Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Endpoints
- Diagnosis of neovascular AMD;
- Age ≥ 60 years;
- Previous anti-VEGF treatment with at least one of the following agents:
- aflibercept, bevacizumab, ranibizumab;
- Poor response to anti-VEGF treatment, as defined above.
- The exclusion criteria were:
- Treatment-naïve patients;
- Patients who received fewer than 3 faricimab injections;
- Patients with other concomitant retinal/macular pathologies that could affect the results;
- Participation in a pivotal faricimab study.
2.2. Treatment Protocol
2.3. Statistical Analysis
3. Results
3.1. Cohort Description
3.2. Functional Outcome
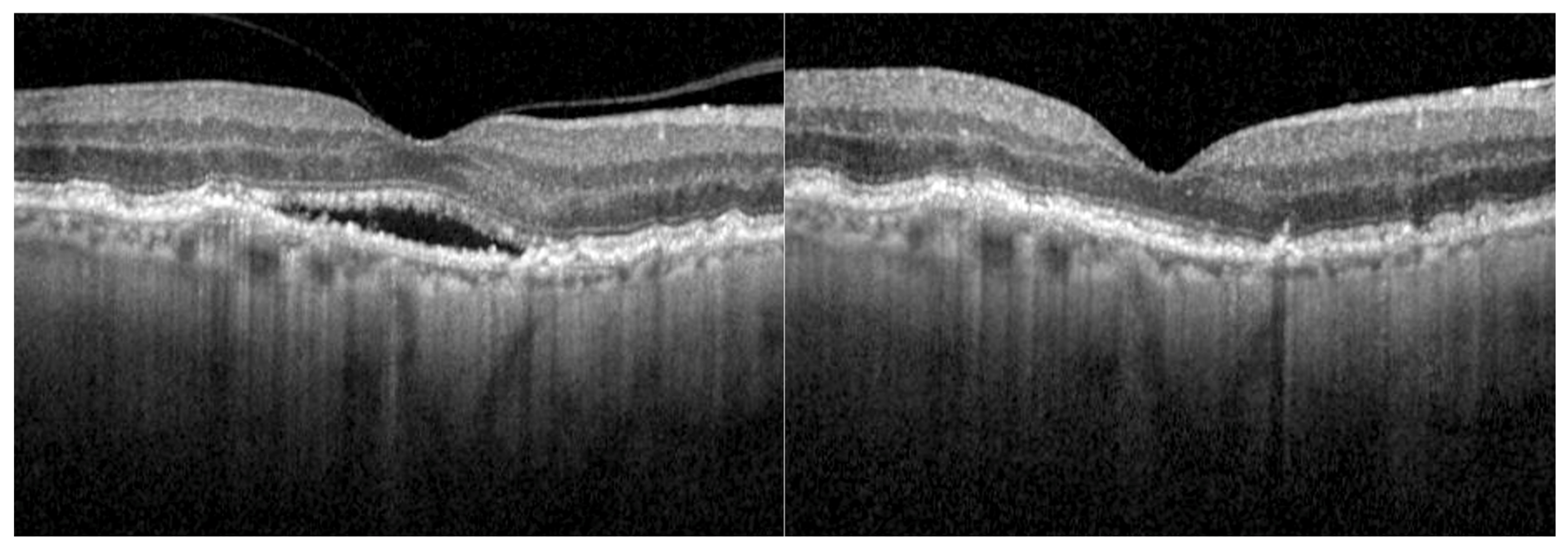
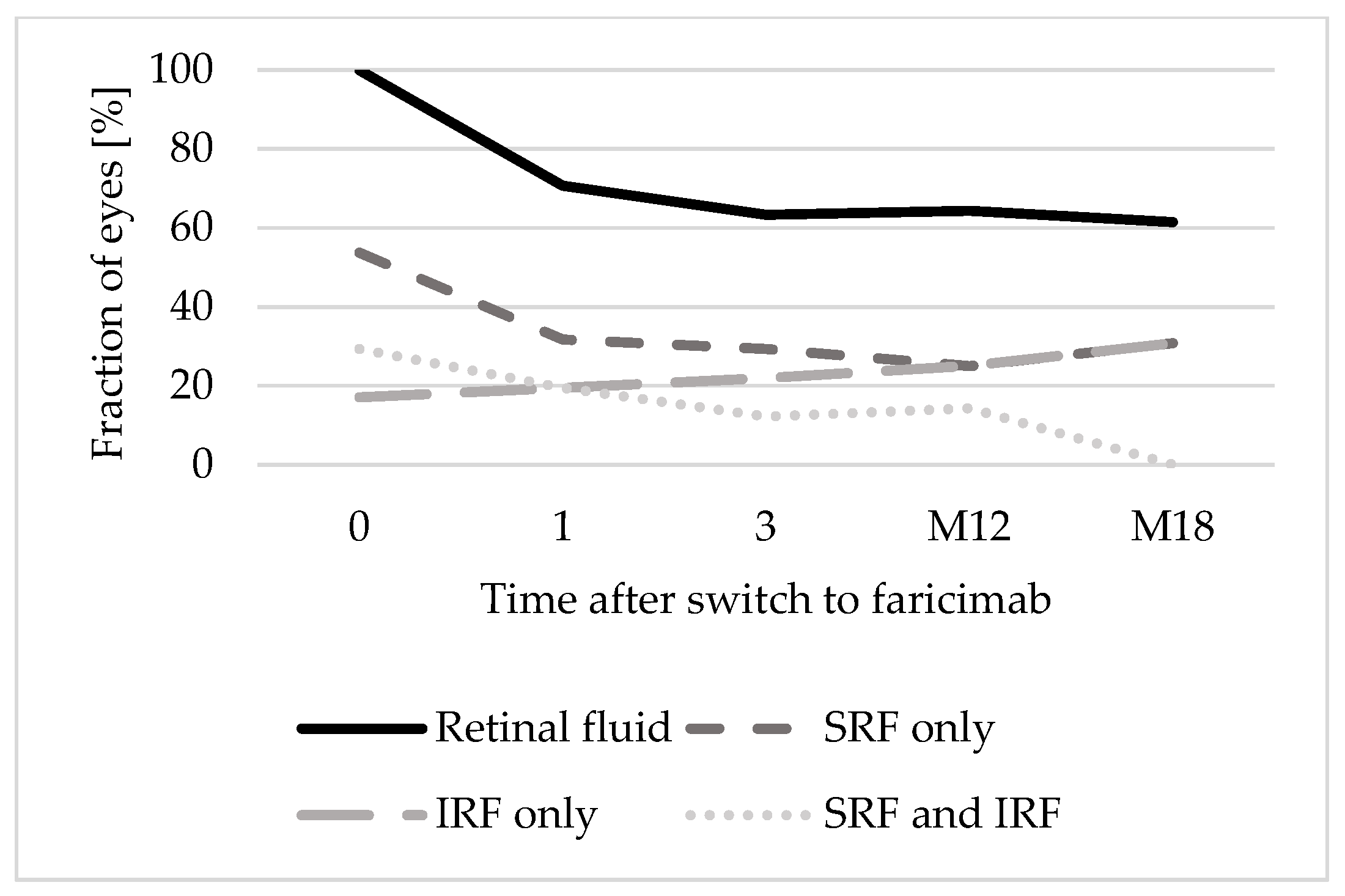
3.3. Morphological Outcome
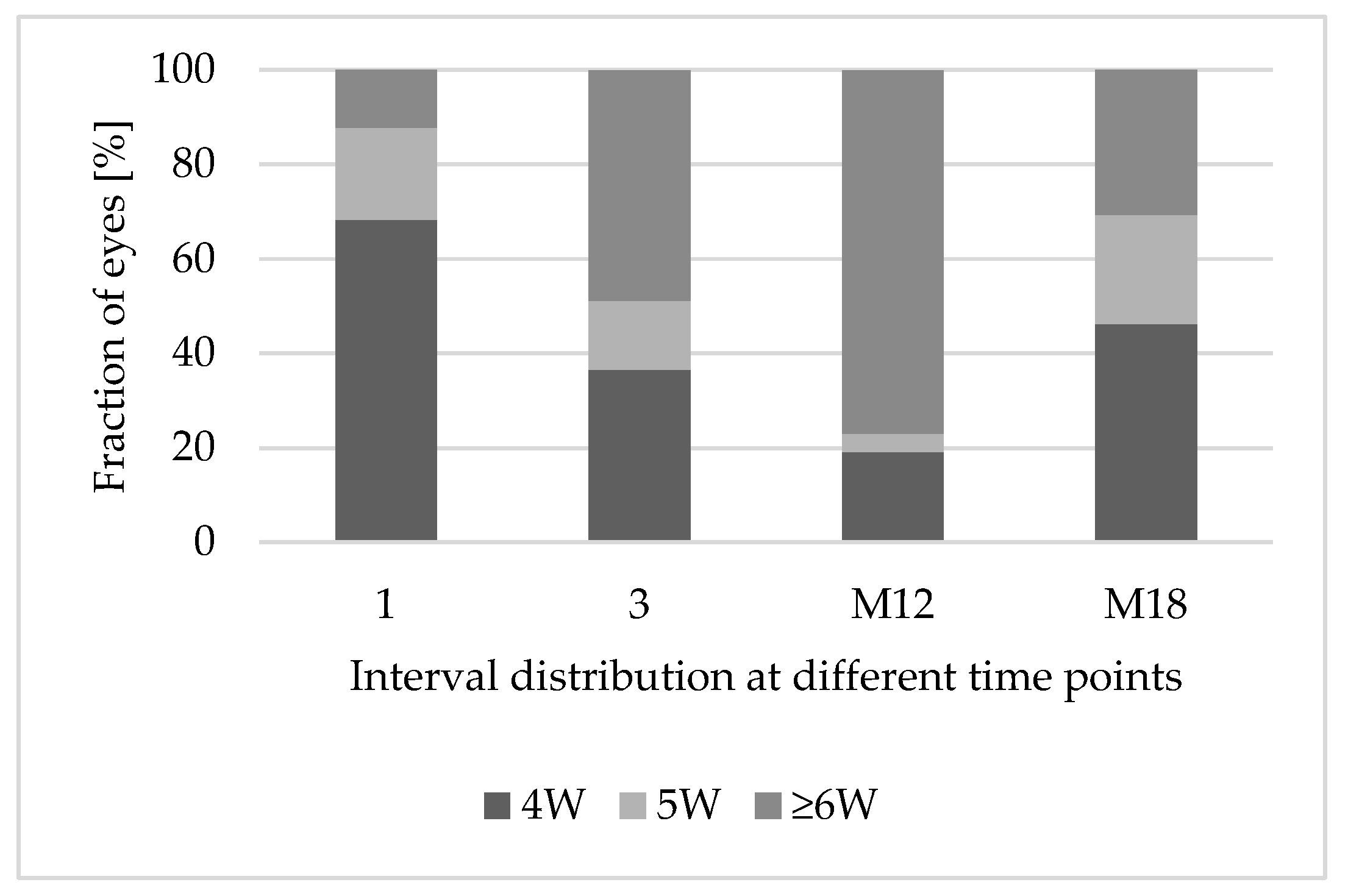
3.4. Treatment Interval Extension
3.5. Prognostic Factors
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (n)AMD | (neovascular) age-related macular degeneration |
| BCVA | best-corrected visual acuity |
| BrM | Bruch’s membrane |
| cRORA | complete RPE and outer retinal atrophy |
| CRT | central retinal thickness |
| ILM | internal limiting membrane |
| IQR | interquartile range |
| IRF | intraretinal fluid |
| ORT | outer retinal tubulations |
| RPE | retinal pigment epithelium |
| SD | standard deviation |
| SD-OCT | spectral domain optical coherence tomography |
| SRF | subretinal fluid |
| T&E | treat and extend regimen |
| VEGF | vascular endothelial growth factor |
References
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Munoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P.; Eye Diseases Prevalence Research, G. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.C.; Wolfs, R.C.; Vingerling, J.R.; Hofman, A.; de Jong, P.T. Age-specific prevalence and causes of blindness and visual impairment in an older population: The Rotterdam Study. Arch. Ophthalmol. 1998, 116, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, J.; Chai, R.; Yuan, S.; Hao, Y. Global burden of low vision and blindness due to age-related macular degeneration from 1990 to 2021 and projections for 2050. BMC Public Health 2024, 24, 3510. [Google Scholar] [CrossRef]
- Belkin, M.; Kalter-Leibovici, O.; Chetrit, A.; Skaat, A. Time trends in the incidence and causes of blindness in Israel. Am. J. Ophthalmol. 2013, 155, 404. [Google Scholar] [CrossRef]
