Longitudinal Ellipsoid Zone Dynamics During Hydroxychloroquine Use
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mavrikakis, I.; Sfikakis, P.P.; Mavrikakis, E.; Rougas, K.; Nikolaou, A.; Kostopoulos, C.; Mavrikakis, M. The Incidence of Irreversible Retinal Toxicity in Patients Treated with Hydroxychloroquine A Reappraisal. Ophthalmology 2003, 110, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine Retinopathy. Eye 2017, 31, 828–845. [Google Scholar] [CrossRef]
- Lally, D.R.; Heier, J.S.; Baumal, C.; Witkin, A.J.; Maler, S.; Shah, C.P.; Reichel, E.; Waheed, N.K.; Bussel, I.; Rogers, A.; et al. Expanded Spectral Domain-OCT Findings in the Early Detection of Hydroxychloroquine Retinopathy and Changes Following Drug Cessation. Int. J. Retin. Vitr. 2016, 2, 18. [Google Scholar] [CrossRef]
- Marmor, M.F. Comparison of Screening Procedures in Hydroxychloroquine Toxicity. Arch. Ophthalmol. 2012, 130, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Hu, J. Effect of Disease Stage on Progression of Hydroxychloroquine Retinopathy. Jama Ophthalmol. 2014, 132, 1105–1112. [Google Scholar] [CrossRef]
- Marmor, M.F.; Melles, R.B. Disparity between Visual Fields and Optical Coherence Tomography in Hydroxychloroquine Retinopathy. Ophthalmology 2014, 121, 1257–1262. [Google Scholar] [CrossRef]
- Greenstein, V.C.; Amaro-Quireza, L.; Abraham, E.S.; Ramachandran, R.; Tsang, S.H.; Hood, D.C. A Comparison of Structural and Functional Changes in Patients Screened for Hydroxychloroquine Retinopathy. Doc. Ophthalmol. 2015, 130, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kellner, S.; Weinitz, S.; Kellner, U. Spectral Domain Optical Coherence Tomography Detects Early Stages of Chloroquine Retinopathy Similar to Multifocal Electroretinography, Fundus Autofluorescence and Near-infrared Autofluorescence. Brit. J. Ophthalmol. 2009, 93, 1444. [Google Scholar] [CrossRef]
