Tribological Properties of Polydopamine-Modified Ag as Lubricant Oil Additives
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
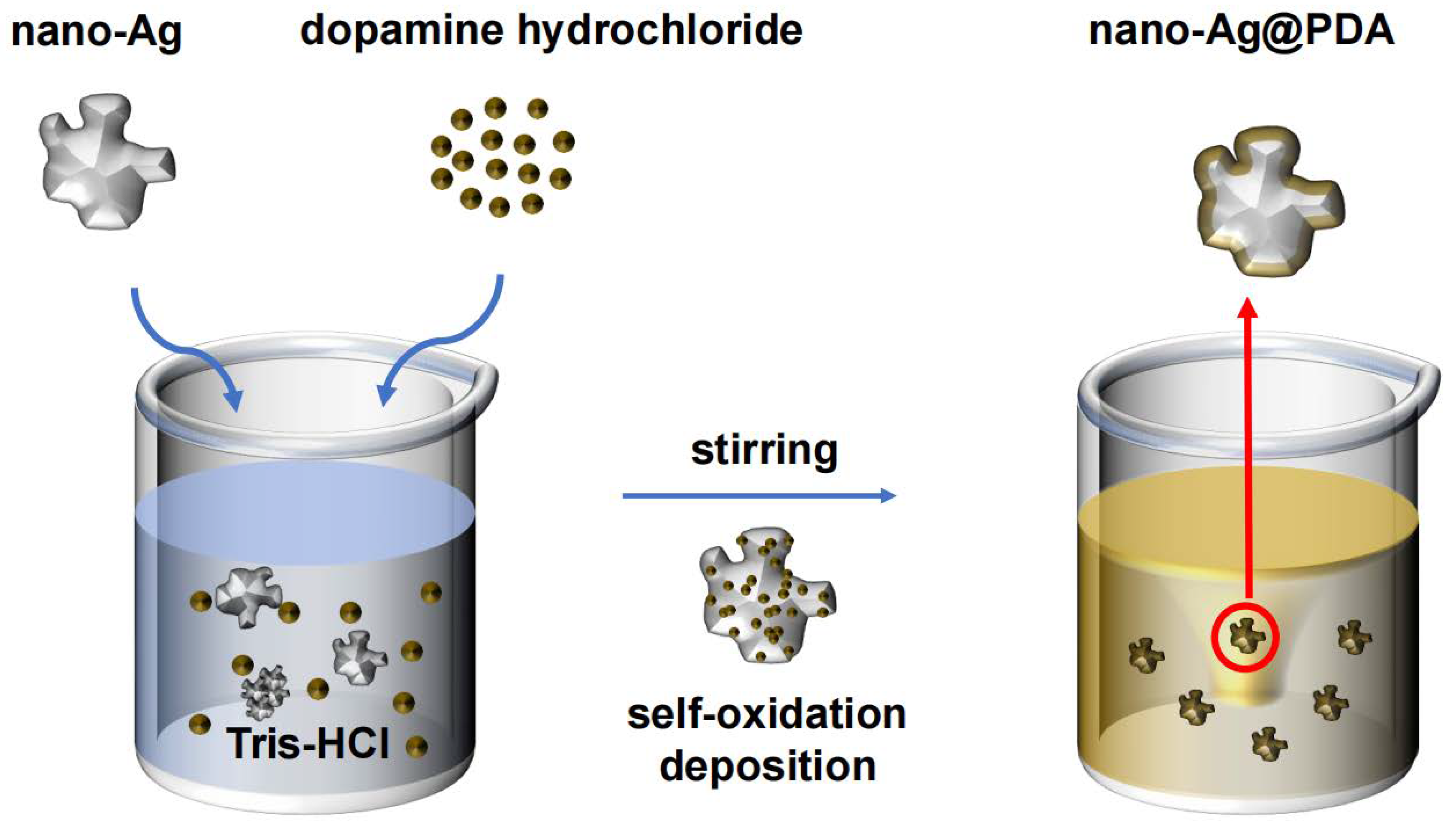
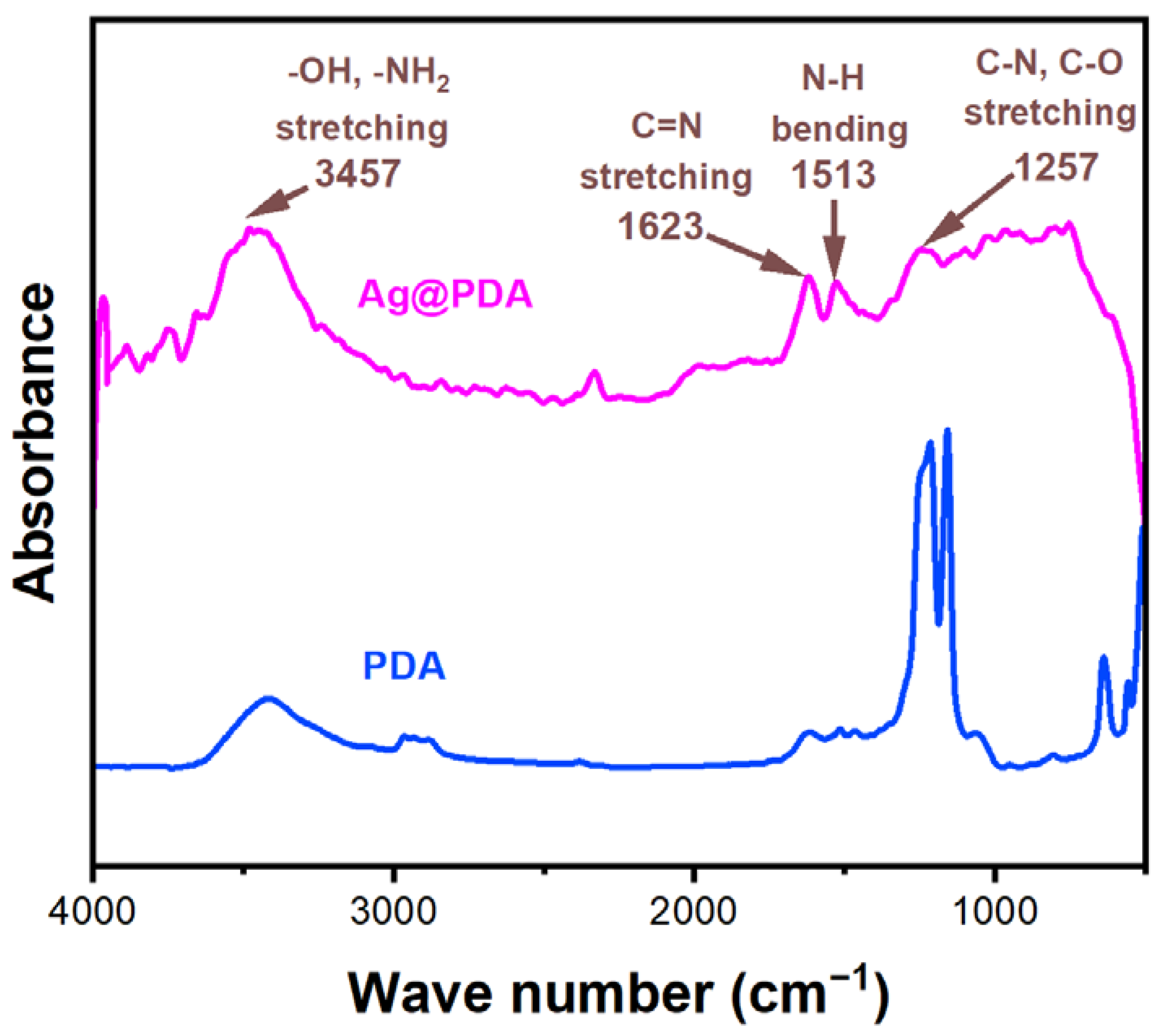
3.1. Synthesis of Ag@PDA
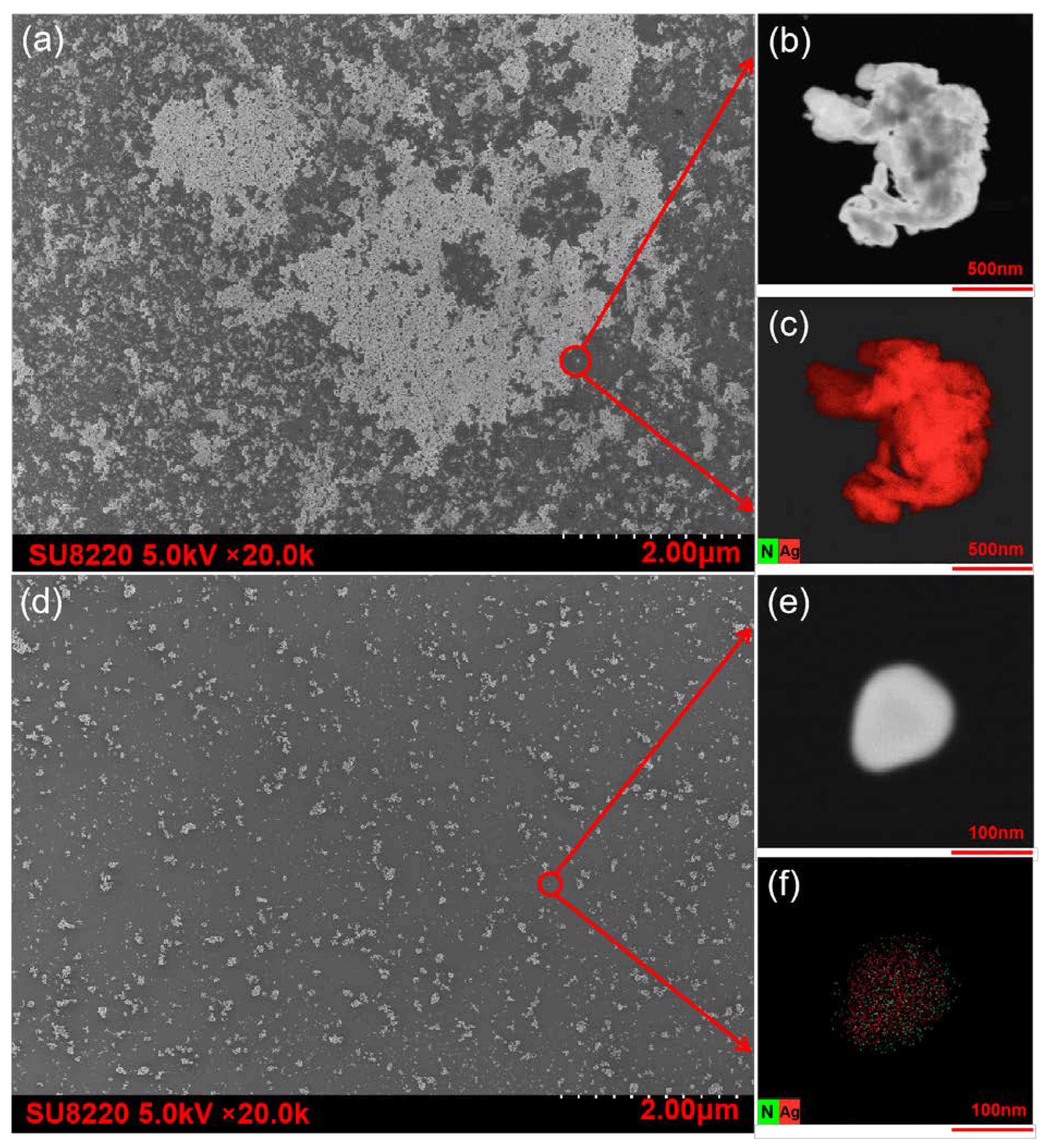
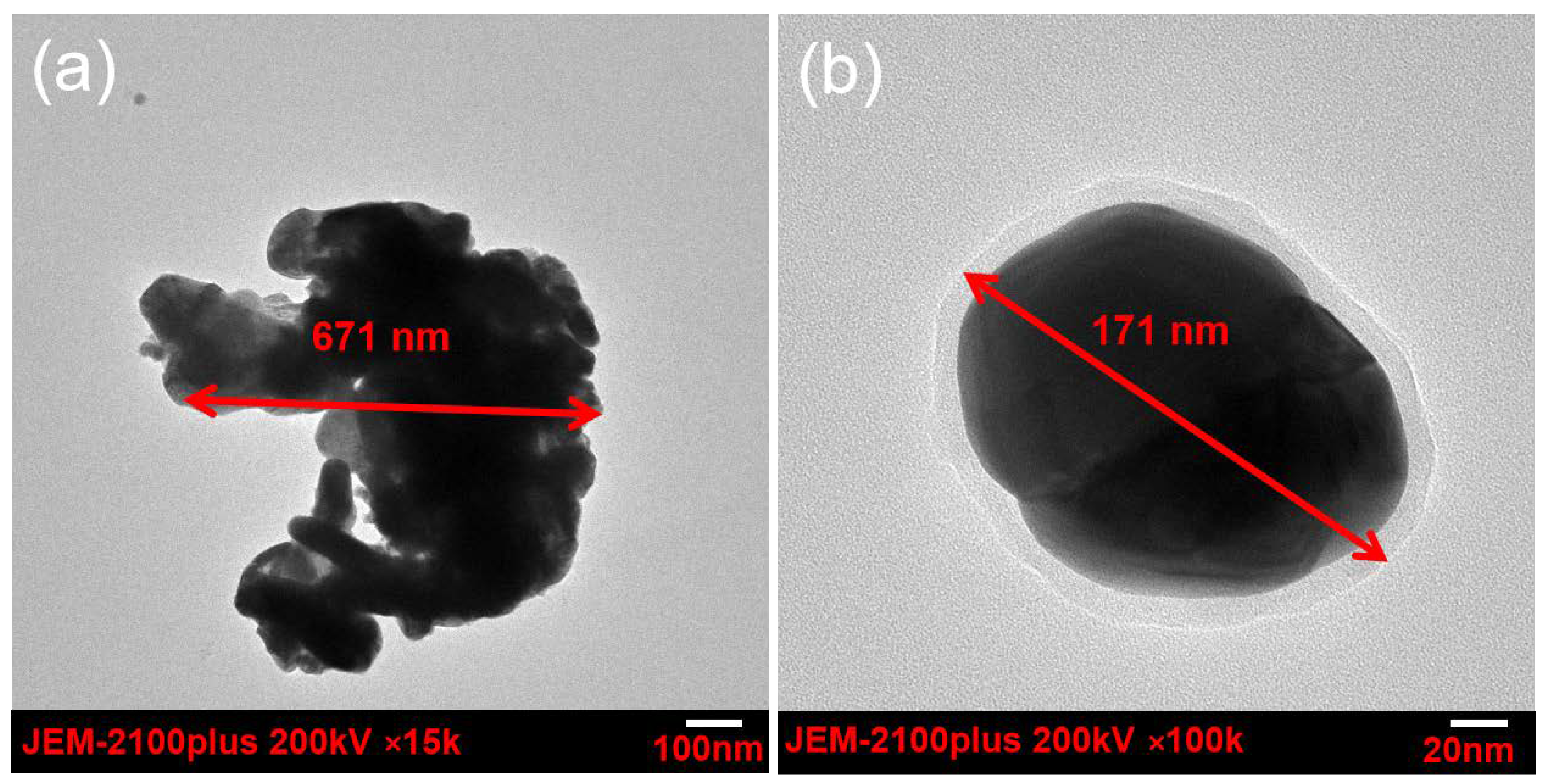
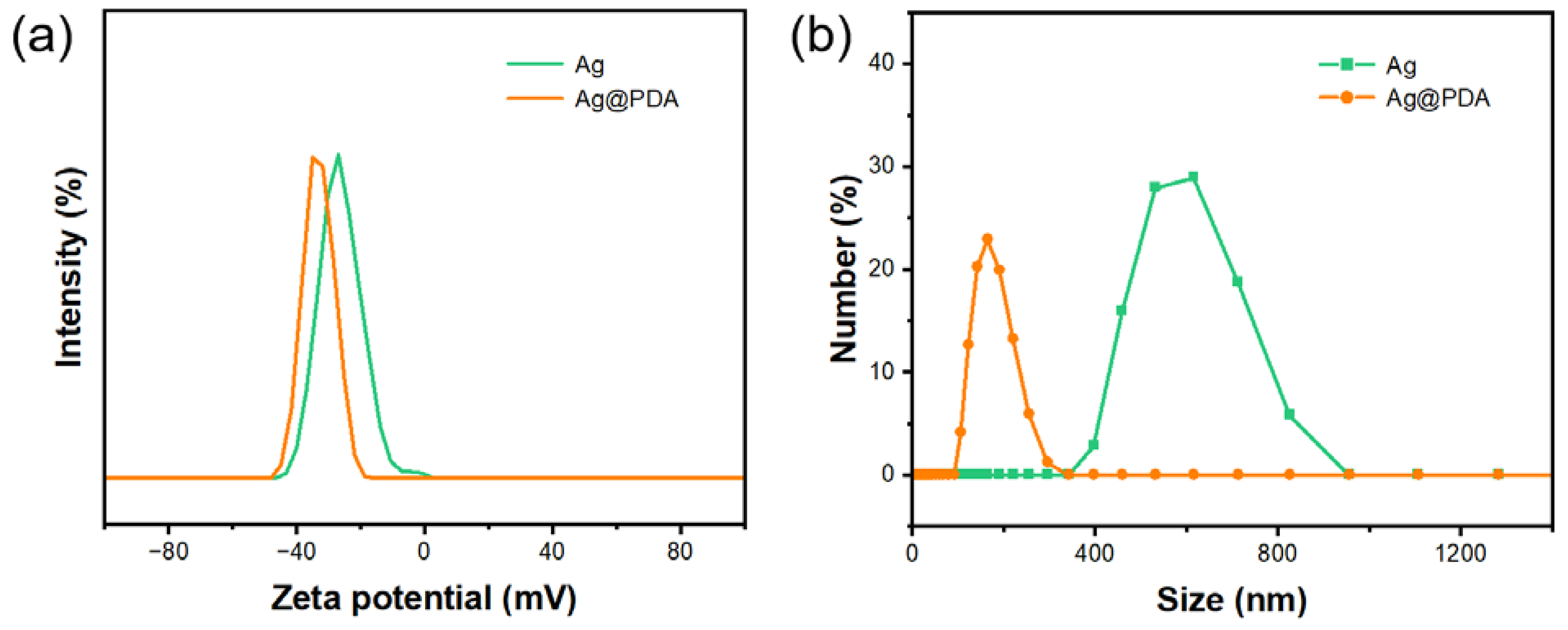
3.2. Dispersibility
3.3. Tribology Performances
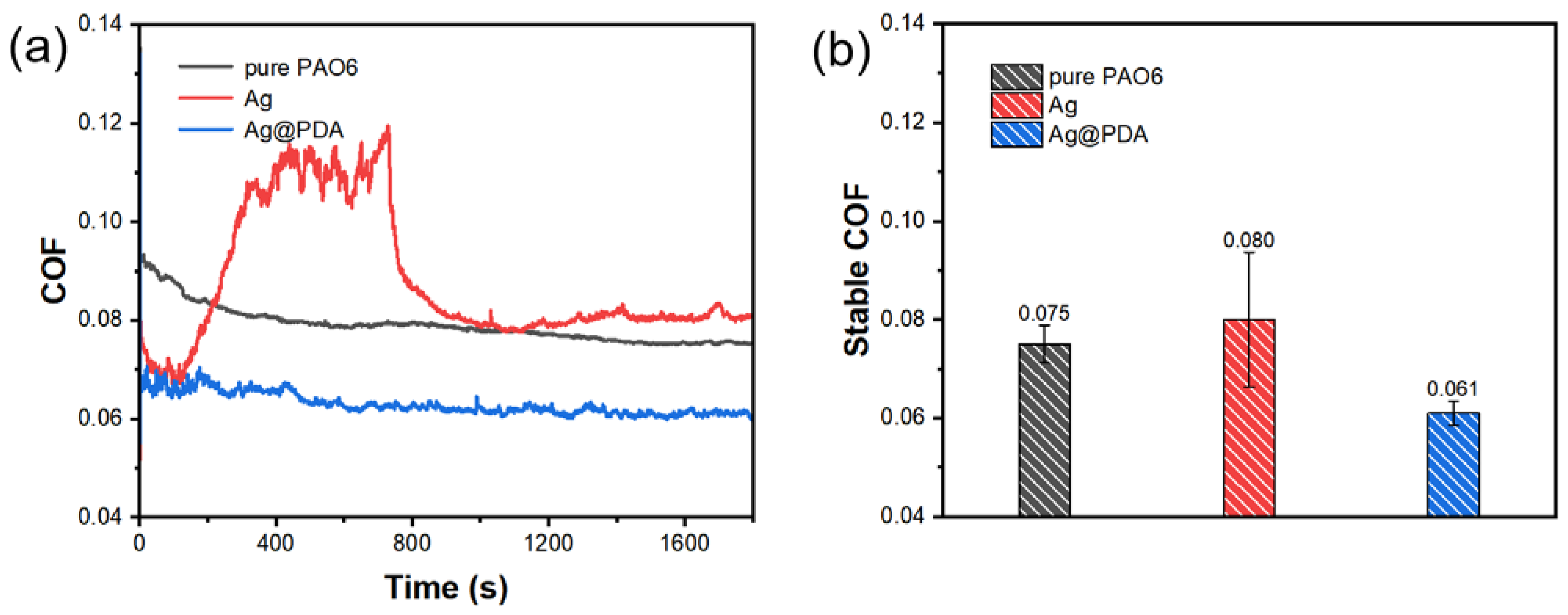
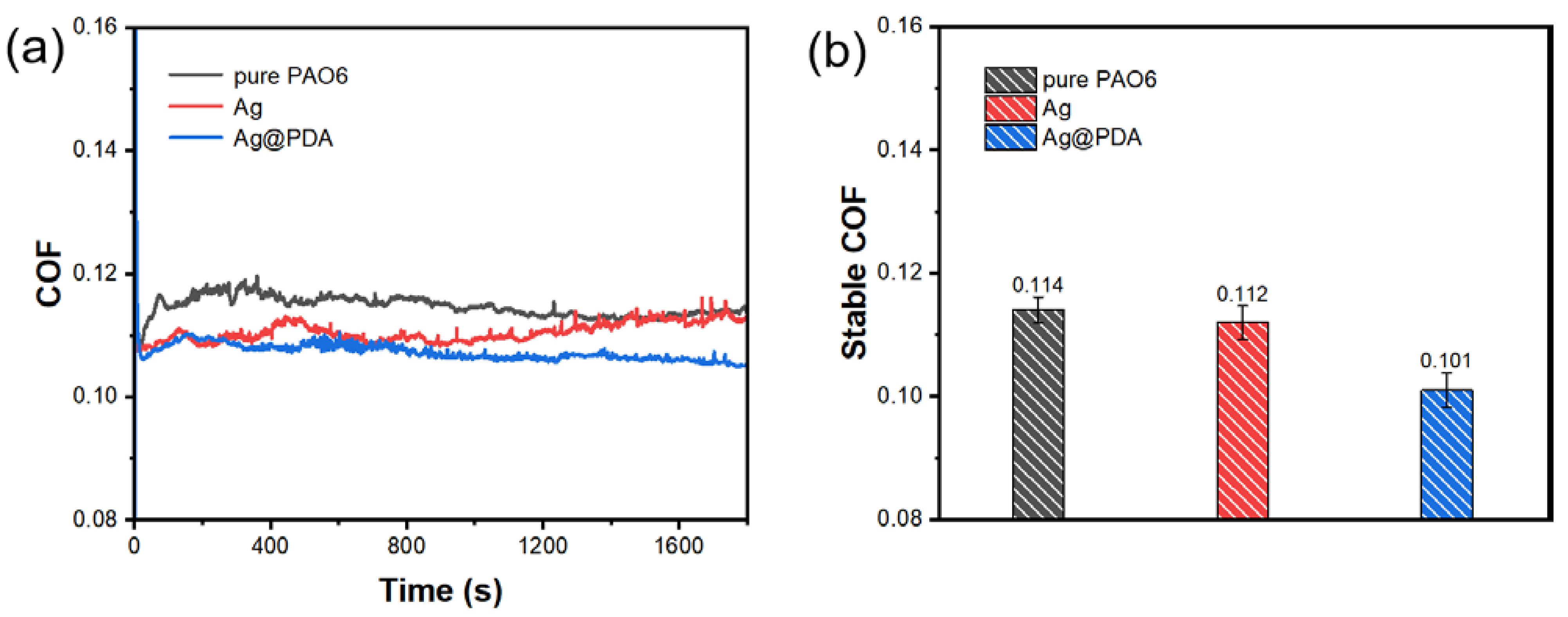
3.3.1. Different Kinds of Additives
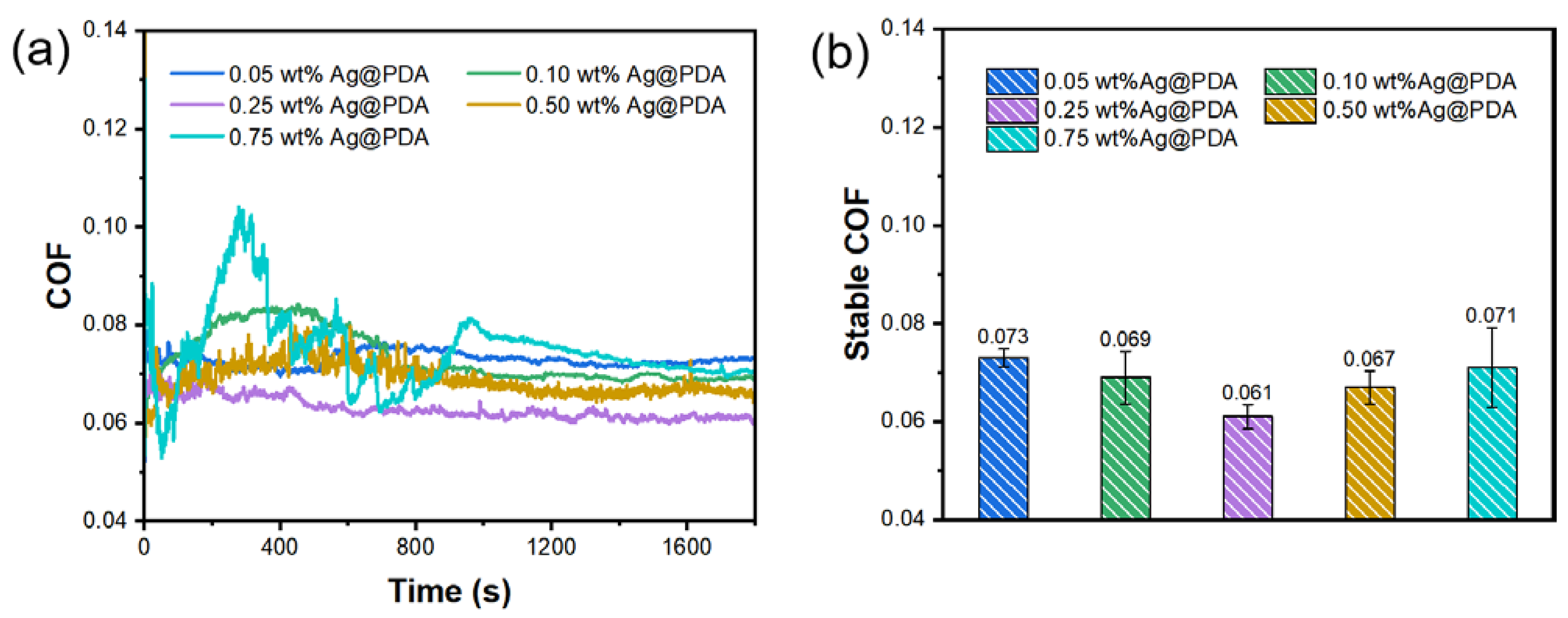
3.3.2. Different Concentrations of Ag@PDA
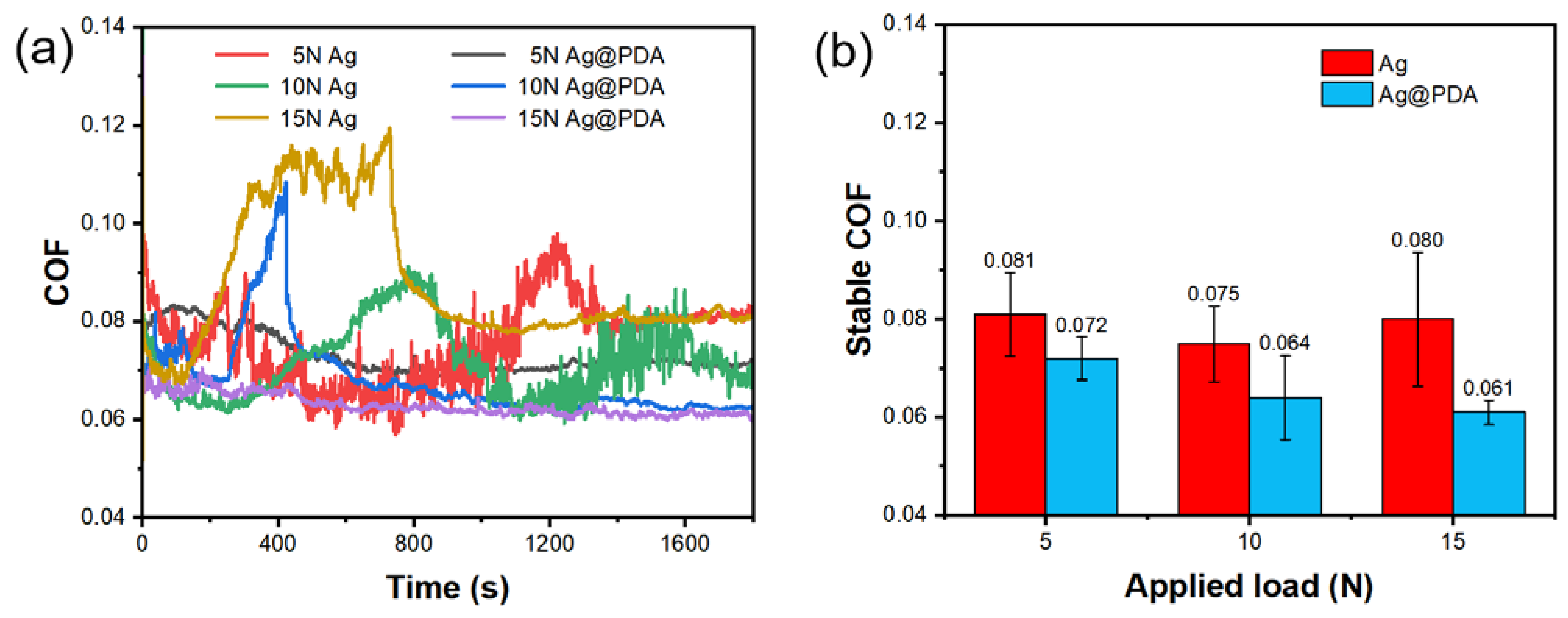
3.3.3. Different Applied Loads
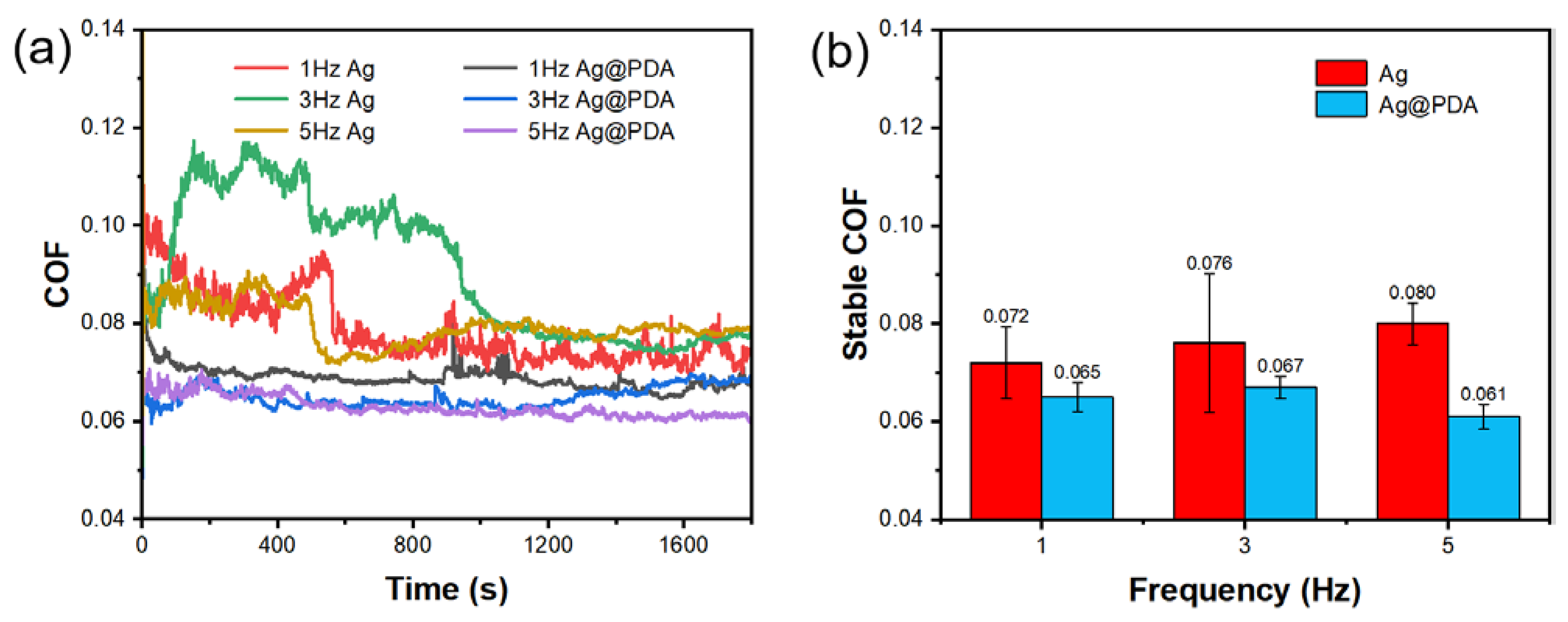
3.3.4. Different Frequencies
3.3.5. Different Friction Pairs
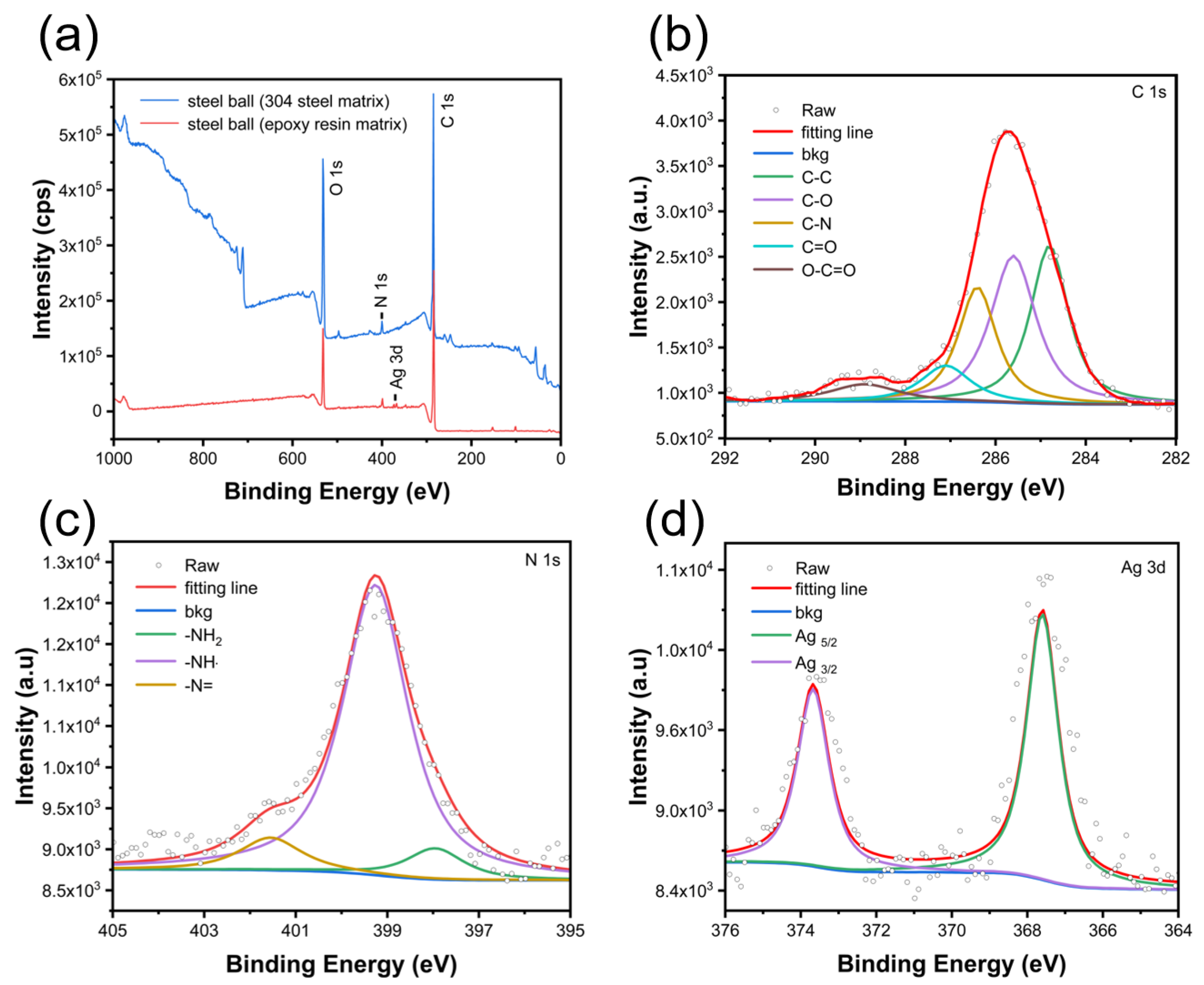
3.3.6. XPS Analysis of Wear Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerni, L.; Raina, A.; Haq, M.I.U. Friction and wear performance of olive oil containing nanoparticles in boundary and mixed lubrication regimes. Wear 2019, 426–427, 819–827. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Gao, H.; Maldonado-Cortés, D.; Vellore, A.; García-Pineda, P.; Montemayor, O.E.; Nava, K.L.; Martini, A. Effects of substrate surface roughness and nano/micro particle additive size on friction and wear in lubricated sliding. Tribol. Int. 2018, 119, 88–98. [Google Scholar] [CrossRef]
