Abstract
During their service, engine oils suffer from various influencing parameters such as thermo-oxidative stress and nitration, hence, the accumulation of degradation products and the entry of contaminants. Accordingly, ICEs need to be able to operate satisfactorily, especially with a degraded lubricant, making it highly recommendable to use such oils for component testing in ICE development. Thus, a new nitrative thermo-oxidative ageing method is presented for closer-to-reality simulation of engine oil alteration with the intention to provide reproducibly aged oils for subsequent bench testing. With this method, a target used oil from field application was replicated and the comparability of oil condition in the lab vs. field regarding oxidation, nitration, additive depletion, and acidification amongst others was verified by conventional and advanced analyses. Special focus was laid on the identification of nitration products, proving them to be predominantly oxidized aromatic species or organophosphates. The presented method gives valuable benefit for the closer-to-reality ageing of engine oils in reasonable time frames with moderate costs and, hence, for the provision of test oils for ICE bench testing enabling rapid engine component assessment.
1. Introduction
The individual passenger car is still dominant for passenger transport, as are medium and heavy commercial vehicles in logistics [1,2]. In addition, there is still a strong reliance on internal combustion engines (ICE) fueled by fossil-based gasoline or diesel [2]. On the other hand, increasingly stringent legal requirements demand stricter emission limits, binding targets for CO2 emission with new and more reliable emission testing under real driving conditions, and improved fuel economy [3,4]. These facts are highly important driving factors for scientific efforts to develop improved ICE technologies with a special focus on elevated environmental friendliness [5]. Strategies to achieve these goals are manifold and include amongst others, (1) the utilization of alternative fuels to reduce carbon emissions, (2) downsizing to small 3- and 4-cylinder engines, (3) the application of advanced engine technologies, (4) development of new exhaust gas treatment systems, or (5) powertrain hybridization [5,6].
For proper operation, the ICE needs sufficient lubricant of suitable quality in terms of viscosity, film-forming properties, heat removal, surface protection, cleaning ability, acid neutralisation, low evaporation tendency, and prevention of noise and vibrations. Selecting the right lubricant should increase fuel efficiency, decrease emissions, and extend service life [7,8]. During engine operation, the engine oil is exposed to harsh conditions, i.e., high temperatures, oxidative environment, gaseous contamination with blow-by gases from combustion, liquid contamination with water, cooling agent, or unburnt fuel, solid contamination with dust, wear particles, or soot, and acidification by degradation products, amongst others [8,9]. These impact factors lead to significant oil condition changes in terms of additive depletion, built-up of degradation products, and contamination [1,10]. Accordingly, ICEs need to be able to operate satisfactorily, especially with a degraded lubricant as this is the predominant condition in everyday operation. Even right after an oil change, a significant amount of used end-of-life lubricant is retained in the engine [4].
Thus, it is highly recommended to use degraded lubricants as test oils in ICE component development as they reflect the real lubrication condition in an engine. However, degraded lubricants are not readily available in sufficient quantity and with a defined, reproducible composition. Hence, artificial alteration methods offer a great possibility to produce degraded test oils for ICE component development.
Amongst all parameters, oxidation is considered to be the main influencing parameter of oil ageing. Thus, a vast investigation has been carried out in recent decades to gain deeper insight into oxidation mechanisms [7,8] as well as antioxidant effectiveness [11]. Frauscher et al. demonstrated the application of labelling oxidation products with the stable isotope 18O together with the identification of oxidation products by gas chromatography coupled with mass spectrometry to elucidate reaction pathways occurring in ester oils with and without antioxidants and fuel components [12,13]. Furthermore, the correlation of oxidation with various oil parameters, such as acidification, base reserve depletion or soot loading was demonstrated [6].
Field studies considering oil oxidation and nitration are common [4,6,14]; cost-efficient sensors [15] and portable spectrometers [16] have been developed in recent years to enable a more accessible oil analysis for machinery operators. Nevertheless, a comprehensive understanding of chemical structures and underlying degradation processes is not available for nitration products although the quantitative determination of nitration is described in several standards [17,18] dating back almost 20 years outlining the importance of this degradation mechanism for a long period of time. Furthermore, nitration has an interesting behaviour among the degradation processes: nitration is reversible as the nitro-products produced in an ICE are thermally unstable and start to decompose above 130 °C [19]. Prior research also showed that nitration products are abundant in gasoline engines and largely absent from diesel engines [6]. This is especially interesting, as diesel engines are characterized by a higher NO2 emission compared to their gasoline counterparts, which shows that atmospheric nitrogen reacts in different ways in diesel and petrol ICEs.
In view of current and future requirements for engine oils, it is essential to understand the degradation processes occurring in the oil during engine operation. To gain comprehensive knowledge of oil damage mechanisms by various load profiles, it is not always possible to run cost-intensive engine bench tests or time-consuming fleet tests. Thus, fundamental research makes use of artificial alteration in the laboratory with the aim of simulating damage patterns comparable to the real engine within reasonable time frames [20].
There is a wide range of standards applying different strains to the oil such as high temperature, oxygen, elevated pressure, or metallic catalysts [21,22,23,24]. However, standard methods are often inadequate for a comprehensive examination of oil ageing because they usually apply limited impact factors to the oils and/or do not prescribe sufficient subsequent analyses to detect the oil condition thoroughly. Thus, the goal of research should be making artificial ageing in the laboratory more realistic by a suitable combination of influencing factors and utilisation of subsequent advanced analysis. For example, Nagy et al. added synthetic fuel components during the artificial ageing of engine oils and compared the resulting altered oils with samples collected from the field [25]. The impact of soot and diesel contamination during the ageing procedure on the friction and wear performance of the respective oils was investigated by Motamen Salehi et al. [26]. The influence of ethanol and its partial combustion products, acetic acid and acetaldehyde, on the lubricant was investigated by adding the respective components continuously during the ageing process [27]. Oil samples from the lab were compared to aliquots sampled from a chassis dynamometer bench test by principal component analysis (PCA) [28]. Using PCA, nitration was identified as the parameter where the two sample types differed most clearly. It could be demonstrated that nitration processes are important contributors to oil ageing in the car but were not considered in the laboratory method.