- Browning, D.J.; Lee, C. Relative Sensitivity and Specificity of 10-2 Visual Fields, Multifocal Electroretinography, and Spectral Domain Optical Coherence Tomography in Detecting Hydroxychloroquine and Chloroquine Retinopathy. Clin. Ophthalmol. 2014, 8, 1389–1399. [Google Scholar] [CrossRef]
- Cukras, C.; Huynh, N.; Vitale, S.; Wong, W.T.; Ferris III, F.L.; Sieving, P.A. Subjective and Objective Screening Tests for Hydroxychloroquine Toxicity. Ophthalmology 2015, 122, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Joung, J.; Lim, H.W.; Lee, B.R. Optical Coherence Tomography Protocols for Screening of Hydroxychloroquine Retinopathy in Asian Patients. Am. J. Ophthalmol. 2017, 184, 11–18. [Google Scholar] [CrossRef]
- Rodriguez-Padilla, J.A.; Hedges, T.R.; Monson, B.; Srinivasan, V.; Wojtkowski, M.; Reichel, E.; Duker, J.S.; Schuman, J.S.; Fujimoto, J.G. High-Speed Ultra–High-Resolution Optical Coherence Tomography Findings in Hydroxychloroquine Retinopathy. Arch. Ophthalmol. 2007, 125, 775–780. [Google Scholar] [CrossRef]
- Chen, E.; Brown, D.M.; Benz, M.S.; Fish, R.H.; Wong, T.P.; Kim, R.Y.; Major, J.C. Spectral Domain Optical Coherence Tomography as an Effective Screening Test for Hydroxychloroquine Retinopathy (the “flying saucer” sign). Clin. Ophthalmol. 2010, 4, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Pasadhika, S.; Fishman, G.A.; Choi, D.; Shahidi, M. Selective Thinning of the Perifoveal Inner Retina as an Early Sign of Hydroxychloroquine Retinal Toxicity. Eye 2010, 24, 756. [Google Scholar] [CrossRef]
- Ugwuegbu, O.; Uchida, A.; Singh, R.P.; Beven, L.; Hu, M.; Kaiser, S.; Srivastava, S.K.; Ehlers, J.P. Quantitative Assessment of Outer Retinal Layers and Ellipsoid Zone Mapping in Hydroxychloroquine Retinopathy. Brit. J. Ophthalmol. 2019, 103, 3. [Google Scholar] [CrossRef]
- Allahdina, A.M.; Chen, K.G.; Alvarez, J.A.; Wong, W.T.; Chew, E.Y.; Cukras, C.A. Longitudinal Changes in Eyes with Hydroxychloroquine Retinal Toxicity. Retina 2019, 39, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.H.; Marmor, M.F. Sequential Changes in Hydroxychloroquine Retinopathy up to 20 Years After Stopping the Drug. Retina 2019, 39, 492–501. [Google Scholar] [CrossRef]
