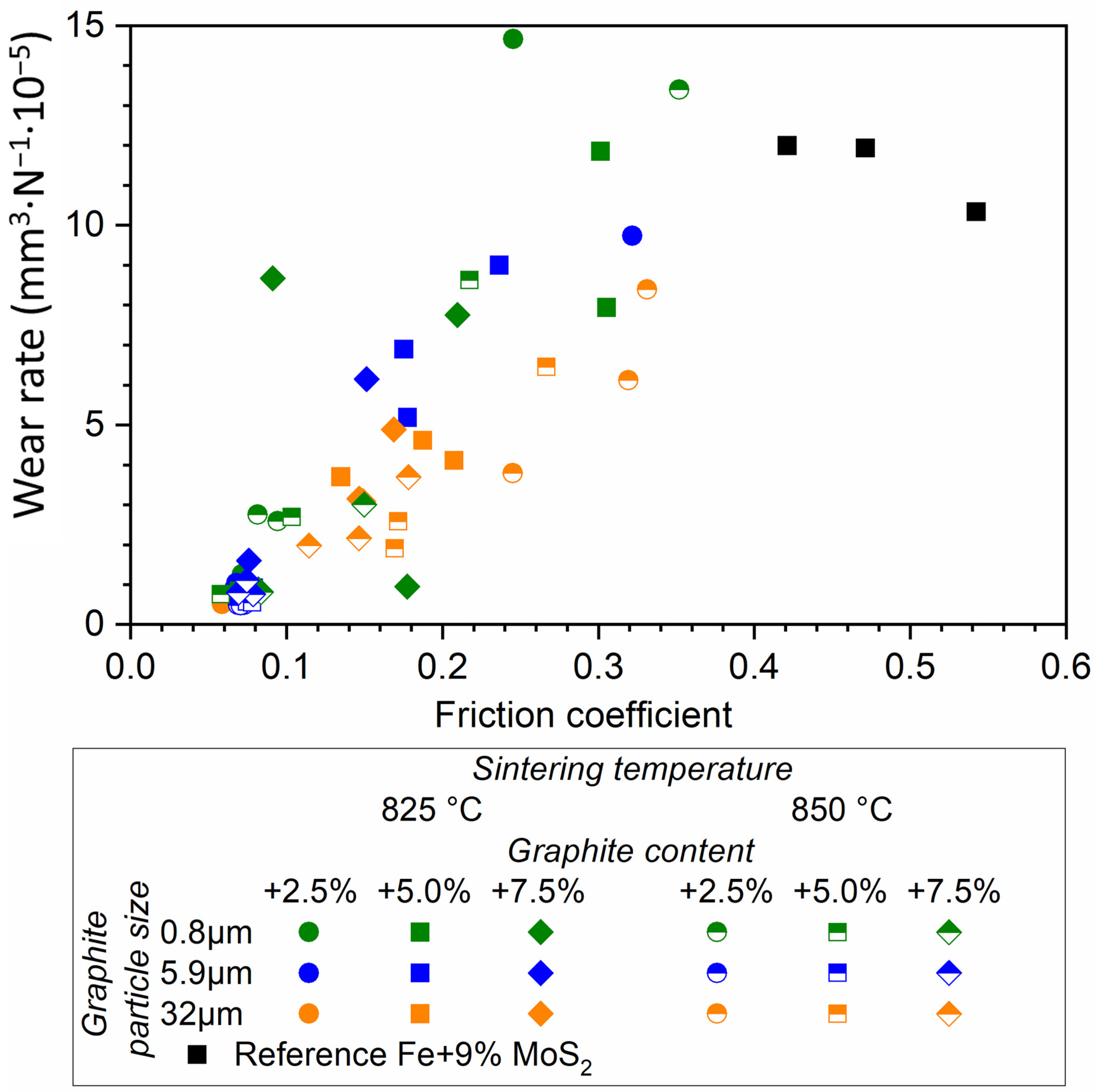
3.3. Tribological Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Colas, G.; Saulot, A.; Michel, Y.; Filleter, T.; Merstallinger, A. Experimental Analysis of Friction and Wear of Self-Lubricating Composites Used for Dry Lubrication of Ball Bearing for Space Applications. Lubricants 2021, 9, 38. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Miyoshi, K. Solid lubrication Fundamentals and Applications, 1st ed.; Taylor & Francis: London, UK, 2001; p. 416. [Google Scholar]
- Busch, C. Solid lubrication. In Lubricants and Lubrication; Wiley-VCH: Weinheim, Germany, 2006; pp. 694–714. [Google Scholar]
- Erdemir, A. Solid lubricants and self-lubricating films. In Modern Tribology Handbook; CRC Press: Boca Raton, FL, USA, 2001; Volume 2, pp. 787–818. [Google Scholar]
- Lansdown, A.R. Molybdenum Disulphide Lubrication; Elsevier: London, UK, 1999; Volume 35, p. 380. [Google Scholar]
- Ludema, K.C. Friction, Wear, Lubrication-a Textbook in Tribology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; p. 294. [Google Scholar]
- Sliney, H.E. Solid lubricants. In Friction, Lubrication and Wear Technology-Metals Handbook; Committee, A.I.H., Ed.; ASM International: Almere, The Netherlands, 1993. [Google Scholar]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 2nd ed.; Butterworth-Heinemann: Waltham, MA, USA, 2001; p. 744. [Google Scholar]
- Kostornov, A.G.; Fushchich, O.I. Sintered antifriction materials. Powder Metall. Met. Ceram. 2007, 46, 503–512. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Copper and Copper Alloys; ASM International: Phoenix, AZ, USA, 2001. [Google Scholar]
- Fedorchenko, I.M. Materials for frictions assemblies. In Powder Metallurgy, Recent Advances; Arunachalam, V.S., Roman, O.V., Eds.; Aspect: London, UK, 1990. [Google Scholar]
- Furlan, K.P.; de Mello, J.D.B.; Klein, A.N. Self-lubricating composites containing MoS2: A review. Tribol. Int. 2018, 120, 280–298. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Gnanamoorthy, R. Microstructure, strength and tribological behavior of Fe–C–Cu–Ni sintered steels prepared with MoS2 addition. J. Mater. Sci. 2007, 42, 4659–4666. [Google Scholar] [CrossRef]
- Maslyuk, V.A. Sintered Composites Based on Stainless Steel. Powder Metall. Met. Ceram. 2000, 39, 549–553. [Google Scholar] [CrossRef]
