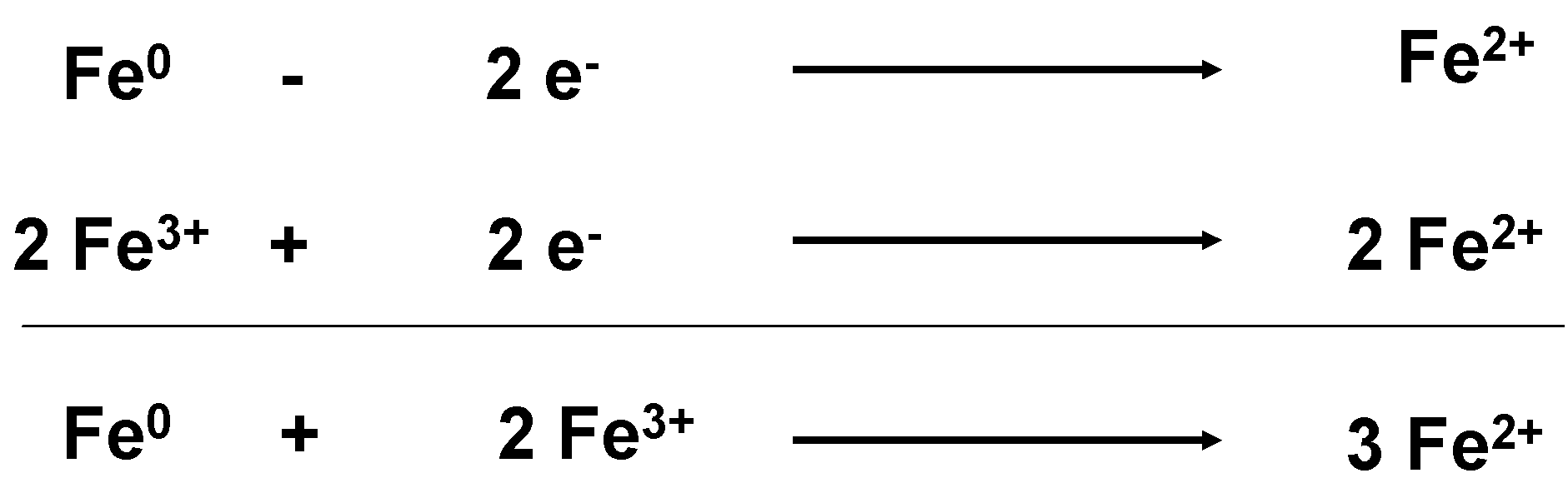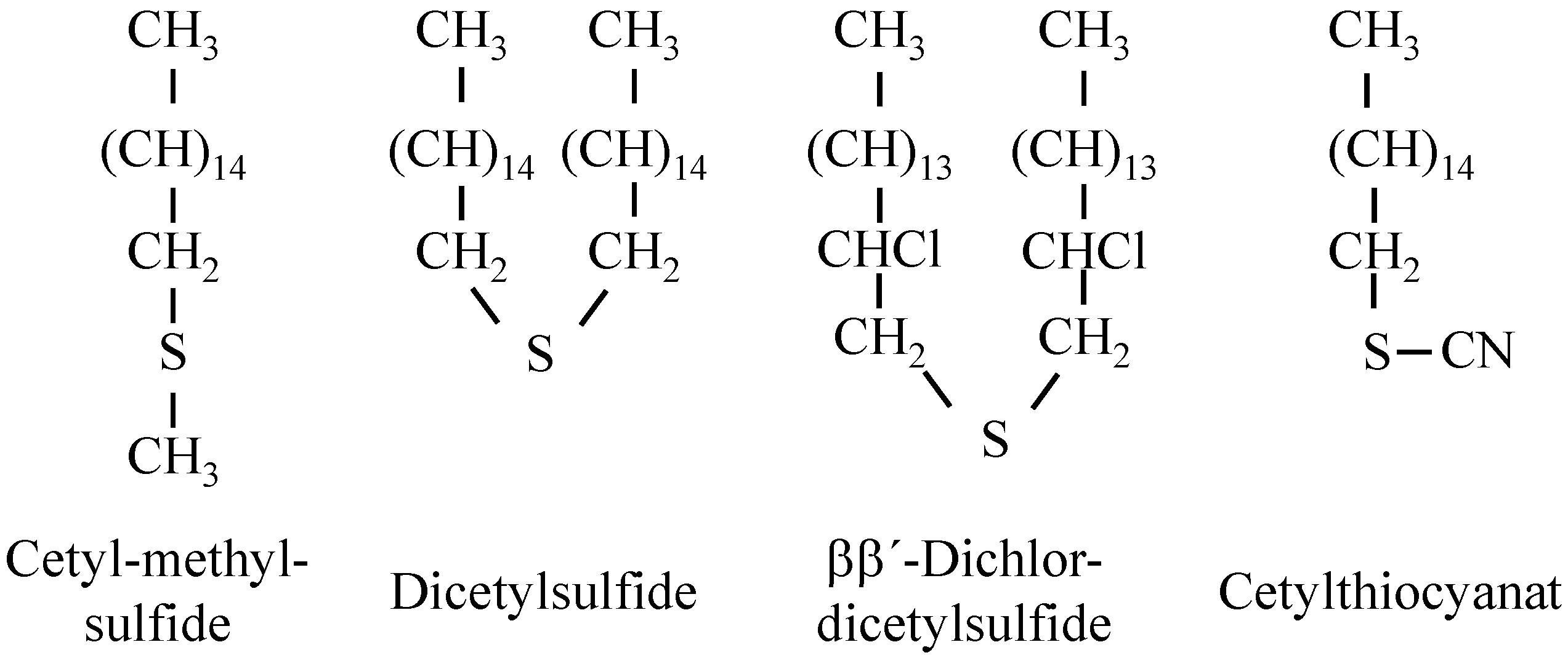1. Introduction
Besides cooling and (in machining processes) chip removal, lubrication is one of the major tasks of metalworking fluids (MWF). The latter is mainly achieved by the specific composition of MWF including oils and different types of additives. To reveal the working mechanisms of those additives, tribological aspects have to be considered. A scientific understanding of the properties of metal surfaces and the additives is crucial to explain the interactions between a MWF and the workpiece material. Furthermore, aspects such as time and temperature must be considered, when discussing the way additives might interact with the workpiece material. Within the last decades, the majority of chemists and tribologists in MWF-research [
1] believed that additives like chlorinated paraffins, sulfurised esters, and polysulfides or phosphorous compounds react with the metal surface in the relatively short contact time of the metalworking process. They were meant to form metal chlorinates, metal sulfides or metal phosphor compounds. From a chemical point of view, these reactions are well possible and reaction layers can in fact be obtained after the process. However, it takes a certain amount of time to form these layers. Time, which is not available in manufacturing processes, which usually are characterized by very high relative velocities. Consequently, the reaction layers found in literature [
1] are likely to be a result of incubation of chemicals at the newly formed metal surfaces after the process. If this is the case, the question, why certain additives have a significant influence on the process arises. The effect obviously cannot be explained by (re)actions taking place seconds, minutes, or hours after the process.
In the following subchapters, the chemical properties of metal surfaces and possible working mechanisms of additives are discussed. Based on these considerations, a new explanation for the lubricating properties of MWF additives is given and supported by experimental results.
1.1. Metal Surfaces from a Chemical Point of View
Remarkably, in the past most tribologists did not properly differentiate between the varying properties of dissimilar metals. As discussed in the following, the chemical surface properties of e.g., steel (carbon steel) and stainless steel are totally different, and have a major influence on the interactions with MWF additives. At a first (macroscopic) view, metals show a homogeneous structure but most metals (steels, copper, aluminum,
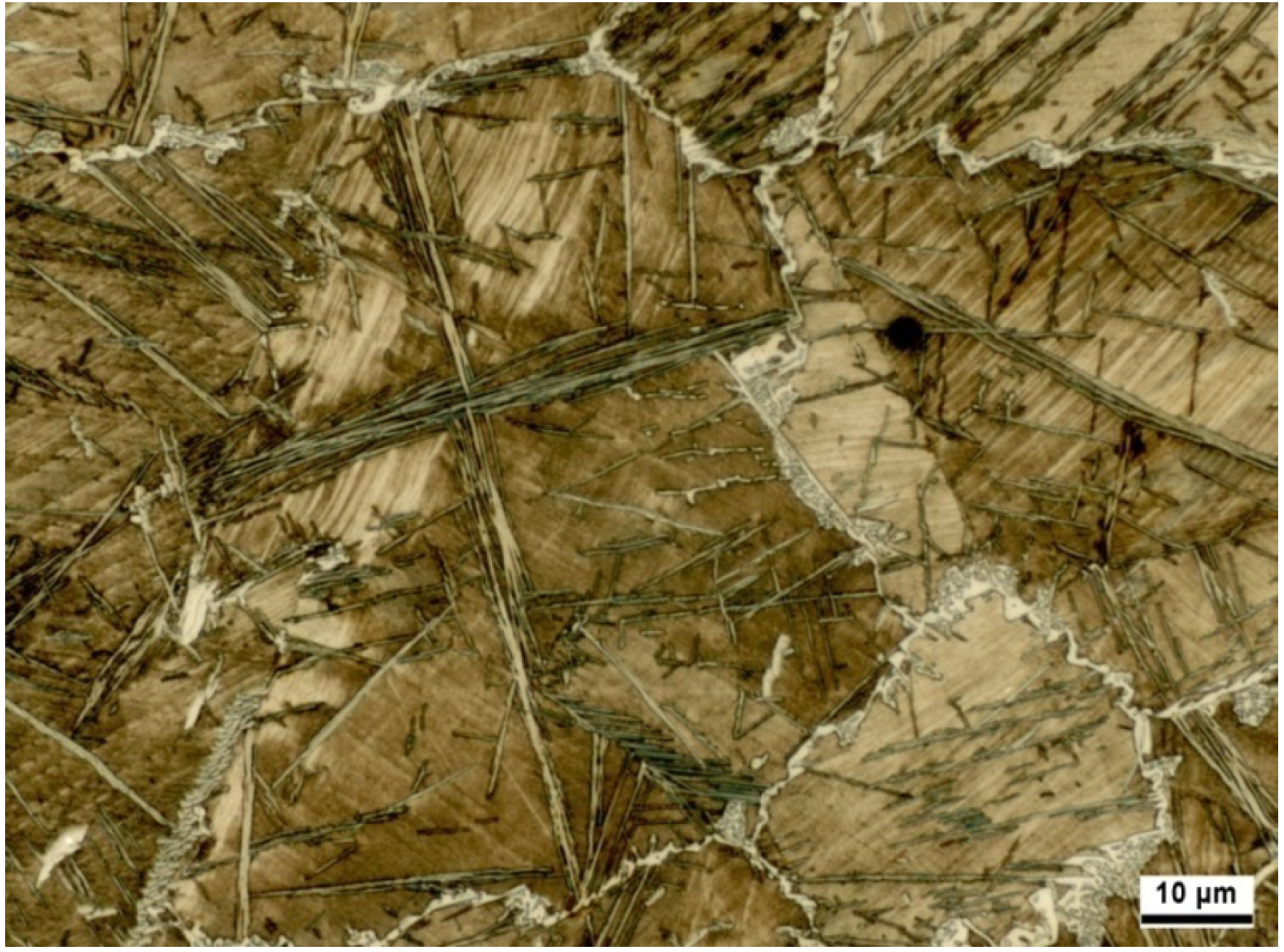
etc.) reveal a polycrystalline microstructure (
Figure 1) with a fixed arrangement of the atoms. According to the kind of metal, the single crystals are set up more or less in a similar way. The prevailing majority of the metals reveal a microstructure according to the cubic or hexagonal crystal system. Cubic crystals can furthermore be subdivided into space-centered and surface-centered crystals. In a common metal, hybrid forms will always form, so usually, no ideal system is given.