- Garrity, S.T.; Jung, J.Y.; Zambrowski, O.; Pichi, F.; Su, D.; Arya, M.; Waheed, N.K.; Duker, J.S.; Chetrit, Y.; Miserocchi, E.; et al. Early Hydroxychloroquine Retinopathy: Optical Coherence Tomography Abnormalities Preceding Humphrey Visual Field Defects. Brit. J. Ophthalmol. 2019, 103, 1600–1604. [Google Scholar] [CrossRef]
- de Sisternes, L.; Hu, J.; Rubin, D.L.; Marmor, M.F. Analysis of Inner and Outer Retinal Thickness in Patients Using Hydroxychloroquine Prior to Development of Retinopathy. JAMA Ophthalmol. 2016, 134, 511. [Google Scholar] [CrossRef]
- de Sisternes, L.; Hu, J.; Rubin, D.L.; Marmor, M.F. Localization of Damage in Progressive Hydroxychloroquine Retinopathy on and off the Drug: Inner Versus Outer Retina, Parafovea Versus Peripheral FoveaRetinal Layers in Progressive HCQ Retinopathy. Investig. Ophth. Vis. Sci. 2015, 56, 3415–3426. [Google Scholar] [CrossRef] [PubMed]
- Modi, Y.S.; Au, A.; Parikh, V.S.; Ehlers, J.P.; Schachat, A.P.; Singh, R.P. Volumetric Single-Layer Inner Retinal Analysis in Patients with Hydroxychloroquine Toxicity. Retina 2016, 36, 1941–1950. [Google Scholar] [CrossRef]
- Arepalli, S.; Srivastava, S.K.; Hu, M.; Kaiser, P.M.; Dukles, N.; Reese, J.L.; Ehlers, J.P.; Lewin, A.S. Assessment of Inner and Outer Retinal Layer Metrics on the Cirrus HD-OCT Platform in Normal Eyes. PLoS ONE 2018, 13, e0203324. [Google Scholar] [CrossRef]
- Itoh, Y.; Vasanji, A.; Ehlers, J.P. Volumetric Ellipsoid Zone Mapping for Enhanced Visualisation of Outer Retinal Integrity with Optical Coherence Tomography. Brit. J. Ophthalmol. 2016, 100, 295. [Google Scholar] [CrossRef]
- Banaee, T.; Singh, R.P.; Champ, K.; Conti, F.F.; Wai, K.; Bena, J.; Beven, L.; Ehlers, J.P. Ellipsoid Zone Mapping Parameters in Retinal Venous Occlusive Disease with Associated Macular Edema. Ophthalmol. Retin. 2018, 2, 836–841. [Google Scholar] [CrossRef]
- Arepalli, S.; Traboulsi, E.I.; Ehlers, J.P. Ellipsoid Zone Mapping and Outer Retinal Assessment in Stargardt Disease. Retina 2018, 38, 1427–1431. [Google Scholar] [CrossRef]
