Interaction between Lubricants Containing Phosphate Ester Additives and Stainless Steels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Supplies
2.2. Sample Preparation
2.3. Gas Chromatography/Mass Spectrometry
2.4. Fourier Transform Infrared Spectroscopy
2.5. Nuclear Magnetic Resonance Spectroscopy
2.6. Raman Spectroscopy
2.7. Scanning Electron Microscopy
3. Results and Discussion
3.1. Analysis of the Residual Lubricant
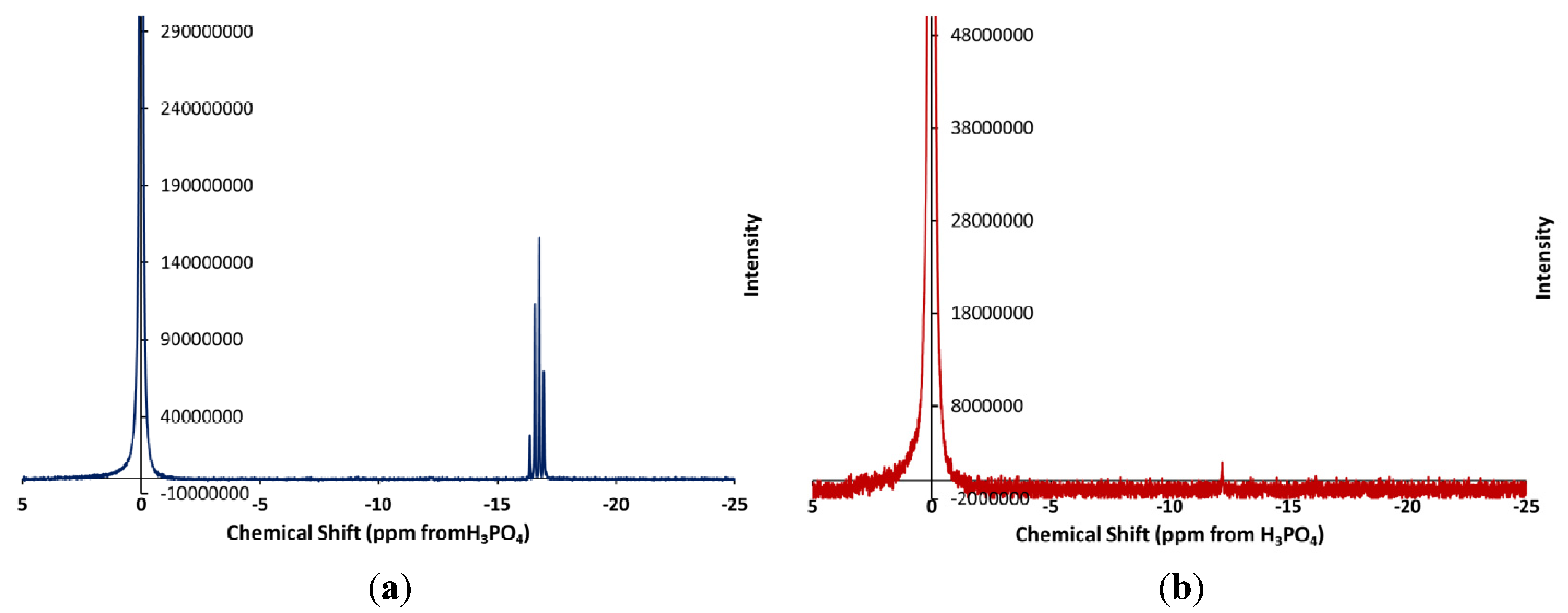
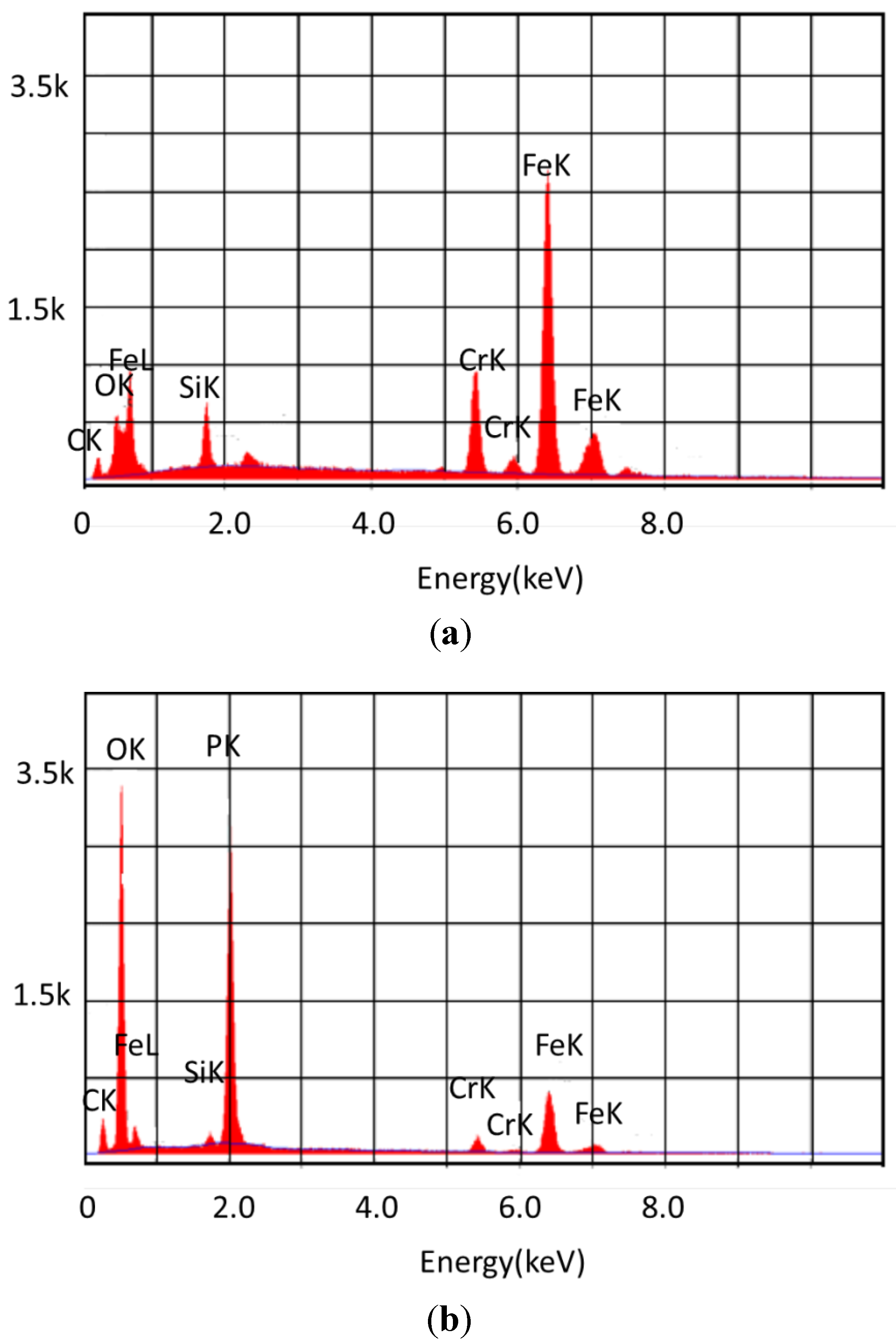
3.2. Analysis of the Solids
| Lubricant | Time (h) | Temperature (°C) | Mass Increase 440C Stainless Steel (g) | Mass Increase Pyrowear 675 Steel (g) |