- Hernandez Battez, A.; Fernandez Rico, J.E.; Navas Arias, A.; Viesca Rodriguez, J.L.; Chou Rodriguez, R.; Diaz Fernandez, J.M. The tribological behaviour of ZnO nanoparticles as an additive to PAO6. Wear 2006, 261, 256–263. [Google Scholar] [CrossRef]
- Gundarneeya, T.P.; Vakharia, D.P. Performance analysis of journal bearing operating on nanolubricants with TiO2, CuO and Al2O3 nanoparticles as lubricant additives. Mater. Today Proc. 2021, 45, 5624–5630. [Google Scholar] [CrossRef]
- Ilie, F.; Covaliu, C. Tribological Properties of the Lubricant Containing Titanium Dioxide Nanoparticles as an Additive. Lubricants 2016, 4, 12. [Google Scholar] [CrossRef]
- Ali, Z.A.A.A.; Takhakh, A.M.; Al-Waily, M. A review of use of nanoparticle additives in lubricants to improve its tribological properties. Mater. Today Proc. 2022, 52, 1442–1450. [Google Scholar] [CrossRef]
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Thampi, A.D.; Prasanth, M.A.; Anandu, A.P.; Sneha, E.; Sasidharan, B.; Rani, S. The effect of nanoparticle additives on the tribological properties of various lubricating oils—Review. Mater. Today Proc. 2021, 47, 4919–4924. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Bicheng, C.; Turkson, R.F.; Qingping, C. Reducing frictional power losses and improving the scuffing resistance in automotive engines using hybrid nanomaterials as nano-lubricant additives. Wear 2016, 364–365, 270–281. [Google Scholar] [CrossRef]
- Szabó, Á.I.; Tóth, Á.D.; Leskó, M.Z.; Hargitai, H. Investigation of the Applicability of Y2O3–ZrO2 Spherical Nanoparticles as Tribological Lubricant Additives. Lubricants 2022, 10, 152. [Google Scholar] [CrossRef]