- Lavine, J.A.; Srivastava, S.K.; Dukles, N.; Reese, J.L.; Ehlers, J.P. Longitudinal Ellipsoid Zone and Subretinal Fluid Mapping Following Ocriplasmin Injection in the Prospective Observational ORBIT Trial. Brit. J. Ophthalmol. 2020, 104, 410. [Google Scholar] [CrossRef]
- Itoh, Y.; Ehlers, J.P. Ellipsoid Zone Mapping and Outer Retinal Characterization After Intravitreal Ocriplasmin. Retina 2016, 36, 2290–2296. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Uchida, A.; Hu, M.; Figueiredo, N.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Boyer, D.S.; Do, D.V.; Gibson, A.; et al. Higher Order Assessment of OCT in Diabetic Macular Edema from the VISTA Study: Ellipsoid Zone Dynamics and the Retinal Fluid Index. Ophthalmol. Retin. 2019, 3, 1056–1066. [Google Scholar] [CrossRef]
- Cakir, A.; Ozturan, Ş.G.; Yildiz, D.; Erden, B.; Bolukbasi, S.; Tascilar, E.K.; Yanmaz, M.N.; Elcioglu, M.N. Evaluation of Photoreceptor Outer Segment Length in Hydroxychloroquine Users. Eye 2019, 33, 1321–1326. [Google Scholar] [CrossRef]
- Kalra, G.; Talcott, K.E.; Kaiser, S.; Ugwuegbu, O.; Hu, M.; Srivastava, S.K.; Ehlers, J.P. Machine Learning–Based Automated Detection of Hydroxychloroquine Toxicity and Prediction of Future Toxicity Using Higher-Order OCT Biomarkers. Ophthalmol. Retin. 2022, 6, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Talcott, K.E.; Kalra, G.; Cetin, H.; Cakir, Y.; Whitney, J.; Budrevich, J.; Reese, J.L.; Srivastava, S.K.; Ehlers, J.P. Automated Evaluation of Ellipsoid Zone At-Risk Burden for Detection of Hydroxychloroquine Retinopathy. J. Pers. Med. 2024, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.; Jayakar, G.; Grisso, P.; Hotaling, N.; Chew, E.Y.; Cukras, C.A. Deep Learning-Based Automatic Detection of Ellipsoid Zone Loss in Spectral-Domain OCT for Hydroxychloroquine Retinal Toxicity Screening. Ophthalmol. Sci. 2021, 1, 100060. [Google Scholar] [CrossRef] [PubMed]
