Abstract
Background: Cerebral desaturation during one-lung ventilation (OLV) in thoracic surgery has been associated with postoperative cognitive dysfunction (POCD). While the adverse effects of low intraoperative regional cerebral oxygen saturation (rScO2) are well documented, the potential clinical value of maintaining supranormal rScO2 levels has not been thoroughly studied. Methods: We conducted a retrospective observational study based on a previously collected cohort from a tertiary university hospital. Adult patients undergoing elective thoracic surgery between January 2019 and December 2022 were included, provided they received lidocaine either intravenously or via a paravertebral block as part of a standardized anesthetic protocol. Patients were divided into the following two groups based on their mean INVOS values 30 min into OLV: those with rScO2 ≥75% (H-INVOS group) and <75% (L-INVOS group). Intraoperative physiological variables, inflammatory biomarkers, cognitive function via the Mini-Mental State Examination, and postoperative outcomes were analyzed. Results: The H-INVOS group exhibited significantly higher preoperative lung function, higher PaO2 and PaCO2 values during OLV, and higher hemoglobin concentrations across all timepoints. They also demonstrated better preservation of cognitive function, lower IL-18 expression at 24 h postoperatively, and shorter hospital stays. There were no statistically significant differences in intraoperative hemodynamics or ventilatory mechanics.
1. Introduction
There is growing evidence of a relationship between regional cerebral oxygen saturation (rScO2) during anesthesia and postoperative cognitive dysfunction (POCD) [1]. This association is particularly relevant in thoracic surgery, where one-lung ventilation (OLV) can compromise cerebral oxygenation [2,3,4]. Consequently, intraoperative rScO2 monitoring has become an important strategy to reduce postoperative neurological injury.
POCD is a multifactorial and clinically significant syndrome, especially in patients over 60 years of age. Because no effective treatment is currently available, prevention is critical [3]. POCD negatively impacts patients’ quality of life, prolongs hospitalization, increases healthcare costs, and is associated with higher postoperative mortality [2].
Unlike arterial oxygen saturation measured by pulse oximetry (SpO2), rScO2 displays a wider normal range. Notably, intraoperative rScO2 reductions are frequent during OLV—even when SpO2 remains within normal limits—and have been associated with an increased risk of POCD [2,5].
The pathophysiology of POCD is thought to involve, among other mechanisms, excessive perioperative inflammation [5,6]. Cerebral hypoxia may trigger neuroinflammatory pathways and disrupt the blood–brain barrier, allowing inflammatory mediators to penetrate cerebral tissue. Similarly, systemic inflammation has been shown to promote neuroinflammation and subsequent neuronal injury [7,8]. Conversely, excessive cerebral oxygenation has also been implicated in the generation of reactive oxygen species and inflammatory mediators, which may contribute to neuronal damage [9].
In this context, intraoperative cerebral oxygenation monitoring has gained increasing attention. While cerebral desaturation is traditionally considered harmful, recent evidence suggests that abnormally elevated rScO2 values may also pose risks. Although the exact threshold of cerebral hyperoxia remains uncertain, studies using jugular venous oxygen saturation have proposed a cutoff of 75% [10]. Consistently, thoracic surgery studies have suI´ve doneggested that cerebral hyperoxia may occur when rScO2 exceeds this threshold [11]. Based on this evidence, we used 75% as a pragmatic cutoff to investigate the potential association between intraoperative cerebral hyperoxia and adverse neurological outcomes. From a personalized medicine perspective, monitoring rScO2 provides real-time, patient-specific information that can guide individualized anesthetic management, moving beyond population-based thresholds and toward tailored perioperative care.
The aim of this study was to evaluate the postoperative impact of maintaining elevated In Vivo Optical Spectroscopy (INVOS) values compared with lower values, with a primary focus on prognostic variables such as length of hospital stay.
2. Materials and Methods
2.1. Study Design
This study is a secondary analysis of data collected within the framework of the original trial (NCT03905837, EudraCT 2016-004271-52), a prospective study conducted under a predefined protocol and approved by the Ethics Committee of Gregorio Marañón Hospital, Madrid, Spain (Chairperson: Dr. Camino Sorabe; protocol code IGMFGG-2016; approval date: 3 April 2018). The original study was conducted in accordance with the Declaration of Helsinki.