- Bloch, S.B.; Larsen, M.; Munch, I.C. Incidence of legal blindness from age-related macular degeneration in denmark: Year 2000 to 2010. Am. J. Ophthalmol. 2012, 153, 209–213.e2. [Google Scholar] [CrossRef]
- Campbell, J.P.; Bressler, S.B.; Bressler, N.M. Impact of availability of anti-vascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch. Ophthalmol. 2012, 130, 794–795. [Google Scholar] [CrossRef]
- Almony, A.; Keyloun, K.R.; Shah-Manek, B.; Multani, J.K.; McGuiness, C.B.; Chen, C.C.; Campbell, J.H. Clinical and economic burden of neovascular age-related macular degeneration by disease status: A US claims-based analysis. J. Manag. Care Spec. Pharm. 2021, 27, 1260–1272. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Sim, S.Y.; Chalkiadaki, E.; Koutsocheras, G.; Nicholson, L.; Sivaprasad, S.; Patel, P.J.; Selvam, S.; Pal, B.; Keane, P.A.; Bhatia, B.; et al. Real-World 1-Year Outcomes of Treatment-Intensive Neovascular Age-Related Macular Degeneration Switched to Faricimab. Ophthalmol. Retin. 2024, 9, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rush, R.B. One-Year Outcomes of Faricimab Treatment for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2023, 17, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Cancian, G.; Paris, A.; Agliati, L.; Rizzato, A.; Clerici, M.; Volpe, G.; Menghini, M.; Grimaldi, G. One-Year Real-World Outcomes of Intravitreal Faricimab for Previously Treated Neovascular Age-Related Macular Degeneration. Ophthalmol. Ther. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.L.; Al-Humaid, S.; Rampakakis, E.; Galic, I.J.; Chen, J.C. Correlation of Visual Acuity with Fibrotic Scar Location in Treated Neovascular Age-Related Macular Degeneration Eyes. Retina 2016, 36, 1324–1330. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Zweifel, S.A.; Engelbert, M.; Laud, K.; Margolis, R.; Spaide, R.F.; Freund, K.B. Outer retinal tubulation: A novel optical coherence tomography finding. Arch. Ophthalmol. 2009, 127, 1596–1602. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group, S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Finger, R.P.; Wiedemann, P.; Blumhagen, F.; Pohl, K.; Holz, F.G. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study—A noninterventional study of ranibizumab treatment for neovascular age-related macular degeneration in Germany. Acta Ophthalmol. 2013, 91, 540–546. [Google Scholar] [CrossRef]