In summary, none of the artificial oil ageing methods currently applied consider nitration processes, although it depicts an important oil parameter in the real system with potential relevance for corrosion behaviour and emissions. Accordingly, the production of artificially aged test oils for ICE component development has to account for this degradation mechanism as well. For this reason, the aim of the study presented at hand was to set up a method for the implementation of nitration mechanisms during artificial oil ageing in the large-scale alteration device. By this new nitrative thermo-oxidative method, a target used oil from the field was replicated with the possibility to use it as test oil in close-to-reality bench testing. In the next step, the comparability of the oil condition from the lab with the target oil condition was verified by means of suitable advanced analyses. Moreover, a special focus was laid on nitration products and their possible decomposition for an in-depth understanding of the origin, propagation, and degradation of nitration compounds.
2. Materials and Methods
2.1. Definition of Target Oil Condition
For the verification of a close-to-reality simulation of engine oil degradation, previously existing knowledge on oil ageing in a real engine was taken as a reference. For this purpose, the target oil condition of the final artificially altered sample was defined based on the final sample of [4], since the applied vehicle is considered to be representative of modern use cases. The corresponding vehicle is equipped with a 1.4 L turbocharged gasoline engine, which is described in detail in [4]. Additionally, the final mileage of the selected target used oil is relatively high, approx. 20,000 km, which is representative of an end-of-life oil condition. Furthermore, since this specific used oil sample was already characterized comprehensively, it offers a very good basis for comparison from an analytical standpoint.
2.2. Engine Oil Selection
To be comparable to the ageing process presented in [4] as well as to its target oil condition, the same commercially available engine oil SAE 5W-30 was selected for artificial alteration. This oil is applicable to both diesel and gasoline engines and meets the specifications for ACEA C3, API SN, and various approvals by vehicle manufacturers, i.e., BMW longlife-04, MB 229.51, MB 229.52, VW 502.00/505.00/505.01, and GM dexos2™. In Table 1, the main physical–chemical properties of the utilized engine oil are presented. Fluctuations in the values in comparison to [4] are due to batch inhomogeneities.

Table 1.
Overview of physical–chemical properties of the applied engine oil.
2.3. Oil Alteration
The novel nitrative thermo-oxidative artificial alteration method is based on the procedure presented in [10,20]. Figure 1 gives a schematic overview of the applied large-scale device.
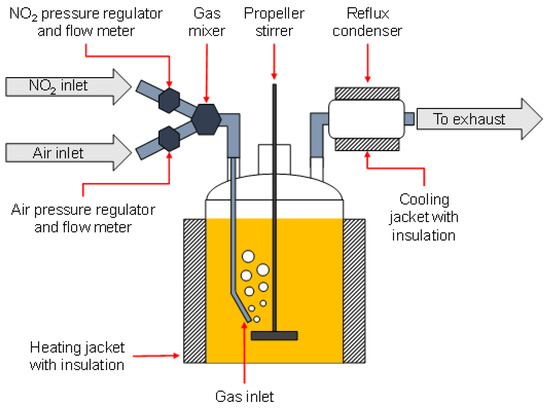
Figure 1.
Schematic set-up of the large-scale artificial alteration device.
180 L of the selected fresh engine oil were kept in a stainless-steel jacketed chemical reactor under continuous stirring by a propeller stirrer at ~480 rpm, tempered between 110 °C and 140 °C, and brought in contact with a metered flow of dried compressed air containing 0–3000 ppm NO2 over a stainless-steel pipe. Experimental parameters of temperature and NO2 contamination in the air flow were continuously adjusted to achieve the pre-defined ratio of oxidation and nitration within the alteration system. The progress of the set points of air flow, NO2 flow, and temperature is depicted in Figure 2. To ensure accurate control and adjustment of the oxidation/nitration ratio, the alteration device (heater and gas flow) was turned off during the weekends. A sample was taken and analyzed by means described in Section 2.5.1 both immediately before the shutdown and immediately after reheating and the start of the gas flow. This enabled the monitoring of the shutdown’s influence on the oil condition.
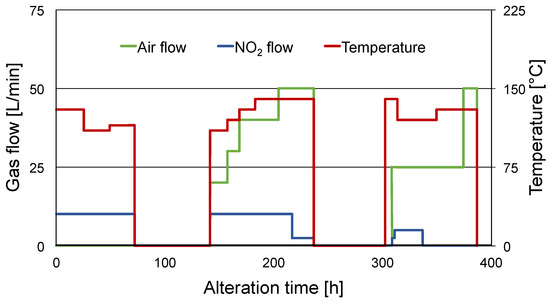
Figure 2.
Progress of set points of air flow, NO2 flow, and temperature.
Throughout the alteration procedure, oil aliquots were sampled at regular intervals for condition monitoring. The results thereof were unceasingly used as feedback to modify the experiment parameters as described above.
2.4. Heat Treatment
Prior research showed that nitration products in used engine oils show a rapid decomposition when heated above 170 °C [19]. Accordingly, an aliquot of the final sample from alteration was subjected to a heat treatment to evaluate whether the nitration products formed during ageing could be decomposed again or removed from the oil, as was the case for real used oil samples in [19]. For this procedure, approximately 120 g of the final sample was stored in an open crystallizing dish at 170 °C in a climate chamber. The oil condition was determined by FT-IR spectroscopy after 1 h, 3 h, and 7 h of heat treatment, respectively.