The objective of this subanalysis is to evaluate the relationship between intraoperative cerebral oxygenation levels and postoperative clinical, inflammatory, and neurocognitive outcomes.
2.2. Study Population
Adult patients scheduled for thoracic surgery between January 2019 and December 2022 were eligible. The original trial enrolled 154 patients randomized into the following three groups: (1) paravertebral lidocaine (2 mg·kg−1·h−1) plus intravenous saline, (2) intravenous lidocaine (1.5 mg·kg−1·h−1) plus paravertebral saline, and (3) intravenous remifentanil (0.1 µg·kg−1·h−1) plus paravertebral saline. The primary endpoint of the parent study was the incidence of postoperative complications according to the Clavien–Dindo classification.
For the present analysis, only patients receiving lidocaine (intravenous infusion or paravertebral block) as part of the standardized anesthetic protocol were included. The control group without lidocaine was excluded to avoid confounding effects on inflammatory profiles. The exclusion criteria were pre-existing cognitive impairment, active neurological disease, emergency thoracic surgery, missing intraoperative INVOS monitoring data, and absence of postoperative cognitive assessments.
2.3. Anesthetic Protocol and Monitoring
No premedication was administered prior to induction. Anesthesia was induced with propofol (2–3 mg/kg), fentanyl (3–5 µg/kg), and rocuronium (1.2 mg/kg). Airway management employed a left-sided double-lumen tube or, in selected cases, a bronchial blocker; placement was confirmed with fiberoptic bronchoscopy. A radial artery catheter was inserted contralateral to the surgical side, and patients were positioned in the lateral decubitus position. A paravertebral catheter was placed at the T5–T6 level.
2.4. Ventilation Strategy
During two-lung ventilation, initial settings included FiO2 0.4, a respiratory rate of 12 breaths/min, a tidal volume (TV) of 8 mL/kg, and optimal PEEP determined via an alveolar recruitment maneuver. With the initiation of one-lung ventilation (OLV), TV was reduced to 4–6 mL/kg, followed by a second recruitment maneuver to reassess the optimal PEEP. FiO2 was titrated to maintain SpO2 > 90%. In cases of hypoxemia (SpO2 < 90% with FiO2 > 0.8), lung isolation device placement was rechecked and continuous positive airway pressure (CPAP) was applied to the dependent lung. If a bronchial blocker was used, reinflation of the collapsed lung was attempted.
2.5. Patient Grouping
Patients were categorized into the following two groups according to their mean INVOS values measured 30 min after OLV initiation: ≥75% (High INVOS group) and <75% (Low INVOS group).
2.6. Assessment of rScO2 and Cognitive Function
Preoperative regional cerebral oxygen saturation (rScO2) was measured immediately prior to induction using bihemispheric INVOS 5100 optodes (Covidien Germany GmbH, Neustadt, Germany). Sensors were applied to the forehead bilaterally and covered with opaque plastic to minimize light interference. The mean of both hemispheres was used for analysis. Anesthetic management was not guided by absolute rScO2 values.
Cognitive function was assessed with the Mini-Mental State Examination (MMSE), administered preoperatively on the ward and repeated 72 h postoperatively. Both absolute values and percentage changes from baseline were analyzed.
2.7. Data Collection
Measurements were obtained at the following three intraoperative timepoints:
- After lateral positioning, prior to OLV initiation.
- Thirty minutes after OLV initiation, once respiratory parameters had stabilized.
- Fifteen minutes after resuming two-lung ventilation.
At each timepoint, arterial blood gases, bronchoalveolar lavage samples, and blood samples for inflammatory biomarkers were collected. Airway pressures and pulmonary compliance were recorded. Hemodynamic variables were obtained via pulse contour analysis (FloTrac), including blood pressure, stroke volume, cardiac output, and stroke volume variation.
The inflammatory biomarkers assessed included TNF-α, IL-1, IL-6, IL-18, IL-10, MMP-2, MMP-3, and MMP-9. Markers of neurological injury were also measured. Additional blood samples for the same analyses were drawn 24 h postoperatively.