- Chaudhary, V.; Mar, F.; Amador, M.J.; Chang, A.; Gibson, K.; Joussen, A.M.; Kim, J.E.; Lee, J.; Margaron, P.; Saffar, I.; et al. Emerging clinical evidence of a dual role for Ang-2 and VEGF-A blockade with faricimab in retinal diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2024. [Google Scholar] [CrossRef]
- Chia, K.J.W.; Gunasekeran, D.V.; Laude, A. The Impact of Switching Anti-Vascular Endothelial Growth Factor Therapy in the Management of Exudative Age-Related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 859–869. [Google Scholar] [CrossRef]
- Schaal, S.; Kaplan, H.J.; Tezel, T.H. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 2008, 115, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Zuber-Laskawiec, K.; Kubicka-Trzaska, A.; Karska-Basta, I.; Pociej-Marciak, W.; Romanowska-Dixon, B. Non-responsiveness and tachyphylaxis to anti-vascular endothelial growth factor treatment in naive patients with exudative age-related macular degeneration. J. Physiol. Pharmacol. 2019, 70, 779–785. [Google Scholar] [CrossRef]
- Raimondi, R.; Falfeli, T.; Bogdanova-Bennet, A.; Varma, D.; Habib, M.; Kotagiri, A.; Steel, D.H.; Grinton, M. Outcomes of Treatment-Resistant Neovascular Age-Related Macular Degeneration Switched from Aflibercept to Faricimab. Ophthalmol. Retina 2023, 8, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.H.; Oh, D.J.; Alderson, S.E.; Bracy, J.; McLeod, M.; Perez, L.I.; Bottini, A.; Chin Yee, D.; Mukkamala, K. Initial Real-World Experience with Faricimab in Treatment-Resistant Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2023, 17, 1287–1293. [Google Scholar] [CrossRef]
- Aljundi, W.; Munteanu, C.; Seitz, B.; Abdin, A.D. Short-term outcomes of intravitreal faricimab for refractory neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2867–2874. [Google Scholar] [CrossRef]
- Wolfrum, P.; Bohm, E.W.; Lorenz, K.; Stoffelns, B.; Pfeiffer, N.; Korb, C.A. Clinical Outcomes Following a Switch of Therapy to Faricimab in Patients Affected by Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2025, 14, 423. [Google Scholar] [CrossRef]
