Abstract
This paper presents an overview on the use of various classes of nanomaterials in lubricant formulations. The following classes of nanomaterials are considered: fullerenes, nanodiamonds, ultradispersed boric acid and polytetrafluoroethylene (PTFE). Current advances in using nanomaterials in engine oils, industrial lubricants and greases are discussed. Results of numerous studies combined with formulation experience of the authors strongly suggest that nanomaterials do indeed have potential for enhancing certain lubricant properties, yet there is a long way to go before balanced formulations are developed.
1. Introduction
The continuing pursuit for better fuel efficiency stands behind many recent advancements in engine technology. “Downsize and charge” has become the major development trend alongside broad acceptance of fuel stratified injection [1]. The introduction of higher power densities (around 65 kW/L and 150 Nm/L in modern diesel engines) raises performance requirements for engine oil.
Nanoadditives open new ways to maximizing lubricant performance [2,3,4]. However, there exists a certain gap, both in attitude and competence, between university researchers and lube industry professionals as their willingness to venture out for new products is concerned. Even though nanomaterials have been around for quite a while, and numerous studies have been carried out showing that nanotechnology can indeed improve the lubrication properties of oils and greases, large-scale market introduction of nano-fortified lubricants is still facing serious technical and legislative obstacles. One practical issue is that lubricant formulations must be balanced with respect to a number of properties. This hinders market entry of nanoadditives. The present communication reviews current advances in using nanomaterials in engine oils, industrial lubricants and greases. Examples are presented to demonstrate the general marketing trend of inappropriately using the word “nano” while overlooking specific technical challenges and failing to develop balanced formulations meeting stringent performance and safety requirements.
2. Results and Discussion
2.1. Fullerenes
Fullerenes are cage molecules which are claimed to enable “rolling” lubrication mechanism. This has never been actually proven. The C60 carbon material has been best studied [5]. Inorganic fullerenes comprise another class of nanomaterials with “fullerene” tag [6,7]. For instance, inorganic fullerene-like material (IF-WS2) nanoparticles can be synthesized by reacting sulfur with tungsten trioxide (WO3) nanoparticles in a hydrogen atmosphere at 500–650 °C [7]. The IF-WS2 nanoparticles have a closed hollow cage structure with an average size of about 50 nm (see Figure 1), which is much larger than the size of the C60 molecule.
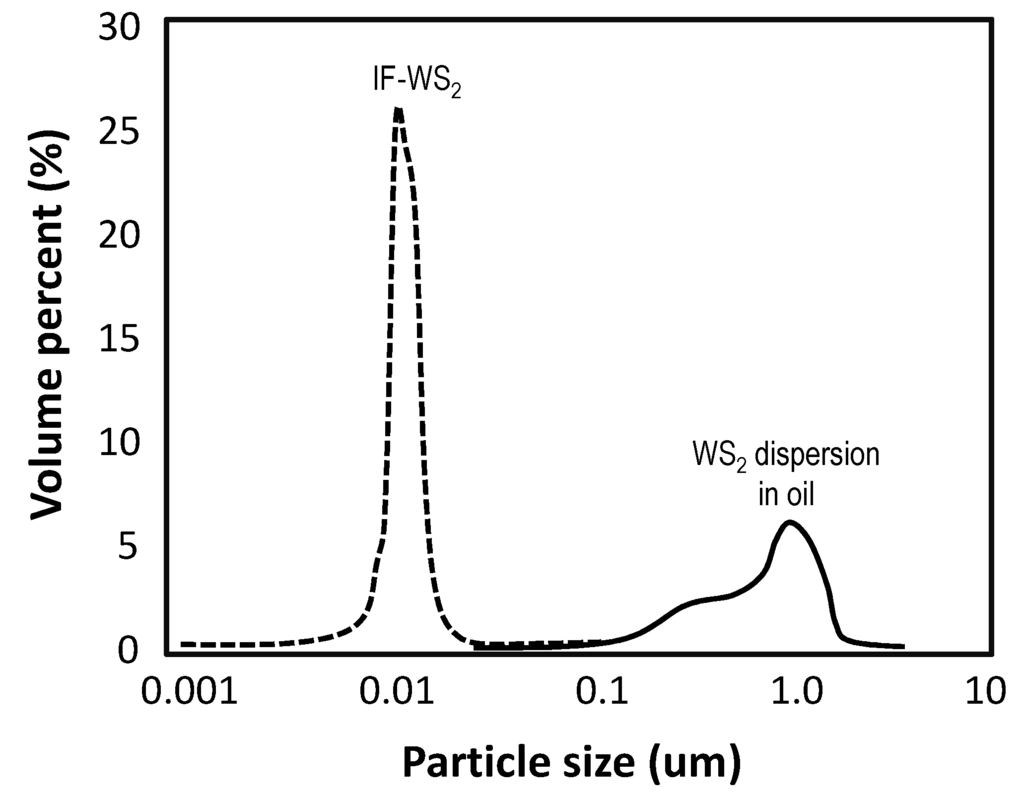
Figure 1.