2.8. Postoperative Course
Patients were transferred to the post-anesthesia care unit and discharged after 24 h if hemodynamic and respiratory parameters were stable. Postoperative complications were assessed at 30 days and classified according to the Clavien–Dindo system [12]. Evaluators were blinded to intraoperative clinical data.
2.9. Statistical Analysis
Data were analyzed with SPSS version 25.0. Continuous variables are reported as medians with interquartile ranges (IQR 25–75), and categorical variables as absolute frequencies and percentages. Between-group comparisons were performed using the Mann–Whitney U test for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. Repeated-measures analysis was applied to evaluate changes in continuous variables over time. Kaplan–Meier analysis was used to compare outcomes between the High and Low INVOS groups. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
A total of 98 patients were included in the analysis. The median age was 67 years (IQR 62–72), and 64% were male. Patients with elevated INVOS values during OLV showed better preoperative pulmonary function test results; however, no significant differences were observed between groups with respect to baseline comorbidities (Table 1).

Table 1.
Baseline characteristics of patients according to INVOS values.
Arterial blood gas analysis during OLV revealed higher PaO2 values and PaO2/FiO2 ratios in patients in the H-INVOS group. Hemoglobin concentrations were also consistently higher at all three assessed time points. No significant differences were observed between groups in terms of mechanical ventilation settings or hemodynamic parameters (Table 2).

Table 2.
Intraoperative physiologic parameters according to INVOS values at different time points.
Patients in the H-INVOS group exhibited consistently higher rScO2 values not only during OLV, but also before and after the OLV phase (Figure 1).
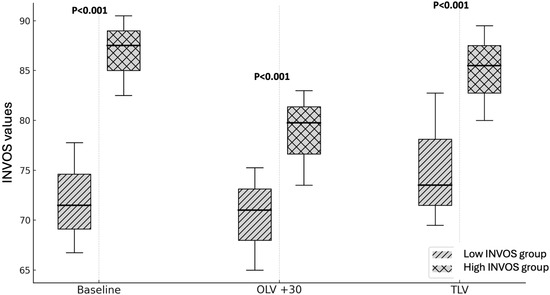
Figure 1.
INVOS values during surgery (high vs. low INVOS groups). OLV + 30: After 30 min of one-lung ventilation; TLV: Two-lung ventilation; p < 0.001 High INVOS vs. Low INVOS groups.
3.2. Inflammatory Biomarkers
Most inflammatory biomarkers were comparable between groups, with the exception of IL-6. Patients in the L-INVOS group had significantly higher IL-6 levels both at the end of surgery and on postoperative day 1 compared with the H-INVOS group (Figure 2, Table 3).
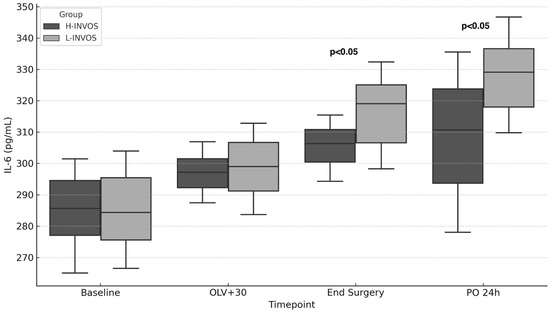
Figure 2.
IL-6 levels over time by INVOS group. Footnote: OLV + 30: After 30 min of one-lung ventilation; TLV: Two-lung ventilation; PO: Postoperative. p < 0.05: High INVOS vs. Low INVOS groups.

Table 3.
Biomarker levels at different time points according to INVOS values.
3.3. Cognitive Outcomes and Length of Hospital Stay
Preoperative MMSE scores were comparable between groups. At 72 h postoperatively, however, MMSE scores were significantly lower in the L-INVOS group compared with the H-INVOS group (30 [IQR 29–30] vs. 29 [28–30], p = 0.018). The percentage decline in MMSE scores was likewise greater in the L-INVOS group (Table 4).

Table 4.
Postoperative outcomes according to INVOS values.
Length of hospital stay was significantly shorter in the H-INVOS group (median 4 [IQR 3–6] days) compared with the L-INVOS group (5 [4–6.5] days; p = 0.019). Although no statistically significant differences were observed in the distribution of Clavien–Dindo complication grades, 71% of patients in the H-INVOS group experienced no complications versus 55% in the L-INVOS group (p = 0.112). All four cases of major postoperative complications occurred in the L-INVOS group. No significant differences were found in the detailed analysis of postoperative complications (Table 4). Kaplan–Meier survival analysis likewise showed no significant differences between groups (log-rank p = 0.27; Figure 3).