2.5. Oil Condition Monitoring
2.5.1. Conventional Oil Analysis
For oil degradation progress detection as well as monitoring and, if necessary, regulating the process parameters based on the current oil condition, sampled aliquots from the artificial alteration were thoroughly analyzed according to the conventional oil parameters presented in Table 2.

Table 2.
Conventional oil analysis.
2.5.2. Advanced Oil Analysis by High-Resolution Mass Spectrometry
The applied MS measurement technique was already utilized in prior research [4,10]; accordingly, only a brief summary is given below.
- Instrument: LTQ Orbitrap XL hybrid tandem high-resolution mass spectrometer (ThermoFisher Scientific, Bremen, Germany).
- Software: Xcalibur version 2.0.7 and Mass Frontier version 8.0 (ThermoFisher Scientific, Bremen, Germany).
The main focus of the MS was the characterization of various degradation products during the alteration process as well as monitoring oil additive depletion.
The samples were diluted to 1:1000. As the solvent, a methanol–chloroform mixture with a ratio of 3:7 was used. The samples were then introduced into the electrospray ionization (ESI) ion source via direct infusion at a flow rate of 5 µL/min. The ESI ion source was operated under the following conditions:
- Source voltage: 3.80 kV,
- Capillary voltage: −35 V,
- Sheath gas flow rate: 55 arb (nitrogen),
- Spray capillary temperature: 275 °C.
The generated ions were transferred to the linear ion trap section, where fragmentation occurred through collision with the applied helium collision gas (also used as a buffer gas). The high-resolution orbitrap detector was then used for the detection of the mass-to-charge ratios at 60,000 resolution measured at full width at half maximum (FWHM). The instrument was operated in negative ion mode, all measurements have a mass accuracy of at least 5 ppm or better.
3. Results and Discussion
3.1. Progress of Alteration Monitored by Conventional Analysis
By means of FT-IR, the important oil parameters oxidation and nitration as well as the depletion of various additives were detected. The progress of these values during the artificial alteration is given in Figure 3 and Figure 4.
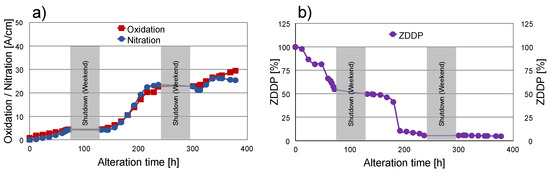
Figure 3.
Progress of alteration determined by FT-IR. (a) Oxidation and nitration; (b) ZDDP antiwear depletion. Note: Grey areas depict shutdowns during weekends.
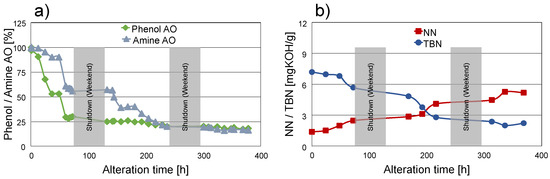
Figure 4.
Progress of alteration. (a) Phenolic and aminic antioxidant depletion; (b) acidification and base reserve depletion. Note: Grey areas depict shutdowns during weekends.
In the first 100 h of alteration, oxidation, as well as nitration, shows only a marginal increase (Figure 3a). Within the next 100 h, a steep rise happens followed by a slight slowing down of the ageing rate in the last third of the ageing process. The oxidation and nitration show very comparable levels during the whole alteration process. This behaviour was intentional, as the target final values are very close to each other, namely 30.2 A/cm for oxidation and 25.0 A/cm for nitration. Accordingly, dynamic control of the alteration parameters was applied, where the oxidation/nitration ratio was kept close to 1:1 externally by the operator to achieve the target oil condition of the reference oil.
Moreover, a significant drop in additive content was detected. The antiwear additive ZDDP degraded during the first 100 h by almost 50% from its initial concentration (Figure 3b). Afterwards, the depletion slowed down for 50 h and, subsequently, dropped down to approximately 5% for the rest of the alteration which can be considered as no remaining ZDDP. As stated in [10], the FT-IR method for concentration determination of ZDDP is not reliable for low ZDDP contents in highly ageing oil samples due to the fact that the measured peak in the area between 1020 cm−1 and 920 cm−1 cannot be exclusively attributed to the original ZDDP but is also influenced by degradation products and, hence, gives false residual values.
Antioxidants also experience a decrease right in the first third of the ageing process, with phenolic AOs falling to 25% of their initial concentration and, hence, degrading much faster than aminic AOs decreasing to 50% (Figure 4a). This phenomenon is well known from the literature [33,34,35], where a synergistic behaviour between sterically hindered phenols and aminic AOs is described based on the regeneration of the aminic compounds by a H radical transfer from the phenolic AO. After the second third of the alteration process, the concentration of aminic AOs decreased analogous to the phenol concentration to a low residual value which is maintained for the rest of the alteration process. This can be attributed to measurement uncertainties since various degradation products might cause spectral interferences with both phenolic and aminic antioxidants. Accordingly, MS was utilized to confirm the findings. Trace amounts of aminic AOs were detected after approx. 170 h of alteration and complete depletion after approx. 215 h. This corresponds to FT-IR findings where the final residual value of the aminic AOs is attained at approx. 230 h underlining the importance of advanced analytical methods such as MS, especially considering analyte selectivity. Nonetheless, trends of AO depletion are shown correctly by FT-IR only with a minor offset. It has to be mentioned that direct measurement of phenolic AOs via MS is problematic utilizing direct infusion, due to conflicts with ZDDP. For this, different techniques can be used such as the methodology presented in [12] but were not applied in this case. However, it can be assumed that the same trends regarding remaining content apply to phenolic as to aminic AOs.