- Slys, I.G.; Perepelkin, A.V.; Fedorchenko, I.M. Structure and properties of sintered stainless steel containing molybdenum disulfide. Sov. Powder Metall. Met. Ceram. 1973, 12, 710–714. [Google Scholar] [CrossRef]
- Šuštaršič, B.; Kosec, L.; Jenko, M.; Leskovšek, V. Vacuum sintering of water-atomised HSS powders with MoS2 additions. Vacuum 2001, 61, 471–477. [Google Scholar] [CrossRef]
- Šuštaršič, B.; Kosec, L.; Dolinšek, S.; Podgornik, B. The characteristics of vacuum sintered M3/2 type HSSs with MoS2 addition. J. Mater. Process. Technol. 2003, 143–144, 98–104. [Google Scholar] [CrossRef]
- Šuštaršič, B.; Kosec, L.; Kosec, M.; Podgornik, B.; Dolinšek, S. The influence of MoS2 additions on the densification of water-atomized HSS powders. J. Mater. Process. Technol. 2006, 173, 291–300. [Google Scholar] [CrossRef]
- Mahathanabodee, S.; Palathai, T.; Raadnui, S.; Tongsri, R.; Sombatsompop, N. Dry sliding wear behavior of SS316L composites containing h-BN and MoS2 solid lubricants. Wear 2014, 316, 37–48. [Google Scholar] [CrossRef]
- Furlan, K.P.; da Costa Gonçalves, P.; Consoni, D.R.; Dias, M.V.G.; de Lima, G.A.; de Mello, J.D.B.; Klein, A.N. Metallurgical Aspects of Self-lubricating Composites Containing Graphite and MoS2. J. Mater. Eng. Perform. 2017, 26, 1135–1145. [Google Scholar] [CrossRef]
- Furlan, K.P.; Prates, P.B.; Andrea dos Santos, T.; Gouvêa Dias, M.V.; Ferreira, H.T.; Rodrigues Neto, J.B.; Klein, A.N. Influence of alloying elements on the sintering thermodynamics, microstructure and properties of Fe–MoS2 composites. J. Alloys Compd. 2015, 652, 450–458. [Google Scholar] [CrossRef]
- Furlan, K.P. Estudo da Sinterização e Evolução Microestrutural de Misturas de Fe-MoS2. Master’s Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2013. [Google Scholar]
- Bueno, P.; Pagnan Furlan, K.; Hotza, D.; Janssen, R. High-temperature stable inverse opal photonic crystals via mullite-sol-gel infiltration of direct photonic crystals. J. Am. Ceram. Soc. 2019, 102, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Furlan, K.P.; Larsson, E.; Diaz, A.; Holler, M.; Krekeler, T.; Ritter, M.; Petrov, A.Y.; Eich, M.; Blick, R.; Schneider, G.A.; et al. Photonic materials for high-temperature applications: Synthesis and characterization by X-ray ptychographic tomography. Appl. Mater. Today 2018, 13, 359–369. [Google Scholar] [CrossRef]