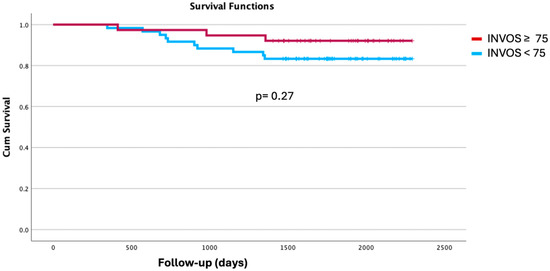
Figure 3.
Kaplan Meier curves. INVOS ≥ 75 vs. <75 during one-lung ventilation.
4. Discussion
Our findings suggest that maintaining intraoperative INVOS values ≥ 75% during OLV with a lung-protective ventilation strategy is associated with improved neurocognitive outcomes, shorter hospital stays, and attenuation of the inflammatory response—particularly reflected by lower IL-6 levels.
4.1. Cerebral Oxygenation and Postoperative Cognitive Function
These results support the hypothesis that adequate cerebral oxygenation during OLV plays a critical role in preserving postoperative cognitive function. Previous studies have shown that rScO2 often declines during OLV—primarily due to reductions in PaO2—in 30% to 100% of patients [4,13,14,15]. Such decreases have been consistently associated with an increased risk of POCD [2,11,16]. Consequently, thoracic anesthesia practice has progressively emphasized not only maintaining adequate peripheral oxygen saturation (SpO2), but also avoiding intraoperative cerebral desaturation, particularly during OLV.
Most published guidelines recommend maintaining rScO2 above 65% during OLV to reduce the risk of POCD. In our study, we investigated the impact of targeting an even higher threshold. This decision was based on the physiological rationale that permissive hypercapnia—a key component of lung-protective ventilation—may enhance cerebral blood flow and thereby increase cerebral tissue oxygenation [17]. We considered the ≥75% threshold both safe for preventing cerebral hypoxia and sufficiently conservative to avoid cerebral hyperemia [11].
Consistent with previous reports, our data showed that patients with higher intraoperative rScO2 values during OLV demonstrated a better postoperative cognitive performance on MMSE assessments, with fewer patients exhibiting scores below 26 points or declines greater than 2 points. Although these differences reached statistical significance, they should be interpreted with caution, as the MMSE—despite its widespread clinical use—remains a relatively crude and nonspecific tool for detecting subtle postoperative cognitive changes. It is characterized by a high specificity but limited sensitivity for identifying POCD.
4.2. Permissive Hypercapnia
Evidence on the impact of rScO2 levels under lung-protective ventilation strategies incorporating low tidal volumes and permissive hypercapnia remains limited. PaCO2 is a potent regulator of cerebral blood flow, inducing cerebral vasodilation, increasing intracranial blood volume, and consequently elevating rScO2. Végh et al. reported that, in normocapnic patients ventilated with a constant PEEP of 5 cmH2O, cerebral desaturation during OLV was uncommon, although 20% of patients still experienced decreases in rScO2 below 60% [18]. By contrast, in our cohort—managed under clear hypercapnic conditions—only 3% of patients had rScO2 values below 60% during OLV. Moreover, the mean reduction in rScO2 observed in our study was comparable to that reported by Végh and colleagues. Therefore, we suggest that their conclusion—that maintaining normocapnia improves cerebral oxygenation—may not be generalizable. Instead, the lower incidence of severe desaturation in our population may largely reflect the use of permissive hypercapnia.
In addition, unlike other studies, we did not find a consistent association between cerebral desaturation and variables such as ventilatory pressures [3,19] and hemodynamic instability [16]. These discrepancies may be explained by differences in study design, particularly the use of lung-protective ventilation with resultant permissive hypercapnia, although the influence of other uncontrolled factors cannot be ruled out.