Furthermore, another observation has to be mentioned. By analysis of samples drawn directly before the weekend shutdown as well as right after the restart, it has been verified that there was no continuation of the ageing processes during the weekend shutdown. This means a great advantage for the operator of the ageing method enabling the user to stop the procedure at any time and still avoid the continuation of uncontrolled ageing in the engine oil.
Due to oxidation, acidic components are built-up during oil degradation which are partly neutralised by basic additives comprising the base reserve in engine oils. Figure 4b contrasts the progress of acidification as NN with the depletion of the base reserve, expressed as TBN. NN and TBN were determined only for selected samples; accordingly, the time resolution of the displayed graphs is lower compared to the FT-IR results.
Right from the beginning, the NN shows a very constant increase from initially 1.4 mg KOH/g to an almost constant level at approximately 5.2 mg KOH/g after 200 h. This level is maintained until the end of the procedure. In contrast, TBN values undergo only a slight decrease from an initial 7.2 mg KOH/g to 6.8 mg KOH/g in the first 50 h followed by an accelerated depletion to a constant low level at 2.0–2.2 mg KOH/g reached after 200 h of alteration and retained until the termination of the alteration process. It has to be mentioned that the remaining constant level of TBN values does not necessarily mean that there is still about 30% of the base reserve in the oil. It is much more probable that these elevated values can be attributed to a limitation in the applied titration method. It is known from the literature that various other components in the oil besides the calcium carbonate base reserve such as dispersant bases, aminic Aos, or other ashless amine additives can be detected by titration according to ASTM D 2896 resulting in false elevated values [36]. Thus, a total depletion of the base reserve after approximately 200 h can be assumed.
Figure 5a displays the content of typical additive elements, namely calcium (Ca), phosphorus (P), sulfur (S), and zinc (Zn) during the alteration. All mentioned elements showed a relatively constant content in the engine oil, with no significant trends indicating elemental loss, e.g., due to deposit formation, or elemental accumulation, e.g., due to base oil evaporation, can be observed. In the case of S, the measured content shows some fluctuations, which can be attributed to the relatively high concentration and the applied measurement method. Nevertheless, no significant changes can be observed during the alteration in the case of S either.
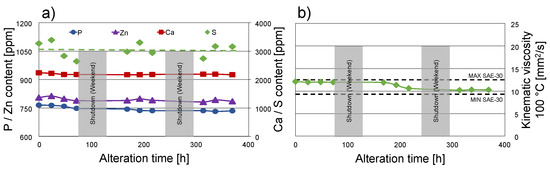
Figure 5.
Progress of alteration. (a) Elemental composition; (b) kinematic viscosity at 100 °C. Note: Grey areas depict shutdowns during weekends.
The kinematic viscosity at 100 °C is depicted in Figure 5b. It was selected as a basis of comparison since the SAE J300 standard defines the range of low-shear-rate kinematic viscosity at this temperature for SAE-30 engine oils [37]. The kinematic viscosity lies at 12.1 mm2/s for the fresh oil and drops to approximately 10.2–10.4 mm2/s towards the end of the procedure. Since both of these values lie within the requirements of a viscosity grade SAE 30 (depicted by the dotted lines) [37], the viscosity change throughout the ageing process can be regarded as not significant. However, this viscosity behaviour was expected since the focus of the alteration has been put on the chemical degradation of the oil. Physical changes such as oil thinning due to shearing of VI improvers or due to dilution by liquid contaminants as well as oil thickening due to polymerization effects or soot accumulation were not considered in the ageing process.
3.2. Comparison with the Target Oil Condition
3.2.1. Conventional Parameters
For a direct comparison of parameters, the same fresh oil was used for the alteration. Although the same product was sourced from the same vendor, some batch inhomogeneities between the two fresh oils are present. Most notably the fresh oil used for the alteration contains molybdenum (Mo), which was not present in the fresh oil used for the field test, which indicates some changes in the utilized additive package. Nevertheless, this does not influence the comparison to a great extent, as such batch-to-batch differences are expected during the operation of lubricated systems.
Figure 6 compares the FT-IR difference spectra of the target used oil and the final altered samples (the respective fresh oil spectra were subtracted). As displayed, the FT-IR spectra are showing a good correlation. Furthermore, the whole fingerprint ranges of the two samples are very well comparable suggesting excellent chemical comparability of the two samples. Fingerprint ranges are typically used for the determination of the identity of a sample, as they are complex, and accordingly hard to interpret. In this regard, a comparison of selected additives was performed by MS instead of FT-IR and is presented in Section 3.2.2.
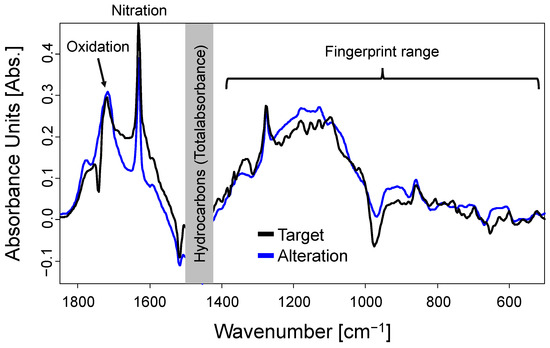
Figure 6.
Comparison of the FT-IR difference spectra of the target used oil and the final altered sample.
