The Role of Orbital Atherectomy for Complex Coronary Calcium Modification: Has It Been Eclipsed?
Abstract
1. Introduction: The Problems of Calcium in PCI
2. Orbital Atherectomy: Mechanism of Action
2.1. Orbital Atherectomy: Clinical Studies
2.1.1. ORBIT I Trial
2.1.2. ORBIT II Trial
2.1.3. OAS Real-World Multicentre Registry
2.1.4. COAST Study
2.1.5. LOAR Registry
2.1.6. DIRO Study
2.1.7. OAS UK Single-Centre Retrospective Study
2.1.8. ECLIPSE Trial
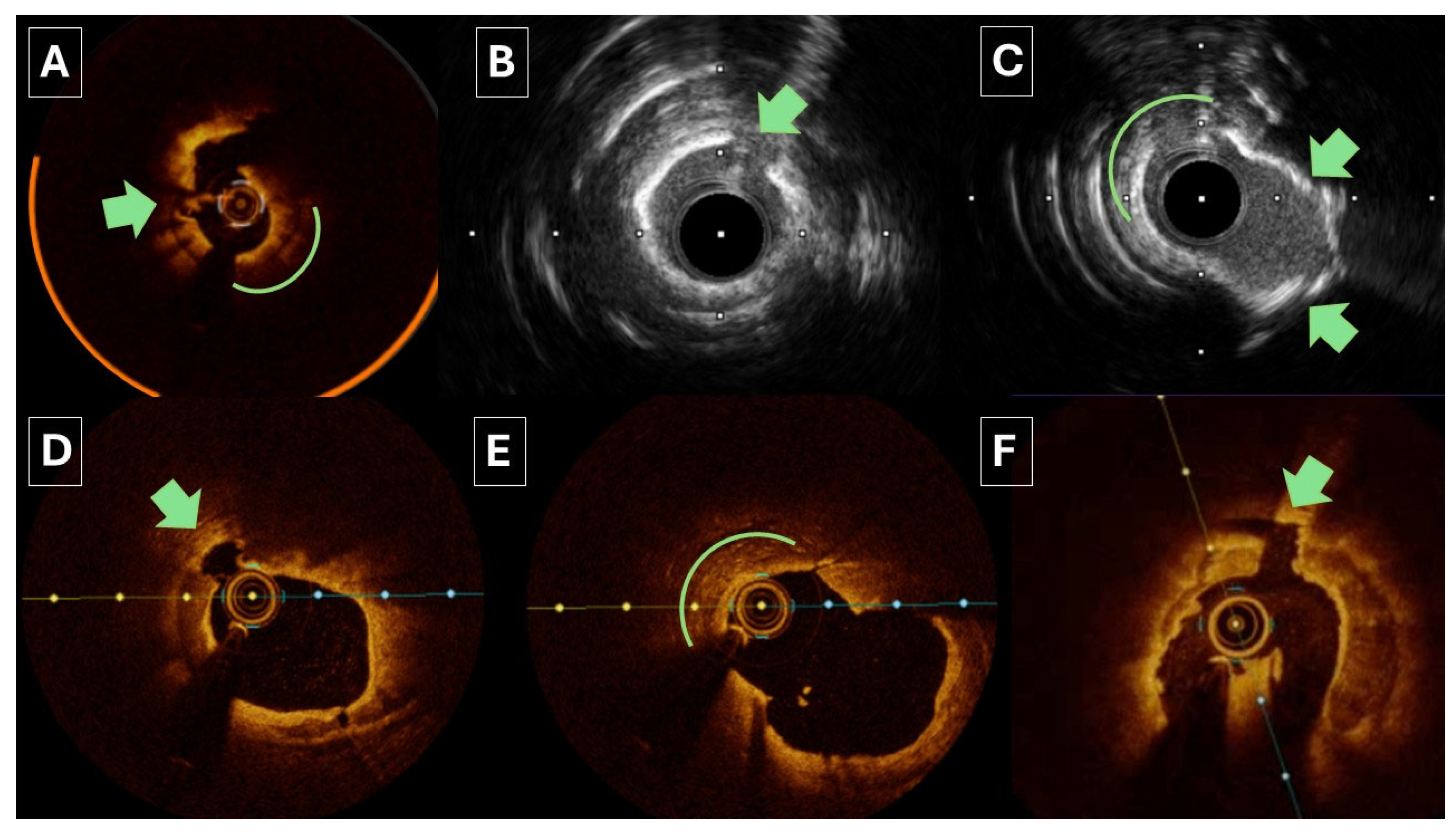
3. Role of Intracoronary Imaging
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashmi, S.; Shah, P.W.; Aherrahrou, Z.; Aikawa, E.; Aherrahrou, R. Beyond the Basics: Unraveling the Complexity of Coronary Artery Calcification. Cells 2023, 12, 2822. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary Artery Calcification: Pathogenesis and Prognostic Implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, J.; Kereiakes, D.J. Calcified plaque modification during percutaneous coronary revascularization. Prog. Cardiovasc. Dis. 2025, 88, 39–52. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef]
- Mosseri, M.; Satler, L.F.; Pichard, A.D.; Waksman, R. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc. Revasc. Med. Mol. Interv. 2005, 6, 147–153. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Okura, H.; Kume, T.; Yamada, R.; Kobayashi, Y.; Fukuhara, K.; Koyama, T.; Nezuo, S.; Neishi, Y.; Hayashida, A.; et al. Impact of target lesion coronary calcification on stent expansion. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 2209–2214. [Google Scholar] [CrossRef]
- Hennessey, B.; Pareek, N.; Macaya, F.; Yeoh, J.; Shlofmitz, E.; Gonzalo, N.; Hill, J.; Escaned, J. Contemporary percutaneous management of coronary calcification: Current status and future directions. Open Heart 2023, 10, e002182. [Google Scholar] [CrossRef]
- Milzi, A.; Simonetto, F.; Landi, A. Percutaneous Revascularization of Thrombotic and Calcified Coronary Lesions. J. Clin. Med. 2025, 14, 692. [Google Scholar] [CrossRef]
- Généreux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Palmerini, T.; Lasalle, L.; Xu, K.; McAndrew, T.; Kirtane, A.; Lansky, A.J.; et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J. Am. Coll. Cardiol. 2014, 63, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Mehran, R.; Mintz, G.S.; Liu, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Leon, M.B.; Serruys, P.W.; et al. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc. Interv. 2020, 13, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Miura, K.; Ikuta, A.; Ohya, M.; Shimada, T.; Osakada, K.; Takamatsu, M.; Taguchi, Y.; Kubo, S.; Tanaka, H.; et al. Prevalence, predictors, and outcomes of in-stent restenosis with calcified nodules. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 17, 1352–1361. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Maehara, A.; Cristea, E.; Witzenbichler, B.; Guagliumi, G.; Brodie, B.; Kellett, M.A.; Dressler, O.; Lansky, A.J.; Parise, H.; et al. Usefulness of minimum stent cross sectional area as a predictor of angiographic restenosis after primary percutaneous coronary intervention in acute myocardial infarction (from the HORIZONS-AMI Trial IVUS substudy). Am. J. Cardiol. 2012, 109, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Serruys, P.W.; Hara, H.; Ono, M.; Gao, C.; Wang, R.; Garg, S.; Sharif, F.; de Winter, R.J.; Mack, M.J.; et al. 10-Year All-Cause Mortality Following Percutaneous or Surgical Revascularization in Patients with Heavy Calcification. JACC Cardiovasc. Interv. 2022, 15, 193–204. [Google Scholar] [CrossRef]
- Copeland-Halperin, R.S.; Baber, U.; Aquino, M.; Rajamanickam, A.; Roy, S.; Hasan, C.; Barman, N.; Kovacic, J.C.; Moreno, P.; Krishnan, P.; et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: Findings from a large multiethnic registry. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 91, 859–866. [Google Scholar] [CrossRef]
- Riley, R.F.; Henry, T.D.; Mahmud, E.; Kirtane, A.J.; Brilakis, E.S.; Goyal, A.; Grines, C.L.; Lombardi, W.L.; Maran, A.; Rab, T.; et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2020, 96, 346–362. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011, 124, e574–e651. [Google Scholar] [CrossRef]
