Geometric Aortic Remodeling and Stent-Graft Migration After TEVAR: Insights from Longitudinal 3D Analysis and Literature Review
Abstract
1. Introduction
2. Longitudinal Geometric Assessment and Clinical Illustration
2.1. Clinical Context and Presentation
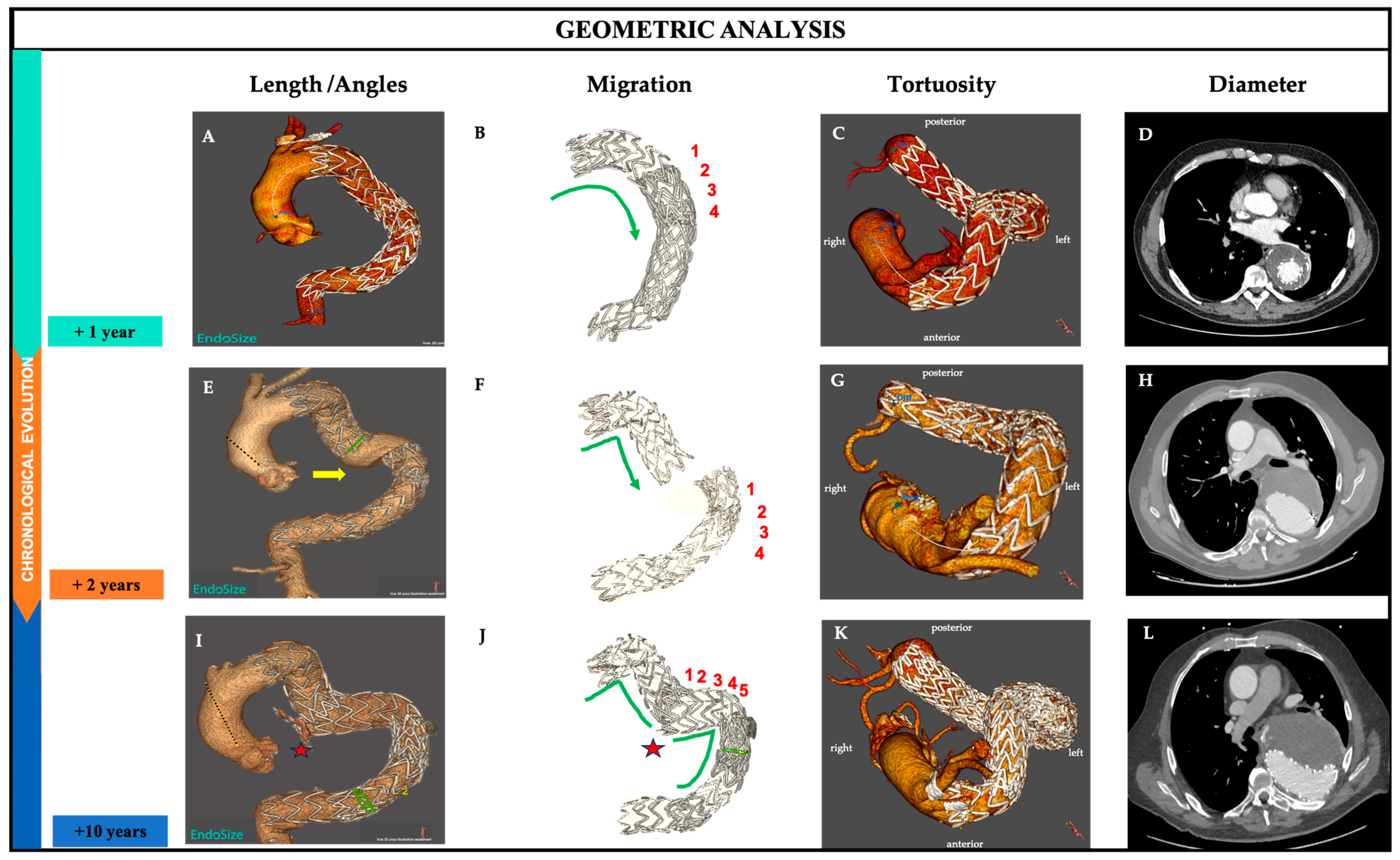
2.2. Definitions
2.3. Image Analysis
2.4. Results
- a.
- Length along the centerline
- b.
- Migration
- c.
- Angles
- d.
- Tortuosity
- e.
