Primary Clitoral Melanoma: Personalized Therapeutic Strategies Informed by Clinical Evidence and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
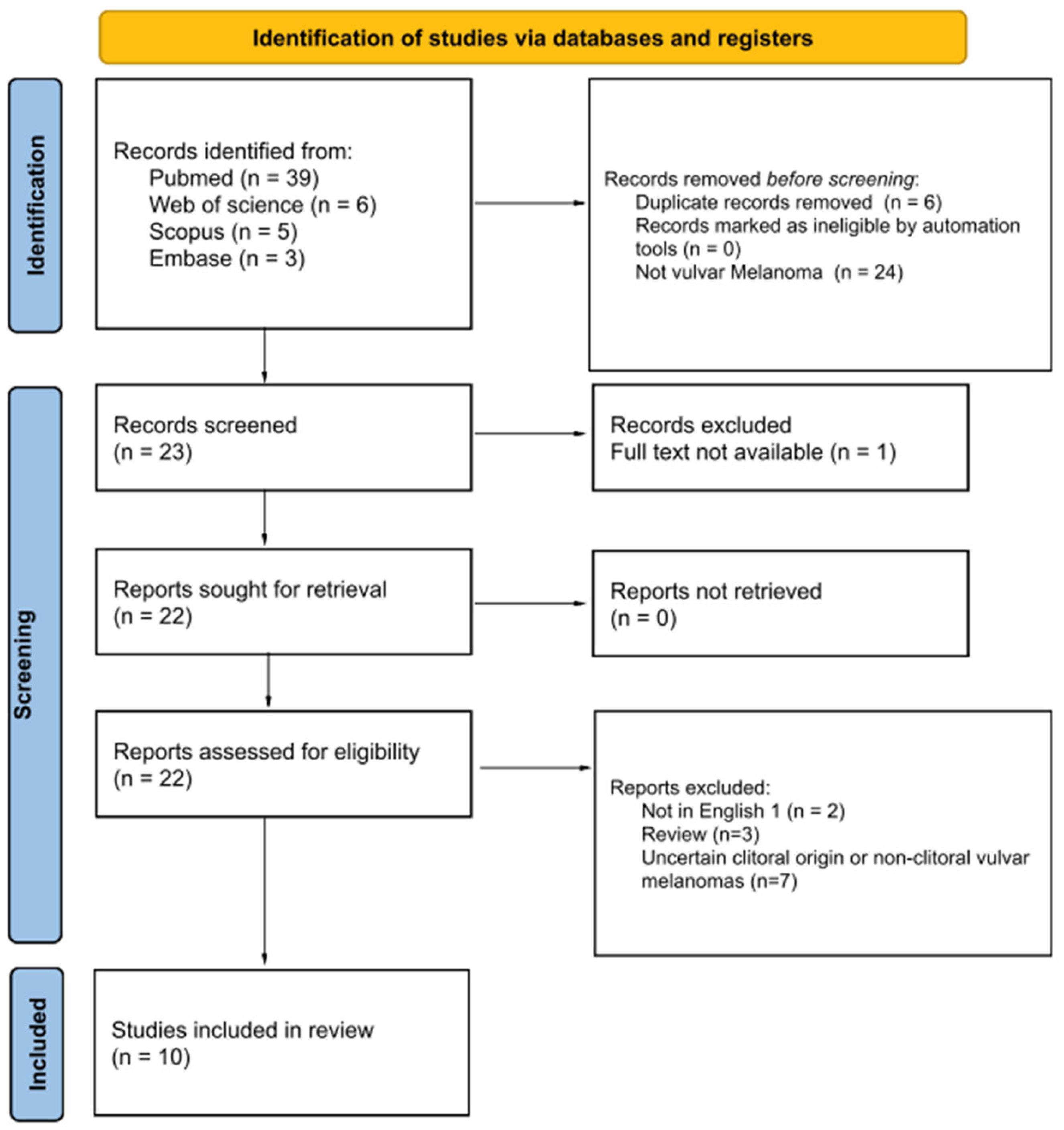
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Risk-of-Bias Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
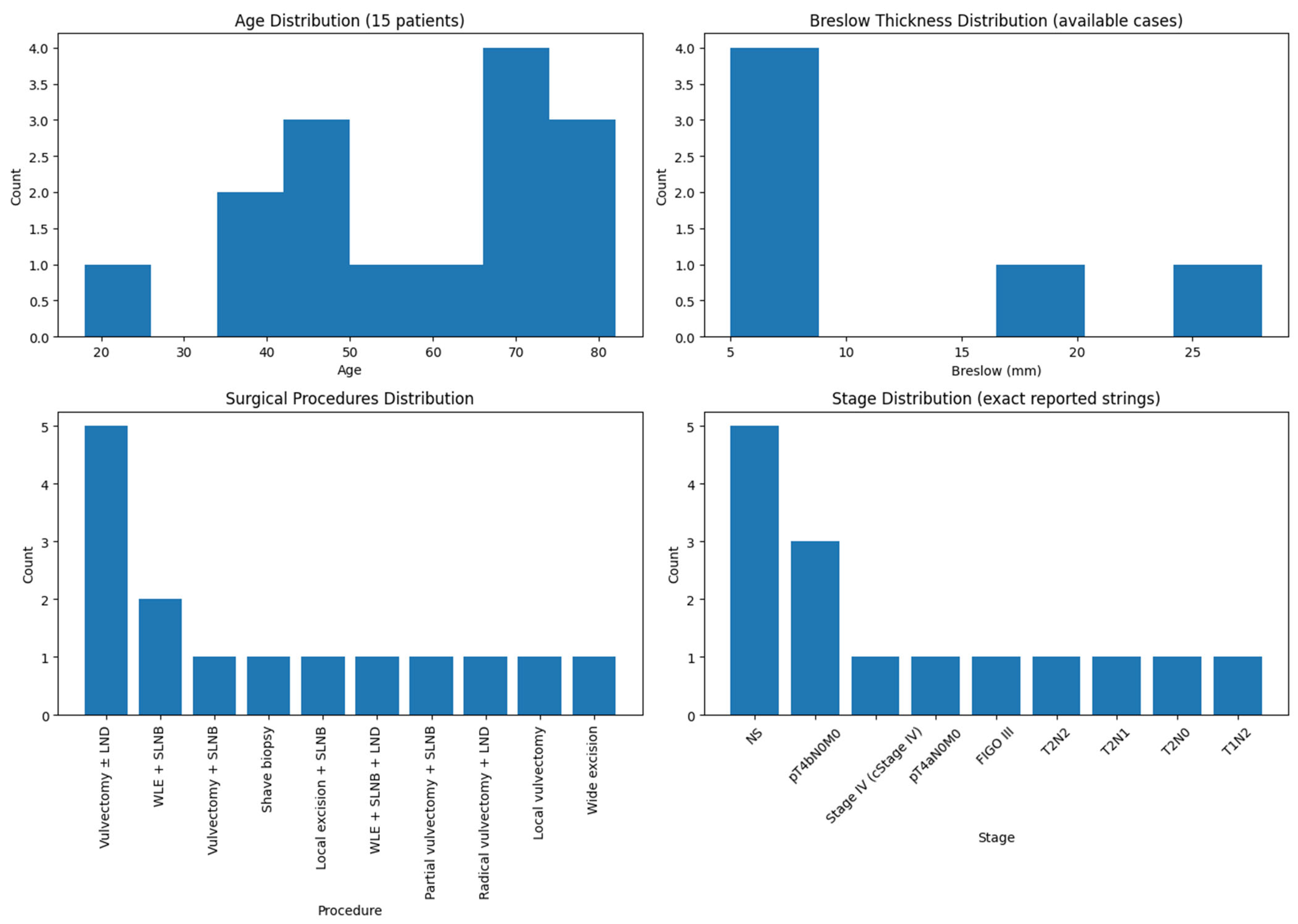
3.1. Patient Characteristics
3.2. Treatment Approaches
3.3. Recurrence and Outcomes
4. Discussion
Case Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLNB | sentinel lymph-node biopsy |
| H&E | Hematoxylin and Eosin |
| Yrs | years |
| FIGO | International Federation of Gynecology and Obstetrics |
| UICC | Union for International Cancer Control, American Joint Committee on Cancer, Tumour–Node–Metastasis |
| AJCC | American Joint Committee on Cancer |
| TNM classification | Tumour–Node–Metastasis |
References
- Testori, A.A.E.; Blankenstein, S.A.; van Akkooi, A.C.J. Primary Melanoma: From History to Actual Debates. Curr. Oncol. Rep. 2019, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.R.; Mehnert, J.M. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cancer Treat. Res. 2016, 167, 295–320. [Google Scholar] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Hamid, O.; Carvajal, R.D. Mucosal Melanoma: Pathogenesis, Clinical Behavior, and Management. Curr. Oncol. Rep. 2012, 14, 441–448. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary Mucosal Melanomas: A Comprehensive Review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Ragnarsson-Olding, B.K.; Kanter-Lewensohn, L.R.; Lagerlöf, B.; Nilsson, B.R.; Ringborg, U.K. Malignant Melanoma of the Vulva in a Nationwide, 25-Year Study of 219 Swedish Females: Clinical Observations and Histopathologic Features. Cancer 1999, 86, 1273–1284. [Google Scholar] [CrossRef]
- Seifried, S.; Haydu, L.E.; Quinn, M.J.; Scolyer, R.A.; Stretch, J.R.; Thompson, J.F. Melanoma of the Vulva and Vagina: Principles of Staging and Their Relevance to Management Based on a Clinicopathologic Analysis of 85 Cases. Ann. Surg. Oncol. 2015, 22, 1959–1966. [Google Scholar] [CrossRef]
