Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies
Abstract
1. Introduction
2. Opportunity Cost and Value Assessment Frameworks
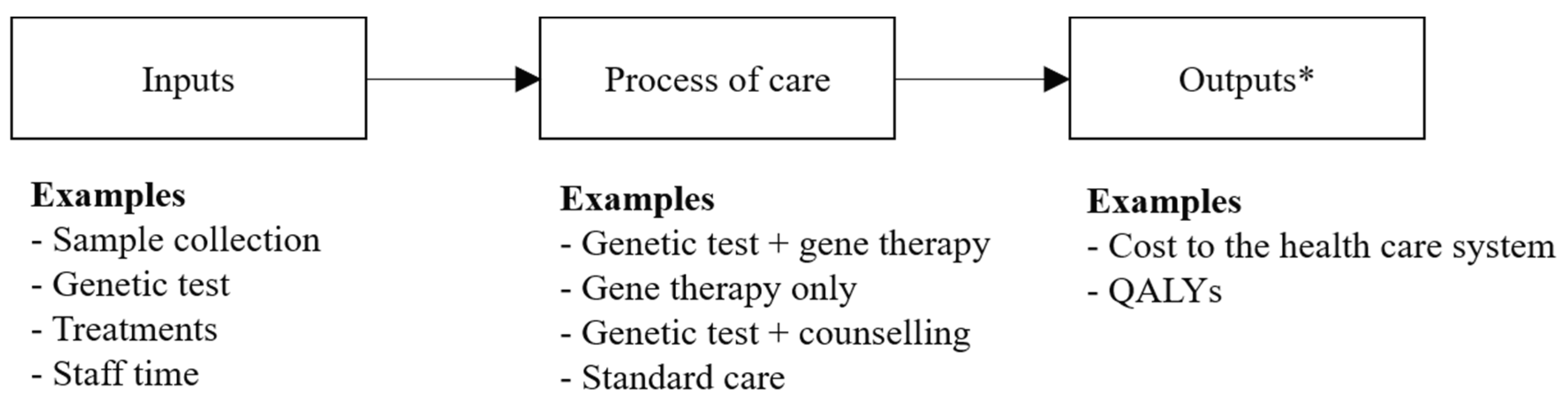
3. Assessing the Value of Genomic-based Diagnostic Tests and Gene Therapies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Available online: http://data.europa.eu/eli/reg/2007/1394/oj (accessed on 20 May 2019).
- Brennan, T.; Wilson, J. The Special Case of Gene Therapy Pricing. Nat. Biotechnol. 2014, 32, 874–876. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Elsanhoury, A.; Reinke, P. Overcoming Challenges Facing Advanced Therapies in the EU Market. Cell Stem Cell 2016, 19, 293–297. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Advanced Therapy Medicinal Products: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products (accessed on 15 February 2019).
- Food and Drug Administration. Cellular & Gene Therapy Products. Available online: https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/default.htm (accessed on 18 February 2019).
- Ylä-Herttuala, S. Glybera’s Second Act: The Curtain Rises on the High Cost of Therapy. Mol. Ther. 2015, 2, 217–218. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Public Statement: Glybera-Expiry of the Marketing Authorisation in the European Union; European Medicines Agency: London, UK, 2017. [Google Scholar]
- European Medicines Agency. Guideline on Compassionate use of Medicinal Products, Pursuant to Article 83 of Regulation (EC) No 726/2004; European Medicines Agency: London, UK, 2007. [Google Scholar]
- Food and Drug Administration. Expanded Access to Investigational Drugs for Treatment Use—Questions and Answers: Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Danzon, P.; Towse, A. The Economics of Gene Therapy and Pharmacogenetics. Value Health 2002, 5, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.; Rémuzat, C.; Auquier, P.; Toumi, M. Gene Therapies Development: Slow Progress and Promising Prospect. J. Mark. Access Health Policy 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Dias, M.; Joo, K.; Kemp, J.; Fialho, S.; da Silva Cunha, A.; Woo, S.; Kwon, Y. Molecular Genetics and Emerging Therapies for Retinitis Pigmentosa: Basic Research and Clinical Perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Mullaney, B.; Bhaskar, S.; Dickerson, J.; Hall, G.; O’Grady, A.; Webster, A.; Ramsden, S.; Black, G. A Paradigm Shift in the Delivery of Services for Diagnosis of Inherited Retinal Disease. J. Med. Genet. 2012, 49, 322–326. [Google Scholar] [CrossRef]
- Payne, K.; Gavan, S.; Wright, S.; Thompson, A. Cost-effectiveness Analyses of Genetic and Genomic Diagnostic Tests. Nat. Rev. Genet. 2018, 19, 235–246. [Google Scholar] [CrossRef]
- UK Genetic Testing Network. Retinal Degeneration Disorders 105 Gene Panel: Postnatal Diagnosis Routine by Sequencing of the Entire Coding Region of Gene (s) at Manchester RGC in 112 Days. Available online: https://ukgtn.nhs.uk/find-a-test/search-by-disorder-gene/details/3834/ (accessed on 19 February 2019).