|---|---|---|---|---|
| Basestock | 24 | 325 | −0.0003 | +0.0004 |
| 24 | 350 | −0.0004 | −0.0002 | |
| 96 | 325 | −0.0006 | −0.0004 | |
| 96 | 350 | −0.0007 | −0.0006 | |
| Basestock +5% Tricresyl Phosphate | 24 | 325 | +0.0004 | +0.0005 |
| 24 | 350 | +0.0008 | +0.0009 | |
| 96 | 325 | +0.0030 | +0.0028 | |
| 96 | 350 | +0.0044 | +0.0032 | |
| Basestock +10% Tricresyl Phosphate | 24 | 325 | +0.0012 | +0.0009 |
| 24 | 350 | +0.0032 | +0.0032 | |
| 96 | 325 | +0.0159 | +0.0158 | |
| 96 | 350 | +0.0215 | +0.0210 |
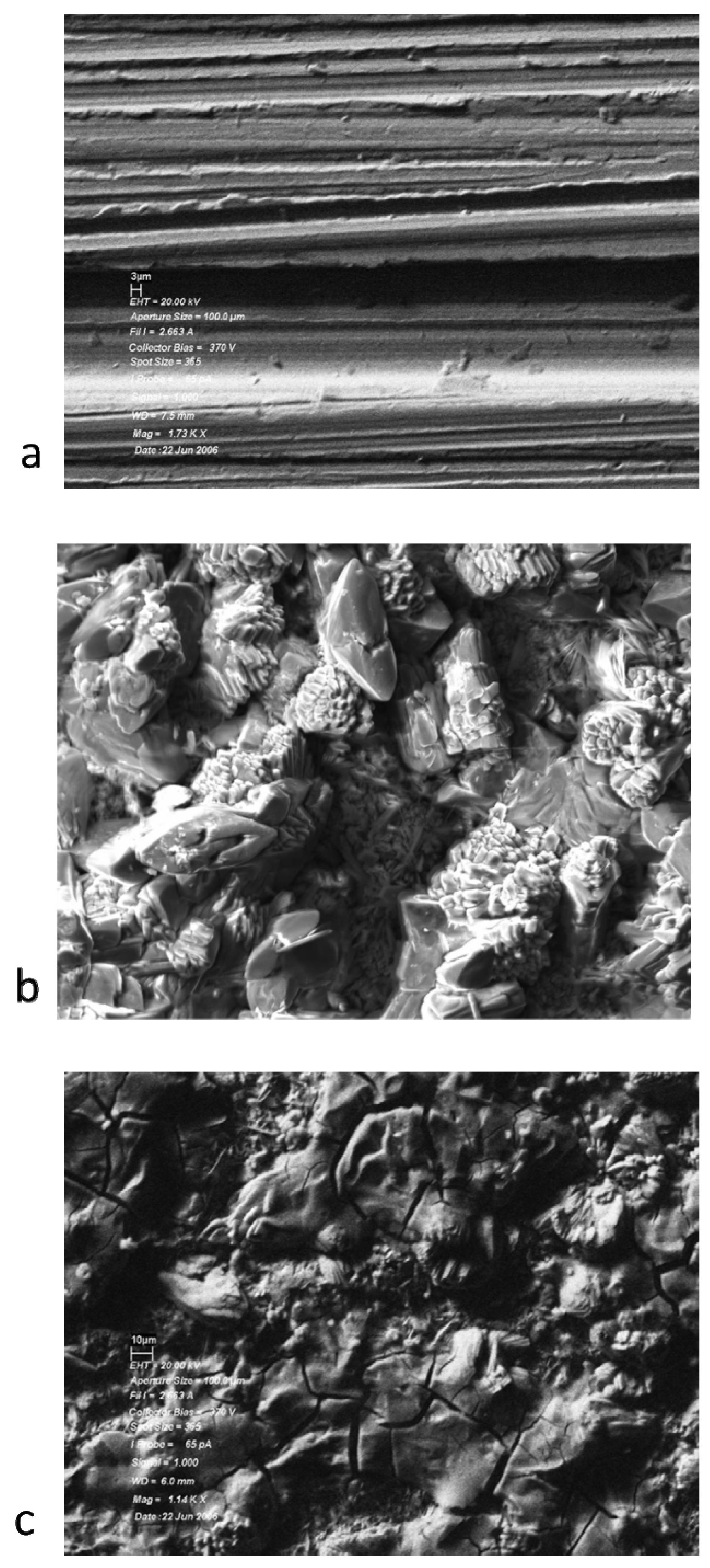
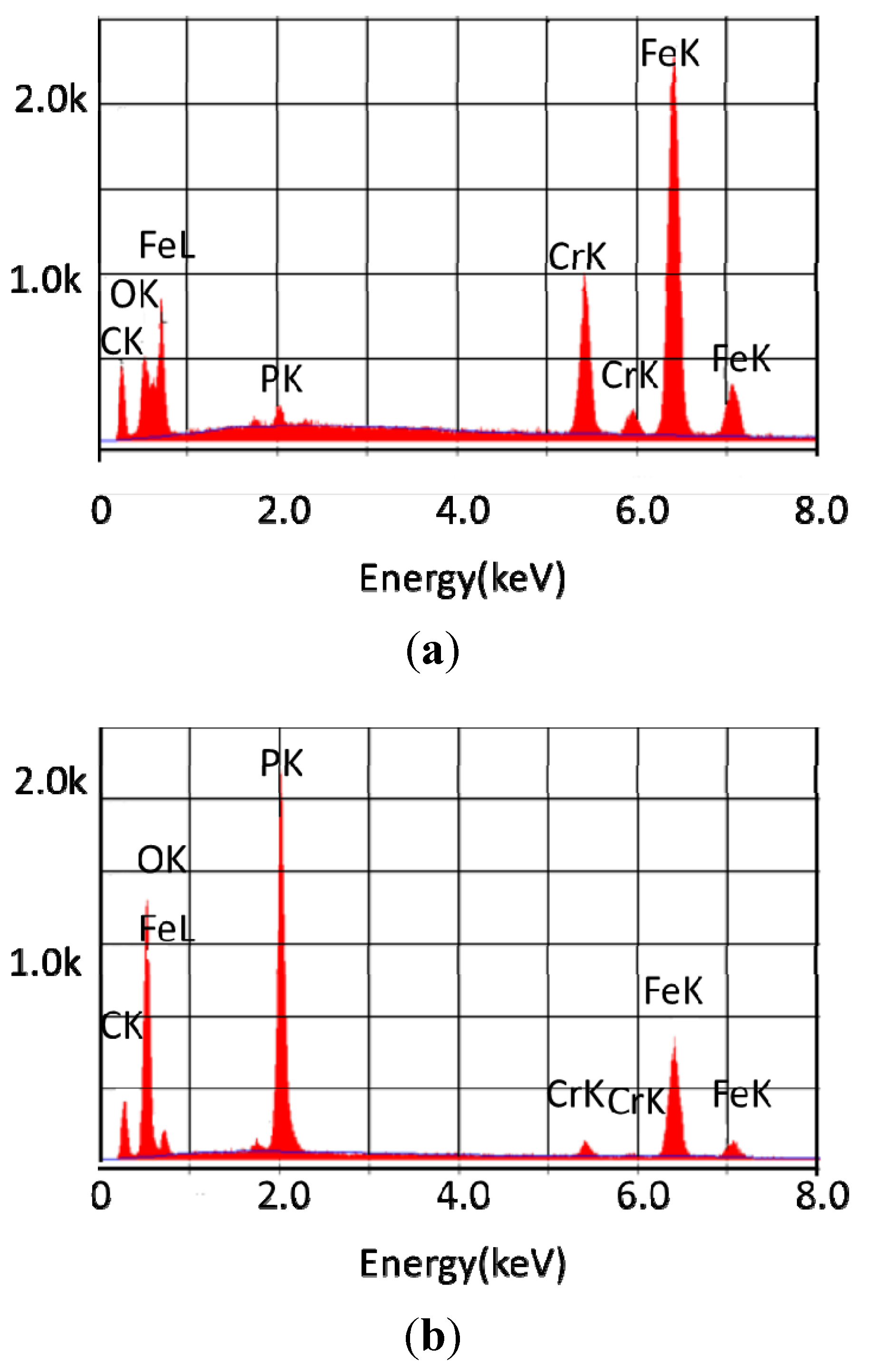