- Kostantinis, S.; Rempakos, A.; Simsek, B.; Karacsonyi, J.; Allana, S.S.; Alexandrou, M.; Gorgulu, S.; Alaswad, K.; Basir, M.B.; Davies, R.E.; et al. Impact of calcium on the procedural techniques and outcomes of chronic total occlusion percutaneous coronary intervention. Int. J. Cardiol. 2023, 390, 131254. [Google Scholar] [CrossRef]
- Onuma, Y.; Tanimoto, S.; Ruygrok, P.; Neuzner, J.; Piek, J.J.; Seth, A.; Schofer, J.J.; Richardt, G.; Wiemer, M.; Carrié, D.; et al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: Two-year angiographic and three-year clinical results from the SPIRIT II study. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2010, 76, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Bolduan, R.W.; Patel, M.R.; Martinsen, B.J.; Azemi, T.; Giugliano, G.; Resar, J.R.; Mehran, R.; Cohen, D.J.; Popma, J.J.; et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2019, 94, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Zhang, Y.-J.; Garg, S.; Iqbal, J.; Valgimigli, M.; Windecker, S.; Mohr, F.W.; Silber, S.; de Vries, T.; Onuma, Y.; et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: A patient-level pooled analysis of 7 contemporary stent trials. Heart Br. Card. Soc. 2014, 100, 1158–1164. [Google Scholar] [CrossRef]
- Shah, M.; Najam, O.; Bhindi, R.; De Silva, K. Calcium Modification Techniques in Complex Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2021, 14, e009870. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415. [Google Scholar] [CrossRef]
- Rawlins, J.; Din, J.N.; Talwar, S.; O’Kane, P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv. Cardiol. 2016, 11, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Généreux, P.; Shlofmitz, R.; Phillipson, D.; Anose, B.M.; Martinsen, B.J.; Himmelstein, S.I.; Chambers, J.W. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc. Revasc. Med. Mol. Interv. 2017, 18, 261–264. [Google Scholar] [CrossRef]
- Bhatt, P.; Parikh, P.; Patel, A.; Chag, M.; Chandarana, A.; Parikh, R.; Parikh, K. Orbital atherectomy system in treating calcified coronary lesions: 3-Year follow-up in first human use study (ORBIT I trial). Cardiovasc. Revasc. Med. Mol. Interv. 2014, 15, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Nef, H.; Escaned, J.; Werner, N.; Banning, A.P.; Hill, J.M.; De Bruyne, B.; Montorfano, M.; Lefevre, T.; Stone, G.W.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt CAD II Study. Circ. Cardiovasc. Interv. 2019, 12, e008434. [Google Scholar] [CrossRef]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W.; et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef]
- Dafaalla, M.; Rashid, M.; Moledina, S.; Kinnaird, T.; Ludman, P.; Curzen, N.; Zaman, S.; Nolan, J.; Mamas, M.A. Characteristics and Outcomes of Patients Who Underwent Coronary Atherectomy in Centers with and without On-Site Cardiac Surgery. Am. J. Cardiol. 2023, 204, 242–248. [Google Scholar] [CrossRef]
- Pesarini, G.; Hellig, F.; Seth, A.; Shlofmitz, R.A.; Ribichini, F.L. Percutaneous coronary intervention for calcified and resistant lesions. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2025, 21, e339–e355. [Google Scholar] [CrossRef]
- Butala, N.M.; Waldo, S.W.; Secemsky, E.A.; Kennedy, K.F.; Spertus, J.A.; Rymer, J.A.; Rao, S.V.; Messenger, J.C.; Yeh, R.W. Use of Calcium Modification During Percutaneous Coronary Intervention After Introduction of Coronary Intravascular Lithotripsy. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101254. [Google Scholar] [CrossRef] [PubMed]
- Sukul, D.; Seth, M.; Madder, R.D.; Basir, M.B.; Menees, D.S.; Kaki, A.; Azzalini, L.; Lee, D.; Gurm, H.S. Contemporary Trends and Outcomes of Intravascular Lithotripsy in Percutaneous Coronary Intervention: Insights from BMC2. JACC Cardiovasc. Interv. 2024, 17, 1811–1821. [Google Scholar] [CrossRef]
- Visinoni, Z.M.; Jurewitz, D.L.; Kereiakes, D.J.; Shlofmitz, R.; Shlofmitz, E.; Ali, Z.; Hill, J.; Lee, M.S. Coronary intravascular lithotripsy for severe coronary artery calcification: The Disrupt CAD I-IV trials. Cardiovasc. Revasc. Med. Mol. Interv. 2024, 65, 81–87. [Google Scholar] [CrossRef]