Figure 1.
Cross-section of a highly alloyed steel revealing its polycrystalline microstructure.
Figure 1.
Cross-section of a highly alloyed steel revealing its polycrystalline microstructure.
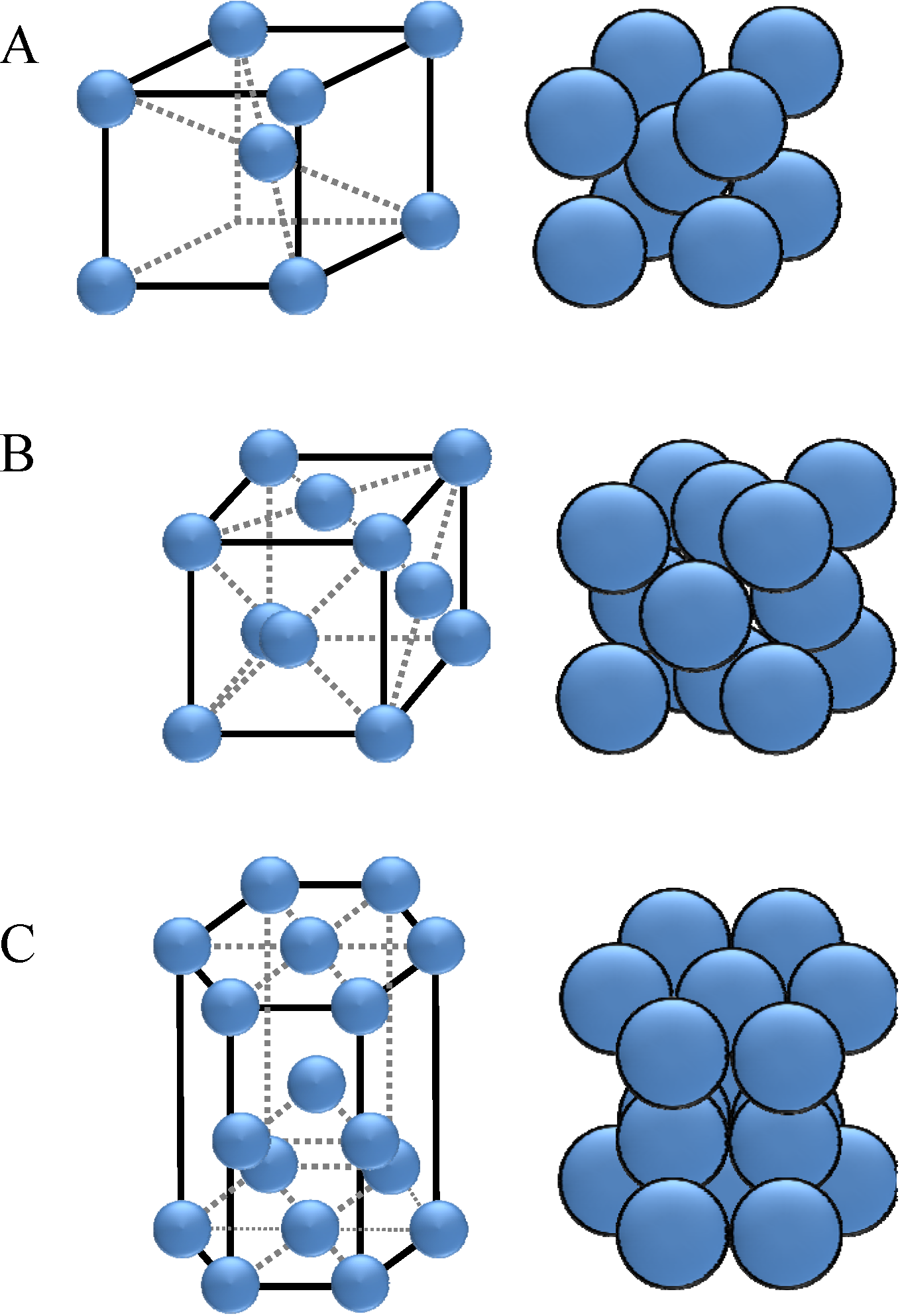
The smallest geometrical entity of a crystal is the elementary cell (
Figure 2). In elementary cells, the atom distances vary in different space directions. Due to this fact, the properties of an elementary cell are also dependent on the orientation of the crystal within the material. This indicates that the ability of the atoms to react with MWF-additives is influenced by the orientation of the crystals.
Figure 2.
Elementary cells: (
A) cubic body-centered; (
B) cubic face-centered; (
C) hexagonal [
2].
Figure 2.
Elementary cells: (
A) cubic body-centered; (
B) cubic face-centered; (
C) hexagonal [
2].
For the working mechanisms in metalworking, defects in the crystal lattice at the surface are of high importance. At any kind of imperfection, microcracks can be formed and penetrated by active (polar) MWF-additives. Kaesche [
3] described the importance of grain boundaries for intercrystalline corrosion processes and pitting corrosion. In the latter case, no additive, but water attacks the material at the grain boundaries.
In addition to microcracks, dislocations also have an influence on the metal surfaces’ ability to interact with MWF-additives. At these defects in the lattice structure, gliding planes can occur. Rehbinder [
4] speaks of so-called “gliding packets” (see below). Klocke [
5] introduces the very clear model of a stack, which consists of lattice layers and gliding planes lying in between (
Figure 3). Though the models are valid for polycrystalline materials as well, it should be mentioned, that both, Rehbinder as well as Klocke have dealt with mono-crystals. The simplified model shown in
Figure 3 is valid for both, forming and cutting as the latter can be considered as a forming process, which forms a chip until it breaks off.
Figure 3.
Gliding phenomena at mono-crystals [
5].
Figure 3.
Gliding phenomena at mono-crystals [
5].