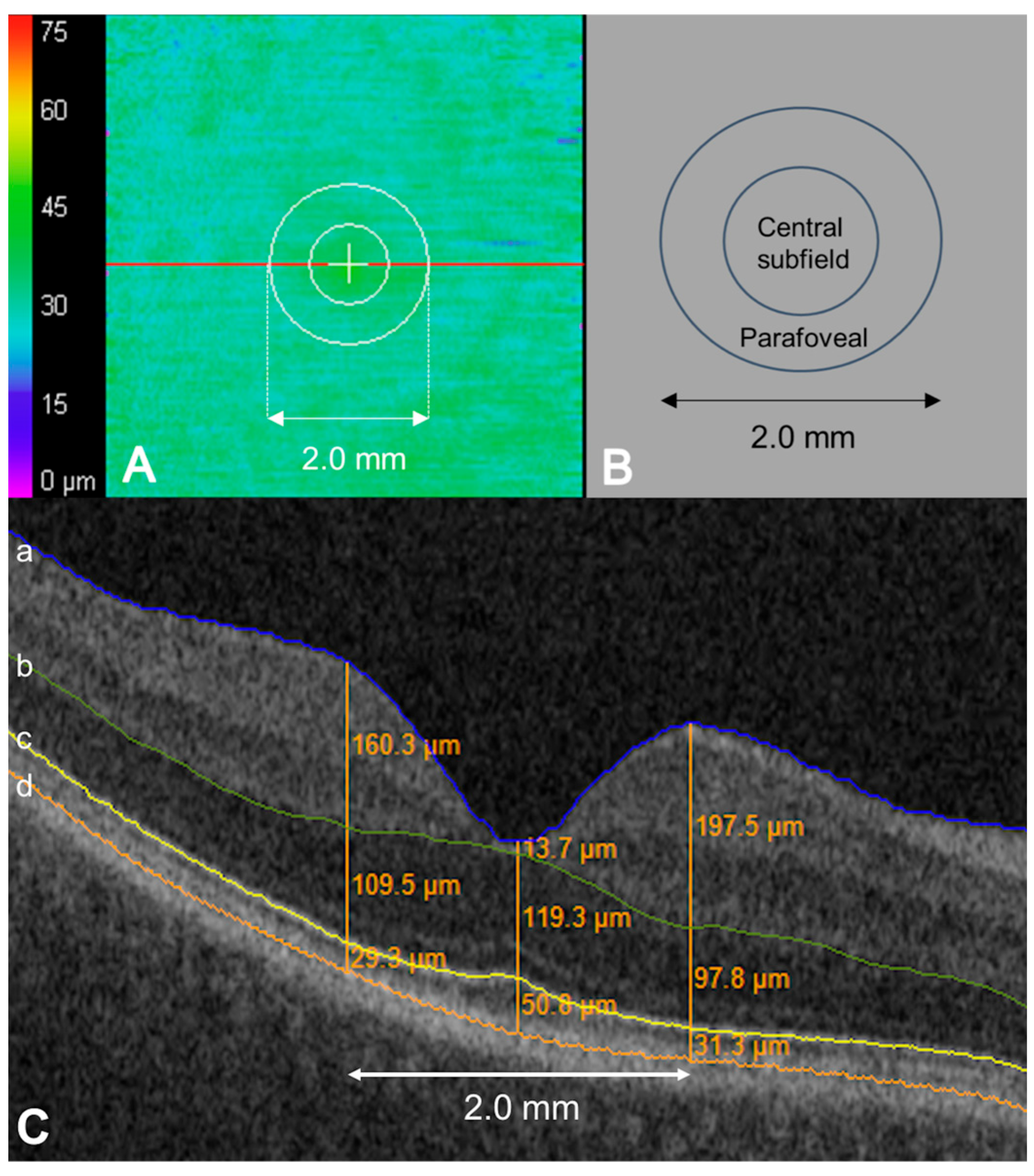

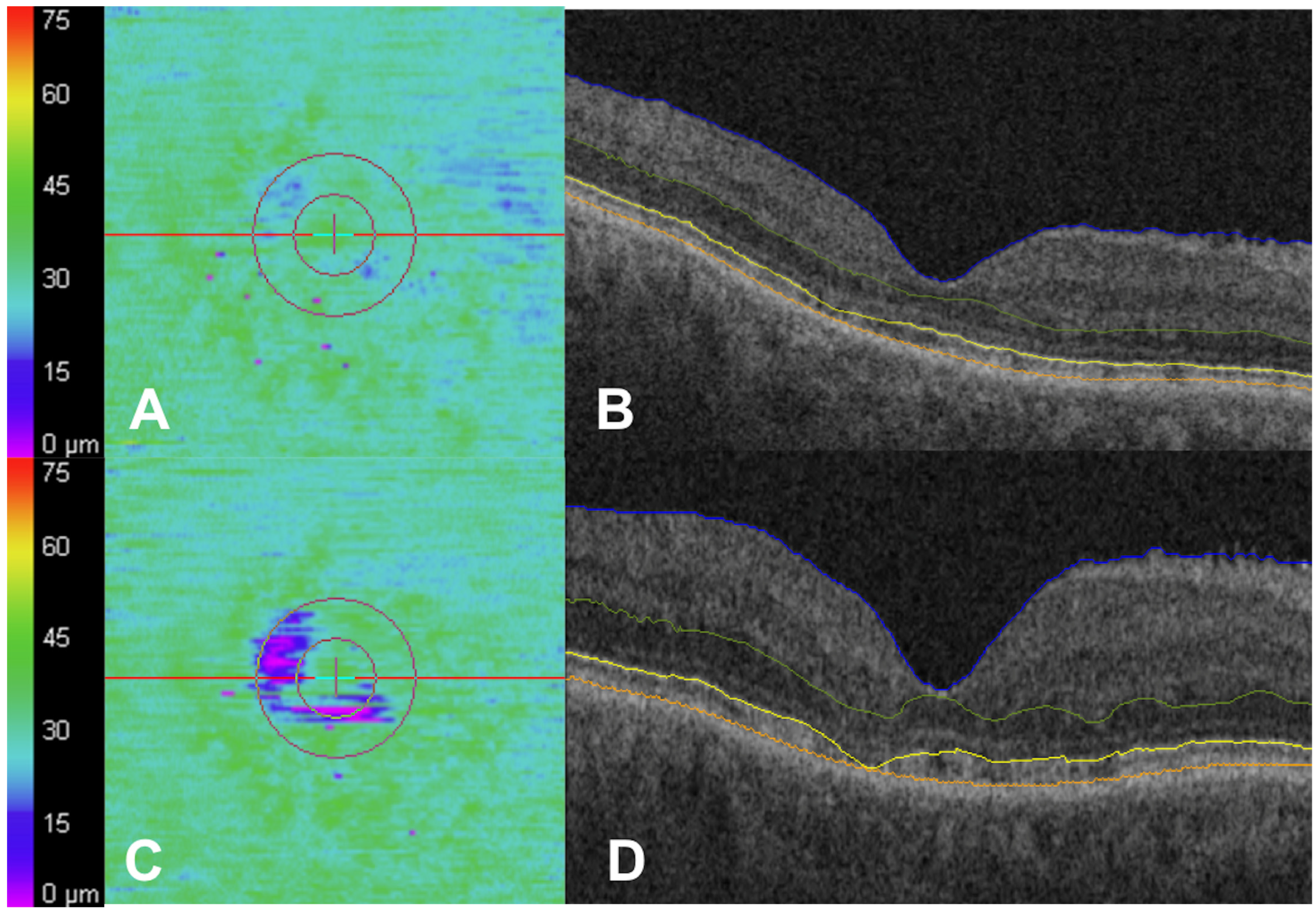
| Baseline Characteristics (n = 373 Eyes) | |
| Age at first OCT, years | |
| Mean ± SD (median, range) | 57.0 ± 12.6 (58, 14–89) |
| Gender | |
| N (percentage) | |
| Female | 322 (86) |
| Male | 51 (14) |
| Ethnicity | |
| N (percentage) | |
| White | 260 (70) |
| Black | 89 (24) |
| Hispanic | 4 (1) |
| Asian | 9 (2) |
| Other | 11 (3) |
| Age HCQ initiated, years | |
| Mean ± SD | 51.5 ± 12.5 |
| Daily HCQ dose, mg | |
| Mean ± SD | 379.38 ± 59.40 |
| HCQ actual body weight dose, mg/kg | |
| Mean ± SD | 4.8 ± 1.5 |
| HCQ ideal body weight dose, mg/kg | |
| Mean ± SD | 6.5 ± 1.5 |
| Time between OCTs, years | |
| Mean ± SD | 3.1 ± 0.9 |
| Cumulative dose at first OCT, kg | |
| Mean ± SD | 0.8 ± 0.5 |
| Duration on HCQ, years | |
| Mean ± SD | |
| First OCT | 5.6 ± 3.7 |
| Second OCT | 8.6 ± 3.8 |
| LogMAR visual acuity | |
| Mean ± SD (median, range) (Snellen equivalent) | |
| First OCT | 0.090 ± 0.17 (0, −0.12–1.3) (Snellen 20/25) |
| Second OCT | 0.103 ± 0.18 (0, −0.12–2.2) (Snellen 20/25) |
| Longitudinal change | 0.013 ± 0.22 (0, −1–1.90) |
| Tamoxifen use | |
| N (percentage) | |
| Yes | 7 (2) |
| No | 366 (98) |
| Kidney disease | |
| N (percentage) | |
| Yes | 24 (6) |
| No | 349 (4) |
| Rheumatoid arthritis | |
| N (percentage) | |
| Yes | 161 (43) |
| No | 212 (57) |
| Lupus | |
| N (percentage) | |
| Yes | 130 (35) |
| No | 243 (65) |
| OCT 1 (Mean ± SD) | OCT 2 (Mean ± SD) | Difference (Mean ± SD) | p-Value (Paired t-Test) | |
| Partial EZ attenuation (EZ -RPE thickness ≤ 20 µm; %) | 3.3 ± 12.2 | 3.9 ± 12.0 | 0.6 ± 9.4 | 0.24 |
| En face percentage of EZ total attenuation (EZ-RPE thickness = 0 µm; %) | 2.1 ± 10.5 | 2.3 ± 9.0 | 0.2 ± 8.0 | 0.60 |
| Central subfield EZ-RPE thickness (µm) | 40.4 ± 4.6 | 40.3 ± 4.6 | −0.12 ± 3.4 | 0.49 |
| Central subfield EZ-RPE volume (mm3) | 0.032 ± 0.004 | 0.032 ± 0.004 | 0.000 ± 0.003 | 0.40 |
| Central subfield EZ-RPE point nasal thickness (0.5 mm from fovea; µm) | 39.1 ± 5.3 | 38.6 ± 6.0 | −0.48 ± 5.6 | 0.10 |