- Wada, H.; Onoda, M.; Nozaki, H.; Kawada, I. The phase relations and homogeneity range of the iron chevrel compound FexMo6S8−y. J. Less Common Met. 1985, 113, 53–63. [Google Scholar] [CrossRef]
- Chen, F.; Feng, Y.; Shao, H.; Zhang, X.; Chen, J.; Chen, N. Friction and wear behaviors of Ag/MoS2/G composite in different atmospheres and at different temperatures. Tribol. Lett. 2012, 47, 139–148. [Google Scholar] [CrossRef]
- Huang, S.; Feng, Y.; Ding, K.; Qian, G.; Liu, H.; Wang, Y. Friction and wear properties of Cu-based self-lubricating composites in air and vacuum conditions. Acta Metall. Sin. (Engl. Lett.) 2012, 25, 391–400. [Google Scholar] [CrossRef]
- Rowe, G.W. Some observations on the frictional behaviour of boron nitride and of graphite. Wear 1960, 3, 274–285. [Google Scholar] [CrossRef]
- GK_GRAPHITE. Graphit Kropfmühl-Graphite Characterisation. Available online: https://www.gk-graphite.com/en/graphite/characterisation/ (accessed on 8 October 2015).
- Pambaguian, L.; Merstallinger, A. Self-lubricating copper matrix composites with high contents of lubricants. In Proceedings of the World Tribology Congress, Vienna, Austria, 3–7 September 2001. [Google Scholar]
- Li, J.L.; Xiong, D.S. Tribological properties of nickel-based self-lubricating composite at elevated temperature and counterface material selection. Wear 2008, 265, 533–539. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Gnanamoorthy, R. Dry sliding friction and wear characteristics of Fe–C–Cu alloy containing molybdenum di sulphide. Mater. Des. 2007, 28, 1135–1141. [Google Scholar] [CrossRef]
- Chen, F.Y.; Feng, Y.; Shao, H.; Li, B.; Qian, G.; Liu, Y.F.; Zhang, X.B. Tribological behaviour of silver based self-lubricating composite. Powder Metall. 2013, 56, 397–404. [Google Scholar] [CrossRef]
- Juszczyk, B.; Malec, W.; Wierzbicki, Ł.; Kulasa, J.; Malara, S.; Czepelak, M.; Cwolek, B. Tribological properties of copper-based composites with lubricating phase particles. Arch. Metall. Mater. 2014, 59, 615–620. [Google Scholar] [CrossRef] [Green Version]


| Sample Name and Description | Composition (vol.%) | |||
|---|---|---|---|---|
| MoS2 | Graphite | Fe | ||
| L_C1a | Fe + C (0.8 µm) + MoS2 | 9.0 | 2.5 | Bal. |
| L_C2a | 9.0 | 5.0 | Bal. | |
| L_C3a | 9.0 | 7.5 | Bal. | |
| L_C0a | 0.0 | 9.0 | Bal. | |
| L_C1b | Fe + C (5.9 µm) + MoS2 | 9.0 | 2.5 | Bal. |
| L_C2b | 9.0 | 5.0 | Bal. | |
| L_C3b | 9.0 | 7.5 | Bal. | |