- Chambers, J.W.; Warner, C.; Cortez, J.; Behrens, A.N.; Wrede, D.T.; Martinsen, B.J. Outcomes after Atherectomy Treatment of Severely Calcified Coronary Bifurcation Lesions: A Single Center Experience. Cardiovasc. Revasc. Med. Mol. Interv. 2019, 20, 569–572. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Jeremias, A.; Shlofmitz, R.; Ali, Z.A. Lesion Preparation with Orbital Atherectomy. Interv. Cardiol. Rev. 2019, 14, 169–173. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Martinsen, B.J.; Lee, M.; Rao, S.V.; Généreux, P.; Higgins, J.; Chambers, J.W.; Kirtane, A.J.; Brilakis, E.S.; Kandzari, D.E.; et al. Orbital atherectomy for the treatment of severely calcified coronary lesions: Evidence, technique, and best practices. Expert Rev. Med. Devices 2017, 14, 867–879. [Google Scholar] [CrossRef]
- Helal, A.; Farooq, M. New Frontiers for Orbital Atherectomy-Crossing an Uncrossable Chronic Total Occlusion. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 105, 115–119. [Google Scholar] [CrossRef]
- Helal, A.; Ehtisham, J.; Shaukat, N. Overcoming Uncrossable Calcified RCA Using Orbital Atherectomy After Failure of Rotational Atherectomy. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 105, 1265–1268. [Google Scholar] [CrossRef]
- Gawalkar, A.A.; Akkineni, K.P.; Vijayvergiya, R.; Karki, P. Advantages of Orbital Atherectomy: A Case Series. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 105, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Diage, T. Evaluation of the Diamondback 360 Coronary Orbital Atherectomy System for treating de novo, severely calcified lesions. Expert Rev. Med. Devices 2014, 11, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Chandra, P.; Choksi, N.; Khanna, P.; Chambers, J. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: The ORBIT I trial. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2013, 81, 1134–1139. [Google Scholar] [CrossRef]
- Généreux, P.; Lee, A.C.; Kim, C.Y.; Lee, M.; Shlofmitz, R.; Moses, J.W.; Stone, G.W.; Chambers, J.W. Orbital Atherectomy for Treating De Novo Severely Calcified Coronary Narrowing (1-Year Results from the Pivotal ORBIT II Trial). Am. J. Cardiol. 2015, 115, 1685–1690. [Google Scholar] [CrossRef]
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518. [Google Scholar] [CrossRef]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.-Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 13, e2182–e2189. [Google Scholar] [CrossRef]
- Chambers, J.W.; Martinsen, B.J.; Sturm, R.C.; Mandair, D.; Valle, J.A.; Waldo, S.W.; Guzzetta, F.; Armstrong, E.J. Orbital atherectomy of calcified coronary ostial lesions. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 100, 553–559. [Google Scholar] [CrossRef]
- Koifman, E.; Garcia-Garcia, H.M.; Kuku, K.O.; Kajita, A.H.; Buchanan, K.D.; Steinvil, A.; Rogers, T.; Bernardo, N.L.; Lager, R.; Gallino, R.A.; et al. Comparison of the Efficacy and Safety of Orbital and Rotational Atherectomy in Calcified Narrowings in Patients Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2018, 121, 934–939. [Google Scholar] [CrossRef]
- Riley, R.F.; Patel, M.P.; Abbott, J.D.; Bangalore, S.; Brilakis, E.S.; Croce, K.J.; Doshi, D.; Kaul, P.; Kearney, K.E.; Kerrigan, J.L.; et al. SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101259. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Gordin, J.S.; Stone, G.W.; Sharma, S.K.; Saito, S.; Mahmud, E.; Chambers, J.; Généreux, P.; Shlofmitz, R. Orbital and rotational atherectomy during percutaneous coronary intervention for coronary artery calcification. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 92, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sotomi, Y.; Shlofmitz, R.A.; Colombo, A.; Serruys, P.W.; Onuma, Y. Patient Selection and Procedural Considerations for Coronary Orbital Atherectomy System. Interv. Cardiol. Rev. 2016, 11, 33–38. [Google Scholar] [CrossRef]
- Lee, M.S.; Martinsen, B.J.; Lee, A.C.; Behrens, A.N.; Shlofmitz, R.A.; Kim, C.Y.; Chambers, J.W. Impact of diabetes mellitus on procedural and one year clinical outcomes following treatment of severely calcified coronary lesions with the orbital atherectomy system: A subanalysis of the ORBIT II study. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 91, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Shlofmitz, E.; Kaplan, B.; Shlofmitz, R. Percutaneous Coronary Intervention in Severely Calcified Unprotected Left Main Coronary Artery Disease: Initial Experience with Orbital Atherectomy. J. Invasive Cardiol. 2016, 28, 147–150. [Google Scholar] [CrossRef]