- Diameter
3. Discussion and Literature Review
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beach, J.M.; Kuramochi, Y.; Brier, C.; Roselli, E.E.; Eagleton, M.J. Durable Outcomes of Thoracic Endovascular Aortic Repair with Zenith TX1 and TX2 Devices. J. Vasc. Surg. 2017, 65, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, J.J. Endoleak Management and Postoperative Surveillance Following Endovascular Repair of Thoracic Aortic Aneurysms. J. Vasc. Surg. 2010, 52, 91S–99S. [Google Scholar] [CrossRef] [PubMed]
- De Masi, M. Thoracic Aorta Remodeling after TEVAR: Monitoring Morphological Parameters to Predict Unfavorable Evolution. J. Surg. 2023, 8, 1840. [Google Scholar] [CrossRef]
- Grassi, V.; Trimarchi, S.; Weaver, F.; De Beaufort, H.W.L.; Azzizzadeh, A.; Upchurch, G.R., Jr.; Piffaretti, G.; Lomazzi, C.; the GREAT participants. Endovascular Repair of Descending Thoracic Aortic Aneurysms—A Mid-Term Report from the Global Registry for Endovascular Aortic Treatment (GREAT). Eur. J. Cardio-Thorac. Surg. 2022, 61, 357–364. [Google Scholar] [CrossRef] [PubMed]
- De León Ayala, I.A.; Cheng, Y.-T.; Chen, S.-W.; Chu, S.-Y.; Nan, Y.-Y.; Liu, K.-S. Outcomes of Type Ia Endoleaks after Endovascular Repair of the Proximal Aorta. J. Thorac. Cardiovasc. Surg. 2022, 163, 2012–2021.e6. [Google Scholar] [CrossRef]
- Nomura, Y.; Kawasaki, R.; Koide, Y.; Okada, T.; Yasumori, K.; Sakamoto, T.; Tanaka, H.; Murakami, H. Aortic Remodeling After Thoracic Endovascular Aortic Repair for Nonacute Uncomplicated Type B Aortic Dissection. Ann. Vasc. Surg. 2024, 99, 209–216. [Google Scholar] [CrossRef]
- Suh, G.-Y.K.; Bondesson, J.; Zhu, Y.D.; Nilson, M.C.; Roselli, E.E.; Cheng, C.P. Ascending Aortic Endograft and Thoracic Aortic Deformation After Ascending Thoracic Endovascular Aortic Repair. J. Endovasc. Ther. 2025, 32, 7–17. [Google Scholar] [CrossRef]
- Kaladji, A.; Lucas, A.; Kervio, G.; Haigron, P.; Cardon, A. Sizing for Endovascular Aneurysm Repair: Clinical Evaluation of a New Automated Three-Dimensional Software. Ann. Vasc. Surg. 2010, 24, 912–920. [Google Scholar] [CrossRef]
- Fillinger, M.F.; Greenberg, R.K.; McKinsey, J.F.; Chaikof, E.L.; Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards. Reporting Standards for Thoracic Endovascular Aortic Repair (TEVAR). J. Vasc. Surg. 2010, 52, 1022–1033.e5. [Google Scholar] [CrossRef]
- Chen, C.-K.; Chou, H.-P.; Chang, Y.-Y.; Shih, C.-C. Elongation of the Aorta after Thoracic Endovascular Aortic Repair: A Longitudinal Study. Int. J. Environ. Res. Public Health 2020, 17, 1205. [Google Scholar] [CrossRef]
- Jordan, W.D.; Desai, N.; Letter, A.J.; Matsumura, J.S. Long-Term Outcomes of the Conformable TAG Thoracic Endoprosthesis in a Prospective Multicenter Trial. J. Vasc. Surg. 2021, 74, 1491–1498. [Google Scholar] [CrossRef]
- Kasirajan, K.; Morasch, M.D.; Makaroun, M.S. Sex-Based Outcomes after Endovascular Repair of Thoracic Aortic Aneurysms. J. Vasc. Surg. 2011, 54, 669–676. [Google Scholar] [CrossRef]
- Piffaretti, G.; Negri, S.; Ferraro, S.; Bossi, M.; Rivolta, N.; Fontana, F.; Castelli, P. Delayed Graft Dislocation after Thoracic Aortic Endovascular Repair. Kathmandu Univ. Med. J. 2014, 12, 97–100. [Google Scholar] [CrossRef][Green Version]