Figure 7 shows the comparison of the target used oil and the final altered sample. Both fresh oils used for the field test and the alteration are also shown to give an overview of the batch inhomogeneities. As displayed, oxidation and nitration are very well comparable (Figure 7a). ZDDP and phenolic as well as aminic antioxidants are showing severe degradation and are almost completely depleted in the target used oil and the final altered sample (Figure 7b,c, respectively). Regarding NN and TBN, the two fresh oil batches show some minor differences, the fresh oil batch used for the alteration displays marginally lower NN and somewhat higher TBN. Nevertheless, the target used oil and the final altered sample are very well comparable regarding NN increase as well as TBN decrease (Figure 7d). For the elemental composition (Figure 7e), the two fresh oil batches show comparable values regarding Ca, P, and Zn. A noteworthy difference between the fresh oil batches is a significantly higher S amount in the fresh oil used for the alteration. Ca, S, P, and Zn stay relatively constant during the on-road utilization and the artificial alteration as well as the target used oil and the final altered sample are well comparable to the respective fresh oil. The kinematic viscosity (at 100 °C in Figure 7f) is in the range defined by the SAE J300 standard [37] for SAE-30 oils (displayed as dotted lines) for all oil samples. Accordingly, they only show negligible changes in the field test as well as in the alteration (Figure 7f). Some minor thinning of the altered sample is observable compared to the respective fresh oil, but differences are expected, as viscosity is influenced by contaminations, most notably soot accumulation and fuel dilution in an internal combustion engine. However, the simulation of those degradation parameters was not in the scope of this work. Table 3 repeats the comparison of all measured conventional values on a numerical basis.
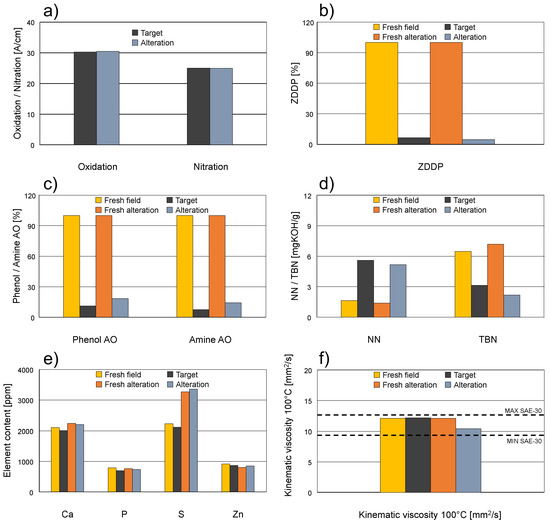
Figure 7.
Comparison of the target used oil and the final altered sample. (a) Oxidation and nitration; (b) ZDDP depletion; (c) phenolic and aminic antioxidant depletion; (d) acidification and base reserve depletion; (e) elemental composition; (f) kinematic viscosity at 100 °C.

Table 3.
Comparison of the physical–chemical properties of the target used oil with the final altered sample.
3.2.2. Advanced Oil Analysis by High-Resolution Mass Spectrometry
Figure 8 displays the comparison of the identified nitration products in the final altered and the target used oil samples. The nitration products are either organophosphate-based (Figure 8a) or aromatic (Figure 8b), and both are showing oxidation in the form of hydroxyl groups. Generally, very similar structures were detected in the final altered as well as the target used oil, indicating good chemical comparability of the nitration. It has to be noted that organophosphate-based species show a higher abundance in the altered oil, while aromatic structures exhibit a higher abundance in the target used oil. As this difference cannot be sufficiently explained based on the available data, further research is needed to investigate the reasons for this dissimilarity.
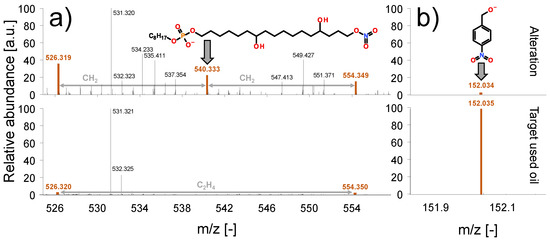
Figure 8.
Comparison of the target used oil and the final altered sample. (a) Phosphate-based nitration products; (b) aromatic nitration products.
High-resolution MS indicates differences in the length of the long alkyl sidechain (C17 alkyl sidechain shown in Figure 8). The organophosphate most likely originates from the degradation of the ZDDP, as it shows a C8 alkyl sidechain as well, which corresponds to the fresh ZDDP additive (please note that the identified species are differing on the long alkyl sidechain). ZDDP degradation is generally faster in petrol engines [6,19], and nitration is also generally higher compared to diesel counterparts [6,19]. The identified structures suggest a close relationship between the degradation of ZDDP and the origination of nitration. Recent studies [6] explain the overall faster additive depletion and degradation product accumulation in petrol engines with the lower air-to-fuel ratio (AFR) in petrol engines leading to the origination of reactive species, as the combustion is less complete. This is particularly occurring at high loads or low engine temperatures where the injection of extra petrol is needed to compensate for the condensation of the fuel on the relatively cold cylinder wall. It seems that the polar nitro groups are reacting predominantly with polar reaction partners, such as ZDDP degradation products or oxidized species. Fewer reactions are shown with nonpolar hydrocarbons, as hydrocarbons with only a nitro group were not identified in a higher abundance in either the altered or the target used oil sample. To accurately describe the possible reaction pathways involved in the origination of nitration products in an internal combustion engine further research with a particular focus on the mechanism is needed.
The degradation of ZDDP is a well-researched topic, presented in detail for the target field test in [4]. To summarize the degradation steps, the dialkyl dithiophosphates are oxidized, producing dialkyl thiophosphates as an intermediary product, and subsequently dialkyl phosphates through further oxidation. Additionally, sulfuric, phosphoric, and nitric acid were observed, and sulfuric and phosphoric acid can partially be attributed to ZDDP degradation [4]. For the displayed comparison, similar oxidation levels were selected from the target field test.
The presented evaluation compares the main ZDDP component in the respective fresh oils. As shown in Figure 9a, the original ZDDP depletes rapidly, only detectable in traces at 10 A/cm oxidation in both the field test as well as in the alteration. This finding once again corresponds to the FT-IR results, which showed the close-to-complete depletion of ZDDP in this oxidation range. Furthermore, the overall very similar depletion of the antiwear additive highlights the comparability of the generated artificially aged sample with the target composition of the field test.