Particle size distributions for IF-WS2 and a regular WS2 dispersion in oil.
Figure 1.
Particle size distributions for IF-WS2 and a regular WS2 dispersion in oil.
Studies suggest that addition of C60 fullerene soot in a lubricant significantly increases the weld load and seizure resistance [8]. C60 fullerene soot and IF-WS2 nanoparticles form much more stable dispersions in hydrocarbons as compared to regular graphite and WS2 powders. Apart from improved dispersion stability, IF-WS2 does not appear to offer any obvious performance benefits over regular WS2 powder. For instance, when used in grease, IF-WS2 scores below regular WS2 in a number of tribological properties, see Table 1.

Table 1.
Effects of WS2 and IF-WS2 on the tribological properties of lubricating grease. The grease used in this study was a food-grade aluminum-complex grease which did not contain any traditional extreme pressure antiwear (EP/AW) additives such as moly and sulfur.
| Tribological characteristics | NLGI 2 food safe grease | Same +5% WS2 | Same +5% IF-WS2 |
|---|---|---|---|
| Four ball wear, mm (ASTM D 2266) | 0.59 | 0.39 | 0.45 |
| Four ball weld, kg (ASTM D 2596) | 315 | 670 | 540 |
| Timken OK load, kg (ASTM D 2509) | 18 | 30 | 24 |
IF-WS2 is marketed as the EP/AW additive for engine oils, gear lubricants and greases [4], yet its applications so far are very limited. Among the chief limiting factors is the uncertainty about the health safety and environmental (HSE) profile of fullerenes. IF-WS2 also has issues with copper corrosion and poor oxidation stability. As a result, IF-WS2 fortified engine oils are likely to fail the International Lubricants Standardization and Approval Committee (ILSAC) GF-2 Sequence L38 and GF-3 Sequence VIII tests. Changes in various performance characteristics of a motor oil due to deployment of IF-WS2 in formulation are shown in Figure 2. Modest improvements (outward arrows) in wear protection (for direct-acting valve trains) and fuel economy are outweighed by degradation (inward arrows) in such pivotal properties as corrosion protection, with a specific risk for main bearing corrosion, oxidative thickening, and emission system durability. IF-WS2 doped oils may cause severe damage to engines with Nikasil cylinder bore coatings, and offer no advantage whatsoever for engines with Alusil bores and roller-follower valve trains.
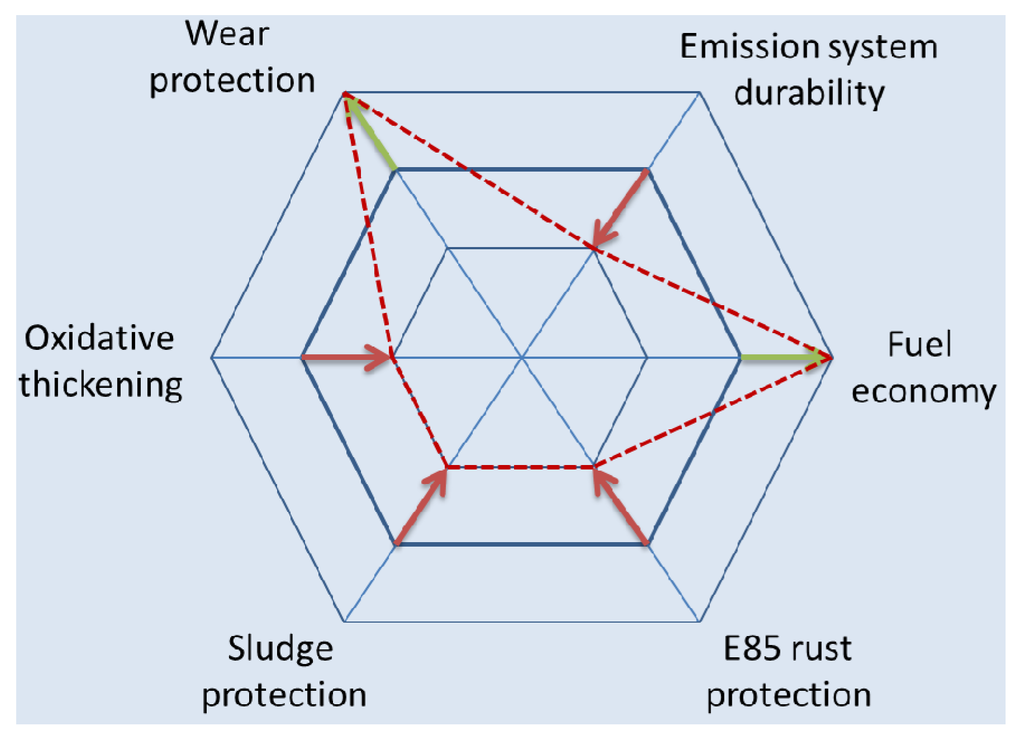
Figure 2.