| Lubricant | Atom% C | Atom% O | Atom% P | Atom% Cr | Atom% Fe |
|---|---|---|---|---|---|
| 440C Stainless Steel | |||||
| Basestock | 40.46 | 15.17 | <1 | 9.68 | 33.26 |
| 5% TCP | 47.86 | 24.68 | 6.38 | 1.25 | 14.35 |
| 10% TCP | 54.97 | 31.25 | 8.32 | 0.58 | 4.53 |
| Pyrowear 675 Steel | |||||
| Basestock | 25.1 | 14.98 | <1 | 8.33 | 39.67 |
| 5% TCP | <5 | 70.97 | 18.77 | 1.3 | 7.75 |
| 10% TCP | <5 | 63.77 | 20.86 | 1.27 | 12.79 |
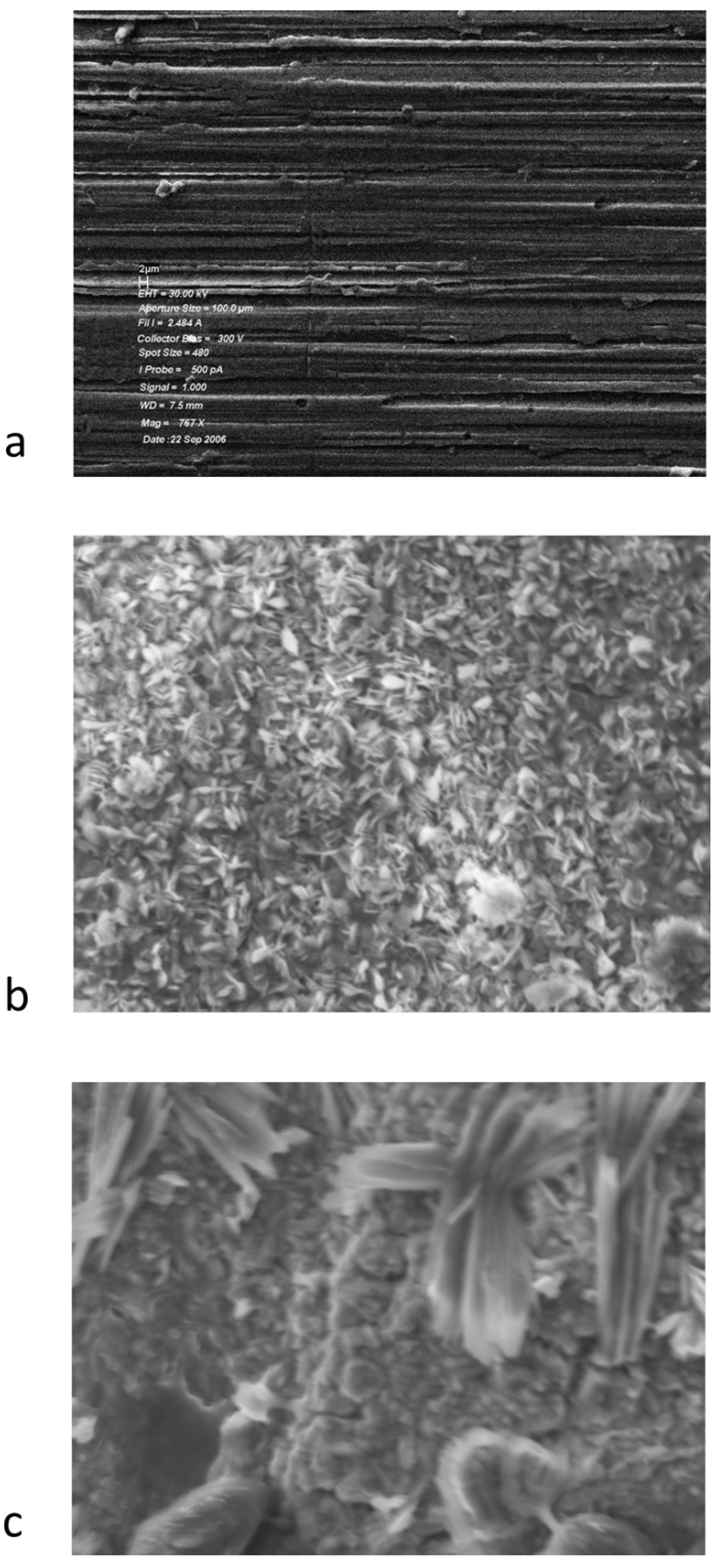

4. Conclusions
References and Notes
- Gschwender, L.J.; Snyder, C.E. Research and development of advanced high-temperature air force Turbine Engine Oil. J. Soc. Tribol. Lubr. Engin. 2000, 56, 20–25. [Google Scholar]