Deeper insights regarding the atomic construction are possible using secondary ion mass spectroscopy (SIMS) and secondary neutral particle-mass spectroscopy (SNMS). Applying these techniques, the surface of an untreated gearwheel is described in [
6] revealing the existence of hydroxyl groups (–OH) at the surface. These OH-groups are bound to iron-atoms. At air humidity of 40% (and more), steels (excluding stainless steel) are covered with a layer of hydroxides and oxides. Stratmann [
7] characterizes the composition of an iron surface with about 25% of γ-FeOOH, 70% of α-FeOOH and a very low quantity of iron oxides. In a more recent work [
8], iron oxides (Fe
3O
4) and iron hydroxides (Fe(OH)
2) are detected at an iron surface. Bhargava
et al. [
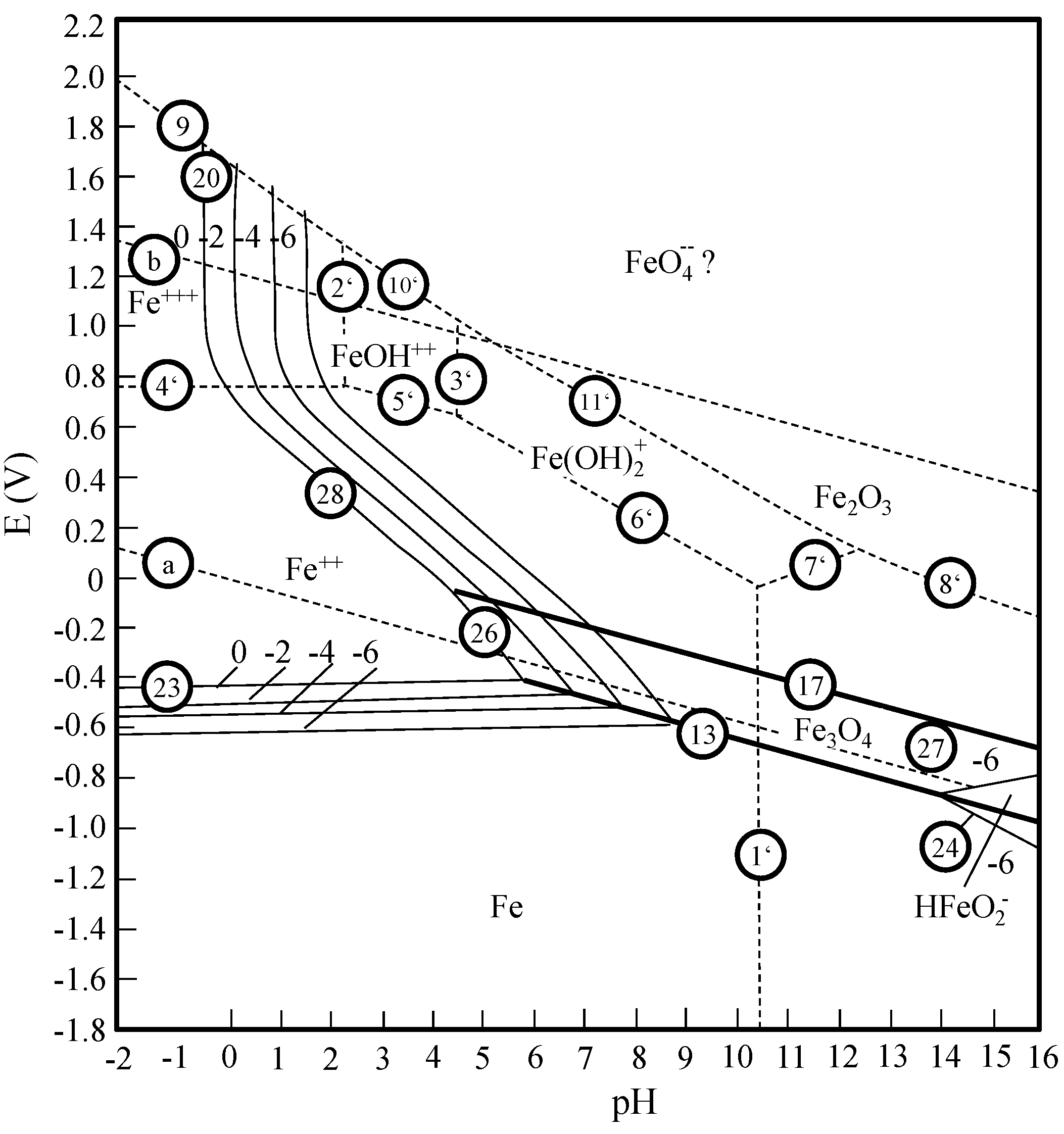
8] ascertain a good compliance with the Pourbaix diagram for the system iron-water (
Figure 4). Iron oxide, iron(II)-, and iron(III)-ions (with hydroxide groups occupied) simultaneously exist in the neutral pH area.
Figure 4.
Pourbaix diagram—system iron-water [
9].
Figure 4.
Pourbaix diagram—system iron-water [
9].
It is well known that stainless steels are covered by a chromium/nickel oxide layer, in changing ratios depending on the alloy. In addition, for aluminum, it is well known that it is covered by a firm sticking oxide skin. For common steel qualities, it is supposed that oxides at the metal surface occur area-wide or island-shaped [
3].
In the past, the working mechanisms of MWF-additives were mainly discussed based on interactions with pure iron [
1]. Furthermore, it has been reported that the additives compete for so-called “(re)active centers” in case of adsorption processes. These “reactive centers” should be “mobile”,
i.e., they are not stationary at a certain iron surface. Unfortunately, the literature situation is very vague regarding the latter aspect. In the discussion to Forbes paper [
10], Hotten states: “Iron is a chameleon—it changes its skin with the surroundings”. Every additive-molecule, which interacts with an atom on the metal surface (reaction or adsorption) changes the electron density at the metal surface. Given that (like in iron) ions can exist in different oxidation states (Fe(II), Fe(III) (and also possibly Fe(0)-atoms)), it is well possible, that by a shift of some electrons, a continuous change of oxidation states among the atoms occurs. This might be a very good explanation for the “mobile reactive centers”-phenomenon.
As described above, the single crystals and the elementary cells can be arranged in different ways. The energetic situation at the metal surface is thereby influenced significantly. Where and how hydroxide groups are located at the metal surface, which area is covered with oxide or not and how fast and in which direction electron movements are possible, depends on the internal structure of the metal. The orientation of the crystals is decisive for the interaction of the metal surface with the MWF-additives. This aspect did not receive consideration in the past. Steels and stainless steels have different chemical properties at the surfaces and for this reason different (re)action behavior regarding additives.
1.2. Additives and Their Interactions with (Fresh) Surfaces
Additives are able to interact with oxides, hydroxide groups or metal ions. In general, additives can be divided into characteristic groups based on the chemical properties of the substances: ionic (e.g., acidic phosphoric acid esters, PEP-additives) and non-ionic substances (e.g., chlorine paraffins, polysulfides). The ionic additives will correspond preferentially with the metal ions. The non-ionic additives should be able to come to an arrangement with the oxide, as well as with the hydroxide groups.
This assumption matches with the observations in industrial practice. In machining of stainless steels, PEP-additives or acidic phosphoric acid esters have a rather limited effect. Here, chlorinated paraffins and sulfur compounds are used successfully. For steels, PEP-additives in combination with sulfur compounds lead to a good performance, but also chlorinated paraffins are very effective additives.
In many metalworking processes, fresh surfaces are generated. If these freshly generated surfaces are not covered by additives immediately, welding (adhesion of workpiece material and tool material) occurs. Trivially, this behavior is called “sticking”. This effect is more distinctive for stainless steels, aluminum and titanium (materials which carry a pure oxide skin), than for steel or copper. Freshly machined metal has the oxidation state (0) and is highly responsive for new bonds or saturation of its surface (at least by adsorption). The oxidation state (0) is an “unstable” state for metals.
Stabilization is achieved by a change of the electric situation in the metal atom. Theoretically, this type of stabilization might also occur due to an internal movement of electrons,
i.e., electrons must be delivered by the atom with oxidation state (0) and be taken up by another atom with an oxidation state >(0). As described above, in steels the oxidation states (II) and (III) exist simultaneously. Atoms with the oxidation state (III) would convert themselves by electron absorption into the state (II), which is more stable (
Figure 5). In the case of stainless steels, aluminum and titanium the internal electron transfer is not possible, as no atoms in the same material are able to take up the electrons. This means that the change of the electronic situation can only occur due to (re)action with external “reaction partners” (MWF-additives, tool material). However, additives are only able to avoid adhesion, when they cover the new surface fast enough.
Figure 5.
Stabilizing of Fe(0) by internal electron transfer.
Figure 5.
Stabilizing of Fe(0) by internal electron transfer.
Nevertheless all theories discussed in literature dealing with the interaction of lubricants with metals assume that it is the additives that play the active role and allow for a longer tool life as well as increased surface quality.
One of the main objectives of this paper is to explain some basic principles regarding the working mechanisms of MWF. As already mentioned, most models described in the past lack of consistency and are limited, as they cover only small aspects of reality. To make progress, it is necessary to compare different theories and models with each other, to discuss the aspects of MWF-metal-interactions in relative terms.
Currently, two different models exist regarding the interaction of lubricants or the MWF-additives within the tribological system. The “old” model of the interaction of additives with metal surfaces is predominantly based on the formation of reaction layers as the tribological effective layer (see
Figure 6). Metal surfaces are assumed to be chemically homogeneous (mostly oxides).
The “new” model mainly negates reactions between additives and metal surfaces in the metal treatment process itself (compare
Section 1.4) and defines adsorption-effects as tribological effective driver of the interaction and metal surfaces are considered to be very differently in the chemical sense [
11]. The “old” model is not able to explain a lot of phenomena’s in metal-working, e.g., forming of stainless steel. The “new” model (see next pages) is able to explain all this.