- Foley, P.J.; Criado, F.J.; Farber, M.A.; Kwolek, C.J.; Mehta, M.; White, R.A.; Lee, W.A.; Tuchek, J.M.; Fairman, R.M. VALOR Investigators Results with the Talent Thoracic Stent Graft in the VALOR Trial. J. Vasc. Surg. 2012, 56, 1214–1221.e1. [Google Scholar] [CrossRef]
- Geisbüsch, P.; Skrypnik, D.; Ante, M.; Trojan, M.; Bruckner, T.; Rengier, F.; Böckler, D. Endograft Migration after Thoracic Endovascular Aortic Repair. J. Vasc. Surg. 2019, 69, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Mell, M.W. Outcome Comparison of Thoracic Endovascular Aortic Repair Performed Outside versus inside Proximal Landing Zone Length Recommendation. J. Vasc. Surg. 2020, 72, 1883–1890. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Melissano, G.; Cambria, R.P.; Dake, M.D.; Mehta, S.; Svensson, L.G.; Moore, R.D.; Zenith TX2 Clinical Trial Investigators. Five-Year Results of Thoracic Endovascular Aortic Repair with the Zenith TX2. J. Vasc. Surg. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Skrypnik, D.; Bischoff, M.S.; Meisenbacher, K.; Kronsteiner, D.B.; Böckler, D. A 10-Year Single-Center Experience with the GORE TAG Conformable Thoracic Stent Graft in the Treatment of Thoracic Aortic Disease. J. Endovasc. Ther. 2022, 29, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Torsello, G.F.; Argyriou, A.; Stavroulakis, K.; Bosiers, M.J.; Austermann, M.; Torsello, G.B.; SURPASS Registry Collaborators. One-Year Results from the SURPASS Observational Registry of the CTAG Stent-Graft with the Active Control System. J. Endovasc. Ther. 2020, 27, 421–427. [Google Scholar] [CrossRef]
- Fairman, A.S.; Beck, A.W.; Malas, M.B.; Goodney, P.P.; Osborne, N.H.; Schermerhorn, M.L.; Wang, G.J. Reinterventions in the Modern Era of Thoracic Endovascular Aortic Repair. J. Vasc. Surg. 2020, 71, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.T.; Mitchell, R.S.; Makaroun, M.S.; Whiting, A.J.; Cardeira, K.R.; Matsumura, J.S. Aortic Neck Morphology after Endovascular Repair of Descending Thoracic Aortic Aneurysms. J. Vasc. Surg. 2006, 43, 26–31. [Google Scholar] [CrossRef]
- Morales, J.P.; Greenberg, R.K.; Morales, C.A.; Cury, M.; Hernandez, A.V.; Lyden, S.P.; Clair, D. Thoracic Aortic Lesions Treated with the Zenith TX1 and TX2 Thoracic Devices: Intermediate- and Long-Term Outcomes. J. Vasc. Surg. 2008, 48, 54–63. [Google Scholar] [CrossRef][Green Version]
- Liffman, K.; Šutalo, I.D.; Lawrence-Brown, M.M.D.; Semmens, J.B.; Aldham, B. Movement and Dislocation of Modular Stent-Grafts Due to Pulsatile Flow and the Pressure Difference Between the Stent-Graft and the Aneurysm Sac. J. Endovasc. Ther. 2006, 13, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, J.W.; Moll, F.L.; Tolenaar, J.L.; Verhagen, H.J.M.; Van Herwaarden, J.A. Validation of a New Standardized Method to Measure Proximal Aneurysm Neck Angulation. J. Vasc. Surg. 2010, 51, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O.; Karthikesalingam, A.; Holt, P.J.; Thompson, M.M.; Sayers, R.; Bown, M.J.; Choke, E.C.; Ma, X. Using Multiple Classifiers for Predicting the Risk of Endovascular Aortic Aneurysm Repair Re-Intervention through Hybrid Feature Selection. Proc. Inst. Mech. Eng. H 2017, 231, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Domanin, M.; Bissacco, D.; Romarowsky, R.M.; Conti, M.; Auricchio, F.; Ferraresi, M.; Trimarchi, S. Drag Forces after Thoracic Endovascular Aortic Repair. General Review of the Literature. Ann. Vasc. Surg. 2021, 75, 479–488. [Google Scholar] [CrossRef]