- Goodchild, C.; Bailey, C.; Soto Hernaez, J.; Ahmed, E.; Salvatore, S. Real world efficacy and durability of faricimab in patients with neovascular AMD (nAMD) who had sub-optimal response to prior anti-VEGF therapy. Eye 2024, 38, 3059–3064. [Google Scholar] [CrossRef]
- Borchert, G.A.; Kiire, C.A.; Stone, N.M.; Akil, H.; Gkika, T.; Fischer, M.D.; Xue, K.; Cehajic-Kapetanovic, J.; MacLaren, R.E.; Charbel Issa, P.; et al. Real-world six-month outcomes in patients switched to faricimab following partial response to anti-VEGF therapy for neovascular age-related macular degeneration and diabetic macular oedema. Eye 2024, 38, 3569–3577. [Google Scholar] [CrossRef]
- Schneider, M.; Bjerager, J.; Hodzic-Hadzibegovic, D.; Klefter, O.N.; Subhi, Y.; Hajari, J. Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2153–2162. [Google Scholar] [CrossRef]
- Cheng, A.M.; Joshi, S.; Banoub, R.G.; Saddemi, J.; Chalam, K.V. Faricimab Effectively Resolves Intraretinal Fluid and Preserves Vision in Refractory, Recalcitrant, and Nonresponsive Neovascular Age-Related Macular Degeneration. Cureus 2023, 15, e40100. [Google Scholar] [CrossRef]
- Bindewald-Wittich, A.; Alkabouni, M.W.; Wolf, A. Optical coherence tomography biomarkers for neovascular age-related macular degeneration: Relevance for the diagnosis, treatment and prognosis. Ophthalmologie 2025, 122, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.J.; Markey, C.M.; Kurstjens, N.P.; Guymer, R.H. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration—A phase IV randomised clinical trial with ranibizumab: The FLUID study. BMC Ophthalmol. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.M.; Philip, A.M.; Leitner, R.; Simader, C.; Langs, G.; Gerendas, B.S.; Schmidt-Erfurth, U. Correlation of 3-Dimensionally Quantified Intraretinal and Subretinal Fluid with Visual Acuity in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Miki, A.; Kamimura, A.; Okuda, M.; Matsumiya, W.; Imai, H.; Kusuhara, S.; Nakamura, M. Short-Term Outcomes of Faricimab Treatment in Aflibercept-Refractory Eyes with Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2023, 12, 5145. [Google Scholar] [CrossRef]
- Szigiato, A.; Mohan, N.; Talcott, K.E.; Mammo, D.A.; Babiuch, A.S.; Kaiser, P.K.; Ehlers, J.P.; Rachitskaya, A.; Yuan, A.; Srivastava, S.K.; et al. Short-Term Outcomes of Faricimab in Patients with Neovascular Age-Related Macular Degeneration on Prior Anti-VEGF Therapy. Ophthalmol. Retina 2024, 8, 10–17. [Google Scholar] [CrossRef]