1.3. The Influence of Temperature (Bowden and Tabor)
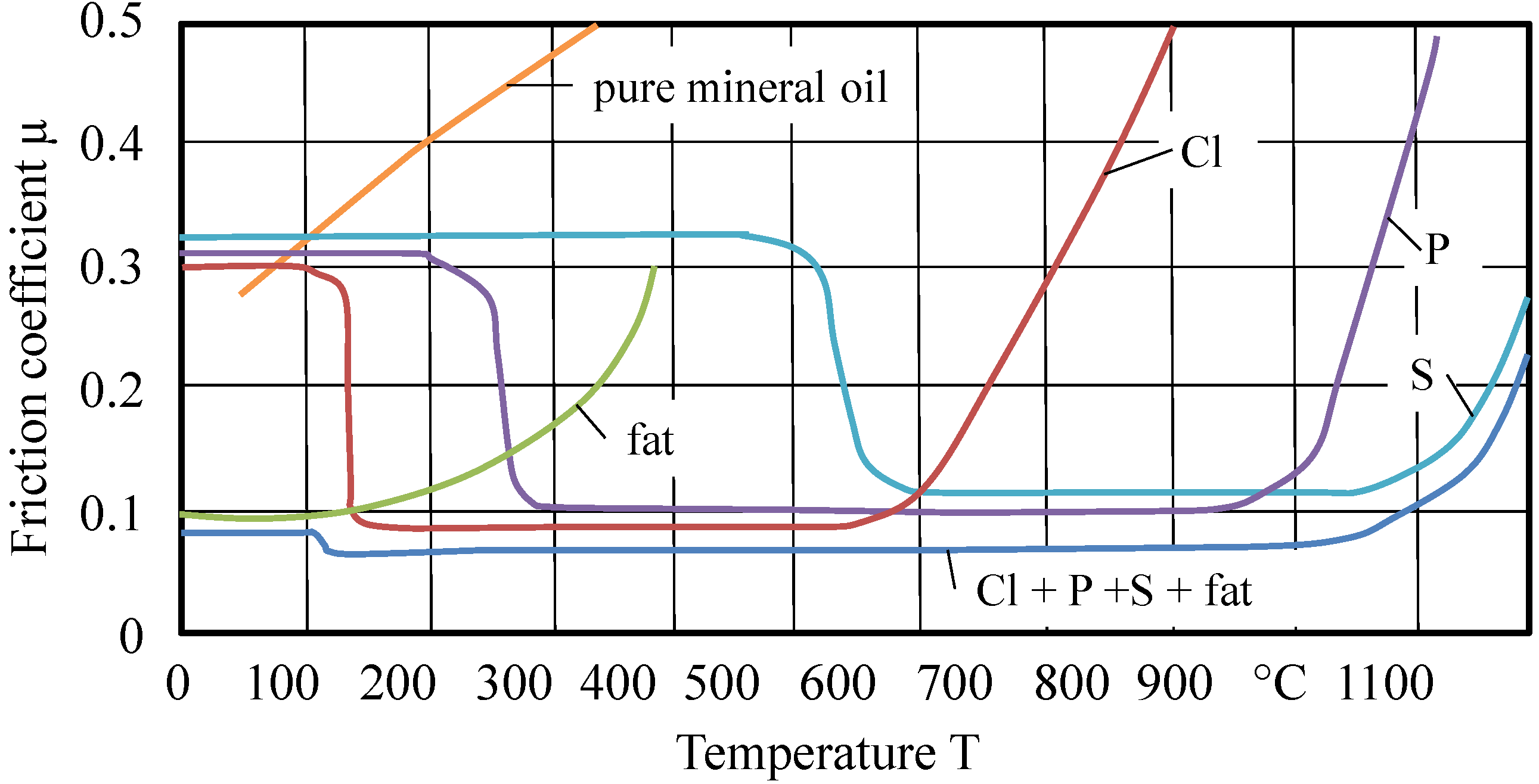
Probably, no monograph has been cited more often in the chemistry of metalworking than “The Friction and Lubrication of Solids” by Bowden & Tabor [
12]. Unfortunately, there is a misleading re-citation of a very important figure from [
12] (
Figure 6), which was not described in that way by Bowden and Tabor.
Figure 6.
Activity areas of additives regarding temperatures [
1].
Figure 6.
Activity areas of additives regarding temperatures [
1].
This figure was re-published often by several authors and modified in an invalid way. Bowden and Tabor generated metal chloride layers by aeration of metal surfaces (steel, copper, and cadmium) with chlorine gas. The so-produced layers are examined tribologically. Up to approximately 300 °C, low friction coefficients rise continuously. Similar values are to be achieved by the coating at metal surfaces with iron chloride solved in ether. Very remarkable is their description of the hydrolytic instability of the chloride layers.
Long chained halogenated paraffins, such as octadecylchloride, cetylbromide and cetyliodide, were examined by Bowden and Tabor. Cited from the original manuscript: “In all cases these compounds gave a low friction and slight surface damage when solid, but increased friction and surface damage above their melting points (approximately 20 °C). This behavior is similar to that of straight-chain paraffins, except that the coefficient of friction is lower. It would appear, therefore, that under the experimental conditions involved there is no reaction between the compounds and the surface to form metallic chloride film. Even at elevated temperatures the friction remains high, showing that the compounds do not react.” [
12]. These compounds are very similar (in structure) to the chlorinated paraffins, which are still in use. The lubricating behavior of organic compounds containing chlorine varies with the stability of the compounds and with the nature of the metal surfaces. Long-chain halides, such as octadecylchloride, are stable even at 300 °C, so that no chloride film is formed. Frictional experiments were carried out using long-chain aliphatic compounds containing sulfur. These compounds, which are described below, are highly polar but are readily soluble in paraffin oil and were usually tested as a one percent solution. Not one of these compounds examined had any appreciable effect on platinum or silver surfaces. The friction remained as high as when paraffin oil itself was used [
12].
This result is a very interesting one, as the sensitivity of silver regarding reactions with sulfur compounds (black coloring) has to be considered. “On steel, copper, and cadmium surfaces, however, it was found that the compounds could be divided into three main classes. The first class consists of long-chain sulfides, such as disulfides and thiocyanates” [
12] (
Figure 7).
Figure 7.
Sulfur compounds, which were investigated by Bowden and Tabor [
12].
Figure 7.
Sulfur compounds, which were investigated by Bowden and Tabor [
12].
“None of these compounds was effective, and it was evident that under the conditions of the experiment there was no chemical reaction with the surfaces. Similar results were obtained with two aromatic sulfur compounds, though there was evidence that at very much higher temperatures they broke down and attacked the surface to form the metal sulfide” [
12]. Though Bowden and Tabor did not specify “higher temperatures”, it can be assumed that they meant temperatures above 300 °C. “The second class consists of sulfur compounds possessing a replaceable hydrogen atom. The compounds investigated included: cetylmercaptan, cetylsulfonic acid, di-thiotridecylic acid, and α-mercaptopalmetic acid. These substances gave indication of reacting with the metal surface to form a sulfide film. Further, although they provided effective lubrication on steel, copper, and cadmium surfaces they were no more effective than paraffin oil alone on silver or platinum. The frictional behavior of these compounds is thus very similar to that of fatty acids and the results suggest that their effectiveness as boundary lubricants is due to their reaction with the metal to form a compound analogous to a metallic soap. ... The third class consists of active compounds not containing a replaceable hydrogen atom, such as dithiocyanates. Dithiocyano-stearic acid prepared from oleic acid and free thiocyanogen provides very good lubrication on steel, the friction remaining low up to 300 °C. This compound is of uncertain composition but a CNS group is present. It is not, however, effective on copper or cadmium. Similar results are obtained with a compound prepared from cetene and thiocyanogen. The behavior of these compounds is obviously not due to sulfide film formation, and the evidence points to the formation of a film of ferric thiocyanate.” [
12].
Phosphorus containing compounds are only discussed briefly. Bowden and Tabor did not discriminate between the discrete classes. “It is probable that the phosphorus compounds often act in a manner similar to that of compounds containing sulfur and chlorine in that surface films are formed which are protective and which, with some metals, may possess a low shear strength.” [
12].
Bowden and Tabor have examined a lot of other, partly very reactive, substances, which are not in use in metalworking fluids any more. These reactive compounds such as, ββ′-dichlordiacetyl selenium indeed lead to metal chloride films. In a conclusion Bowden and Tabor published the following misleading sentence: “It is not suggested that the compounds described in this and the preceding part are necessarily good practical “extreme pressure” additives, but that the action of these compounds is similar to that of conventional “EP” additives” [
12].
There is no doubt about the lubricating effect of sulfide, chloride or thiocyanic layers, only the way of their synthesis should be questioned. Bowden and Tabor have used reaction pathways, which are not realistic in metalworking. This does not reduce the real reward of Bowden and Tabor; the authors were able to identify (very precisely) the temperature at which sulfide or chloride layers will have their tribological breakdown. But in summary, Bowden and Tabor have never originated a diagram with the contents of
Figure 6.
1.4. Time in Metalworking Processes
Chemical reactions are able to use different pathways, depending on the type of the reaction partners. Radical and ionic reactions run very fast. Breaking a molecular bonding and its subsequent recombination with molecule parts of the reaction partner needs significantly more time. Regarding the working mechanism of MWF-additives, a very important question is which amount of time would be available for the assumed interaction between the additive and the metal surface in real manufacturing processes. Depending on the process (e.g., typically broaching works at a cutting speed of 6–30 m/min; turning at a cutting speed of 150–300 m/min and grinding at 30–100 m/s) the time for the generation of 1 mm nascent surface is to be found within the range of 0.5 to 2 ms.
The classical rules of the reaction kinetics can be applied only in a limited way in metalworking. They assume that with the concentration of the reaction partner (educt) and with rising temperature, the reaction velocity increases. In metalworking, one educt is the activated metal surface (microcracks, dislocations, fresh surfaces generated by cutting or surface enlargement while forming), the other educt are the additives in metalworking fluids. The first step in the direction of a possible reaction is the adsorption of the additives to the metal surface. This step depends on the kind of the molecular structure of the metal surface and the kind of the additives, which will find a “docking station” or not. Between the additives, which have the same basic principle, there is a kind of competitive situation.
Adsorption and desorption are depending on temperature, i.e., with rising temperature adsorbed molecules tend to desorb. In the end, the electric double layer is responsible for the polarization of additives and with this, for the adsorption. This paper intends to give new insights, revealing that a “real” chemical reaction is of lower meaning for the metalworking itself.
1.5. The Rehbinder-Effect
Rehbinder [
4] discovered a reduction of the needed power in forming processes of solid bodies due to the influence of surface-active substances. He performed drawing experiments applying monocrystals of different metals, which he grew especially for these experiments. The monocrystals were divided into three identical parts. The first workpiece was drawn in air, the second in pure paraffinic oil and the third in paraffinic oil, which includes 0.2% of a surface-active substance. He obtained identical results in air and pure paraffinic oil. The results with the surface-active substances were considerably better. Rehbinder himself writes in the preface to his monograph: With these investigations one succeeded in ascertaining a row of new phenomena which contains by the interaction on the basis of adsorption forces between a formed metal and the surrounding medium, the surface-active materials, were caused. The lowering of the yield point of metallic mono-crystals, the specific structural changes of the formed metal, the rise of flow rate of metals, the lowering of the fatigue strength and the facilitation of forming process of metals by the electric capillary effect (translated from the German original text) [
4].