4.3. Cerebral Oxygen Threshold
In thoracic anesthesia practice, the effective interpretation of cerebral oximetry requires clear and standardized thresholds that can be applied consistently across patients. One of the main limitations of rScO2 monitoring is the absence of a simple, universally accepted cutoff values for clinically significant desaturation—values that would prompt corrective interventions during OLV, such as increasing FiO2, applying CPAP to the non-dependent lung, or performing recruitment maneuvers. However, indiscriminate application of these strategies carries risks, including oxidative stress, surgical field interference, and barotrauma.
This challenge is compounded by wide interindividual variability in rScO2 values, influenced by factors such as age, cerebral anatomy, cranial bone thickness, systemic perfusion, and head positioning. Consequently, many authors advocate for using relative rScO2 changes during OLV—commonly defined as reductions ≥15–20% from baseline—as markers of clinically relevant desaturation [14,16,20,21]. Others support absolute thresholds [2,13] or recommend a combined approach that incorporates both absolute and relative criteria [22]. From a practical standpoint, we consider that relying solely on relative changes may complicate bedside decision making and fails to provide a straightforward clinical signal to guide intervention.
4.4. SpO2 vs. rScO2
In our study, SpO2 values did not differ significantly between groups. Nevertheless, patients in the H-INVOS group demonstrated superior arterial oxygenation and gas exchange, as evidenced by higher PaO2/FiO2 ratios. These findings reinforce the concept that pulse oximetry alone is not a reliable surrogate for monitoring cerebral oxygenation.
4.5. Neuroinflammation and Its Role in Postoperative Cognitive Dysfunction
POCD is multifactorial in origin, with perioperative inflammation recognized as a major pathogenic contributor. Thoracic surgery elicits a substantial systemic inflammatory response, driven both by surgical trauma and by OLV-induced lung injury [23,24]. It is well established that the perioperative release of proinflammatory mediators compromises blood–brain barrier integrity, triggering endothelial dysfunction and the infiltration of peripheral immune cells into the brain parenchyma. These processes activate astrocytes and microglia, leading to neuronal dysfunction, memory impairment, and ultimately POCD [25,26]. Interleukin-6 (IL-6) has been identified as a key cytokine in postoperative brain injury, modulating neuronal plasticity and synaptic activity in the hippocampus and cerebral cortex—regions critical for executive function [27].
Wang et al. demonstrated that the correction of cerebral desaturation events was associated with reduced perioperative inflammatory markers and lower rates of POCD [28]. Additional studies have shown that peripheral inflammation can lead to neuroinflammation and subsequent cerebral tissue damage [29,30].
In our study, preoperative inflammatory biomarkers did not differ significantly between groups. However, patients with lower intraoperative rScO2 values during OLV exhibited higher IL-6 levels at the end of surgery and again 24 h later [25,31,32]. Conversely, reduced IL-6 expression in patients with higher cerebral oxygenation suggests that a more stable perfusion and oxygenation environment may attenuate this neuroinflammatory cascade.
It should be noted, however, that this subanalysis included only patients who received perioperative lidocaine, a drug with well-documented anti-inflammatory properties. Therefore, we cannot exclude the possibility that lidocaine contributed to the absence of significant differences in other neuroinflammatory markers.
4.6. Cerebral Oximetry as a Systemic Indicator and Predictor of Recovery
The extracerebral significance of regional cerebral oxygen saturation (rScO2) monitoring during thoracic surgery remains incompletely understood. In our study, patients with lower intraoperative rScO2 values during OLV experienced a longer hospital stay. This finding has important clinical implications, as prolonged hospitalization increases the risk of nosocomial complications and is unequivocally associated with higher healthcare costs.
Although rScO2 monitoring was initially developed to optimize cerebral outcomes, the brain may serve as a sentinel or “index organ”, reflecting global tissue oxygenation. Given the brain’s robust autoregulatory capacity, it may be the last organ to manifest signs of compromised perfusion or oxygen delivery. This suggests that rScO2 thresholds currently considered acceptable may, in fact, need to be set at supranormal levels to adequately reflect systemic oxygenation. Accordingly, significant cerebral desaturations may imply concurrent desaturation in other organs, potentially contributing to the broad range of adverse perioperative outcomes described in prior studies [14,21].