- Pippel, E.; Woltersdorf, J.; Pockl, G.; Lichtenegger, G. Microstructure and nanochemistry of carbide precipitates in high-speed steel S6-5-2-5. Mater. Charact. 1999, 43, 41–55. [Google Scholar] [CrossRef]
- Hetzner, D.W.; van Geertruyden, W. Crystallography and metallography of carbides in high alloy steels. Mater. Charact. 2008, 59, 825–841. [Google Scholar] [CrossRef]
- Brusilovskii, B.A.; Shashko, A.Y. An X-ray study of carbides in the working layer of cold-rolling rolls. Metal Sci. Heat Treat. 2001, 43, 180–182. [Google Scholar] [CrossRef]
- Saba, C.S.; Forster, N.H. Reactions of aromatic phosphate esters with metals and their oxides. Tribol. Lett. 2002, 12, 135–146. [Google Scholar] [CrossRef]
- Hils, J.E.; Johnson, D.W.; Benin, V. Computational Investigations of the Interactions between Phosphate Esters and Metal. In Proceedings of 42nd Central Regional Meeting of the American Chemical Society, Dayton, OH, USA, 16–19 June 2010.
- Fernandez-Torres, L.C.; Zhao, X.; Kim, B.; Perry, S.S. Chemical Modification Influence on the Frictional Properties of Small Model Lubricant Molecules Adsorbed on VC(100). In Proceedings of 225th ACS National Meeting, New Orleans, LA, USA, 23–27 March 2003.