Rehbinder’s explanation for these phenomena was the stabilization of microcracks, which always exist in metals (monocrystals and polycrystals). This means that the surface-active substances interact with the fresh surfaces by preventing a re-welding of microcracks. This effect leads to shorter chips in cutting. In forming operations, the surface-active substances are stabilizing the so-called gliding packets of the material.
Generally, every aspect of metalworking is considered to be strongly influenced by surface-active substances. In the end, the origin of electric double layers in microcracks, transfer or in interfaces between crystals in polycrystalline systems is the driving force for the accumulation (adsorption) of polar additives (surface-active materials).
4. Results
4.1. Survey with Different Esters (E)
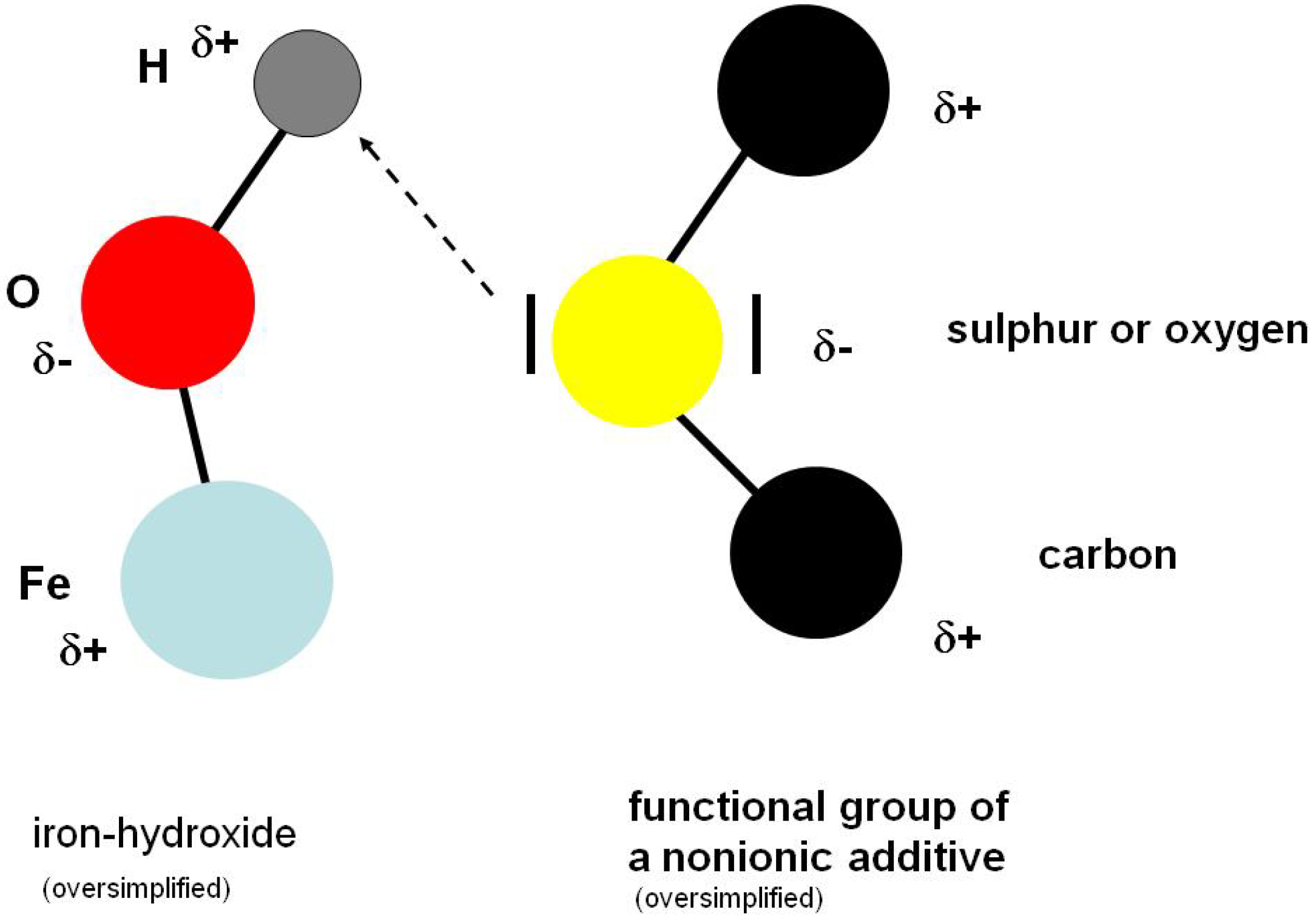
As a general basis, a class of additives was selected which is known to allow for excluding chemical reaction, namely esters (
Table 1). According to the “old” and also to the “new” theory, this class of substances should work purely based on adsorption. The new theory assumes that adsorption on metal atoms in oxidic groups as well as hydrogen bridge bonds with the hydrogen atoms of the hydroxide groups on the metal surface should be possible.
Table 1.
Brugger-values of mixtures ester in mineral oil.
Table 1.
Brugger-values of mixtures ester in mineral oil.
| | Chemistry of ester | Viscosity of mixtures at 40 °C (mm2/s) | Brugger-value 100Cr6-specimen (N/mm2) | Brugger-value 1.4305-specimen (N/mm2) |
|---|
| E1 | triglyceride I (synthetic) | 16.2 | 26.4 | 15.6 |
| E2 | triglyceride II (synthetic) | 16.8 | 28.1 | 15.4 |
| E3 | trimethylol-propanester | 30.9 | 23.5 | 18.9 |
| E4 | triglyceride III (native) | 26.6 | 22 | 18.1 |
| E5 | complex ester | 120.1 | 48.6 | 21.5 |
| E6 | electrical treated native ester | 256.7 | 43.7 | 21.5 |
| E7 | fatty acid ester I | 13.4 | 24.3 | 17.8 |
| E8 | fatty acid ester II | 6.8 | 33.5 | 17 |
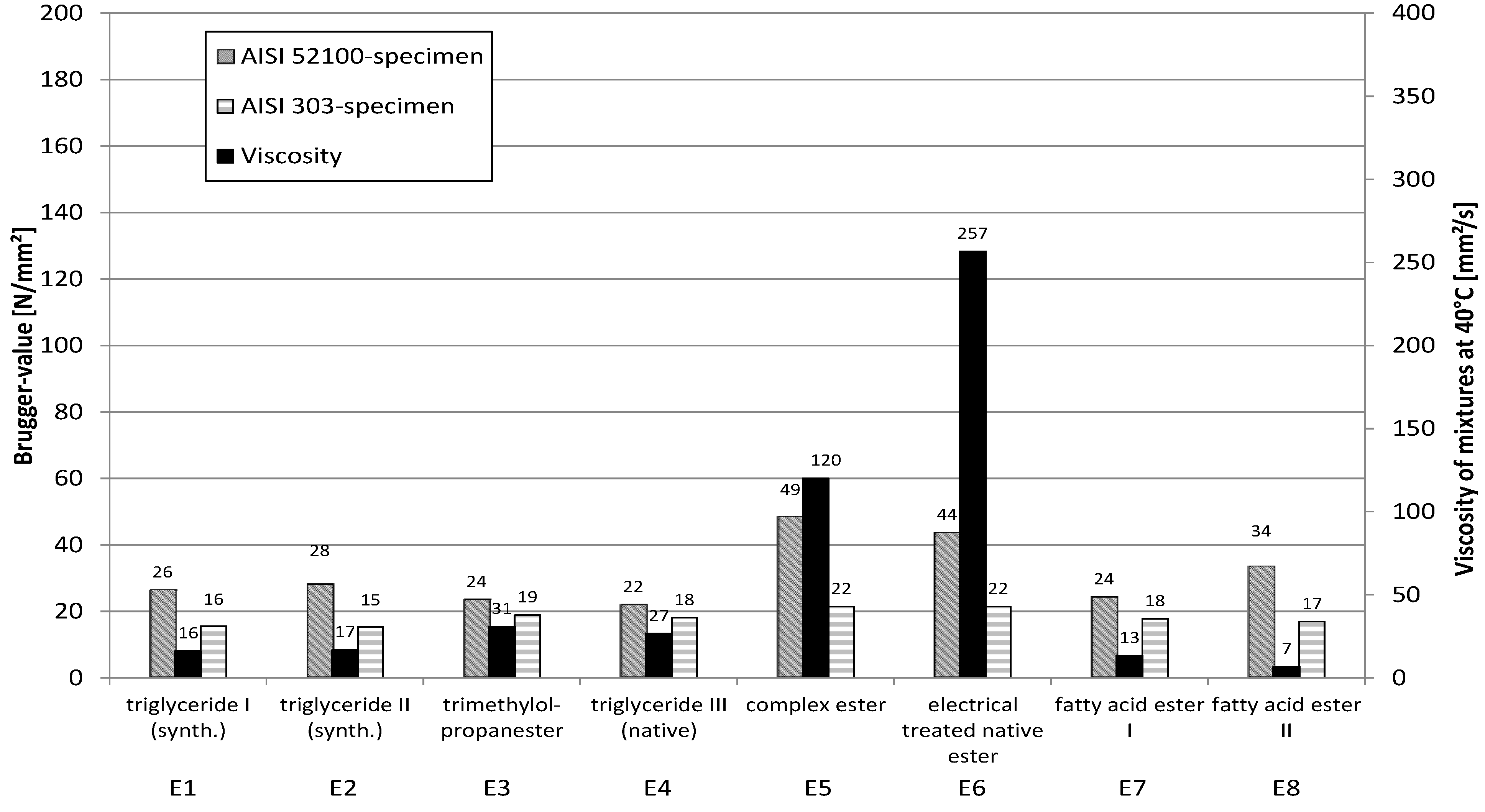
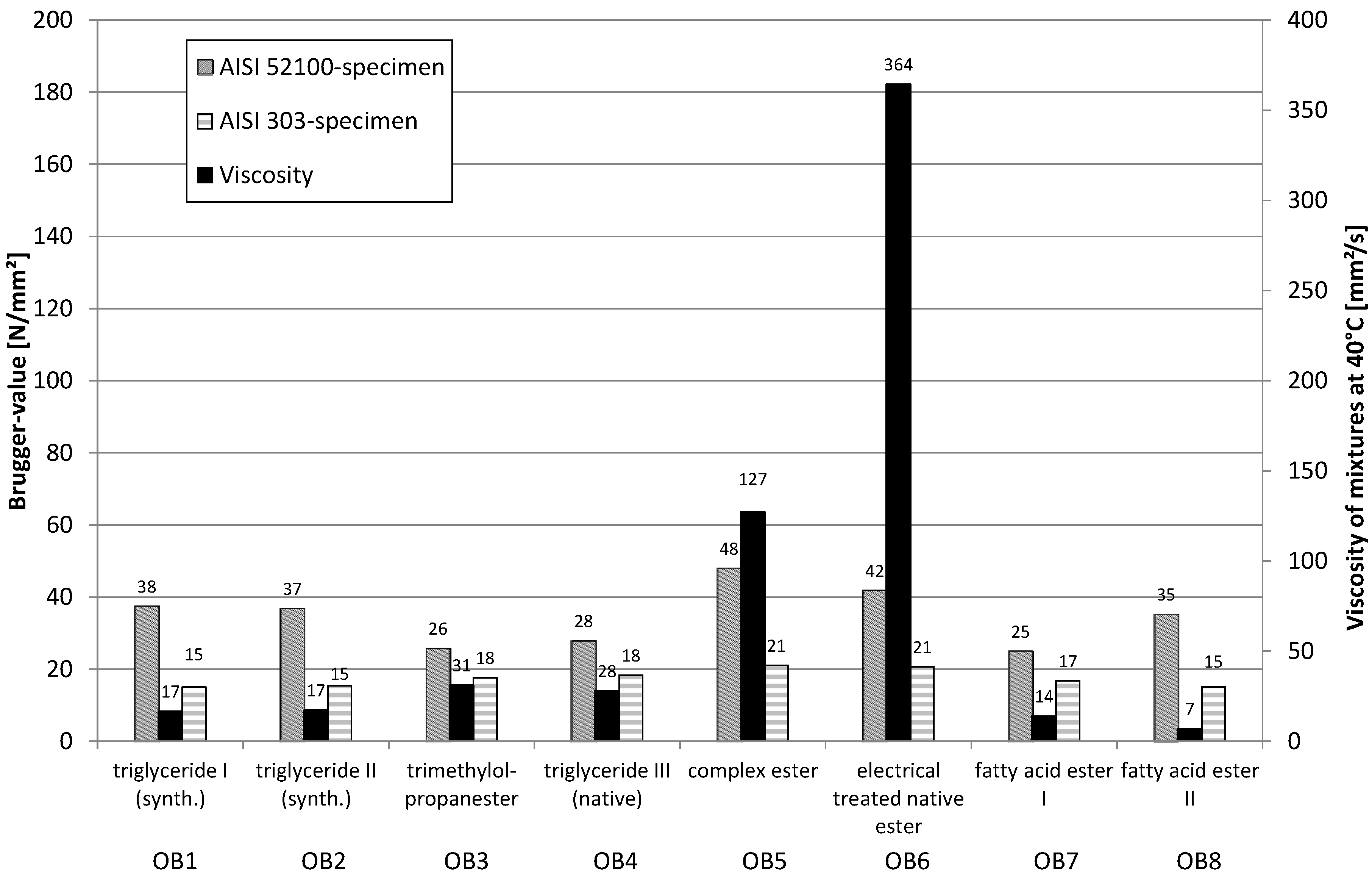
Different types of esters (triglycerides, fattyacid ester and complex ester) (
Table 1), which differ in viscosity and stereochemistry in the ester group were used.