| Central subfield EZ-RPE point temporal thickness (0.5 mm from fovea; µm) | 37.9 ± 5.7 | 37.7 ± 5.7 | −0.2 ± 6.0 | 0.49 |
| Parafoveal EZ-RPE thickness (µm) | 36.8 ± 3.8 | 36.6 ± 3.9 | −0.2 ± 3.0 | 0.35 |
| Parafoveal EZ-RPE volume (mm3) | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.00 ± 0.01 | 0.39 |
| Parafoveal EZ-RPE point nasal thickness (1 mm from fovea; µm) | 35.6 ± 5.1 | 35.1 ± 5.6 | −0.5 ± 5.9 | 0.08 |
| Parafoveal EZ-RPE point temporal thickness (1 mm from fovea; µm) | 35.1 ± 5.9 | 35.1 ± 5.6 | 0.0 ± 6.1 | 0.96 |
| EZ-RPE volume (mm3) | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.0 ± 0.1 | 0.53 |
| Patients with Increase in partial EZ Attenuation of 4% or greater (n = 34) | Patients with < 4% Increase in partial EZ Attenuation (n = 339) | p-Value (Paired t-Test) | |
| Age HCQ initiated, years Mean ± SD | 61.6 ± 13.0 | 50.5 ± 12.1 | <0.001 |
| Age at first OCT, years Mean ± SD | 68.0 ± 12.8 | 55.9 ± 12.1 | <0.001 |
| Age at second OCT, years Mean ± SD | 71.0 ± 13.3 | 59.0 ± 12.3 | <0.001 |
| Cumulative HCQ dose at first OCT, kg Mean ± SD | 0.86 ± 0.58 | 1.28 ± 0.59 | 0.24 |
| Cumulative HCQ dose at second OCT, kg Mean ± SD | 1.31 ± 0.58 | 1.14 ± 0.74 | 0.12 |
| Actual body weight HCQ dose, mg/kg Mean ± SD | 5.11 ± 1.19 | 4.78 ± 1.56 | 0.15 |
| Ideal body weight HCQ dose, mg/kg Mean ± SD | 6.97 ± 1.38 | 6.50 ± 1.56 | 0.07 |
| LogMAR visual acuity, first OCT Mean ± SD | 0.20 ± 0.24 | 0.08 ± 0.16 | 0.01 |
| LogMAR visual acuity, second OCT Mean ± SD | 0.20 ± 0.25 | 0.09 ± 0.17 | 0.02 |
| Patients with Increase in partial EZ Attenuation > 4% (n = 34; Mean ± SD) | Patients with <4% Increase in partial EZ Attenuation (n = 339; Mean ± SD) | p-Value (Paired t-Test) | |
| Partial EZ attenuation (Longitudinal change; EZ-RPE thickness ≤ 20 µm; %) | 17.6 ± 168 | −1.1 ± 6.2 | <0.001 |
| Total EZ attenuation (Longitudinal change; EZ-RPE thickness = 0 µm; total loss; %) | 11.8 ± 14.7 | −0.9 ± 5.9 | <0.001 |