- Lee, M.S.; Shlofmitz, E.; Nguyen, H.; Shlofmitz, R.A. Outcomes in Diabetic Patients Undergoing Orbital Atherectomy System. J. Interv. Cardiol. 2016, 29, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Meraj, P.; Jauhar, R.; Sethi, S.S.; Shlofmitz, R.A.; Lee, M.S. Safety of orbital atherectomy in patients with left ventricular systolic dysfunction. J. Interv. Cardiol. 2017, 30, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Shlofmitz, E.; Mansourian, P.; Sethi, S.; Shlofmitz, R.A. Gender-Based Differences in Outcomes After Orbital Atherectomy for the Treatment of De Novo Severely Calcified Coronary Lesions. J. Invasive Cardiol. 2016, 28, 440–443. [Google Scholar]
- Lee, M.S.; Shlofmitz, E.; Lluri, G.; Shlofmitz, R.A. Outcomes in Elderly Patients with Severely Calcified Coronary Lesions Undergoing Orbital Atherectomy. J. Interv. Cardiol. 2017, 30, 134–138. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, A.C.; Shlofmitz, R.A.; Martinsen, B.J.; Hargus, N.J.; Elder, M.D.; Généreux, P.; Chambers, J.W. ORBIT II sub-analysis: Impact of impaired renal function following treatment of severely calcified coronary lesions with the Orbital Atherectomy System. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2017, 89, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Martinsen, B.J.; Shlofmitz, R.; Shlofmitz, E.; Lee, A.C.; Chambers, J. Orbital atherectomy treatment of severely calcified coronary lesions in patients with impaired left ventricular ejection fraction: One-year outcomes from the ORBIT II study. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 329–337. [Google Scholar] [CrossRef]
- Lee, M.S.; Shlofmitz, E.; Shlofmitz, R.; Sahni, S.; Martinsen, B.; Chambers, J. Outcomes After Orbital Atherectomy of Severely Calcified Left Main Lesions: Analysis of the ORBIT II Study. J. Invasive Cardiol. 2016, 28, 364–369. [Google Scholar]
- Lee, M.S.; Shlofmitz, E.; Kaplan, B.; Alexandru, D.; Meraj, P.; Shlofmitz, R. Real-World Multicenter Registry of Patients with Severe Coronary Artery Calcification Undergoing Orbital Atherectomy. J. Interv. Cardiol. 2016, 29, 357–362. [Google Scholar] [CrossRef]
- Redfors, B.; Sharma, S.K.; Saito, S.; Kini, A.S.; Lee, A.C.; Moses, J.W.; Ali, Z.A.; Feldman, R.L.; Bhatheja, R.; Stone, G.W. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification: Coronary Orbital Atherectomy System Study (COAST). Circ. Cardiovasc. Interv. 2020, 13, e008993. [Google Scholar] [CrossRef]
- Rola, P.; Włodarczak, S.; Barycki, M.; Furtan, Ł.; Jastrzębski, A.; Kędzierska, M.; Doroszko, A.; Lesiak, M.; Włodarczak, A. Safety and Efficacy of Orbital Atherectomy in the All-Comer Population: Mid-Term Results of the Lower Silesian Orbital Atherectomy Registry (LOAR). J. Clin. Med. 2023, 12, 5842. [Google Scholar] [CrossRef]
- Kirtane, A.J.; Généreux, P.; Lewis, B.; Shlofmitz, R.A.; Dohad, S.; Choudary, J.; Dahle, T.; Pineda, A.M.; Shunk, K.; Maehara, A.; et al. Orbital atherectomy versus balloon angioplasty before drug-eluting stent implantation in severely calcified lesions eligible for both treatment strategies (ECLIPSE): A multicentre, open-label, randomised trial. Lancet 2025, 405, 1240–1251. [Google Scholar] [CrossRef]
- Helal, A.; Ahmad, N.; Bajmmal, O.; Ehtisham, J.; Hogrefe, K.; Raju, P.; Sharman, D.; Shaukat, N.; Farooq, M. Orbital Atherectomy in Calcified Coronary Lesions: A 1-Year Retrospective Observational Outcome Study. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 105, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Egami, Y.; Nohara, H.; Kawanami, S.; Sugae, H.; Kawamura, A.; Ukita, K.; Matsuhiro, Y.; Nakamura, H.; Yasumoto, K.; et al. Direct Comparison of Rotational vs. Orbital Atherectomy for Calcified Lesions Guided by Optical Coherence Tomography. JACC Cardiovasc. Interv. 2023, 16, 2125–2136. [Google Scholar] [CrossRef]
- Lee, T.; Ashikaga, T.; Nozato, T.; Kaneko, M.; Miyazaki, R.; Okata, S.; Nagase, M.; Horie, T.; Terui, M.; Kishigami, T.; et al. Predictors of coronary artery injury after orbital atherectomy as assessed by optical coherence tomography. Int. J. Cardiovasc. Imaging 2023, 39, 1367–1374. [Google Scholar] [CrossRef]
- ECLIPSE: A Large-Scale, Randomized Trial of Orbital Atherectomy vs. Conventional Balloon Angioplasty in Severely Calcified Coronary Arteries Prior to DES Implantation. TCTMD.com. October 29. 2024. Available online: https://www.tctmd.com/slide/eclipse-large-scale-randomized-trial-orbital-atherectomy-vs-conventional-balloon-angioplasty (accessed on 28 April 2025).