- Hernández Battez, A.; González, R.; Viesca, J.L.; Fernández, J.E.; Díaz Fernández, J.M.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Singh, Y.; Rahim, E.A.; Singh, N.K.; Sharma, A.; Singla, A.; Palamanit, A. Friction and wear characteristics of chemically modified mahua (madhuca indica) oil based lubricant with SiO2 nanoparticles as additives. Wear 2022, 508–509, 204463. [Google Scholar] [CrossRef]
- Ahmad, U.; Raza Naqvi, S.; Ali, I.; Saleem, F.; Taqi Mehran, M.; Sikandar, U.; Juchelková, D. Biolubricant production from castor oil using iron oxide nanoparticles as an additive: Experimental, modelling and tribological assessment. Fuel 2022, 324, 124565. [Google Scholar] [CrossRef]
- Mariño, F.; López, E.R.; Arnosa, Á.; González Gómez, M.A.; Piñeiro, Y.; Rivas, J.; Alvarez-Lorenzo, C.; Fernández, J. ZnO nanoparticles coated with oleic acid as additives for a polyalphaolefin lubricant. J. Mol. Liq. 2022, 348, 118401. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. [Google Scholar] [CrossRef]
- Hong, F.T.; Schneider, A.; Sarathy, S.M. Enhanced lubrication by core-shell TiO2 nanoparticles modified with gallic acid ester. Tribol. Int. 2020, 146, 106263. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, F.; Barber, G.C.; Zou, Q.; Wang, J.; Guo, S.; Yuan, Y.; Jiang, Q. Role of nano-sized materials as lubricant additives in friction and wear reduction: A review. Wear 2022, 490-491, 204206. [Google Scholar] [CrossRef]