Changes in the performance spectrum of ILSAC GF-5 oil top-treated with IF-WS2 emulsion.
Figure 2.
Changes in the performance spectrum of ILSAC GF-5 oil top-treated with IF-WS2 emulsion.
2.2. Nanodiamonds
The term is usually used to describe ultradispersed diamonds produced by detonation of hexagen or trinitrotoluene in a closed camber [9]. The average particle size is 4 to 6 nm. As a lubricant additive, nanodiamonds are claimed to embed into the sliding surfaces rendering them more resistant to wear, or alternatively, enable “rolling lubrication” between the surfaces, thus reducing friction and wear [10,11]. For instance, Chou and Lee have observed reduction in pin-on-disk tests for lubricants doped with 50 to 150 ppm nanodiamonds [10], see Figure 3.
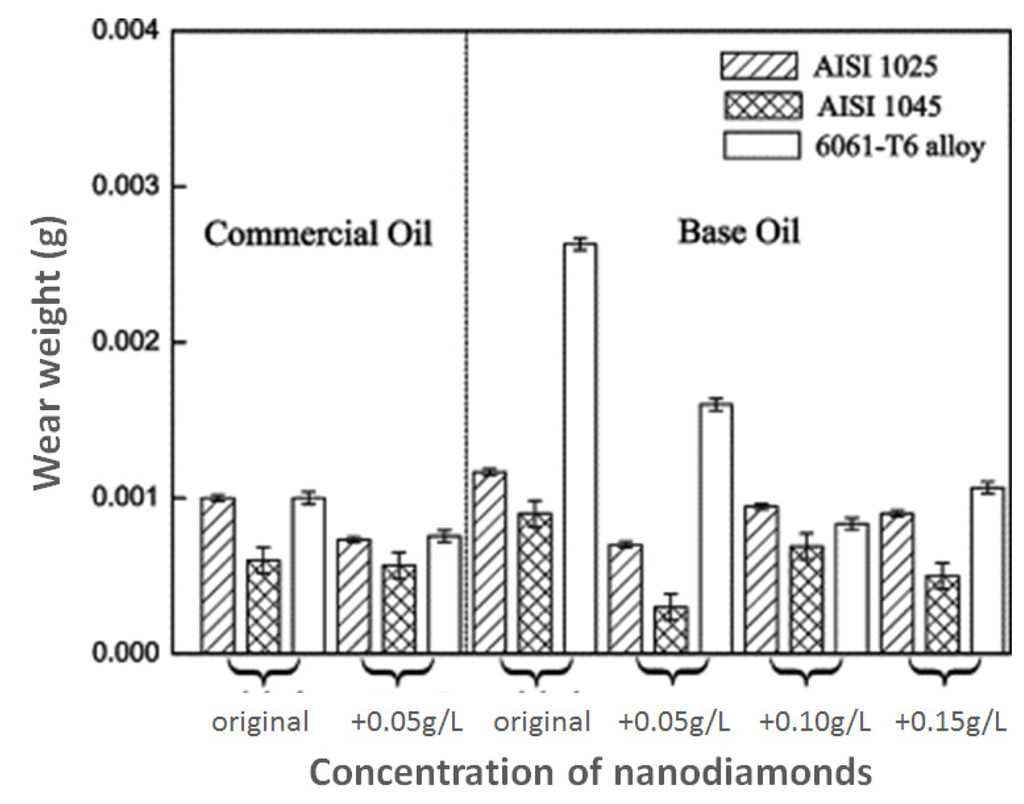
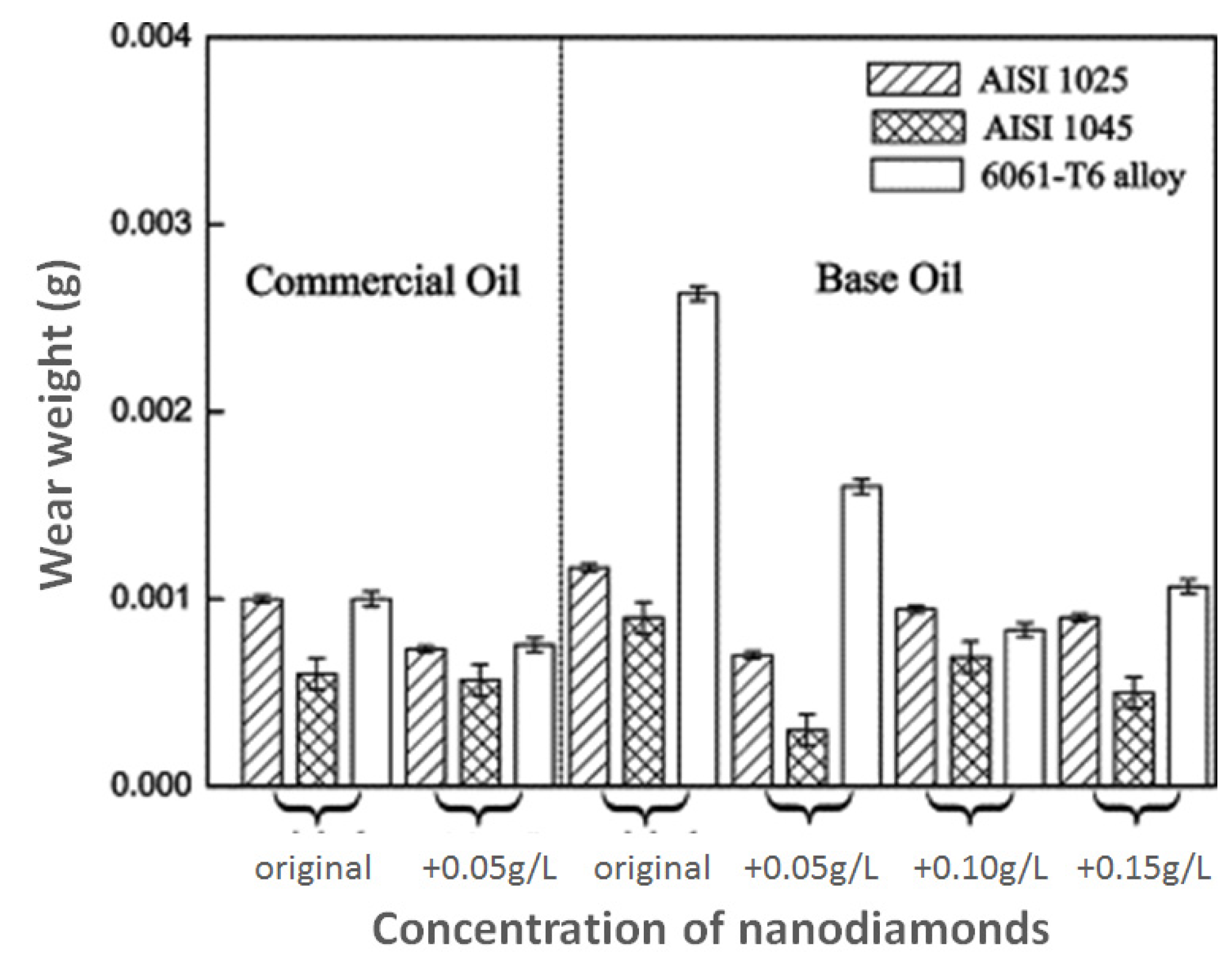
Figure 3.
Wear of rotating disk in pin-on-disk tests carried out by Chou and Lee [10]. The pin was made of carbon chromium steel. The rotating disks were made of AISI 1045 steel, AISI 1025 steel and 6061-T6 aluminum alloy. Note that pin wear was not quantified by the authors.
Figure 3.