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Intravascular imaging-guided coronary drug-eluting stent implantation: An updated network meta-analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J.; et al. Intravascular Imaging During Percutaneous Coronary Intervention: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Malaiapan, Y.; Leung, M.; White, A.J. The role of intravascular ultrasound in percutaneous coronary intervention of complex coronary lesions. Cardiovasc. Diagn. Ther. 2020, 10, 1371–1388. [Google Scholar] [CrossRef]
- Araki, M.; Di Mario, C.; Guagliumi, G.; Kastrati, A.; Escaned, J.; Joner, M.; Johnson, T.W.; Räber, L.; Adriaenssens, T.; Prati, F.; et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat. Rev. Cardiol. 2022, 19, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Andreasen, L.N.; Neghabat, O.; Laanmets, P.; Kumsars, I.; Bennett, J.; Olsen, N.T.; Odenstedt, J.; Hoffmann, P.; Dens, J.; et al. OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N. Engl. J. Med. 2023, 389, 1477–1487. [Google Scholar] [CrossRef]
- Witzenbichler, B.; Maehara, A.; Weisz, G.; Neumann, F.-J.; Rinaldi, M.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; Duffy, P.L.; Brodie, B.R.; et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: The assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation 2014, 129, 463–470. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Martinsen, B.; Lee, M.; Généreux, P.; Behrens, A.; Kumar, G.; Puma, J.; Shlofmitz, R.; Chambers, J. Utilizing intravascular ultrasound imaging prior to treatment of severely calcified coronary lesions with orbital atherectomy: An ORBIT II sub-analysis. J. Interv. Cardiol. 2017, 30, 570–576. [Google Scholar] [CrossRef]
- Shin, D.; Hong, D.; Singh, M.; Lee, S.H.; Sakai, K.; Dakroub, A.; Malik, S.; Maehara, A.; Shlofmitz, E.; Stone, G.W.; et al. Intravascular imaging-guided percutaneous coronary intervention for heavily calcified coronary lesions: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2024, 40, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Laudani, C.; Occhipinti, G.; Spagnolo, M.; Greco, A.; Rochira, C.; Agnello, F.; Landolina, D.; Mauro, M.S.; Finocchiaro, S.; et al. Coronary Angiography, Intravascular Ultrasound, and Optical Coherence Tomography for Guiding of Percutaneous Coronary Intervention: A Systematic Review and Network Meta-Analysis. Circulation 2024, 149, 1065–1086. [Google Scholar] [CrossRef]
- Kurogi, K.; Ishii, M.; Ikebe, S.; Kaichi, R.; Mori, T.; Komaki, S.; Yamamoto, N.; Yamanaga, K.; Arima, Y.; Yamamoto, E.; et al. Optical coherence tomography-versus intravascular ultrasound-guided stent expansion in calcified lesions. Cardiovasc. Interv. Ther. 2022, 37, 312–323. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2025, 85, 2135–2237. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-Y.; Ahn, J.-M.; Yun, S.-C.; Hur, S.-H.; Cho, Y.-K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.-W.; Won, H.; et al. Optical Coherence Tomography-Guided or Intravascular Ultrasound-Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial. Circulation 2023, 148, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Gao, X.-F.; Ge, Z.; Kong, X.-Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.-L.; Lin, S.; Lu, Q.-H.; Wang, X.-Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Sugizaki, Y.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Malik, S.; Dakroub, A.; et al. A Revised Optical Coherence Tomography-Derived Calcium Score to Predict Stent Underexpansion in Severely Calcified Lesions. JACC Cardiovasc. Interv. 2025, 18, 622–633. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Tsoulios, A.; Cohen, D.J.; Mintz, G.S.; et al. Impact of Eruptive vs. Noneruptive Calcified Nodule Morphology on Acute and Long-Term Outcomes After Stenting. JACC Cardiovasc. Interv. 2023, 16, 1024–1035. [Google Scholar] [CrossRef]
- Sotomi, Y.; Cavalcante, R.; Shlofmitz, R.A.; Suwannasom, P.; Tateishi, H.; Tenekecioglu, E.; Zheng, Y.; Abdelghani, M.; de Winter, R.J.; Wykrzykowska, J.J.; et al. Quantification by optical coherence tomography imaging of the ablation volume obtained with the Orbital Atherectomy System in calcified coronary lesions. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016, 12, 1126–1134. [Google Scholar] [CrossRef]
- Yamamoto, M.H.; Maehara, A.; Kim, S.S.; Koyama, K.; Kim, S.-Y.; Ishida, M.; Fujino, A.; Haag, E.S.; Alexandru, D.; Jeremias, A.; et al. Effect of orbital atherectomy in calcified coronary artery lesions as assessed by optical coherence tomography. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2019, 93, 1211–1218. [Google Scholar] [CrossRef]