- Huang, Y.; Han, S.; Liu, S.; Wang, Y.; Li, J. Preparation and tribological properties of surface-modified calcium borate nanoparticles as additive in lubricating oil. Ind. Lubr. Tribol. 2014, 66, 143–150. [Google Scholar] [CrossRef]
- Bogdan, D.; Grosu, I.-G.; Filip, C. How thick, uniform and smooth are the polydopamine coating layers obtained under different oxidation conditions? An in-depth AFM study. Appl. Surf. Sci. 2022, 597, 153680. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Zhang, M.; Dong, Z.; Zhang, K. Deposition of Ag nanoparticles on polydopamine-functionalized CNTs for improving the tribological properties of PPESK composites. Compos. Part A Appl. Sci. Manuf. 2022, 153, 106709. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, D.; Chen, X.; Yao, W.; Wang, Y.; Zheng, Z.; Tan, H.; Zhang, Y. Mussel-inspired polydopamine-modified cellulose nanocrystal fillers for the preparation of reinforced and UV-shielding poly (lactic acid) films. J. Mater. Res. Technol. 2022, 19, 4350–4359. [Google Scholar] [CrossRef]
- Chen, G.; Jin, B.; Zhao, J.; Li, Y.; He, Y.; Luo, J. Efficient one-pot synthesis of mussel-inspired Cu-doped polydopamine nanoparticles with enhanced lubrication under heavy loads. Chem. Eng. J. 2021, 426, 131287. [Google Scholar] [CrossRef]
- Dong, S.; Xiao, G.; Chen, C.; Yang, Z.; Chen, C.; Wang, Q.; Lin, L. Polydopamine enwrapped titanium dioxide-assisted dispersion of graphene to strength fire resistance of intumescent waterborne epoxy coating. Prog. Org. Coat. 2021, 157, 106291. [Google Scholar] [CrossRef]