Wear of rotating disk in pin-on-disk tests carried out by Chou and Lee [10]. The pin was made of carbon chromium steel. The rotating disks were made of AISI 1045 steel, AISI 1025 steel and 6061-T6 aluminum alloy. Note that pin wear was not quantified by the authors.
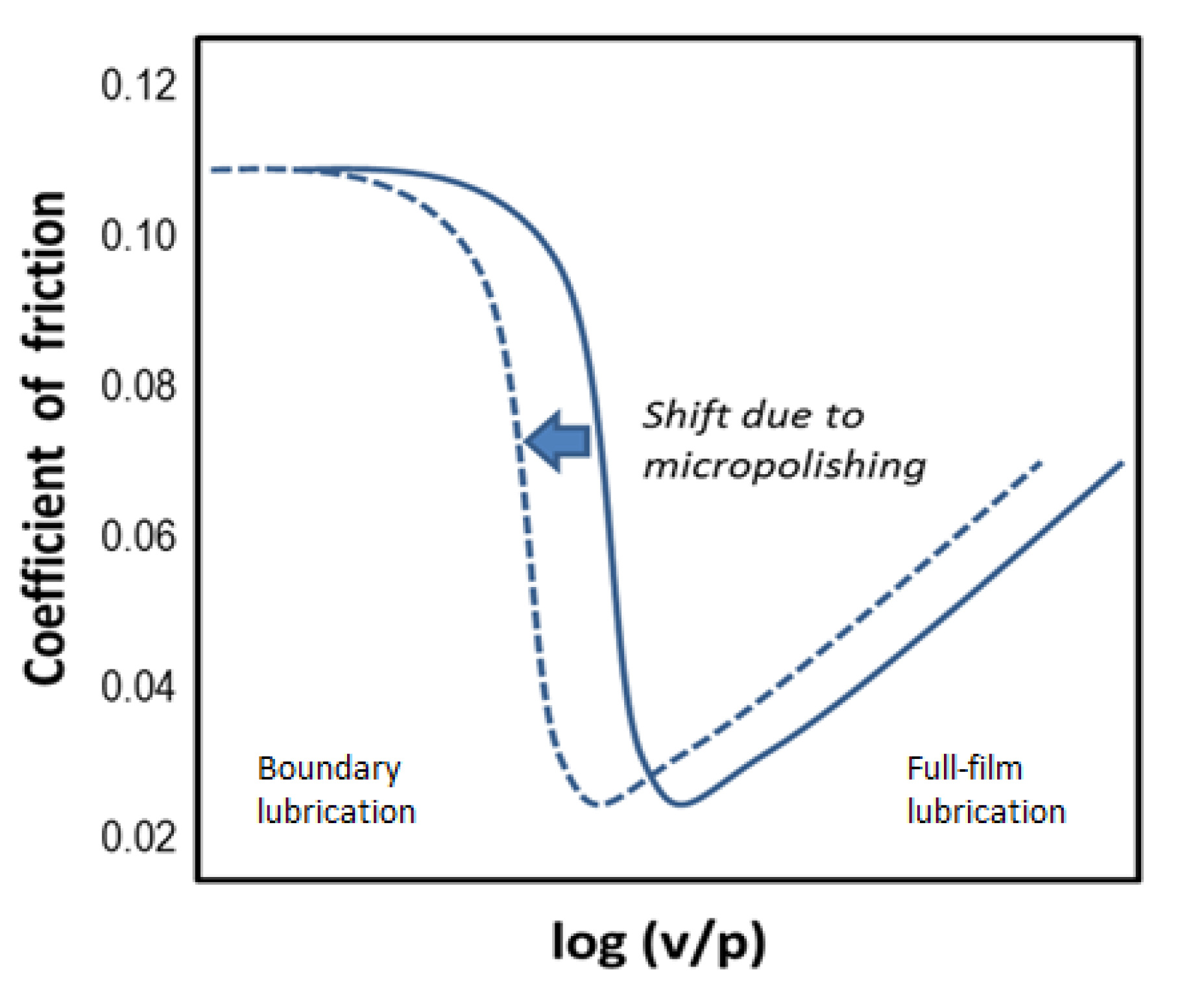
However, our studies and formulation experience have led us to a different conclusion regarding the EP/AW efficiency of nanodiamonds: The fact that a reduction in friction is observed when nanodiamonds are added to lubricant formulations is consistent with their micropolishing effect resulting in faster running-in and smoother mating surfaces. A similar effect has been observed for carbon nanohorns [12]. As a result of it, the transition from full-film to boundary lubrication occurs at a lower velocity-to-pressure ratio, and the Stribeck diagram is shifted to the left (see Figure 4).