- Ali, Z.A.; Shin, D.; Vijayvergiya, R.; Gawalkar, A.A.; Shlofmitz, R.A.; Alfonso, F.; Calligaris, G.; Canova, P.; Sakai, K.; Price, M.J.; et al. Optical coherence tomography- vs. angiography-guided coronary stent implantation in calcified lesions: The ILUMIEN IV trial. Eur. Heart J. 2025, 46, 3201–3210. [Google Scholar] [CrossRef]
- Jurado-Román, A.; Gómez-Menchero, A.; Rivero-Santana, B.; Amat-Santos, I.J.; Jiménez-Valero, S.; Caballero-Borrego, J.; Ojeda, S.; Miñana, G.; Gonzálvez-García, A.; Tébar-Márquez, D.; et al. Rotational Atherectomy, Lithotripsy, or Laser for Calcified Coronary Stenosis: The ROLLER COASTR-EPIC22 Trial. JACC Cardiovasc. Interv. 2025, 18, 606–618. [Google Scholar] [CrossRef]
- Amabile, N.; Rangé, G.; Landolff, Q.; Bressollette, E.; Meneveau, N.; Lattuca, B.; Levesque, S.; Boueri, Z.; Adjedj, J.; Casassus, F.; et al. OCT vs. Angiography for Guidance of Percutaneous Coronary Intervention of Calcified Lesions. JAMA Cardiol. 2025, 10, 666–675. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Chen, L.; Chen, M. Intensive plaque modification with rotational atherectomy and cutting balloon before drug-eluting stent implantation for patients with severely calcified coronary lesions: A pilot clinical study. BMC Cardiovasc. Disord. 2016, 16, 112. [Google Scholar] [CrossRef]
- Furuichi, S.; Tobaru, T.; Asano, R.; Watanabe, Y.; Takamisawa, I.; Seki, A.; Sumiyoshi, T.; Tomoike, H. Rotational atherectomy followed by cutting-balloon plaque modification for drug-eluting stent implantation in calcified coronary lesions. J. Invasive Cardiol. 2012, 24, 191–195. [Google Scholar]
- Sharma, S.K.; Mehran, R.; Vogel, B.; Hooda, A.; Sartori, S.; Hanstein, R.; Feng, Y.; Shlofmitz, R.A.; Jeremias, A.; Spirito, A.; et al. Rotational atherectomy combined with cutting balloon to optimise stent expansion in calcified lesions: The ROTA-CUT randomised trial. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2024, 20, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Badr, S.; Ben-Dor, I.; Dvir, D.; Barbash, I.M.; Kitabata, H.; Minha, S.; Pendyala, L.K.; Loh, J.P.; Torguson, R.; Pichard, A.D.; et al. The state of the excimer laser for coronary intervention in the drug-eluting stent era. Cardiovasc. Revasc. Med. Mol. Interv. 2013, 14, 93–98. [Google Scholar] [CrossRef]
- Jawad-Ul-Qamar, M.; Sharma, H.; Vetrugno, V.; Sandhu, K.; Ludman, P.F.; Doshi, S.N.; Townend, J.N.; Osheiba, M.; Zaphiriou, A.; Khan, S.Q. Contemporary use of excimer laser in percutaneous coronary intervention with indications, procedural characteristics, complications and outcomes in a university teaching hospital. Open Heart 2021, 8, e001522. [Google Scholar] [CrossRef]
- Protty, M.B.; Gallagher, S.; Farooq, V.; Sharp, A.S.P.; Egred, M.; O’Kane, P.; Kinnaird, T. Combined use of rotational and excimer lASER coronary atherectomy (RASER) during complex coronary angioplasty-An analysis of cases (2006-2016) from the British Cardiovascular Intervention Society database. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, E911–E918. [Google Scholar] [CrossRef]
- Takahashi, T.; Menegus, M.; Choi, H.; Bliagos, D.; Assafin, M.; Rauch, J.; Johnson, M.; Greenberg, M.; Khaliq, A.; Scotti, A.; et al. Complementary Utility of Intravascular Lithotripsy with Atherectomy for Severely Calcified Coronary Stenoses in Contemporary Practice. J. Invasive Cardiol. 2023, 35, E46–E54. [Google Scholar] [CrossRef]
- Hinton, J.; O’Kane, P. Combination Tools for Calcium Modification from RASER to Orbitalshock. Interv. Cardiol. Rev. Res. Resour. 2024, 19, e18. [Google Scholar] [CrossRef]
- Kachi, D.; Lee, T.; Terui, M.; Nagase, M.; Misawa, T.; Miyazaki, R.; Kaneko, M.; Nagata, Y.; Nozato, T.; Ashikaga, T. The impact of novel scoring balloon and cutting balloon after orbital atherectomy on severely calcified coronary lesion as assessed by optical coherence tomography. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2024, 104, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sardella, G.; Stefanini, G.; Leone, P.P.; Boccuzzi, G.; Fovero, N.T.; Van Mieghem, N.; Giacchi, G.; Escaned, J.; Fineschi, M.; Testa, L.; et al. Coronary Lithotripsy as Elective or Bail-Out Strategy After Rotational Atherectomy in the Rota-Shock Registry. Am. J. Cardiol. 2023, 198, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.; Varma, R.; Din, J.; Kodoth, V.; Talwar, S.; O’Kane, P. “RotaShock”—A Revolution in Calcium Modification: Long-term Follow-up from a Single High-volume Centre. Interv. Cardiol. 2025, 20, e08. [Google Scholar] [CrossRef]