- Food and Drug Administration. In Vitro Companion Diagnostic Devices: Guidance for Industry and Food and Drug Administration Staff; Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Scheerens, H.; Malong, A.; Bassett, K.; Boyd, Z.; Gupta, V.; Harris, J.; Mesick, C.; Simnett, S.; Stevens, H.; Gilbert, H.; et al. Current Status of Companion and Complementary Diagnostics: Strategic Considerations for Development and Launch. Clin. Transl. Sci. 2017, 10, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Payne, K. Fish and Chips all Round? Regulation of DNA-based Genetic Diagnostics. Health Econ. 2009, 18, 1233–1236. [Google Scholar] [CrossRef]
- Mattocks, C.; Morris, M.; Matthijs, G.; Swinnen, E.; Corveleyn, A.; Dequeker, E.; Müller, C.; Pratt, V.; Wallace, A.; EuroGentest Validation Group. A Standardized Framework for the Validation and Verification of Clinical Molecular Genetic Tests. Eur. J. Hum. Gen. 2010, 18, 1276–1288. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Regulation of Genetic Tests. Available online: https://www.genome.gov/10002335/regulation-of-genetic-tests/ (accessed on 19 February 2019).
- UK Genetics Testing Network. Genetic Test Evaluation. Available online: https://ukgtn.nhs.uk/resources/genetic-test-evaluation/ (accessed on 2 April 2019).
- Basu, A. Financing Cures in the United States. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 1–4. [Google Scholar] [CrossRef][Green Version]
- Touchot, N.; Flume, M. The Payers’ Perspective on Gene Therapies. Nat. Biotechnol. 2015, 33, 902–904. [Google Scholar] [CrossRef]
- Drummond, M.; Sculpher, M.; Claxton, K.; Stoddart, G.; Torrance, G. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Claxton, K.; Martin, S.; Soares, M.; Rice, N.; Spackman, E.; Hinde, S.; Devlin, N.; Smith, P.; Sculpher, M. Methods for the Estimation of the National Institute for Health and Care Excellence Cost-effectiveness Threshold. Health Technol. Assess 2015, 19, 1–503. [Google Scholar] [CrossRef]
- McCabe, C.; Claxton, K.; Culyer, A. The NICE Cost-effectiveness Threshold: What it is and what that Means. Pharmacoeconomics 2008, 26, 733–744. [Google Scholar] [CrossRef]
- Mandelblatt, J.; Ramsey, S.; Lieu, T.; Phelps, C. Evaluating Frameworks That Provide Value Measures for Health Care Interventions. Value Health 2017, 20, 185–192. [Google Scholar] [CrossRef]
- Willke, R.; Neumann, P.; Garrison, L.; Ramsey, S. Review of Recent US Value Frameworks—A Health Economics Approach: An ISPOR Special Task Force Report [6]. Value Health 2018, 21, 155–160. [Google Scholar] [CrossRef]
- Angelis, A.; Lange, A.; Kanavos, P. Using Health Technology Assessment to Assess the Value of New Medicines: Results of a Systematic Review and Expert Consultation Across Eight European Countries. Eur J. Health Econ. 2018, 19, 123–152. [Google Scholar] [CrossRef]
- Schnipper, L.; Davidson, N.; Wollins, D.; Blayney, D.; Dicker, A.; Ganz, P.; Hoverman, J.; Langdon, R.; Lyman, G.; Meropol, N.; et al. Updating the American Society of Clinical Oncology Value Framework: Revisions and Reflections in Response to Comments Received. J. Clin. Oncol. 2016, 34, 2925–2934. [Google Scholar] [CrossRef]
- Cherny, N.; Sullivan, R.; Dafni, U.; Kerst, J.; Sobrero, A.; Zielinski, C.; de Vries, E.; Piccart, M. A Standardised, Generic, Validated Approach to Stratify the Magnitude of Clinical Benefit that can be Anticipated from Anti-cancer Therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diagnostics Assessment Programme Manual; National Institute for Health and Care Excellence: Manchester, UK, 2011. [Google Scholar]
- National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal; National Institute for Health and Care Excellence: London, UK, 2013. [Google Scholar]
- National Institute for Health and Care Excellence. Interim Process and Methods of the Highly Specialised Technologies Programme: Updated to Reflect 2017 Changes; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Institute for Clinical and Economic Review. Overview of the ICER Value Assessment Framework and Update for 2017-2019; Institute for Clinical and Economic Review: Boston, MA, USA, 2017. [Google Scholar]