- Huang, K.; Cao, X.; Kong, L.; Lu, Z.; Zhang, G.; Ding, Q.; Hu, H. Effect of Ag content on friction and wear properties of Ag and V co-doped CrN coatings at 25–700 °C. Ceram. Int. 2021, 47, 35021–35028. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Wang, J.; Li, H.; Huang, J.; Xiong, D. Frictional properties of silver over-coated on surface textured tantalum interlayer at elevated temperatures. Surf. Coat. Technol. 2019, 365, 189–199. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Su, F.; Li, Z.; Chen, Y.; Xu, Z. Microstructure and tribological performance of adaptive MoN–Ag nanocomposite coatings with various Ag contents. Wear 2022, 488–489, 204170. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Z.; Liu, C.; Zhao, L.; Ni, J.; Li, Y.; Shao, X.; Wang, C. The synthesis and tribological properties of Ag/polydopamine nanocomposites as additives in poly-alpha-olefin. Tribol. Int. 2017, 114, 282–289. [Google Scholar] [CrossRef]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z.; Tetyana, P.; Hlatshwayo, T.; Mlambo, M.; et al. Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arab. J. Chem. 2022, 15, 103798. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Zhang, Y.; He, F.; Jing, L.; He, X. Fabrication of magnetic alginate beads with uniform dispersion of CoFe2O4 by the polydopamine surface functionalization for organic pollutants removal. Appl. Surf. Sci. 2016, 389, 567–577. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Li, X.; Li, D.; Zhu, M. Hydroxyl-terminated ionic liquids functionalized graphene oxide with good dispersion and lubrication function. Tribol. Int. 2020, 148, 106350. [Google Scholar] [CrossRef]