- Yarusi, B.B.; Jagadeesan, V.S.; Hussain, S.; Jivan, A.; Tesch, A.; Flaherty, J.D.; Schimmel, D.R.; Benzuly, K.H. Combined Coronary Orbital Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified Coronary Stenoses: The First Case Series. J. Invasive Cardiol. 2022, 34, E210–E217. [Google Scholar] [CrossRef]
- McLaughlin, T.J.; Sachdeva, R.; Kumar, G. First United States Experience with Rota-Shock: A Case Series. Cardiovasc. Revasc. Med. Mol. Interv. 2022, 40S, 209–213. [Google Scholar] [CrossRef]
- Naeem-Shaukat, Z.; Ehtisham, J.; Shaukat, N.; Helal, A. Saline-Enhanced Optical Coherence Tomography (OCT) and Combined Orbital Atherectomy and Intravascular Lithotripsy in Complex Right Coronary Artery Stenosis with Chronic Kidney Disease. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2025, 105, 1183–1187. [Google Scholar] [CrossRef]
- Rozenbaum, Z.; Takahashi, T.; Kobayashi, Y.; Bliagos, D.; Menegus, M.; Colombo, A.; Latib, A. Contemporary technologies to modify calcified plaque in coronary artery disease. Prog. Cardiovasc. Dis. 2021, 69, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.F.; Mui, C.Y.; Chung, T.-S. TCTAP C-037 Orbitotripsy in Emergent PCI: The Combined Use of Orbital Atherectomy and Intravascular Lithrotripsy for Heavily Calcified Coronary Lesions in a Patient Presented with Acute ST-Segment Elevation Myocardial Infarction. JACC 2023, 81 (Suppl. 16), S138–S140. [Google Scholar] [CrossRef]
- Yasumura, K.; Koshy, A.N.; Vinayak, M.; Vengrenyuk, Y.; Minatoguchi, S.; Krishnamoorthy, P.; Hooda, A.; Sharma, R.; Kapur, V.; Sweeny, J.; et al. Rotational, orbital atherectomy and intravascular lithotripsy for coronary calcified nodules: Insights from optical coherence tomography. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2024, 104, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Florek, K.; Bartoszewska, E.; Biegała, S.; Klimek, O.; Malcharczyk, B.; Kübler, P. Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions. J. Clin. Med. 2023, 12, 7246. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Kirtane, A.J.; Kandzari, D.E.; Armstrong, E.J.; Krucoff, M.W.; Redfors, B.; Ben-Yehuda, O.; Lerew, D.R.; Ali, Z.A.; Maehara, A.; et al. Randomized evaluation of vessel preparation with orbital atherectomy prior to drug-eluting stent implantation in severely calcified coronary artery lesions: Design and rationale of the ECLIPSE trial. Am. Heart J. 2022, 249, 1–11. [Google Scholar] [CrossRef]
- PCR EuroPCR 2024: Primary Outcomes from PINNACLE I Clinical Trial Establish Safety and Effectiveness of Elixir Medical’s Hertz Contact Intravascular Lithotripsy System for Calcium Fragmentation in Moderate to Severe Calcified Coronary Artery Lesions. Available online: https://www.pcronline.com/News/Press-releases/2024/EuroPCR-2024-Primary-outcomes-from-PINNACLE-I-clinical-trial-establish-safety-and-effectiveness-of-Elixir-Medical-s-Hertz-Contact-Intravascular-Lithotripsy-System-for-calcium-fragmentation-in-moderate-to-severe-calcified-coronary-artery-lesions (accessed on 1 August 2025).
- Shockwave Medical, Inc. Prospective, Multicenter, Single-Arm, Investigational Device Exemption (IDE) Study of the Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave Javelin Coronary IVL Catheter (FORWARD CAD IDE Study). In clinicaltrials.gov.; 2025. Available online: https://clinicaltrials.gov/study/NCT06662500 (accessed on 1 August 2025).

| Parameter | Rotational Atherectomy (RA) | Excimer Laser Coronary Atherectomy (ELCA) | Intravascular Lithotripsy (IVL) | Orbital Atherectomy (OA) |
|---|---|---|---|---|
| Device | Rotablator Rotational Atherectomy System | Spectranetics CVX-300 Excimer Laser System | Shockwave IVL System | Diamondback 360 Coronary Orbital Atherectomy System |
| Mechanism of Action | Rotational Diamond-tipped burr spins concentrically on the wire Differential cutting ablates calcified plaque | Laser Pulsed ultraviolet laser (308 nm) photoablation, results in vaporisation of plaque Photochemical, photothermal, photomechanical (microbubble formation) | Shockwave Pulsed sonic/acoustic shockwaves fracture calcified plaque intramurally | Orbital Eccentrically mounted diamond-coated crown uses centrifugal force to orbit Differential sanding ablates calcified plaque |
| Burr/Crown Size | 1.25–2.50 mm (Burr) | Catheters with various tip sizes, available with concentric and eccentric tip designs | 2.50–4.00 mm (Balloon) | 1.25 mm (Crown) |
| Guidewire | 0.009”/0.014” tip RotaWire Guide Wires | 0.014” Guidewire | 0.014” Guidewire | 0.012”/0.014” tip ViperWire Advance Coronary Guide Wire |
| Ablation Speed | Variable | Adjustable laser energy settings | N/A | 80,000 and 120,000 rpm |
| Ability to ablate forward and backward | No, front-cutting, mono-directional | No, forward emission of laser energy for vaporisation | Circumferential | Yes, bi-directional |