Figure 13 specifies the type of ester and the Brugger-values of esters in a mixture with 60% naphthenic base oil. The relatively moderate Brugger-values using stainless steel (AISI 303, 1.4305) as a workpiece material indicate a rather low adsorption at this surface material. The complex esters E5 and E6 are an exception. Here, obviously another electron density distribution is present within the molecule. Possibly even the higher viscosity has an effect here. Esters E1 and E2 show the lowest values. Both contain exclusively full-saturated fatty acid residues and therefore can only use the oxygen atoms of the ester groups as a binding site.
Using carbon steel (AISI 52100, 100Cr6) the type of ester obviously has a big influence on the resulting values. The different values are a hint for the sterical influence of the hydrocarbon groups at the ester group. Multiple bonds in the acid residues of the ester groups are also able to form hydrogen bridge bonds.
The mixtures of the complex esters E5 and E6 lead to clearly higher Brugger values with carbon steel, because they have more binding sites in the molecule and thereby more “bonds” with the metal surface can be built up. In this investigation, the influence of the viscosity cannot be totally excluded. Nevertheless, the viscosities of the mixtures seem to be of lower importance, as higher viscosities do not always lead to higher Brugger values.
To get a deeper understanding regarding the impact of esters, these were combined with other additives in the next survey. Polysulfides, which prefer oxidic groups and only to a minor extent hydrogen bridge bonds, and overbased sulfonates, which prefer ionic bonds, were used.
4.2. Mixtures of Esters and Polysulfides (S)
The addition of the polysulfide should limit the chance of adsorption of esters, because the polysulfide should preferentially interact with the metal atoms of the oxidic groups on the metal surface and thereby block these for the ester.
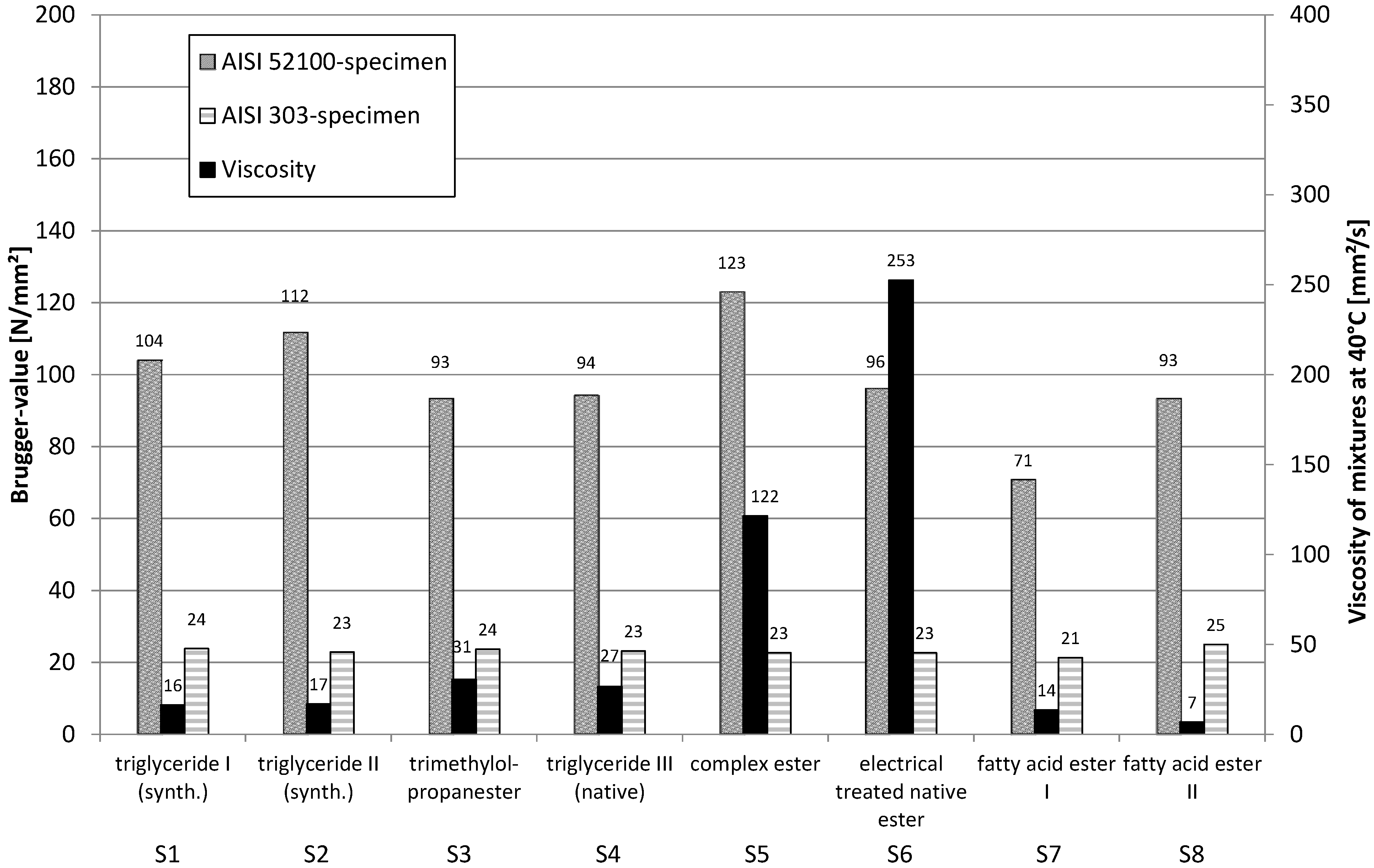
Surprisingly, all Brugger values using AISI 52100 (100Cr6) as a workpiece material, clearly are increased by the addition of only 2% of polysulfide (
Figure 14). This means that esters and the polysulfides do not compete for the same surface areas, but are complementary in their effects. This gives prove to the fact that esters work preferentially, based on hydrogen bridge bonds, and not based on adsorption to the oxidic groups.