- Ashraf, M.; Banaee, T.; Silva, F.Q.; Singh, R.P. Switching Anti-Vascular Endothelial Growth Factors in Refractory Neovascular Age-Related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retina 2018, 49, 166–170. [Google Scholar] [CrossRef]
- Khanani, A.M.; Kotecha, A.; Chang, A.; Chen, S.J.; Chen, Y.; Guymer, R.; Heier, J.S.; Holz, F.G.; Iida, T.; Ives, J.A.; et al. TENAYA and LUCERNE: Two-Year Results from the Phase 3 Neovascular Age-Related Macular Degeneration Trials of Faricimab with Treat-and-Extend Dosing in Year 2. Ophthalmology 2024, 131, 914–926. [Google Scholar] [CrossRef]
- Li, Y.; Chong, R.; Fung, A.T. Association of Occlusive Retinal Vasculitis with Intravitreal Faricimab. JAMA Ophthalmol. 2024, 142, 489–491. [Google Scholar] [CrossRef]
- Thangamathesvaran, L.; Kong, J.; Bressler, S.B.; Singh, M.; Wenick, A.S.; Scott, A.W.; Arevalo, J.F.; Bressler, N.M. Severe Intraocular Inflammation Following Intravitreal Faricimab. JAMA Ophthalmol. 2024, 142, 365–370. [Google Scholar] [CrossRef]
- Metrangolo, C.; Donati, S.; Mazzola, M.; Fontanel, L.; Messina, W.; D’Alterio, G.; Rubino, M.; Radice, P.; Premi, E.; Azzolini, C. OCT Biomarkers in Neovascular Age-Related Macular Degeneration: A Narrative Review. J. Ophthalmol. 2021, 2021, 9994098. [Google Scholar] [CrossRef]
- Han, J.M.; Han, J.; Ko, J.; Jung, J.; Park, J.I.; Hwang, J.S.; Yoon, J.; Jung, J.H.; Hwang, D.D. Anti-VEGF treatment outcome prediction based on optical coherence tomography images in neovascular age-related macular degeneration using a deep neural network. Sci. Rep. 2024, 14, 28253. [Google Scholar] [CrossRef]

| Demographic and Baseline Characteristic | |
|---|---|
| Number of eyes | 41 |
| Number of patients | 39 |
| Age in years (mean ± SD) | 80.5 ± 8.1 |
| Gender (male/female) | 21/18 |
| Laterality (left/right) | 21/20 |
| Lens status (pseudophakic/phakic) | 32/9 |
| BCVA (logMAR) (median; IQR) | 0.4 (0.3–0.6) |
| Injections before switch (median; IQR) | 53.0 (32.0–73.0) |
| Last substance administered Aflibercept/Bevacizumab/Ranibizumab | 13/13/15 |
| Total of different substances administered before switch 1/2/3 | 6/10/25 |
| OCT Characteristic | N (%) |
|---|---|
| Reticular drusen | 4 (9.8) |