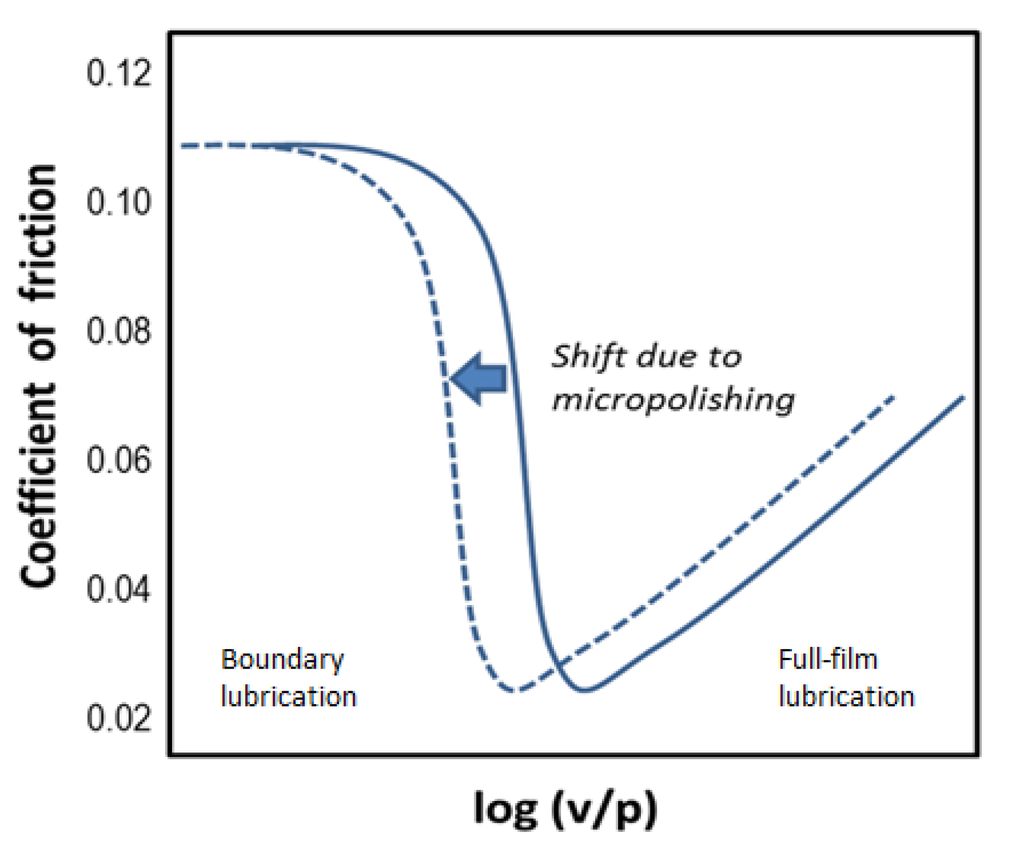
Figure 4.
Shift in the Stribeck diagram due to the micropolishing effect of nanodiamonds. Here, v is the sliding velocity and p is the contact pressure. Transition from full-film lubrication to boundary lubrication occurs at a v/p ratio when the lubricant film thickness becomes smaller than the average asperity height. Since the micropolished surface (broken line) has lower asperities than the original surface (solid line), the transition will occur as a lower v/p ratio.
Figure 4.
Shift in the Stribeck diagram due to the micropolishing effect of nanodiamonds. Here, v is the sliding velocity and p is the contact pressure. Transition from full-film lubrication to boundary lubrication occurs at a v/p ratio when the lubricant film thickness becomes smaller than the average asperity height. Since the micropolished surface (broken line) has lower asperities than the original surface (solid line), the transition will occur as a lower v/p ratio.
The micropolishing effect of nanodiamonds becomes insignificant in the case of aged oil, where wear rate and steady-state surface roughness are dominated by other factors, such as oil contamination. Furthermore, since the abrasiveness of nanodiamonds does not go away after the initial running-in period, there is a risk for excessive wear over a longer period of time (Figure 5). Analysis of oils from engines run with engine oils doped by nanodiamonds (available as aftermarket oil treatment packages) reveals unusually high levels of wear metals such as aluminum, copper and chromium, indicative of accelerated wear of bearings and piston rings. Nanodiamonds may also alter the tribology of finger roller follower valvetrain, increasing the risk of roller skidding. On the other hand, the micropolishing effect of nanodiamonds in engine oil seems to improve surface finish of certain components after the running-in. Therefore, nanodiamonds may prove useful in running-in oil formulations, yet more studies are needed to discern possible unintended consequences.