- Murzaku, E.C.; Penn, L.A.; Hale, C.S.; Pomeranz, M.K.; Polsky, D. Vulvar Nevi, Melanosis, and Melanoma: An Epidemiologic, Clinical, and Histopathologic Review. J. Am. Acad. Dermatol. 2014, 71, 1241–1249. [Google Scholar] [CrossRef]
- Wang, D.; Xu, T.; Zhu, H.; Dong, J.; Fu, L. Primary Malignant Melanomas of the Female Lower Genital Tract: Clinicopathological Characteristics and Management. Am. J. Cancer Res. 2020, 10, 4017–4037. [Google Scholar]
- Košt’álová, M.; Košt’ál, M.; Ettler, K.; Hadži Nikolov, D.; Jandová, E.; Šimková, M. Melanoma Clitoridis. Int. J. Dermatol. 2007, 46, 393–395. [Google Scholar] [CrossRef]
- Nagarajan, P.; Curry, J.L.; Ning, J.; Piao, J.; Torres-Cabala, C.A.; Aung, P.P.; Ivan, D.; Ross, M.I.; Levenback, C.F.; Frumovitz, M.; et al. Tumor Thickness and Mitotic Rate Robustly Predict Melanoma-Specific Survival in Patients with Primary Vulvar Melanoma: A Retrospective Review of 100 Cases. Clin. Cancer Res. 2017, 23, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, 89. [Google Scholar] [CrossRef]
- Iwasaki, H.; Itoh, S.; Iseda, N.; Tsutsui, Y.; Izumi, T.; Bekki, Y.; Yoshiya, S.; Ito, T.; Toshima, T.; Nakahara, T.; et al. Robot-Assisted Laparoscopic Hepatectomy for Liver Metastasis from Clitoral Malignant Melanoma: A Case Report. Surg. Case Rep. 2024, 10, 258. [Google Scholar] [CrossRef]
- Fuchs, E.; Khanijow, A.; Garcia, R.L.; Goff, B.A. Imiquimod treatment of vulvar melanoma in situ invading the urethra. Gynecol. Oncol. Rep. 2021, 38, 100875. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Ajebo, E.M.; Diamond, D.; Powell, M.; Belcher, M. Primary vulvar melanoma in an adolescent patient. Pediatr. Dermatol. 2023, 40, 749–750. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Chmielowski, B.; Kang, Y.; Raman, S.; Memarzadeh, S. Management of Clitoral Melanoma Presenting as an Exophytic Clitoral Mass: A Case Report and Review of the Literature. Curr. Oncol. 2021, 28, 4264–4272. [Google Scholar] [CrossRef]
- White, B.M.; Vartevan, A.; Harris, J.; Scott, C.; Eber, D. Stage Two Malignant Melanoma of the Clitoris: A Case Report. Cureus 2019, 11, e4530. [Google Scholar] [CrossRef]
- Takahashi, C.; Uehara, J.; Ohishi, Y.; Honma, M.; Ishida-Yamamoto, A.; Iwasaki, T.; Takahashi, I.; Iizuka, H. Malignant Melanoma on Female Clitoris with Bidirectional Upper and Lower Lymphatic Flow. J. Dermatol. 2015, 42, 842–844. [Google Scholar] [CrossRef]