| L_C0b | 0.0- | 9.0 | Bal. | |
| L_C1c | Fe + C (32 µm) + MoS2 | 9.0 | 2.5 | Bal. |
| L_C2c | 9.0 | 5.0 | Bal. | |
| L_C3c | 9.0 | 7.5 | Bal. | |
| L_C0c | 0.0 | 9.0 | Bal. | |
| Sample | Sintering Temperature (°C) | |
|---|---|---|
| 825 | 850 | |
| Fe + 9% MoS2 | Fe α 06-0696 * MoS2 37-1492 FeMo2S4 71-0379 | Fe α 06-0696 FeMo2S4 71-0379 Fe1.25Mo6S7.7 37-1442 FeS 80-1027 |
| Fe + 9% MoS2 + 5.0% C (0.8 µm) | Fe α 06-0696 Graphite 26-1080 MoS2 37-1492 Fe1.25Mo6S7.7 37-1442 | |
| Fe + 9% MoS2 + 5.0% C (5.9 µm) | Fe α 06-0696 Graphite 26-1080 MoS2 37-1492 Fe1.25Mo6S7.7 37-1442 | Fe α 06-0696 Graphite 26-1080 MoS2 37-1492 Fe1.25Mo6S7.7 37-1442 FeMo2S4 71-0379 |
| Fe + 9% MoS2 + 5.0% C (32 µm) | Fe α 06-0696 Graphite 26-1080 MoS2 37-1492 FeMo2S4 71-0379 | |
| Author (Year) | Base Matrix | Composition (w.%) | MoS2 | Graphite | Lowest COF |
|---|---|---|---|---|---|
| This work | Fe | Fe | 9 vol.% | 2.5 to 7.5 vol.% | 0.07 |
| Dhanasekaran and Gnanamoorthy (2007) [16] | Fe | Fe + 0.6%C + 2.5%Cu + 3%Ni | 3 to 5 w.% | 0.0 | 0.30 |
| Dhanasekaran and Gnanamoorthy (2007) [35] | Fe | Fe + 0.6%C + 2.5%Cu | 3 to 5 w.% | 0.0 | 0.30 |
| Li and Xiong (2008) [34] | Ni | Ni + 20%Cr + W + Fe | 5, 10 and 15 w.% | 3 w.% | 0.20 |
| Chen et al. (2012) [29] | Ag | Ag | 15 vol.% | 5 vol.% | 0.14 |
| Chen et al. (2013) [36] | Ag | Ag | 0 to 20 vol.% | 0 to 20 vol.% | 0.12 |
| Huang et al. (2012) [30] | Cu | Cu | 0 to 30 w.% | 0 to 30 w.% | 0.30 |
| Juszczyk et al. (2014) [37] | Cu | Cu + 10%Sn | 5 to 20 w.% | 5 to 20 w.% | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lima, G.A.; Klein, A.N.; Furlan, K.P. Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties. Lubricants 2022, 10, 142. https://doi.org/10.3390/lubricants10070142
De Lima GA, Klein AN, Furlan KP. Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties. Lubricants. 2022; 10(7):142. https://doi.org/10.3390/lubricants10070142
Chicago/Turabian StyleDe Lima, Gabriel Araujo, Aloisio Nelmo Klein, and Kaline Pagnan Furlan. 2022. "Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties" Lubricants 10, no. 7: 142. https://doi.org/10.3390/lubricants10070142
APA StyleDe Lima, G. A., Klein, A. N., & Furlan, K. P. (2022). Controlling the Solid-State Reaction in Fe-MoS2 Self-Lubricating Composites for Optimized Tribological Properties. Lubricants, 10(7), 142. https://doi.org/10.3390/lubricants10070142