- Fernandez-Torres, L.C.; Kim, B.I.; Perry, S.S. The frictional response of VC(100) surfaces: Influence of 1-octanol and 2,2,2-trifluoroetnanol adsorption. Tribol. Lett. 2003, 15, 43–50. [Google Scholar] [CrossRef]
- Fernández-Torres, L.; Zhao, X.; Chen, Z.; Salmeron, C.; Perry, S. Influence of ethyl acetate and alkyl phosphate adsorption on the frictional properties of VC(100). Tribol. Lett. 2005, 18, 207–213. [Google Scholar] [CrossRef]
- Snyder, C.E.; Gschwender, L.J. Trends Toward Synthetic Lubricants in Aerospace. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; Rudnick, L.R., Ed.; CRC Press: Boca Raton, Florida, FA, USA, 2006; Volume 10, pp. 811–815. [Google Scholar]
- Snyder, C.E.; Gschwender, L.J.; Sharma, S.K. Long-Term Additive Trends in Aerospace Applications. In Lubricant Additives: Chemistry and Applications; Rudnick, L.R., Ed.; CRC Press: Boca Raton, Florida, FA, USA, 2009; pp. 637–644. [Google Scholar]
- Trivedi, H.K.; Forster, N.H.; Rosado, L. Rolling contact fatigue evaluation of advanced bearing steels with and without the oil anti-wear additive tricresyl phosphate. Tribol. Lett. 2011, 41, 597–605. [Google Scholar] [CrossRef]
- Cutler, J.N.; Sanders, J.H.; Zabinski, J.S.; John, P.J.; McCutchen, J.R.; Kasten, L.S.; Tan, K.H. Surface chemistry of new lubrication systems for high-speed spacecraft bearings. Tribol. Lett. 2000, 8, 17–23. [Google Scholar] [CrossRef]
- Johnson, D.W.; Hils, J.E.; Forster, N. Interaction of polyol esters and phosphate esters with metal carbides. Tribol. Lett. 2011, 42, 223–232. [Google Scholar] [CrossRef]
- Standard Reference Data Program. National Institute of Standards and Technology, Gaithersburg, MD, Standard Reference Database 1A. Available online: http://www.nist.gov/srd/nist1a.cfm (accessed on 6 May 2013).
- McLafferty, F.W. Interpretation of Mass Spectra; Mill Valley: California, CA, USA, 1980; pp. 119–167. [Google Scholar]
- Litzow, M.R.; Spalding, T.R. Mass Spectrometry of Inorganic and Organometallic Compounds; Elsevier: Amsterdam, The Netherland, 1973; pp. 321–439. [Google Scholar]
- Gorenstein, D.G. Dependence of 31P chemical shifts on oxygen-phosphorus-oxygen bond angles in phosphate esters. J. Am. Chem. Soc. 1975, 97, 898–900. [Google Scholar] [CrossRef]
- Johnson, D.W.; Morrow, S.; Forster, N.H.; Saba, C.S. Vapor phase lubrication: Reaction of phosphate ester vapors with iron and steel. Chem. Mater. 2002, 14, 3867–3875. [Google Scholar]
- Molt, K.; Behmer, D.; Pohl, M. Different techniques for determining the coating weight of phosphate layers on galvanized steel by means of FT-IR spectrometry. Fresenius J. Anal. Chem. 1997, 358, 36–41. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: West Suffix, UK, 2004. [Google Scholar]
- Johnson, D.W. Application of raman spectroscopy to lubricants, lubricated surfaces and lubrication phenomena. Spectroscopy 2011, 26, 46–50. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johnson, D.W.; Bachus, M.; Hils, J.E. Interaction between Lubricants Containing Phosphate Ester Additives and Stainless Steels. Lubricants 2013, 1, 48-60. https://doi.org/10.3390/lubricants1020048
Johnson DW, Bachus M, Hils JE. Interaction between Lubricants Containing Phosphate Ester Additives and Stainless Steels. Lubricants. 2013; 1(2):48-60. https://doi.org/10.3390/lubricants1020048
Chicago/Turabian StyleJohnson, David W., Matthew Bachus, and John E. Hils. 2013. "Interaction between Lubricants Containing Phosphate Ester Additives and Stainless Steels" Lubricants 1, no. 2: 48-60. https://doi.org/10.3390/lubricants1020048
APA StyleJohnson, D. W., Bachus, M., & Hils, J. E. (2013). Interaction between Lubricants Containing Phosphate Ester Additives and Stainless Steels. Lubricants, 1(2), 48-60. https://doi.org/10.3390/lubricants1020048