| Central subfield EZ-RPE thickness (Longitudinal change; µm) | −0.87 ± 3.24 | −0.05 ± 3.39 | 0.17 |
| Central subfield EZ-RPE volume (Longitudinal change; mm3) | −0.001 ± 0.003 | 0.000 ± 0.003 | 0.09 |
| Central subfield EZ-RPE point nasal thickness (Longitudinal change; 0.5 mm from fovea; µm) | −2.9 ± 7.5 | −0.2 ± 5.4 | 0.05 |
| Central subfield EZ-RPE point temporal thickness (Longitudinal change; 0.5 mm from fovea; µm) | −0.8 ± 8.9 | −0.2 ± 5.7 | 0.68 |
| Parafoveal EZ-RPE thickness (Longitudinal change; µm) | −1.58 ± 3.05 | 0.00 ± 2.95 | 0.006 |
| Parafoveal EZ-RPE volume (Longitudinal change; mm3) | −0.005 ± 0.010 | 0.000 ± 0.009 | 0.004 |
| Parafoveal EZ-RPE point nasal thickness (Longitudinal change; 1 mm from fovea; µm) | −2.9 ± 8.8 | −0.3 ± 5.5 | 0.10 |
| Parafoveal EZ-RPE point temporal thickness (Longitudinal change; 1 mm from fovea; µm) | −0.81 ± 8.07 | 0.10 ± 5.91 | 0.53 |
| EZ-RPE volume (Longitudinal change; mm3) | −0.05 ± 0.09 | 0.01 ± 0.08 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matar, K.; Talcott, K.E.; Ugwuegbu, O.; Hu, M.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Longitudinal Ellipsoid Zone Dynamics During Hydroxychloroquine Use. J. Pers. Med. 2025, 15, 416. https://doi.org/10.3390/jpm15090416
Matar K, Talcott KE, Ugwuegbu O, Hu M, Srivastava SK, Reese JL, Ehlers JP. Longitudinal Ellipsoid Zone Dynamics During Hydroxychloroquine Use. Journal of Personalized Medicine. 2025; 15(9):416. https://doi.org/10.3390/jpm15090416
Chicago/Turabian StyleMatar, Karen, Katherine E. Talcott, Obinna Ugwuegbu, Ming Hu, Sunil K. Srivastava, Jamie L. Reese, and Justis P. Ehlers. 2025. "Longitudinal Ellipsoid Zone Dynamics During Hydroxychloroquine Use" Journal of Personalized Medicine 15, no. 9: 416. https://doi.org/10.3390/jpm15090416
APA StyleMatar, K., Talcott, K. E., Ugwuegbu, O., Hu, M., Srivastava, S. K., Reese, J. L., & Ehlers, J. P. (2025). Longitudinal Ellipsoid Zone Dynamics During Hydroxychloroquine Use. Journal of Personalized Medicine, 15(9), 416. https://doi.org/10.3390/jpm15090416