- Esteban, P.P.; Jenkins, A.T.A.; Arnot, T.C. Elucidation of the mechanisms of action of Bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloids Surf. B Biointerfaces 2016, 139, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Deng, Y.; Chen, S.; Liang, W.; Zhang, L.; Wei, X.; Gao, S.; Wan, Y. Carbon quantum dots doped with silver as lubricating oil additive for enhancing tribological performance at various temperatures. Appl. Surf. Sci. 2022, 599, 154029. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Hu, Y.; Hou, B. Tribological performance and conductive capacity of Ag coating under boundary lubrication. Tribol. Int. 2017, 110, 161–172. [Google Scholar] [CrossRef]
- Kumara, C.; Luo, H.; Leonard, D.N.; Meyer, H.M.; Qu, J. Organic-Modified Silver Nanoparticles as Lubricant Additives. ACS Appl. Mater. Interfaces 2017, 9, 37227–37237. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Wang, B.; Huang, Z.; Lu, H. Ultrahigh yield synthesis of mesoporous carbon nanoparticles as a superior lubricant additive for polyethylene glycol. Dalton Trans. 2020, 49, 5283–5290. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Su, F.; Xu, X.; Chu, P.K. 2D black phosphorus dotted with silver nanoparticles: An excellent lubricant additive for tribological applications. Chem. Eng. J. 2020, 392, 123631. [Google Scholar] [CrossRef]