The mechanism linking higher cerebral oxygenation with improved clinical recovery may involve preserved cognitive function, which facilitates early mobilization, adherence to postoperative treatment, and overall functional rehabilitation. Although the overall incidence of postoperative complications was similar between groups, there was a trend toward fewer pulmonary complications and a lower frequency of Clavien–Dindo grade ≥2 events in patients with higher INVOS values during OLV.
These findings are consistent with those of Kazan et al., who associated persistently low cerebral oxygenation with an increased risk of respiratory complications [14]. In line with Roberts’ observations, we propose that rScO2 may function as a marker of global recovery potential, capturing perioperative vulnerability not adequately reflected in conventional classifications such as Clavien–Dindo. In this sense, rScO2 may be considered not only as a neurological monitoring parameter, but also as a biomarker for personalized perioperative medicine, helping to stratify risk, predict recovery trajectories, and guide individualized interventions.
5. Limitations
This study has several limitations. First, it was a retrospective subanalysis, with the inherent methodological constraints of such a design. Although strict inclusion criteria were applied and the non-lidocaine group was excluded to minimize pharmacological confounding, residual confounding cannot be entirely ruled out. Second, while the sample size was sufficient to detect differences in primary outcomes such as MMSE scores, it may have been underpowered to identify differences in less frequent outcomes, including major complications and mortality. Third, although the MMSE is widely used, it has a limited sensitivity for detecting subtle postoperative cognitive changes. Moreover, we did not include systematic monitoring for potential complications associated with elevated rScO2 values, nor did we employ a comprehensive neuropsychological battery that would have allowed for a more detailed assessment of the specific cognitive domains affected.
6. Conclusions
Although the optimal absolute rScO2 threshold remains a matter of debate, our findings suggest that higher intraoperative values may help identify patients at lower risk of complications and support faster recovery. Beyond its neurological relevance, rScO2 may also serve as a surrogate marker of global physiological status, particularly in the setting of lung-protective strategies such as permissive hypercapnia. Prospective studies are warranted to confirm these observations and to determine whether rScO2-guided interventions can improve perioperative outcomes. Our results suggest that incorporating cerebral oximetry into routine perioperative care could contribute to a more personalized approach, in which anesthetic and ventilatory strategies are adapted to the patient’s unique physiological response, ultimately improving outcomes.
Author Contributions
Conceptualization: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; methodology: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; software: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; validation: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; formal analysis, I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; investigation: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; resources: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V., and P.P.; data curation: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V., and P.P.; writing—original draft preparation: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; writing—review and editing: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; visualization: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; supervision: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; project administration: I.G., F.d.l.G., J.H., A.R., E.d.l.F., D.M.-G., C.A.C., S.H., E.C., C.S., E.V. and P.P.; funding acquisition I.G., F.d.l.G., C.S. and E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto de Salud Carlos III. Spanish Ministry of Health in the 2018 call (PI18/01305).
Institutional Review Board Statement