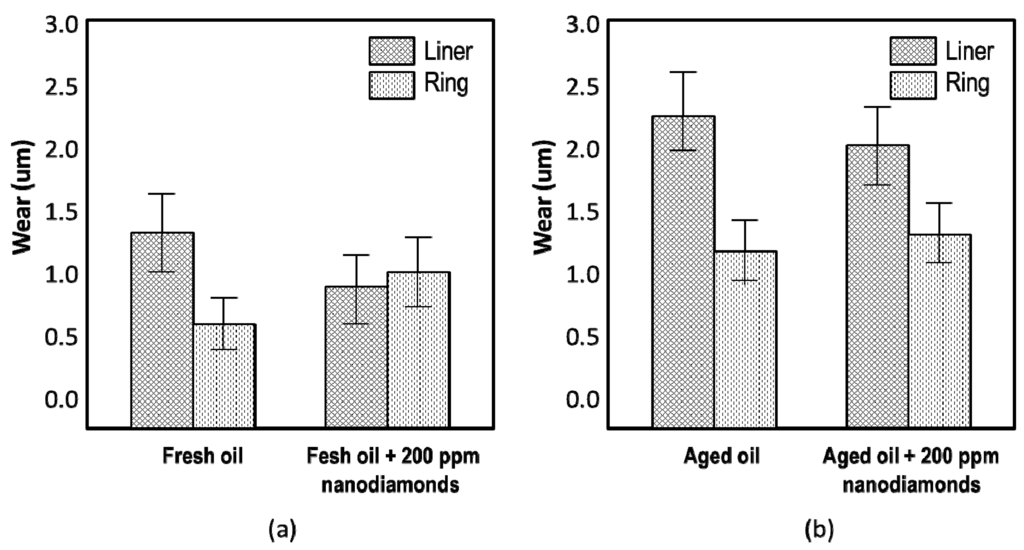
Figure 5.
Wear of piston ring and cylinder liner lubricated by SAE 30 engine oil with and without nanodiamonds. A reciprocating ring-on-liner tester was used under the following conditions: Test duration 1 h, frequency 20 Hz, load 360 N, lubrication: (a) Fresh SAE 30 engine oil; (b) Pre-oxidized SAE 30 engine oil diluted with 10 wt% diesel fuel and “contaminated” by 0.2 wt% particulate matter containing a mix of soot, quartz, alumina and kaolinite; oil temperature 90 °C. Note the increased ring wear with nanodiamond-doped fresh oil (a).
Figure 5.
Wear of piston ring and cylinder liner lubricated by SAE 30 engine oil with and without nanodiamonds. A reciprocating ring-on-liner tester was used under the following conditions: Test duration 1 h, frequency 20 Hz, load 360 N, lubrication: (a) Fresh SAE 30 engine oil; (b) Pre-oxidized SAE 30 engine oil diluted with 10 wt% diesel fuel and “contaminated” by 0.2 wt% particulate matter containing a mix of soot, quartz, alumina and kaolinite; oil temperature 90 °C. Note the increased ring wear with nanodiamond-doped fresh oil (a).
2.3. Boric Acid
In the not so distant past, boric acid used to be a common additive in metal-working fluid (MWF) formulations thanks to its excellent EP/AW properties and bacteriostatic and bactericidal actions. Nowadays, it has been largely phased out from MWFs because of HSE concerns. However, some recent studies mention “boron-based nanoparticulate lubrication additives that can drastically lower friction and wear in a wide range of industrial and transportation applications”, indicating renewed interest in boric acid. By replacing sulfur and phosphorous, boron additives are hoped to eliminate the main sources of environmentally hazardous emissions and wastes [13].
Unfortunately, there are quite a few technical hurdles to mar that optimism. First of all, boric acid has no antioxidant effect, so it cannot replace zinc dithiophosphate (ZDDP). Second, boric acid is not compatible with some essential lubricant additives, specifically with the total base number (TBN) buffer in the engine oil, which may lead to corrosion and sludge problems. For instance, engine oils containing boric acid are likely to fail ASTM D 6557 and D 6593 (ILSAC GF-3 Sequence VG) tests.