- Institute for Clinical and Economic Review. Modifications to the ICER Value Assessment Framework for Treatments for Ultra-rare Diseases; Institute for Clinical and Economic Review: Boston, MA, USA, 2017. [Google Scholar]
- Paulden, M.; Stafinski, T.; Menon, D.; McCabe, C. Value-based Reimbursement Decisions for Orphan Drugs: A Scoping Review and Decision Framework. Pharmacoeconomics 2015, 33, 255–269. [Google Scholar] [CrossRef]
- Paulden, M. Recent Amendments to NICE’s Value-based Assessment of Health Technologies: Implicitly Inequitable? Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 239–242. [Google Scholar] [CrossRef]
- Hettle, R.; Corbett, M.; Hinde, S.; Hodgson, R.; Jones-Diette, J.; Woolacott, N.; Palmer, S. The Assessment and Appraisal of Regenerative Medicines and Cell Therapy Products: An Exploration of Methods for Review, Economic Evaluation and Appraisal. Health Technol. Assess 2017, 21, 1–204. [Google Scholar] [CrossRef]
- Medical Research Council. Developing and Evaluating Complex Interventions: New Guidance; Food and Drug Administration: London, UK, 2008. [Google Scholar]
- Payne, K.; McAllister, M.; Davies, L. Valuing the Economic Benefits of Complex Interventions: When Maximising Health is not Sufficient. Health Econ. 2013, 22, 258–271. [Google Scholar] [CrossRef]
- Gavan, S.; Thompson, A.; Payne, K. The Economic Case for Precision Medicine. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Zimmermann, M.; Lubinga, S.; Banken, R.; Rind, D.; Cramer, G.; Synnott, P.; Chapman, R.; Khan, S.; Carlson, J. Cost Utility of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease. Value Health 2019, 22, 161–167. [Google Scholar] [CrossRef]
- Sculpher, M.; Claxton, K.; Pearson, S. Developing a Value Framework: The Need to Reflect the Opportunity Costs of Funding Decisions. Value Health 2017, 20, 234–239. [Google Scholar] [CrossRef]
- Bryant, L.; Christopher, D.; Giles, A.; Hinderer, C.; Rodriguez, J.; Smith, J.; Traxler, E.; Tycko, J.; Wojno, A.; Wilson, J. Lessons Learned from the Clinical Development and Market Authorization of Glybera. Hum. Gene Ther. Clin. Dev. 2013, 24, 55–64. [Google Scholar] [CrossRef]
- Hampson, G.; Towse, A.; Pearson, S.; Dreitlein, W.; Henshall, C. Gene Therapy: Evidence, Value and Affordability in the US Health Care System. J. Comp. Eff. Res. 2018, 7, 15–28. [Google Scholar] [CrossRef]
- Corbett, M.; Webster, A.; Hawkins, R.; Woolacott, N. Innovative Regenerative Medicines in the EU: A Better Future in Evidence? BMC Medicine 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Claxton, K.; Ginnelly, L.; Sculpher, M.; Philips, Z.; Palmer, S. A Pilot Study on the use of Decision Theory and Value of Information Analysis as Part of the NHS Technology Assessment Programme. Health Technol. Assess 2004, 8, 1–118. [Google Scholar] [CrossRef]
- Sculpher, M.; Drummond, M.; Buxton, M. The Iterative use of Economic Evaluation as Part of the Process of Health Technology Assessment. J. Health Serv. Res. Policy 1997, 2, 26–30. [Google Scholar] [CrossRef] [PubMed]
| Condition | Therapy | Administration | Status | Price Per Dose |
|---|---|---|---|---|
| Vision loss | Luxturna (voretigene neparvovec-rzyl) | In vivo | Licensed 2017 | USD$850,000 |
| Lipoprotein lipase deficiency | Glybera (alipogene tiparvovec) | In vivo | Licensed 2012; withdrawn 2017 | €1.1million |
| Severe combined immunodeficiency | Strimvelis (autologous CD34+) | Ex vivo | Licensed 2016 | €594,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavan, S.P.; Lu, C.Y.; Payne, K. Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies. J. Pers. Med. 2019, 9, 28. https://doi.org/10.3390/jpm9020028
Gavan SP, Lu CY, Payne K. Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies. Journal of Personalized Medicine. 2019; 9(2):28. https://doi.org/10.3390/jpm9020028
Chicago/Turabian StyleGavan, Sean P., Christine Y. Lu, and Katherine Payne. 2019. "Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies" Journal of Personalized Medicine 9, no. 2: 28. https://doi.org/10.3390/jpm9020028
APA StyleGavan, S. P., Lu, C. Y., & Payne, K. (2019). Assessing the Joint Value of Genomic-Based Diagnostic Tests and Gene Therapies. Journal of Personalized Medicine, 9(2), 28. https://doi.org/10.3390/jpm9020028