This study received provisional approval on 5 March 2018 and final approval on 3 April 2018 from the Ethics Committee of Gregorio Marañon Hospital in Madrid, Spain (Chairperson Dra Camino Sorabe) (IRB protocol number: IGMFGG-2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions (regarding patient confidentiality).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CPAP | Continuous positive airway pressure |
| FiO2 | Inspired fraction oxygen |
| H-INVOS | High-INVOS |
| IL | Interleuquine |
| INVOS | In Vivo Optical Spectroscopy |
| L-INVOS | Low-INVOS |
| MMPs | Metalloproteinases |
| MMSE | Mini-Mental State Examination |
| OLV | One-lung ventilation |
| PEEP | Positive end-expiratory pressure |
| POCD | Postoperative cognitive dysfunction |
| rScO2 | Regional cerebral oxygen saturation |
| SpO2 | Peripherical saturation oxygen |
| TNF | Tumor necrosis factor |
| TV | Tidal volume |
References
- Qiu, L.; Ma, Y.; Ge, L.; Zhou, H.; Jia, W. Efficacy of Cerebral Oxygen Saturation Monitoring for Perioperative Neurocognitive Disorder in Adult Noncardiac Surgical Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. World Neurosurg. 2025, 194, 123570. [Google Scholar] [CrossRef]
- Roberts, M.L.; Lin, H.M.; Tinuoye, E.; Cohen, E.; Flores, R.M.; Fischer, G.W.; Weiner, M.M. The Association of Cerebral Desaturation During One-Lung Ventilation and Postoperative Recovery: A Prospective Observational Cohort Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 542–550. [Google Scholar] [CrossRef]
- Teng, P.; Liu, H.; Xu, D.; Feng, X.; Liu, M.; Wang, Q. Effect of optimizing cerebral oxygen saturation on postoperative delirium in older patients undergoing one-lung ventilation for thoracoscopic surgery. J. Int. Med. Res. 2024, 52, 03000605241274604. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D.; Johnson, G.A.; Rehman, S.; Fisher, R.; Caron, N. Cerebral oxygenation monitoring using near infrared spectroscopy during one-lung ventilation in adults. J. Minimal Access Surg. 2008, 4, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Knaak, C.; Vorderwülbecke, G.; Spies, C.; Piper, S.K.; Hadzidiakos, D.; Borchers, F.; Brockhaus, W.-R.; Radtke, F.M.; Lachmann, G. C-reactive protein for risk prediction of post-operative delirium and post-operative neurocognitive disorder. Acta Anaesthesiol. Scand. 2019, 63, 1282–1289. [Google Scholar] [CrossRef]
- Lammers-Lietz, F.; Akyuz, L.; Feinkohl, I.; Lachmann, C.; Pischon, T.; Volk, H.D.; von Häfen, C.; Yürek, F.; Winterer, G.; Spies, C.D. Interleukin 8 in postoperative delirium—Preliminary findings from two studies. Brain Behav. Immun. Health 2022, 20, 100419. [Google Scholar] [CrossRef]
- Tastan, B.; Heneka, M.T. The impact of neuroinflammation on neuronal integrity. Immunol. Rev. 2024, 327, 8–32. [Google Scholar] [CrossRef]
- Knethen, V.; Kanda, N.; Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Simon Machado, R.; Mathias, K.; Joaquim, L.; de Quadros, R.W.; Rezin, G.T.; Petronilho, F. Hyperoxia and brain: The link between necessity and injury from a molecular perspective. Neurotox Res. 2024, 42, 25. [Google Scholar] [CrossRef]
- Tsaousi, G.; Tramontana, A.; Yamani, F.; Bilotta, F. Cerebral Perfusion and Brain Oxygen Saturation Monitoring with: Jugular Venous Oxygen Saturation, Cerebral Oximetry, and Transcranial Doppler Ultrasonography. Anesth. Clin. 2021, 39, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, W.; Mu, D.L.; Zhao, X.; Li, X.Y.; Wang, D.X.; Jia, H.-Q.; Dai, F.; Meng, L. Association between Cerebral Desaturation and Postoperative Delirium in Thoracotomy with One-Lung Ventilation: A Prospective Cohort Study. Anesth. Analg. 2021, 133, 176–186. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef]
- Brinkman, R.; Amadeo, R.J.J.; Funk, D.J.; Girling, L.G.; Grocott, H.P.; Mutch, W.A.C. Cerebral oxygen desaturation during one-lung ventilation: Correlation with hemodynamic variables. Can. J. Anesth. 2013, 60, 660–666. [Google Scholar] [CrossRef]
- Kazan, R.; Bracco, D.; Hemmerling, T.M. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br. J. Anaesth. 2009, 103, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, K.; Okutai, R. Cerebral desaturation during single-lung ventilation is negatively correlated with preoperative respiratory functions. J. Cardiothorac. Vasc. Anesth. 2011, 25, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, K. The Effect of One-Lung Ventilation on Cerebral Oxygenation and Neurocognitive Functions. J. Cardıo Vasc. Thoracıc Anaesth. Intensıve Care Socıety 2022, 28, 7–14. [Google Scholar] [CrossRef]