Figure 13.
Brugger-values of mixtures ester in mineral oil.
Figure 13.
Brugger-values of mixtures ester in mineral oil.
Figure 14.
Brugger-values of mixtures ester and polysulfide in mineral oil.
Figure 14.
Brugger-values of mixtures ester and polysulfide in mineral oil.
The (compared to
Figure 13) higher values using stainless steel (AISI 303, 1.4305) as a workpiece material are clearly due to the adsorptive effect of the polysulfide, as a chemical reaction of the polysulfide with the high-grade steel surface can be excluded.
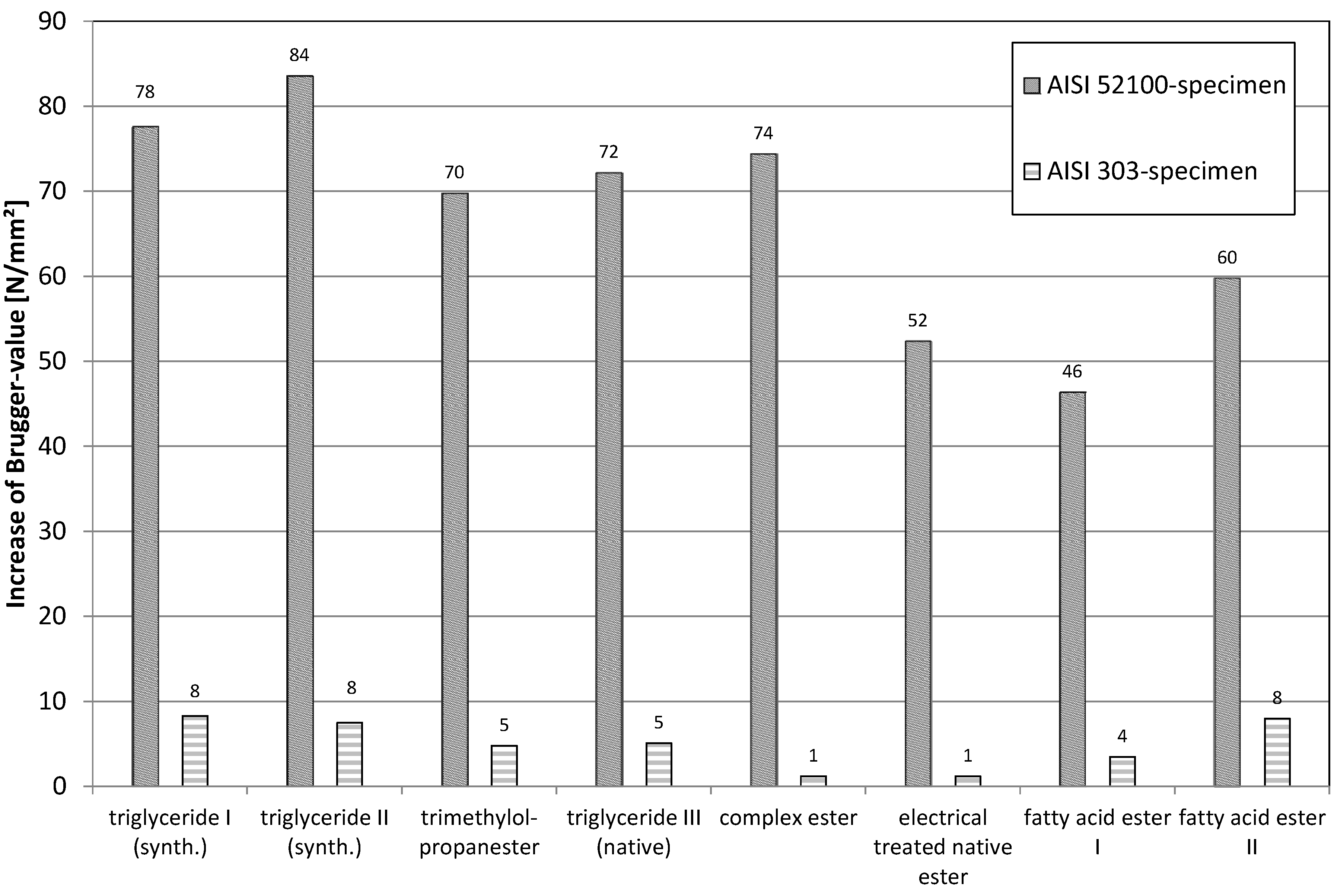
In
Figure 15, the change of the Brugger-values caused by addition of 2% polysulfide is given. As already described before the Brugger-values of the AISI 52100-specimens are clearly higher. However, the amount of increase based on the addition of polysulfides looks different. This is even more obvious for the values, which were obtained for the specimens made of AISI 303. The increase of the Brugger values of ester mixture S5 and S6 (AISI 303-specimens) are almost within the margin of error of the Brugger method. This means that both complex esters E5 and E6 obviously block the docking places, which are also needed by the polysulfide.
Figure 15.
Increase of Brugger-values of ester mixtures caused by addition of polysulfides in mineral oil.
Figure 15.
Increase of Brugger-values of ester mixtures caused by addition of polysulfides in mineral oil.
A similar behavior is also observed (even if not as clearly) regarding the ester, which contains the multiple bonds in the acid residues groups. The same effect is also to be obtained looking at the values, which were determined using the AISI 52100-specimens. As the content of oxidic groups at these surfaces is considerably lower than at the purely oxidic stainless steel surface, the effects are, of course, lower.
4.3. Mixtures of Esters and Overbased Sulfonates (OB)
Comparable to the survey presented in
Section 4.2, the influence of overbased sulfonates was investigated. The Brugger-values using carbon steel as a workpiece material show a minor improvement. The values using stainless steel reveal no influence of overbased sulfonates at all. The reason for this can be found considering the ionic character of overbased sulfonates, which are not able to interact with the purely oxidic surface of stainless steel (
Figure 16).
Figure 16.
Brugger-values of ester mixtures and overbased sulfonates in mineral oil.
Figure 16.
Brugger-values of ester mixtures and overbased sulfonates in mineral oil.
The low increase of the values at carbon steel shows that overbased sulfonates do not obviously hinder the interaction of esters based on the hydrogen bridge bond. The third kind of possible interactions (ionically) leads to a low improvement of the Brugger-values compared to the pure mixtures of ester in mineral oil (E1–E8).
4.4. Investigations Using a Complex Matrix (M)
To support the “new” theory it is necessary to examine mixtures with more than two components. Thus, mixtures were established, which get closer to the reality in MWF. The following mixtures of additives were tested:
(X)% base oil (mineral oil)
(A)% polysulfide—relevant for oxidic groups
(B)% ester—relevant for hydroxidic groups
(C)% overbased sulfonates—relevant for ionic parts on metal surface
In this case, the exact concentrations of additives are subject to secret protection. The result of A + B + C+ X equals 100%. This means that in case one component is not included in the mixture, base oil was used to fill up to 100%. The suitable formula arises from
Table 2. It shows all possible combinations of the mixtures and their results at the Brugger machine with a AISI 52100-specimen as well as the welding loads of the four ball machine (ASTM D2596) with AISI 52100 balls.
Brugger-values and welding loads correlate very well to each other, which refers to the same active mechanism of the additives with the surfaces of the specimens (AISI 52100). Investigations of the same material combination (AISI 52100-ball/AISI 52100-disc) with the oscillation-fretting-machine (standard terms, not shown here) showed no correlation. It is obvious that the parameters of the oscillation-fretting-machine must be adapted, as the results of the Brugger device as well as the welding loads correlate well with reality.
Table 2.
Brugger-values and welding loads of the complex matrix.
Table 2.
Brugger-values and welding loads of the complex matrix.