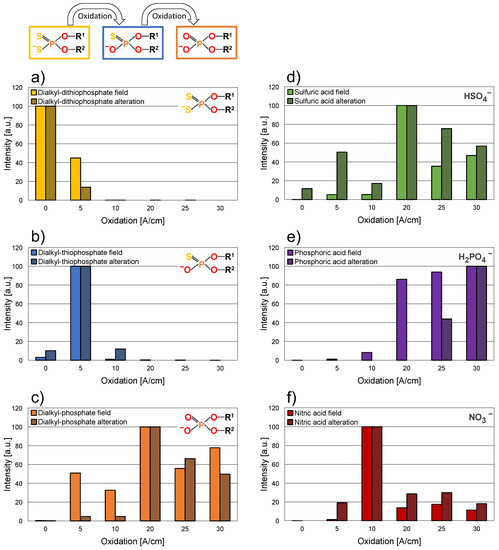
Figure 9.
Comparison of the target field test and the alteration. (a) Dialkyl dithiophosphates; (b) dialkyl thiophosphates; (c) dialkyl phosphates; (d) sulfuric acid; (e) phosphoric acid; (f) nitric acid.
The first degradation products, dialkyl thiophosphates (Figure 9b) are originating rapidly, peaking at around 5 A/cm oxidation, and subsequently decomposing rapidly. Again, the target field test and the alteration are showing very similar trends. Dialkyl phosphates originating from further oxidation of the dialkyl thiophosphates are detectable from 5 A/cm oxidation in both the target field test and the alteration. The target field test shows a somewhat higher relative abundance in the low oxidation regime, up to approx. 10 A/cm oxidation, but the overall trends are comparable, especially from 20 A/cm oxidation onward. In general, dialkyl phosphates are detectable at higher oxidation in a greater relative abundance, after the depletion of dialkyl dithiophosphates and thiophosphates, and remain in a higher relative abundance in both the target field test as well as in the alteration. (Please note that some batch inhomogeneity was identified in the alkylation of the ZDDP in the fresh oils.) During the field test, dihexyl dithiophosphate (m/z 297.111) was observed as the main component, for the fresh oil batch used for the alteration propyl-octyl dithiophosphate (m/z 283.097) was found to be the most prevalent. Accordingly, “dialkyl thiophosphates” are referring to dihexyl thiophosphate (m/z 281.134) in the target fields test and propyl-octyl thiophosphate (m/z 267.120) in the alteration, similarly, “dialkyl phosphates” are referring to dihexyl phosphate (m/z 265.156) and propyl-octyl phosphate (m/z 251.142), respectively.
Furthermore, the inorganic acids sulfuric, phosphoric, and nitric acid are shown in Figure 9d,e. Sulfuric acid is detectable at a somewhat lower, and nitric acid at a slightly higher, oxidation in the case of the alteration. Nevertheless, the overall trends are very comparable and underline the chemical comparability of the used and artificially altered oil samples.
3.3. Heat Treatment and Depletion of Nitration Products
A heat treatment of the final altered oil sample was performed to investigate the depletion of nitration products at elevated temperatures. Prior research showed that depletion of the nitration products starts above 170 °C in used engine oils [19], so the temperature of the heat treatment was chosen accordingly. Figure 10 displays the changes in oxidation and nitration (measured by FT-IR) during the heat treatment. As shown, nitration depletes rapidly during the heat treatment, and only a very minor residual nitration is visible after 7 h. Comparatively, only a minor increase in oxidation is measured, which shows that the underlying chemical changes mostly affect the nitration products. This corresponds to the findings reported in [19], where similarly a minor oxidation increase and a close-to-complete depletion of nitration were seen. Accordingly, the depletion of nitration products at elevated temperatures is very similar to that of used oils.
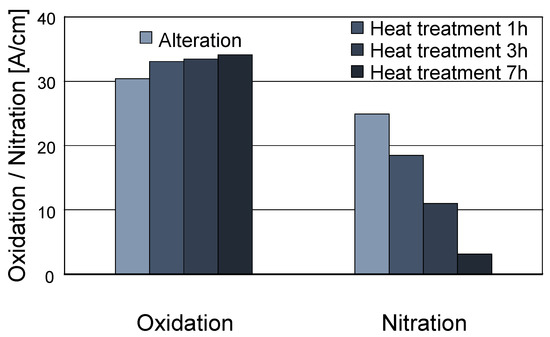
Figure 10.
Changes in oxidation and nitration due to heat treatment at 170 °C.
Figure 11 shows the mass spectra corresponding to the identified homologue-series of nitration products during the heat treatment. The identified structures are not detectable after the heat treatment, which corresponds to the findings of FT-IR regarding the depletion of nitration products.
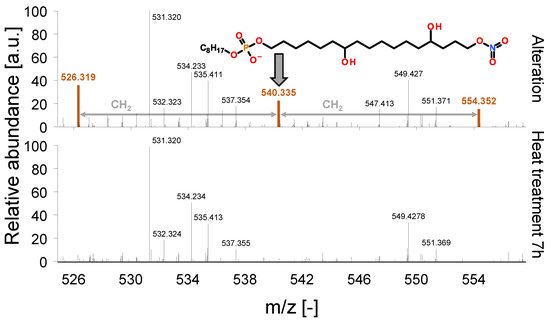
Figure 11.
Depletion of nitration structures.
4. Conclusions
The aim of the study in hand was the implementation of a novel nitrative thermo-oxidative method for artificial oil ageing to simulate close-to-reality engine oil degradation to enable the production of degraded oils with the pre-defined condition to be applied as test oils for ICE development.