| Retinal fluid | 41 (100) |
| SRF and IRF | 12 (29.3) |
| SRF only | 22 (53.7) |
| IRF only | 7 (17.1) |
| ORTs | 5 (12.2) |
| Subfoveal disciform scar | 24 (58.5) |
| Sub-RPE | 21 |
| Subretinal | 0 |
| Mixed sub-RPE and subretinal | 3 |
| cRORA foveal/extrafoveal | 9/15 (22.0/36.6) |
| Subretinal hemorrhage | 0 |
| Central retinal thickness (µm) (median; IQR) | 365.0 (292.5–453) |
| SRF/IRF M12 (N = 18) | No SRF/IRF M12 (N = 10) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 81.22 ± 8.03 | 79.6 ± 7.52 | 0.605 |
| Sex (female) | 8 | 6 | 0.622 |
| BCVA (logMAR) (median; IQR) | 0.3 (0.2–0.53) | 0.45 (0.3–0.5) | 0.555 |
| Years until switch (median; IQR) | 6.5 (2–7.25) | 6.5 (2.75–8.0) | 0.944 |
| Injections until switch (median; IQR) | 64.5 (30.25–81.0) | 51.5 (31.5–61.5) | 0.245 |
| CRT at baseline (µm) (median; IQR) | 396.25 (320.5–585.25) | 333.5 (276.5–400.38) | 0.051 |
| Presence of reticular drusen at baseline | 2 | 2 | 0.520 |
| Presence of SRF at baseline | 14 | 9 | 0.418 |
| Presence of IRF at baseline | 10 | 3 | 0.194 |
| Presence of ORTs at baseline | 2 | 1 | 0.927 |
| Presence of fibrosis at baseline | 13 | 4 | 0.094 |
| Presence of foveal cRORA at baseline | 2 | 3 | 0.211 |
| Presence of extrafoveal cRORA at baseline | 6 | 3 | 0.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löw, K.; Sitnilska, V.; Tang, Y.; Lammert, J.Q.; Krohne, T.U.; Altay, L. Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration. J. Pers. Med. 2025, 15, 189. https://doi.org/10.3390/jpm15050189
Löw K, Sitnilska V, Tang Y, Lammert JQ, Krohne TU, Altay L. Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration. Journal of Personalized Medicine. 2025; 15(5):189. https://doi.org/10.3390/jpm15050189
Chicago/Turabian StyleLöw, Katrin, Vasilena Sitnilska, Yuhe Tang, Jeany Q. Lammert, Tim U. Krohne, and Lebriz Altay. 2025. "Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration" Journal of Personalized Medicine 15, no. 5: 189. https://doi.org/10.3390/jpm15050189
APA StyleLöw, K., Sitnilska, V., Tang, Y., Lammert, J. Q., Krohne, T. U., & Altay, L. (2025). Real-Life Treatment Intervals and Morphological Outcomes Following the Switch to Faricimab Therapy in Neovascular Age-Related Macular Degeneration. Journal of Personalized Medicine, 15(5), 189. https://doi.org/10.3390/jpm15050189