| | Mixture | Brugger-value AISI 52100-specimen (N/mm²) | Four-ball welding load AISI 52100-balls (N) |
|---|
| M1 | 100% base oil | 14.5 | <2000 |
| M2 | (X + A + B)% base oil/(C)% overbased sulfonate | 55 | 2200 |
| M3 | (X + B + C)% base oil/(A)% polysulfide | 75 | 2800 |
| M4 | (X + A + C)% base oil/(B)% ester | 25.5 | <2000 |
| M5 | (X + B)% base oil/(A)% polysulfide/(C)% overbased sulfonate | 86 | 3600 |
| M6 | (X + A)% base oil/(B)% ester/(C)% overbased sulfonate | 56 | <2000 |
| M7 | (X + C)% base oil/(A)% polysulfide/(B)% ester | 129 | 3000 |
| M8 | (X)% base oil/(A)% polysulfide/(B)% ester/(C)% overbased sulfonate | 177 | 4200 |
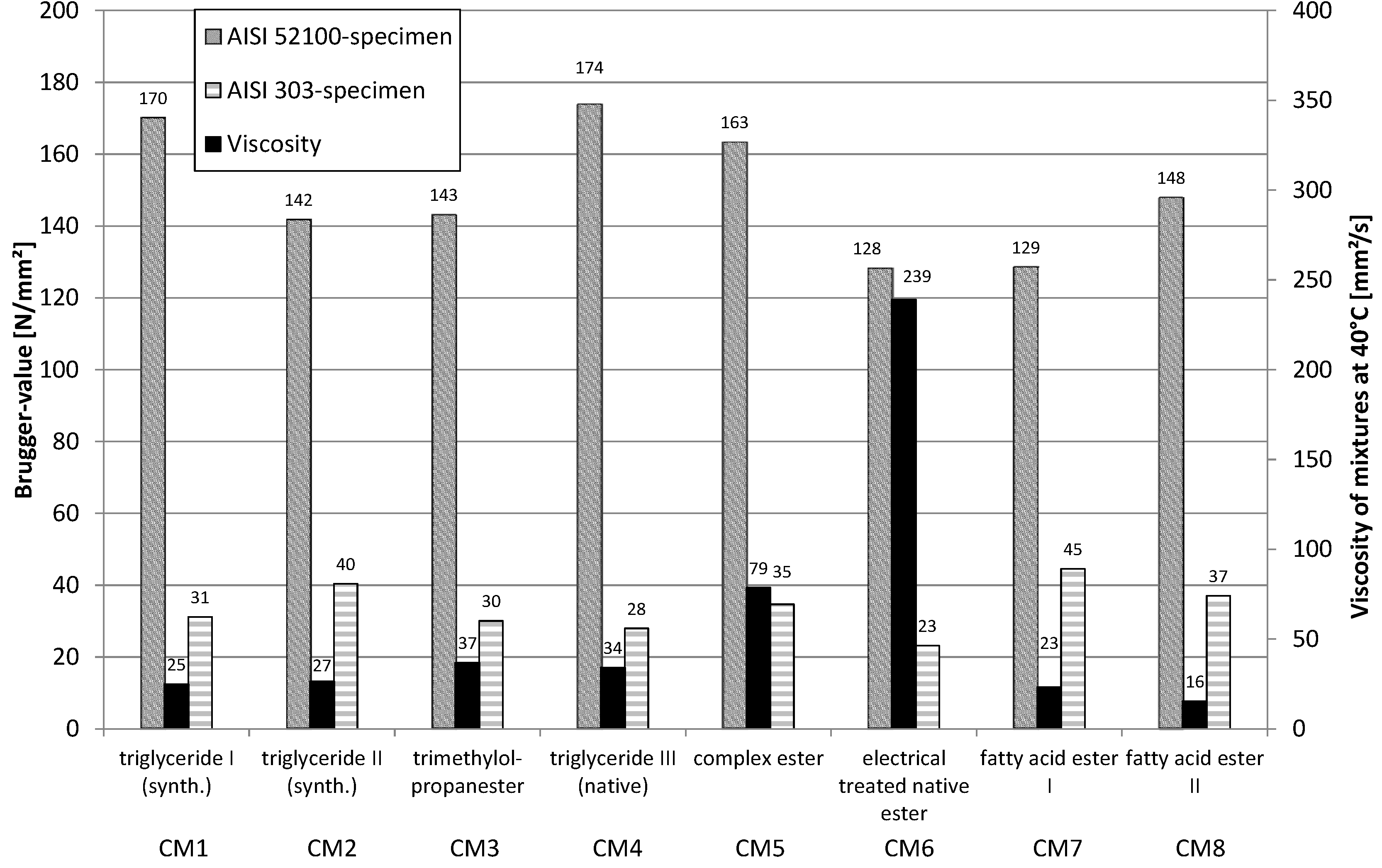
4.5. Continuative Investigations Using the Complete Matrix (CM)
In the following, some results are presented, which are achieved by application of the “ideal” matrix from
Section 4.4 (M8). The concentrations of the mixtures CM1 to CM8 (
Figure 17) are always kept constant, so only the type of the ester is varied. Which of the ester types has a clear influence is evident. The expected effects already described above can be also observed here.
Figure 17.
Brugger-values of trials with complete matrix-variation of ester.
Figure 17.
Brugger-values of trials with complete matrix-variation of ester.
The best values are achieved by using the triglycerides on carbon steel. The results achieved show the dependence of the performance of the esters on their stereochemistry, meaning the way how the oxygen atoms of the esters are able to approach the hydrogen atoms of the hydroxides. The same effect can be observed for stainless steel specimens.
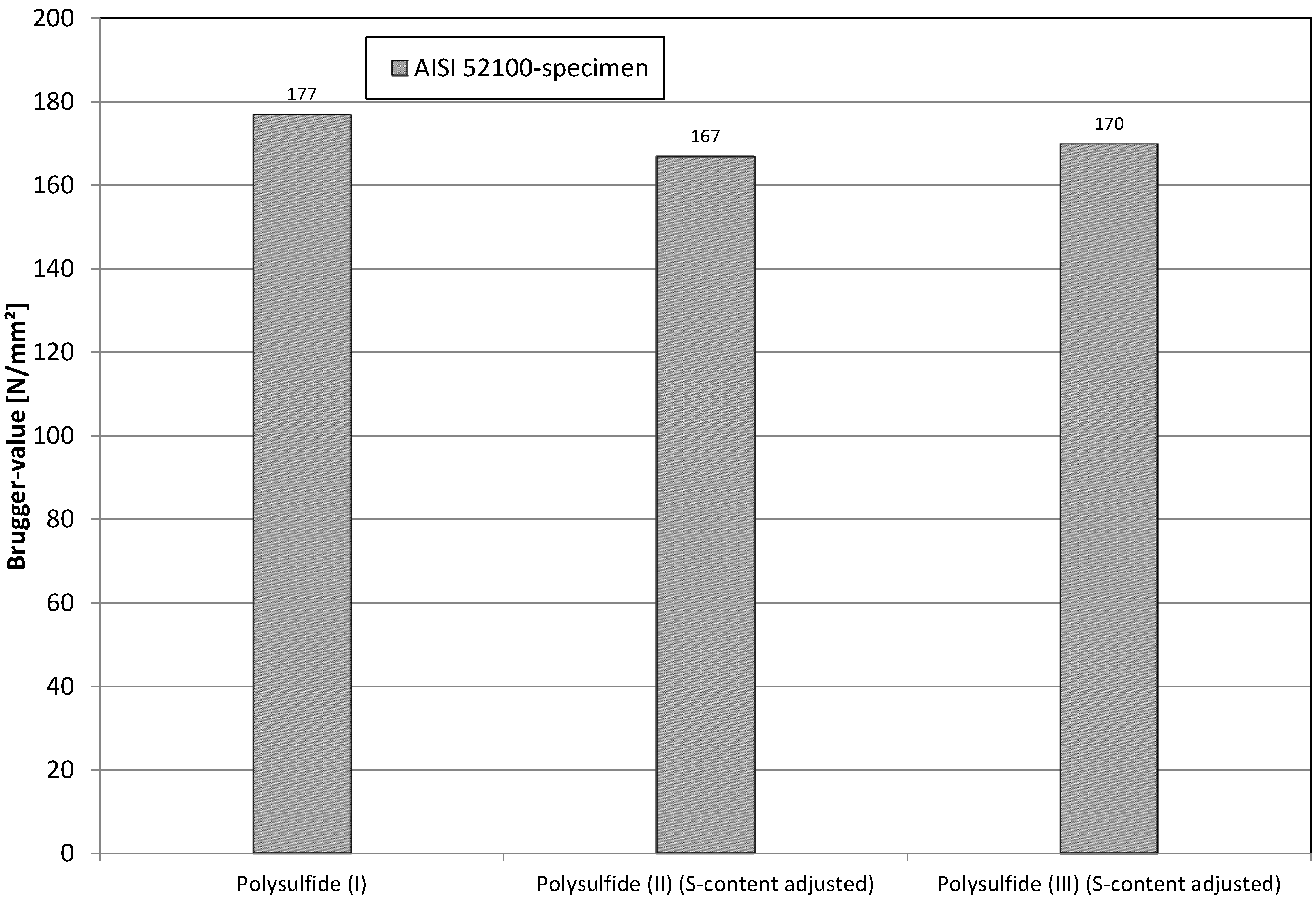
In a subsequent approach, the active polysulfide was replaced by a different active polysulfide and, in a third trial, by an inactive polysulfide. Attention was paid to the fact that the sulfur-content remained constant within the mixture. As the results in
Figure 18 show, the change of the polysulfide has no significant influence on the results. This means that the performance of the lubricant does not depend on the activity of the polysulfide, but only on the sulfur content itself. This was observed before in [
11] and was reconfirmed here.
Figure 18.
Results of replacement of polysulfides.
Figure 18.
Results of replacement of polysulfides.
The values given in
Figure 18 are of course only valid in the matrix described in
Section 4.4. This means that the free electron pairs in the sulfur-atoms of the polysulfides are able to interact only with the metal atoms of the oxidic groups of the metal surface, as the hydroxides are blocked with ester and overbased sulfonates. If the hydroxides were also available, a movement of the results should be obtained. These interrelationships were examined by Huesmann and described in [
14]. It was shown that polysulfides in “undisturbed” surroundings (no other additives) are able to have a weak interaction with the hydroxides.
5. Conclusions
This paper discusses the possible interactions of chemical substances in lubricants (additives) with metal surfaces. Based on theoretical considerations, the main working mechanism (excluding ionic reactions) is expected to be adsorption. By interacting with the atoms of the metal surface, the additives should change the electric properties of the workpiece material itself and thereby influence further (re)actions indirectly, which makes the whole process very complex. Furthermore, a steric impact of additives (covering of the surface influences the probability/possibility of further (re)actions) must be considered.
The results presented here, prove that the theory of homogeneous chemical properties of metal surfaces is not valid any more. The consideration of hydroxides and oxides at the surface leads to a better predictability of the behavior of additives or their mixtures. Including chemically differently built up surfaces (here, AISI 52100 and AISI 303) in the models, predictions of the behavior of additives or their mixtures in practice is possible. So-called synergisms or antagonisms can be explained casually with the different affinity of additives to certain surface structures.
Future work should point out, how other additives, e.g., wear protection additives (AW), influence the results. Based on the considerations and the new model presented here, a deeper scientific understanding of the interaction (functionality) of these additives will be achieved.