- Zeng, Q.; Yu, F.; Dong, G. Superlubricity behaviors of Si3N4/DLC Films under PAO oil with nano boron nitride additive lubrication. Surf. Interface Anal. 2013, 45, 1283–1290. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Zhu, M. Amine-terminated ionic liquid modified graphene oxide/copper nanocomposite toward efficient lubrication. Appl. Surf. Sci. 2019, 491, 105–115. [Google Scholar] [CrossRef]
- Miao, G.; Li, F.; Gao, Z.; Xu, T.; Miao, X.; Ren, G.; Song, Y.; Li, X.; Zhu, X. Ag/Polydopamine Coated Textile for Enhanced Liquid/Liquid Mixtures Separation and Dye Removal. Iscience 2022, 25, 104213. [Google Scholar] [CrossRef]
- Yao, A.; Yan, Y.; Tan, L.; Shi, Y.; Zhou, M.; Zhang, Y.; Zhu, P.; Huang, S. Improvement of filtration and antifouling performance of cellulose acetate membrane reinforced by dopamine modified cellulose nanocrystals. J. Membr. Sci. 2021, 637, 119621. [Google Scholar] [CrossRef]
- Chen, W.; Yu, Y.; Sui, X.; Zhu, S.; Huang, G.; Yang, J. Tribological behavior of the low-pressure cold sprayed (Cu-5Sn)/Al2O3-Ag solid-lubricating coating in artificial seawater. Surf. Coat. Technol. 2020, 403, 126359. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Bai, M. Effect of Ag nanoparticles additive on the tribological behavior of multialkylated cyclopentanes (MACs). Wear 2009, 266, 627–631. [Google Scholar] [CrossRef]
- Raza, S.; Raza, M.; Zada, S.; Li, X.; Liu, C. Fabrication of biomass-derived polymer with dopamine and Ag nanoaggregates: Prevention of the biofilm of bacteria and catalytic degradation of organic dyes. Eur. Polym. J. 2021, 157, 110635. [Google Scholar] [CrossRef]