The time-resolved analysis of the alteration showed increasing oxidation and nitration with close-to-complete depletion of ZDDP as well as the phenolic and aminic antioxidants in 200 h. Furthermore, the increasing oxidation provoked significant acidification of the engine oil sample and a close-to-complete depletion of the base reserve additives. Comparatively, elemental composition and kinematic viscosity only displayed minor changes.
Comparison of the target used oil and the final altered sample on basis of conventional oil condition parameters showed a similar degradation process in the laboratory to on-road utilization. Oxidation, nitration, antioxidant and antiwear additive depletion, NN and TBN and elemental composition were almost identical in the target used oil and the final altered sample, apart from the S content which can be traced back to inhomogeneities of the fresh oil batches.
Mass spectrometry showed that the nitration products are predominantly oxidized aromatic species or organophosphates (ZDDP degradation products) in the final altered sample. This finding is especially interesting, as petrol engines are characterized by higher nitration and faster ZDDP degradation compared to diesel counterparts, which corresponds to the identified structures containing both nitro and organophosphate groups. Identical nitration products were detected in the target used oil, although the ratio of aromatic and organophosphate-based nitration products differed from the final altered sample. Degradation of the ZDDP additive was generally comparable in the alteration and the target field test, where similar relative abundances of residual original ZDDP, as well as dialkyl thiophosphates and dialkyl phosphates, were observed at the same oxidation levels. Accordingly, the final altered and target used oils showed good comparability regarding additive degradation and the resulting chemical structures, even on the molecular level.
Results of the heat treatment of the final altered oil proved, as known from the literature, that nitration products are not thermally stable and highlighted that further research is needed to completely understand the underlying chemistry of this degradation process.
Concluding, the final altered and the target used oils showed high chemical comparability regarding all conventional oil parameters, as well as the structure of nitration and ZDDP degradation products. Considering that the alteration was completed within 3 weeks while the corresponding field test took place over 10 months these findings highlight the time and cost efficiency of close-to-reality oil degradation Furthermore, tailor-made altered oil can be produced on a scale suitable to supply test oils for the industry for product development purposes.
Author Contributions
Conceptualization: C.B., A.A. and M.F.; methodology: C.B., A.A., A.R. and M.F.; formal analysis: A.A. and A.R.; investigation: C.B., A.A. and M.F.; resources: M.F.; data curation: A.A.; writing—original draft preparation: C.B., A.A. and A.R.; writing—review and editing: C.B. and M.F.; visualization: A.A.; supervision: M.F.; project administration: C.B.; funding acquisition: M.F. All authors have read and agreed to the published version of the manuscript.
Funding
Presented results were realized in research projects with financial support from the participating project partners and the Austrian COMET program (Project InTribology, No. 872176). The COMET program is funded by the Austrian Federal Government and concerning InTribology by the provinces of Lower Austria and Vorarlberg.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forschung & Lehre: Geht das Zeitalter des Autos zu Ende? Available online: https://www.forschung-und-lehre.de/zeitfragen/geht-das-zeitalter-des-autos-zu-ende-1831 (accessed on 12 July 2022).
- European Automobile Manufacturers’ Association (ACEA): ACEA Report–Vehicles in Use–Europe 2022. Available online: https://www.acea.auto/files/ACEA-report-vehicles-in-use-europe-2022.pdf (accessed on 12 July 2022).
- European Comission: Emissions in the Automotive Sector. Available online: https://ec.europa.eu/growth/sectors/automotive-industry/environmental-protection/emissions-automotive-sector_en (accessed on 12 July 2022).
- Dörr, N.; Agocs, A.; Besser, C.; Ristic, A.; Frauscher, M. Engine Oils in the Field: A Comprehensive Chemical Assessment of Engine Oil Degradation in a Passenger Car. Trib. Lett. 2019, 67, 68. [Google Scholar] [CrossRef]
- Nagy, A.L.; Agocs, A.; Ronai, B.; Raffai, P.; Rohde-Brandenburger, J.; Besser, C.; Dörr, N. Rapid Fleet Condition Analysis through Correlating Basic Vehicle Tracking Data with Engine Oil FT-IR Spectra. Lubricants 2021, 9, 114. [Google Scholar] [CrossRef]
- Agocs, A.; Nagy, A.L.; Tabakov, Z.; Perger, J.; Rohde-Brandenburger, J.; Schandl, M.; Besser, C.; Dörr, N. Comprehensive assessment of oil degradation patterns in petrol and diesel engines observed in a field test with passenger cars–Conventional oil analysis and fuel dilution. Tribol. Int. 2021, 161, 107079. [Google Scholar] [CrossRef]
- Sejkorova, M.; Hurtova, I.; Jilek, P.; Novak, M.; Voltr, O. Study of the Effect of Physicochemical Degradation and Contamination of Motor Oils on Their Lubricity. Coatings 2021, 11, 60. [Google Scholar] [CrossRef]
- Wei, L.; Chen, S.; Sun, X.; Jin, Y.; Jia, D.; Li, J.; Duan, H. Study on comprehensive degradation stability of special and nonspecific motor oils. Lubr. Sci. 2021, 33, 92–99. [Google Scholar] [CrossRef]
- Mang, T.; Dresel, W. Lubricants and Lubrication, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of engine oils with defined degree of degradation by means of a large scale artificial alteration method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- Frauscher, M.; Agocs, A.; Besser, C.; Rögner, A.; Allmaier, G.; Dörr, N. Time-Resolved Quantification of Phenolic Antioxidants and Oxidation Products in a Model Fuel by GC-EI-MS/MS. Energy Fuels 2020, 34, 2674–2682. [Google Scholar] [CrossRef]
- Frauscher, M.; Besser, C.; Dörr, N.; Allmaier, G. Oxidation products of ester-based oils with and without antioxidant identified by stable isotope labelling and mass spectrometry. Appl. Sci. 2017, 7, 396. [Google Scholar] [CrossRef]
- Frauscher, M.; Besser, C.; Allmaier, G.; Dörr, N. Elucidation of oxidation and degradation products of oxygen containing fuel components by combined use of a stable isotopic tracer and mass spectrometry. Anal. Chim. Acta 2017, 993, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kral, J., Jr.; Konecny, B.; Kral, J.; Madac, K.; Fedorko, G.; Molnar, V. Degradation and chemical change of longlife oils following intensive use in automobile engines. Measurement 2014, 50, 34–42. [Google Scholar] [CrossRef]
- Shinde, H.M.; Bewoor, A.K. Evaluating petrol engine oil deterioration through oxidation and nitration parameters by low-cost IR sensor. Appl. Petrochem. Res. 2022, 10, 83–94. [Google Scholar] [CrossRef]
- Eralytics GmbH: Used-Oil Analysis Using a Portable FTIR Spectrometer. Available online: https://eralytics.com/wp-content/uploads/ERASPEC-OIL-article-PIN.pdf (accessed on 12 July 2022).