- Anderloni, M.; Schuind, S.; Salvagno, M.; Donadello, K.; Peluso, L.; Annoni, F.; Taccone, F.S.; Bogossian, E.G. Brain Oxygenation Response to Hypercapnia in Patients with Acute Brain Injury. Neurocrit Care 2024, 40, 750–758. [Google Scholar] [CrossRef]
- Végh, T.; Szatmári, S.; Juhász, M.; László, I.; Vaskó, A.; Takács, I.; Szegedi, L.; Fülesdi, B. One-lung ventilation does not result in cerebral desaturation during application of lung protective strategy if normocapnia is maintained. Acta Physiol. Hung. 2013, 100, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lv, S.; Xiao, Q.; Zhang, Y.; Yi, W.; Bai, Y.; Lu, K.; Bermea, K.C.; Semel, J.; Yang, X.; et al. Effects of positive end-expiratory pressure on regional cerebral oxygen saturation in elderly patients undergoing thoracic surgery during one-lung ventilation: A randomized crossover-controlled trial. BMC Pulm Med. 2024, 24, 120. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, A.; Besir, A.; Kutanis, D.; Erturk, E.; Tugcugil, E.; Saylan, S. The effect of different anesthesia techniques on cerebral oxygenation in thoracic surgery. Cir. Y Cir. (Engl. Ed.) 2022, 90 (Suppl. S1), 52–60. [Google Scholar]
- Li, S.; Zhang, J.; Hu, J.; Li, L.; Liu, G.; Zheng, T.; Wang, F.; Liu, L.; Li, G. Association of regional cerebral oxygen saturation and postoperative pulmonary complications in pediatric patients undergoing one-lung ventilation: A propensity score matched analysis of a prospective cohort study. Front. Pediatr. 2022, 10, 1077578. [Google Scholar] [CrossRef]
- Egawa, J.; Inoue, S.; Nishiwada, T.; Tojo, T.; Kimura, M.; Kawaguchi, T.; Taniguchi, S.; Furuya, H.; Kawaguchi, M. Effets des agents anesthésiques sur l’issue cognitive postopératoire précoce et l’équilibre peropératoire d’oxygène cérébral chez les patients subissant une chirurgie pulmonaire: Une étude clinique randomisée. Can. J. Anesth. 2016, 63, 1161–1169. [Google Scholar] [CrossRef]
- De La Gala, F.; Piñeiro, P.; Reyes, A.; Vara, E.; Olmedilla, L.; Cruz, P.; Garutti, I. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br. J. Anaesth. 2017, 119, 655–663. [Google Scholar] [CrossRef]
- Bruinooge, A.J.G.; Mao, R.; Gottschalk, T.H.; Srinathan, S.K.; Buduhan, G.; Tan, L.; Halayko, A.J.; Kidane, B. Identifying biomarkers of ventilator induced lung injury during one-lung ventilation surgery: A scoping review. J. Thorac. Dis. 2022, 14, 4506–4520. [Google Scholar] [CrossRef]
- Zhang, S.; Tao Xjun Ding, S.; Feng Xwei Wu Fqin Wu, Y. Associations between postoperative cognitive dysfunction, serum interleukin-6 and postoperative delirium among patients after coronary artery bypass grafting: A mediation analysis. Nurs. Crit. Care 2024, 29, 1245–1252. [Google Scholar] [CrossRef]
- Xiao, M.Z.; Liu, C.X.; Zhou, L.G.; Yang, Y.; Wang, Y. Postoperative delirium, neuroinflammation, and influencing factors of postoperative delirium: A review. Medicine 2023, 102, E32991. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochem. Int. 2024, 181, 105900. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, M.; Wang, P.; Fang, P. Goal-directed therapy based on rScO2 monitoring in elderly patients with one-lung ventilation: A randomized trial on perioperative inflammation and postoperative delirium. Trials 2022, 23, 687. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Chen, W.; Kong, G.; Wei, L.; Xie, Y. Peripheral inflammation and neurocognitive impairment: Correlations, underlying mechanisms, and therapeutic implications. Front. Aging Neurosci. 2023, 15, 1305790. [Google Scholar] [CrossRef]
- Taylor, J.; Wu, J.G.; Kunkel, D.; Parker, M.; Rivera, C.; Casey, C.; Naismith, S.; Teixeira-Pinto, A.; Maze, M.; Pearce, A.R.; et al. Resolution of elevated interleukin-6 after surgery is associated with return of normal cognitive function. Br. J. Anaesth. 2023, 131, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Barreto Chang, O.L.; Maze, M. Defining the role of Interleukin-6 for the development of perioperative neurocognitive disorders: Evidence from clinical and preclinical studies. Front. Aging Neurosci. 2023, 14, 1097606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).