- Sarojini, S.; Vanathi Vijayalakshmi, R. XPS studies on silver ion conducting solid electrolyte SbI3-Ag2MoO4. Mater. Today Proc. 2022, 68, 314–318. [Google Scholar] [CrossRef]

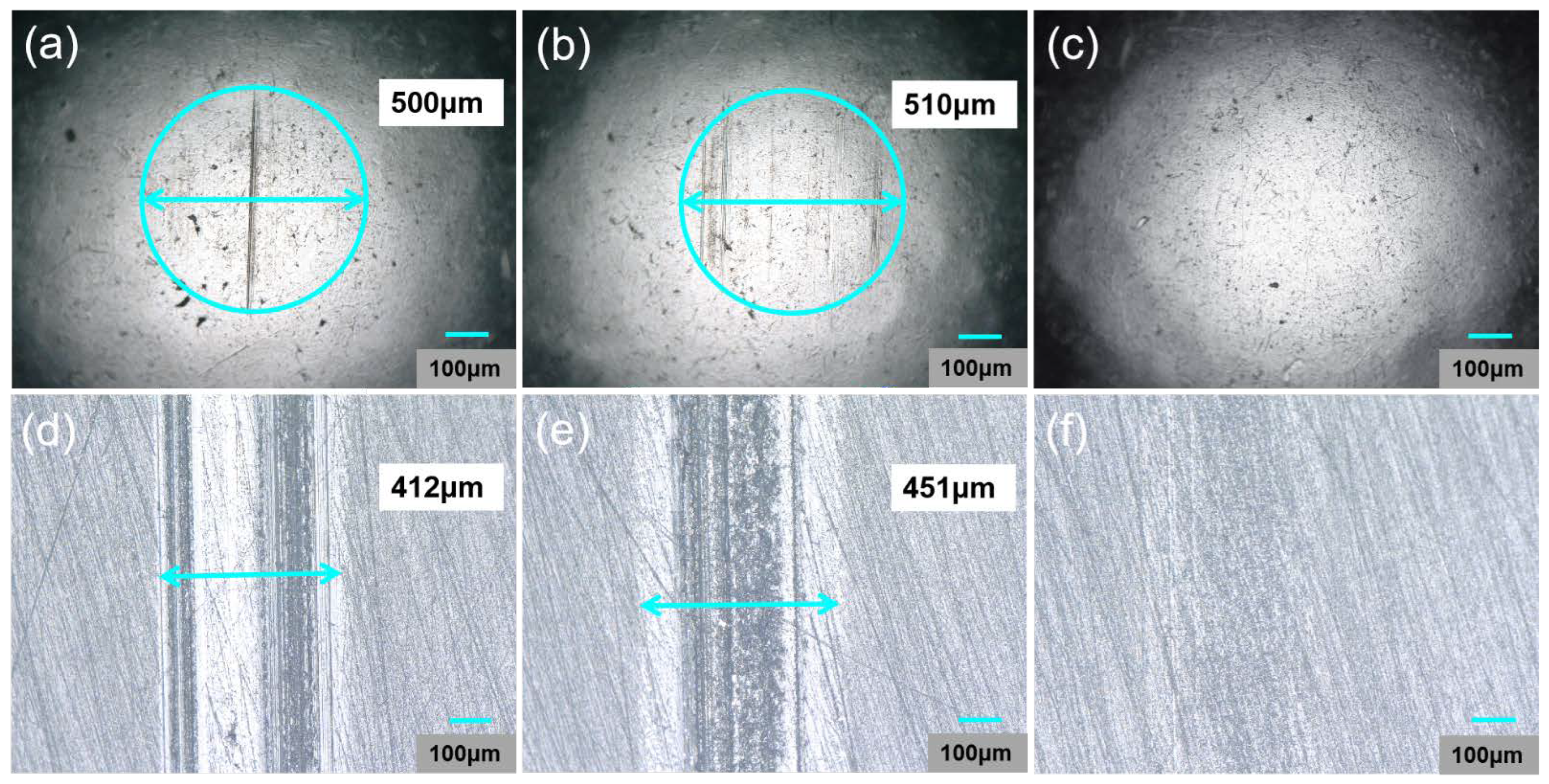
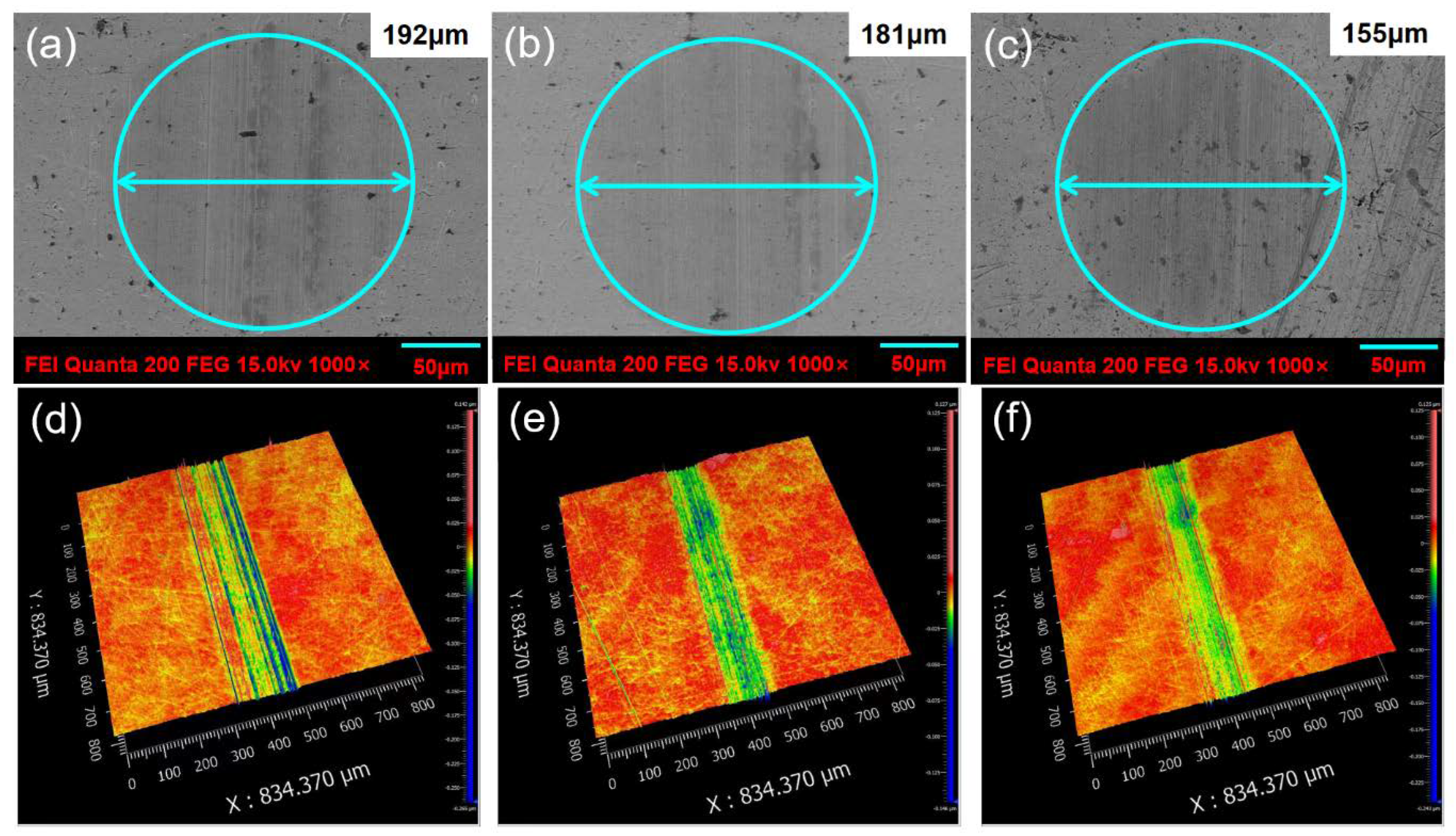
| Samples | Wear Scar Width (μm) | Wear Scar Depth (μm) |
|---|---|---|
| Pure PAO6 | 173 | 0.078 |
| 0.25 wt% Ag + PAO6 | 149 | 0.058 |
| 0.25 wt% Ag@PDA + PAO6 | 139 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cheng, J.; Lu, C.; Chen, H.; Xie, G.; Zhang, L.; Luo, J. Tribological Properties of Polydopamine-Modified Ag as Lubricant Oil Additives. Lubricants 2022, 10, 343. https://doi.org/10.3390/lubricants10120343
Zhang Y, Cheng J, Lu C, Chen H, Xie G, Zhang L, Luo J. Tribological Properties of Polydopamine-Modified Ag as Lubricant Oil Additives. Lubricants. 2022; 10(12):343. https://doi.org/10.3390/lubricants10120343
Chicago/Turabian StyleZhang, Yanxin, Jun Cheng, Changfeng Lu, Hao Chen, Guoxin Xie, Lin Zhang, and Jianbin Luo. 2022. "Tribological Properties of Polydopamine-Modified Ag as Lubricant Oil Additives" Lubricants 10, no. 12: 343. https://doi.org/10.3390/lubricants10120343
APA StyleZhang, Y., Cheng, J., Lu, C., Chen, H., Xie, G., Zhang, L., & Luo, J. (2022). Tribological Properties of Polydopamine-Modified Ag as Lubricant Oil Additives. Lubricants, 10(12), 343. https://doi.org/10.3390/lubricants10120343