- DIN 51453; Testing of Lubricants–Determination of Oxidation and Nitration of Used Motor Oils–Infrared Spectrometric Method. Deutsches Institut für Normung: Berlin, Germany, 2004.
- ASTM E 2412; Standard Practice For Condition Monitoring Of Used Lubricants By Trend Analysis Using Fourier Transform Infrared (FT-IR) Spectrometry. ASTM International: West Conshohocken, PA, USA, 2018.
- Agocs, A.; Budnyk, S.; Frauscher, M.; Ronai, B.; Besser, C.; Dörr, N. Comparing oil condition in diesel and gasoline engines. Ind. Lubr. Tribol. 2020, 72, 1033–1039. [Google Scholar] [CrossRef]
- Agocs, A.; Budnyk, S.; Besser, C.; Ristic, A.; Frauscher, M.; Ronai, B.; Dörr, N. Production of Used Engine Oils with Defined Degree of Degradation in a Large-scale Device: Correlation of Artificially Altered Oils with Field Samples. ACTA Tech. Jaurinensis 2020, 13, 131–150. [Google Scholar] [CrossRef]
- CEC L-48-A00; Oxidation Stability of Lubricating Oils Used in Automotive Transmissions by Artificial Ageing. Co-Ordinating European Council for the Development of Performance Tests for Fuels, Lubricants and other Fluids: Brussels, Belgium, 2014.
- ASTM D 6186; Standard Test Method for Oxidation Induction Time of Lubricating Oils by Pressure Differential Scanning Calorimetry (PDSC). ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D 943; Standard Test Method for Oxidation Characteristics of Inhibited Mineral Oils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D 2272; Standard Test Method for Oxidation Stability of Steam Turbine Oils by Rotating Pressure Vessel. ASTM International: West Conshohocken, PA, USA, 2014.
- Nagy, A.L.; Rohde-Brandenburger, J.; Zsoldos, I. Artificial Aging Experiments of Neat and Contaminated Engine Oil Samples. Lubricants 2021, 9, 63. [Google Scholar] [CrossRef]
- Motamen Salehi, F.; Morina, A.; Neville, A. The effect of soot and diesel contamination on wear and friction of engine oil pump. Tribol. Int. 2017, 115, 285–296. [Google Scholar] [CrossRef]
- Besser, C.; Schneidhofer, C.; Dörr, N.; Novotny-Farkas, F.; Allmaier, G. Investigation of long-term engine oil performance using lab-based artificial ageing illustrated by the impact of ethanol as fuel component. Tribol. Int. 2012, 46, 174–182. [Google Scholar] [CrossRef]
- Besser, C.; Dörr, N.; Novotny-Farkas, F.; Varmuza, K.; Allmaier, G. Comparison of engine oil degradation observed in laboratory alteration and in the engine by chemometric data evaluation. Tribol. Int. 2013, 65, 37–47. [Google Scholar] [CrossRef]
- DIN ISO 6618:2015–07; Petroleum Products and Lubricants–Determination of Acid or Base Number–Colour-Indicator Titration Method. Deutsches Institut für Normung: Berlin, Germany, 2015.
- DIN ISO 3771:1985–04; Petroleum Products; Total Base Number; Perchloric Acid Potentiometric Titration Method. Deutsches Institut für Normung: Berlin, Germany, 1985.
- ASTM D 7042; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D 2270; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken: PA, USA, 2016.
- Gatto, V.J.; Moehle, W.E.; Cobb, T.W.; Schneller, E.R. Oxidation fundamentals and its application to turbine oil testing. J. ASTM Int. 2006, 3, 1–20. [Google Scholar] [CrossRef]
- Soleimani, M.; Dehabadi, L.; Wilson, L.D.; Tabil, L.G. Antioxidants Classification and Applications in Lubricants. In Tribology, Lubricants and Additives; Johnson, D., Ed.; InTechOpen: London, UK, 2018; pp. 23–42. [Google Scholar]
- Kassler, A.; Pittenauer, E.; Dörr, N.; Allmaier, G. Development of an accelerated artificial ageing method for the characterization of degradation products of antioxidants in lubricants by mass spectrometry. Eur. J. Mass Spectrom. 2019, 25, 300–323. [Google Scholar] [CrossRef] [PubMed]
- Agiral, A.; Zalatan, D.; Sutor, P. Understanding total base number measurement. In Proceedings of the STLE 73th Annual Meeting & Exhibition, Minneapolis, MN, USA, 20–24 May 2018. [Google Scholar] [CrossRef]
- SAE J300; Engine Oil Viscosity Classification. SAE International: Warrendale, PA, USA, 2021.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).