- Piura, B.; Rabinovich, A.; Dgani, R. Malignant Melanoma of the Vulva: Report of Six Cases and Review of the Literature. Eur. J. Gynaecol. Oncol. 1999, 20, 182–186. [Google Scholar]
- Cascinelli, N.; Di Re, F.; Lupi, G.; Balzarini, G.P. Malignant Melanoma of the Vulva. Tumori J. 1970, 56, 345–352. [Google Scholar] [CrossRef]
- Janovski, N.A.; Marshall, D.; Taki, I. Malignant Melanoma of the Vulva. Am J Obstet Gynecol 1962, 84, 523–536. [Google Scholar] [CrossRef]
- Piura, B. Management of Primary Melanoma of the Female Urogenital Tract. Lancet Oncol. 2008, 9, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Kottschade, L.A.; Grotz, T.E.; Dronca, R.S.; Salomao, D.R.; Pulido, J.S.; Wasif, N.; Jakub, J.W.; Bagaria, S.P.; Kumar, R.; Kaur, J.S.; et al. Rare Presentations of Primary Melanoma and Special Populations. Am. J. Clin. Oncol. 2014, 37, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, V.E.; Chan, J.K.; Shin, J.Y.; Berek, J.S.; Osann, K.; Kapp, D.S. Vulvar Melanoma. Obstet. Gynecol. 2007, 110, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Weikel, W.; Hofmann, M.; Steiner, E.; Knapstein, P.G.; Koelbl, H. Reconstructive Surgery Following Resection of Primary Vulvar Cancers. Gynecol. Oncol. 2005, 99, 92–100. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Di Donato, V.; Bracchi, C.; Marchetti, C.; Tomao, F.; Palaia, I.; Perniola, G.; Muzii, L. Modified Gluteal Fold Advancement V-Y Flap for Vulvar Reconstruction after Surgery for Vulvar Malignancies. Gynecol. Oncol. 2014, 132, 125–129. [Google Scholar] [CrossRef]
- Rambhia, P.H.; Scott, J.F.; Vyas, R.; Gerstenblith, M.R. Genitourinary Melanoma; NCBI: Bethesda, MD, USA, 2018; ISBN 9780994438157. [Google Scholar]
- Luna-Ortiz, K.; Aguilar-Romero, M.; Villavicencio-Valencia, V.; Zepeda-Castilla, E.; Vidrio-Morgado, H.; Peteuil, N.; Mosqueda-Taylor, A. Comparative Study between Two Different Staging Systems (AJCC TNM VS BALLANTYNE’S) for Mucosal Melanomas of the Head & Neck. Med. Oral. Patol. Oral. Cir. Bucal 2016, 21, e425-30. [Google Scholar] [CrossRef]