| Continuous blood flow during ablation | No | Yes | Yes | Yes |
| Particle Size | 5–10 µm | <10 µm | Not applicable | 2 µm |
| Power Source | Pneumatic system | Laser generator: Spectranetics CVX-300 | Electrical generator | Electronic system |
| Study | Study Design | Year | Population | Study Duration | Entry Criteria | Primary Endpoint (s) | Key Findings |
|---|---|---|---|---|---|---|---|
| ORBIT I | Prospective, single-arm, multicentre | 2013 | 50 | 6 months | De novo calcified coronary lesions determined by angiography or IVUS | Device performance, procedural success, MACE | Device success 98%, procedural success 94%, in-hospital MACE 4%, 6-month MACE 8% |
| ORBIT II | Prospective, single-arm, multicentre | 2014–2016 | 443 | 2 years | Severely calcified coronary lesions, determined by angiography only 92%, or based on IVUS 8% | 30-day MACE, 1-year MACE, 2-year MACE, procedural success | 30-day MACE 10.4%, 1-year MACE 16.4%, 2-year MACE 19.4%, procedural success 88.9% |
| Lee et al. Real-world Multicentre Registry | Retrospective, multicentre | 2016 | 458 | 30 days | Severe CAC, enrolled based on the presence of radio-opacities on angiography 100% | 30-day MACCE | 30-day MACCE 1.7%, all-cause mortality 1.3%, MI 1.1%, TVR 0% |
| COAST | Prospective, multicentre, single-arm | 2020 | 100 | 1 year | Severely calcified coronary lesions, determined by angiography only 65%, by IVUS 21%, or determined by OCT 14% | Procedural success, 30-day MACE | Procedural success 85%, 30-day MACE 15% |
| LOAR | Prospective, single-arm | 2023 | 96 | 6 months | Severe calcification, determined by angiography or IVUS | In-hospital MACCE, 6-month MACCE | In-hospital MACCE 5.2%, 6-month MACCE 10.4% |
| DIRO | Prospective, randomised | 2023 | 100 | 8 months | De novo calcified lesions, assessed by OCT (90% in RA group, 88% in OA group) or angiographically (10% in RA group, 12% in OA group) | Stent expansion, procedural outcomes | RA group had greater stent expansion (99.5% vs. 90.6%) |
| Helal et al. UK Single-centre Study | Retrospective, single-centre | 2025 | 53 | 1 year | Severely calcified stenosis in a native coronary artery on angiography 54.7%, IVUS 18.9% or OCT 26.4% | Procedural success, 30-day MACE | Procedural success 98.1%, 30-day MACE 5.7% |
| ECLIPSE | Prospective, randomised, multicentre | 2025 | 2005 | 2 years | Severely calcified coronary lesions, confirmed angiographically (97.1% of lesions in OA group, 97.0% in balloon angioplasty group), or by OCT | Target vessel failure, in-stent minimal cross-sectional area | No significant difference in 1-year target vessel failure or minimal stent area between OA and balloon angioplasty groups |
| Study | Year | Patients (N) | Dissection (%) | Perforation (%) | Slow Flow/No Reflow (%) | Abrupt Closure (%) | In-Hospital MACE (%) | 30-Day MACE (%) | 30-Day TVR/TLR (%) |
|---|---|---|---|---|---|---|---|---|---|
| ORBIT I | 2013 | 50 | 12 a | 2.0 | 0 | 0 | 4 | 6 | 2.0 |
| ORBIT II | 2014–2016 | 443 | 3.4 b | 1.8 | 0.9 | 1.8 | 9.8 | 10.4 | 1.4 |
| Lee et al. Real-world Multicentre Registry | 2016 | 458 | 0.9 | 0.7 | 0.7 | NS | NS | 1.7 c | 0.0 |
| COAST | 2020 | 100 | 2.0 | 2.0 | 2.0 | 3.0 | 14 | 15 | 1.0 |
| LOAR | 2023 | 96 | NS | 1.0 | 2.0 | 1.0 | 5.2 c | NS | NS |
| DIRO | 2023 | 100 | 2.0 in OA | 0.0 in RA, 2.0 in OA | 6.0 in RA, 4.0 in OA | NS | NS | NS | 0.0 in both groups |
| Helal et al. UK Single-centre Study | 2025 | 53 | 13.2 | 0.0 | 13.2 | NS | 1.9 | 5.7 | 0.0 |
| ECLIPSE | 2025 | 2005 | 6.9 in OA b, 6.3 in BA b | 1.8 in OA, 1.0 in BA | 1.7 in OA, 0.5 in BA | 0.6 in OA, 0.2 in BA | NS | NS | 1.5 in OA, 1.2 in BA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khullar, N.; Singh, T.; O’Kane, P.; Hinton, J. The Role of Orbital Atherectomy for Complex Coronary Calcium Modification: Has It Been Eclipsed? J. Pers. Med. 2025, 15, 414. https://doi.org/10.3390/jpm15090414
Khullar N, Singh T, O’Kane P, Hinton J. The Role of Orbital Atherectomy for Complex Coronary Calcium Modification: Has It Been Eclipsed? Journal of Personalized Medicine. 2025; 15(9):414. https://doi.org/10.3390/jpm15090414
Chicago/Turabian StyleKhullar, Natasha, Trisha Singh, Peter O’Kane, and Jonathan Hinton. 2025. "The Role of Orbital Atherectomy for Complex Coronary Calcium Modification: Has It Been Eclipsed?" Journal of Personalized Medicine 15, no. 9: 414. https://doi.org/10.3390/jpm15090414
APA StyleKhullar, N., Singh, T., O’Kane, P., & Hinton, J. (2025). The Role of Orbital Atherectomy for Complex Coronary Calcium Modification: Has It Been Eclipsed? Journal of Personalized Medicine, 15(9), 414. https://doi.org/10.3390/jpm15090414