- Raffort, J.; Adam, C.; Carrier, M.; Ballaith, A.; Coscas, R.; Jean-Baptiste, E.; Hassen-Khodja, R.; Chakfé, N.; Lareyre, F. Artificial Intelligence in Abdominal Aortic Aneurysm. J. Vasc. Surg. 2020, 72, 321–333.e1. [Google Scholar] [CrossRef]

| Geometrical Parameters | +1 Year | +2 Years | +10 Years |
|---|---|---|---|
| Thoracic Aorta (TA) | |||
| Length TA (mm) | 388.0 | 435.0 | 503.0 |
| Angles TA (°) | 154.73 | 159.14 | 159.94 |
| Tortuosity TA | 2.74 | 3.29 | 3.83 |
| Stent Graft (SG) | |||
| Length SG (mm) | 208.0 | 238.0 | 295.0 |
| Tortuosity SG | 1.31 | 1.56 | 1.84 |
| Angles SG (°) | 111.23 | 102.22 | 114.4 |
| Migration (n stents) | 0 stents | 4 stents | 5 stents |
| Aneurysm Diameter (mm) | 81.6 | 74.9 | 100.5 |
| Study | Migration Rate, % | Follow-Up, y | Time of Migration, m | Reintervention Rate % n Reintervention/n Migration | Morbidity Rate, % n Morbidity/n Migration | Mortality Rate, % n Deaths/n Migration |
|---|---|---|---|---|---|---|
| Beach et al. [1] | 11 (22/200) | 0–9.3 | 29 | 40.9 (9/23) | 13.6 (3/22) | 4.5 (1/22) |
| Jordan et al. [11] | 1 (6/66) | 1–5 | 2, 24, 36, 60 | 0 | 16.7 (1/6) | 0 |
| Kasirajan et al. [12] | 0.23 (1/421) | 0–1 | 12 | ND | ND | ND |
| Piffaretti et al. [13] | 1.7 (2/117) | 0–12 | 12 | 16.7 (2/12) | 16.7 (2/12) | 0 |
| Foley et al. [14] | 3.1 (6/195) | 0–5 | 12, 24, 36, 48, 60 | 16.7 (1/6) | 16.7 (1/6) | 0 |
| Geisbüsch et al. [15] | 7.3 (9/123) | 0.5–10 | 8, 11, 14, 22, 30, 32, 43, 49, 64 | 55.5 (5/9) | 44.4 (4/9) | 11.1 (1/9) |
| Yoon and Mell [16] | 1.6 (1/63) | 4.8 | 14 | 100 (1/1) | 100 (1/1) | 0 |
| Matsumura et al. [17] | 7.6 (12/158) | 0–5 | 3.2 ± 1.7 years | 16.7 (2/12) | 16.7 (2/12) | 0 |
| Skrypnik et al. [18] | 2.6 (5/194) | 0.5–10 | 12, 19, 31, 43, 64 | 80 (4/5) | 100 (5/5) | 40 (2/5) |
| Torsello et al. [19] | 0.8 (1/127) | 0–1 | 12 | 100 (1/1) | 100 (1/1) | 0 |
| Fairman et al. [20] | 2.9 (3/105) | 0–1 | 12, 24, 60 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Masi, M.; Guivier-Curien, C.; Gaudry, M.; Jacquier, A.; Piquet, P.; Deplano, V. Geometric Aortic Remodeling and Stent-Graft Migration After TEVAR: Insights from Longitudinal 3D Analysis and Literature Review. J. Pers. Med. 2025, 15, 393. https://doi.org/10.3390/jpm15080393
De Masi M, Guivier-Curien C, Gaudry M, Jacquier A, Piquet P, Deplano V. Geometric Aortic Remodeling and Stent-Graft Migration After TEVAR: Insights from Longitudinal 3D Analysis and Literature Review. Journal of Personalized Medicine. 2025; 15(8):393. https://doi.org/10.3390/jpm15080393
Chicago/Turabian StyleDe Masi, Mariangela, Carine Guivier-Curien, Marine Gaudry, Alexis Jacquier, Philippe Piquet, and Valérie Deplano. 2025. "Geometric Aortic Remodeling and Stent-Graft Migration After TEVAR: Insights from Longitudinal 3D Analysis and Literature Review" Journal of Personalized Medicine 15, no. 8: 393. https://doi.org/10.3390/jpm15080393
APA StyleDe Masi, M., Guivier-Curien, C., Gaudry, M., Jacquier, A., Piquet, P., & Deplano, V. (2025). Geometric Aortic Remodeling and Stent-Graft Migration After TEVAR: Insights from Longitudinal 3D Analysis and Literature Review. Journal of Personalized Medicine, 15(8), 393. https://doi.org/10.3390/jpm15080393