- Baderca, F.; Cojocaru, S.; Lazăr, E.; Lăzureanu, C.; Lighezan, R.; Alexa, A.; Raica, M.; Nicola, T. Amelanotic Vulvar Melanoma: Case Report and Review of the Literature. Rom. J. Morphol. Embryol. 2008, 49, 219–228. [Google Scholar]
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert. Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef]

| Study (Year) | Age | Stage | Breslow (mm) | Immunohistochemical Positivity—Pathogenic Mutations | Initial Surgery | Adjuvant Therapy | Recurrence Site(s) | Recurrence Treatment | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Iwasaki et al. 2024 [13] | 82 | pT4bN0M0, pStage IIC | 18, ulcerated | / | Wide local excision + sentinel node biopsy | None | 4 yrs: right lung; 9 yrs: liver | Pneumectomy + Pembrolizumab; Hepatectomy + Pembrolizumab | 9 years |
| Fuchs et al. 2021 [14] | 69 | NS | / | / | Simple Vulvectomy + radical vulvectomy and bilateral inguinal/femoral sentinel lymph node dissection | None | 6 yrs: vulva 7 yrs: vulva 11 yrs: vulva 12 yrs: vulva + periurethral area 16 yrs: periurethral area | Partial vulvectomy + topical imiquimod Nivolumab | 17 years |
| Li et al. 2021 [15] | 18 | cStage IV | / | SOX positivity—NRAS and PTEN mutation | Shave biopsy | Dacarbazine | / | / | Death after 41 days |
| Szlachta-McGinn et al. 2021 [16] | 52 | pT4bN0M0; pStage IIC | 28, ulcerated | SOX10, S100, HMB45, MART1 positivity—PD-L1 mutation | Local excision (positive margins) → sentinel node biopsy and wide local excision after 6 weeks | Pembrolizumab | None reported | None | NS |
| White et al. 2019 [17] | 67 | pT4aN0M0; pStage IIB | 8, not ulcerated | Melan-A and S-100 positivity | Wide local excision + sentinel node biopsy after 12 weeks | None | None reported | None | 1 year 3 months |
| Takahashi et al. 2015 [18] | 66 | Not specified | 7.5 | / | Wide local excision + sentinel node biopsy + left inguinal lymphadenectomy | β-interferon | None reported | None | 6 months |
| Kost’álová et al. 2007 [10] | 77 | pT4bN0M0; pStage IIC | 5, ulcerated | / | Partial vulvectomy + sentinel node biopsy | None | None reported | None | 9 months |