Recently, it has been proposed to combine electrochemical boriding with the use of colloidal boron nitride for improving tribological performance of drivetrain components in advanced wind turbines [14]. This may be a promising future application of this new technology.
2.4. Polytetrafluoroethylene (PTFE)
PTFE has a well-defined footstep in the lubrication engineering with impressive performance profile in greases, chain oils, dry-film lubricants, etc. [15,16]. Recognition of potential to reduce friction and wear has led to use of PTFE as a dry-film lubricant and friction modifier long before the buzzword “nano” has come into daily use. PTFE-fortified oils and greases are known to exhibit higher welding loads, higher load wear indexes, and reduced stick-slip. Though PTFE nanodispersions are used in a number of aftermarket engine treatment products, the use of PTFE in engine oils is rather limited because of inherent instability of PTFE dispersions in oil, the risk of oil filter clogging, as well as difficulties with recycling. As a matter of fact, the application of PTFE in engine oils is discouraged even by the major PTFE producer, DuPont Company [16].
3. Conclusions
Nanomaterials have potential for enhancing certain lubricant properties, yet there is a long way to go before balanced formulations are developed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhmud, B. Pursuit for better fuel economy: Reducing engine friction helps maxing out miles per gallon. Veh. Compon. 2012, 5, 18–21. [Google Scholar]
- Kolesnikov, V.I.; Myasnikova, N.A.; Volnyanko, E.N.; Ermakov, S.F.; Sychev, A.P.; Sychev, A.A. Lubricants with ceramic nanoadditives and wear-resistant surface structures of heavy-duty frictional joints. Russ. Eng. Res. 2011, 31, 454–457. [Google Scholar] [CrossRef]
- Gitis, N.V. Tribology Testing of Lubricating Oils with Nano-Additives. In Proceedings of 16th International Colloquium Tribology, TAE, Stuttgart, Germany, 15–17 January 2008; p. 78.
- Zak, Z.; Fleischer, N.; Zarbuv, M.; Drangai, L.; Genut, M. Nanomaterials for gear lubrication solutions. Available online: http://www.gearsolutions.com/media//uploads/assets//PDF/Articles/Dec_09/1209_Nano.pdf (accessed on 18 November 2013).
- Kroto, H.W.; Heath, J.R.; O’Brien, C.S.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Zink, N.; Pansiot, J.; Kieffer, J.; Therese, H.A.; Panthöfer, M.; Rocker, F.; Kolb, U.; Tremel, W. Selective synthesis of hollow and filled fullerene-like (IF) WS2 nanoparticles via metal-organic chemical vapor deposition. Chem. Mater. 2007, 19, 6391–6400. [Google Scholar] [CrossRef]
- Titov, A. Effect of Fullerene Containing Lubricants on Wear Resistance of Machine Components in Boundary Lubrication; Department of Mechanical Engineering, The New Jersey Institute of Technology: Newark, NJ, USA, 2004. [Google Scholar]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar]
- Chou, C.C.; Lee, S.H. Tribological behavior of nanodiamond-dispersed lubricants on carbon steels and aluminum alloy. Wear 2010, 269, 757–762. [Google Scholar] [CrossRef]
- Puzyr, A.P.; Burov, A.E.; Selyutin, G.E.; Voroshilov, V.A.; Bondar, V.S. Modified nanodiamonds as antiwear additives to commercial oils. Tribol. Trans. 2012, 55, 149–154. [Google Scholar] [CrossRef]
- Zin, V.; Agresti, F.; Barison, S.; Colla, L.; Pagura, C.; Fabrizio, M. Investigation on Tribological Properties of Nanolubricants with Carbon Nano-Horns as Additives at Different Temperatures. In Proceedings of the World Tribology Congress, Torino, Italy, 9–13 September 2013.
- Canter, N. Boron nanotechnology-based lubricant additive. Tribol. Lubr. Technol. 2009, 65, 12–13. [Google Scholar]
- Greco, A.; Mistry, K.; Sista, V.; Eryilmaz, O.; Erdemir, A. Friction and wear behaviour of boron based surface treatment and nano-particle lubricant additives for wind turbine gearbox applications. Wear 2011, 271, 1754–1760. [Google Scholar] [CrossRef]
- Heatwole, O. Presenting PTFE: A Potent Resin, a Well-Kept Secret; QMI/Ever-Wear, Inc.: Lakeland, FL, USA, 1991. [Google Scholar]
- Ebnesajjad, S. Fluoropolymer Additives; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 6. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