| Piura et al. 1999 [19] | 46 | FIGO Stage III | 5 | / | Radical vulvectomy + distal urethra resection + bilateral lymphadenectomy | None | 6 yrs: vulvar; 10 yrs: vagina; 11 yrs: vagina; 12 yrs: widespread | Multiple local excisions + vaginal wall brachytherapy | 12 years (death) |
| Cascinelli et al. 1970 [20] | 1. 46 2. 71 3. 39 4. 74 5. 49 | 1.T2N2 2.T2N1 3.NS 4.T2N0 5.T1N2 | NS | / | Cases 1, 2, 4, 5: Vulvectomy ± bilateral lymph node dissection; Case 3: Radiotherapy | Lipidol 1311 | / | / | 1: 1y5m (death); 2: 1y8m (death); 3: 9y (alive); 4: 4y (other cause); 5: 1y (alive) |
| Janovski et al. 1962 [21] | 59 & 37 | NS | NS | / | 1: Local vulvectomy; 2: Wide excision | None | 1: 1 yr mons pubis; 2y inguinal nodes| 2: 9 months inguinal node; 2 yrs inguinal node | 1: Lymphadenectomy (refused further treatment); 2: Lymph node excision | 1: 2y4m (death); 2: 18y |
| Category | Key Points/Strategies |
|---|---|
| Epidemiology & Presentation |
|
| Pathology & Disease Characteristics |
|
| Surgical Management |
|
| Nodal Assessment |
|
| Immunohistochemical positivity Pathogenic mutations |
|
| Adjuvant & Systemic Therapy |
|
| Staging Challenges |
|
| Follow-up & Prognosis |
|
| Research Needs |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Pitsillidi, A.; Vona, L.; Stabile, G.; Noé, G. Primary Clitoral Melanoma: Personalized Therapeutic Strategies Informed by Clinical Evidence and Systematic Review. J. Pers. Med. 2026, 16, 70. https://doi.org/10.3390/jpm16020070
Pitsillidi A, Vona L, Stabile G, Noé G. Primary Clitoral Melanoma: Personalized Therapeutic Strategies Informed by Clinical Evidence and Systematic Review. Journal of Personalized Medicine. 2026; 16(2):70. https://doi.org/10.3390/jpm16020070
Chicago/Turabian StylePitsillidi, Anna, Laura Vona, Guglielmo Stabile, and Günter Noé. 2026. "Primary Clitoral Melanoma: Personalized Therapeutic Strategies Informed by Clinical Evidence and Systematic Review" Journal of Personalized Medicine 16, no. 2: 70. https://doi.org/10.3390/jpm16020070
APA StylePitsillidi, A., Vona, L., Stabile, G., & Noé, G. (2026). Primary Clitoral Melanoma: Personalized Therapeutic Strategies Informed by Clinical Evidence and Systematic Review. Journal of Personalized Medicine, 16(2), 70. https://doi.org/10.3390/jpm16020070







