Abstract
Parkinson’s disease involves widespread neurodegeneration that extends far beyond the basal ganglia, giving rise to a diverse range of non-motor symptoms that frequently emerge before motor onset. These include autonomic dysfunction, cognitive decline, neuropsychiatric disturbances, sleep-related disorders, and sensory deficits. Here, we synthesize current evidence on the anatomical, neurochemical, and network-level mechanisms that drive these symptoms, and we examine how they shape disease progression and clinical heterogeneity. We highlight the limitations of dopamine-centric models and advocate for a framework that treats non-motor symptoms as the disorder’s primary, mechanistically distinct features. We also discuss how emerging technologies—such as multi-omic profiling, artificial intelligence, and network neuroscience—enable earlier identification, stratification of non-motor phenotypes, and the development of precision-based therapeutic strategies. Recognizing non-motor symptoms as central to Parkinson’s disease redefines how the disorder should be diagnosed, studied, and treated.
1. Introduction
Parkinson’s disease (PD) has long been defined by its cardinal motor features—tremor, rigidity, and bradykinesia. However, a growing body of evidence shows that non-motor symptoms often precede motor onset by years and contribute more significantly to long-term disability and reduced quality of life. These symptoms—including sensory alterations, autonomic dysfunction, sleep-related disorders, neuropsychiatric features, and cognitive decline—are not ancillary, but integral to PD pathophysiology. Their emergence correlates with dysfunction across multiple neurotransmitter systems, including serotonergic, noradrenergic, and cholinergic networks, extending the scope of PD beyond dopamine depletion in the basal ganglia [1,2,3,4,5,6].
Neuropathological studies have traced the origins of PD-related neurodegeneration to extranigral regions. Lewy bodies, first described in 1912, and later models by Braak and colleagues positioned α-synuclein pathology in the olfactory bulb, the dorsal motor nucleus of the vagus, and autonomic ganglia as early events in disease progression [2,3,4,5,6,7,8]. These observations help explain why hyposmia, REM sleep behavior disorder, and autonomic failure often appear long before motor symptoms. However, not all patients follow Braak’s proposed caudorostral staging. Moreover, α-synuclein burden does not always correlate with neurodegeneration or clinical severity, suggesting that other mechanisms—such as mitochondrial dysfunction, neuroinflammation, and gut–brain axis disruption—also drive disease progression [9,10].
Recognition of the primacy of non-motor symptoms in PD has prompted a reexamination of traditional clinical frameworks. While previous reviews have examined specific symptom domains, few have offered an integrated synthesis grounded in anatomical, neurochemical, and therapeutic perspectives (Figure 1 and Figure 2). This review highlights converging mechanisms across symptom clusters, examines their temporal and pathological trajectories, and assesses how they impact diagnosis and treatment.
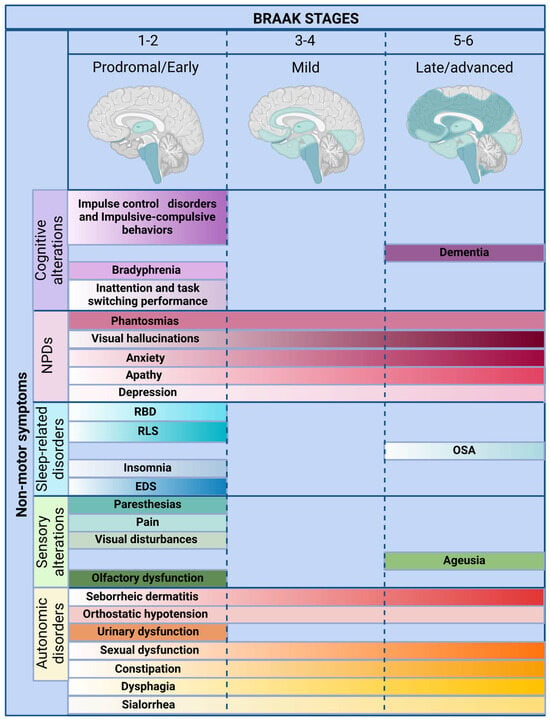
Figure 1.
Temporal emergence of non-motor symptoms in Parkinson’s disease relative to Braak staging. The evolving trajectory of non-motor symptoms across Braak stages 1 through 6, aligned with the ascending neuroanatomical propagation of α-synuclein pathology. Symptoms are grouped by domain—autonomic, sensory, sleep-related, neuropsychiatric, and cognitive—and plotted according to their earliest reported onset in prodromal, early, and advanced disease stages. A color gradient represents the intensification of each symptom over time. Early autonomic signs (e.g., constipation, orthostatic hypotension) and REM sleep behavior disorder typically precede motor onset, highlighting their diagnostic and predictive relevance. Mid-stage features such as apathy and visual hallucinations reflect advancing pathology, while cognitive decline and dementia dominate in later stages. This temporal map underscores the value of early symptom recognition for diagnosis, prognosis, and disease stratification. Abbreviations: EDS, excessive daytime sleepiness; NPDs, neuropsychiatric disorders; OSA, obstructive sleep apnea; RBD, REM sleep behavior disorder; RLS, restless leg syndrome. Figure created with BioRender.com, accessed on 5 February 2025. A color gradient represents the intensification of each symptom over time.
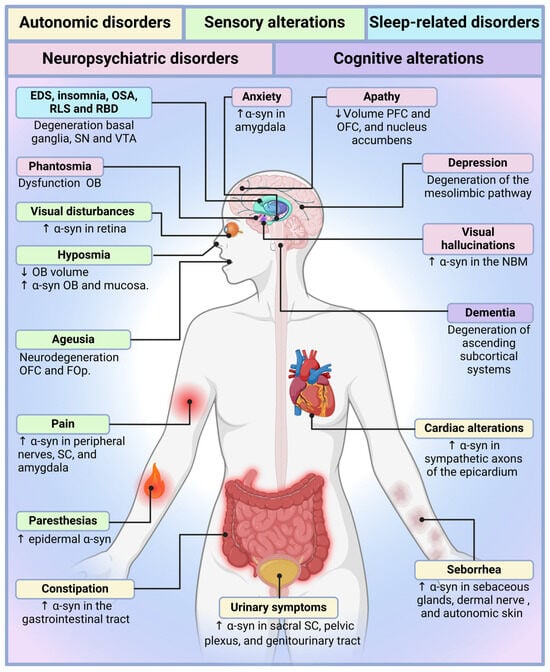
Figure 2.
Neuroanatomical substrates and mechanistic underpinnings of non-motor symptoms in Parkinson’s disease. This illustration maps the brain and peripheral regions implicated in non-motor manifestations of Parkinson’s disease, integrating structural and neurochemical changes with clinical symptom domains. α-Synuclein accumulation and neuronal loss in brain stem nuclei (e.g., dorsal motor nucleus of the vagus, locus coeruleus), limbic areas (e.g., amygdala, orbitofrontal cortex), and cholinergic centers (e.g., nucleus basalis of Meynert) disrupt circuits involved in autonomic regulation, emotional processing, arousal, memory, and sleep–wake transitions. Degeneration in the hypothalamic and prefrontal regions contributes to apathy, anxiety, and thermoregulatory failure, while dysfunction in the olfactory bulb, spinal cord, and peripheral autonomic ganglia gives rise to sensory and visceral symptoms. The convergence of central and peripheral pathology reinforces the need to reconceptualize Parkinson’s disease as a network-level, multisystem disorder rather than a focal basal ganglia syndrome. Abbreviations: ↑ = Increased; ↓ = Decreased; α-syn, α-synuclein; EDS, excessive daytime sleepiness; FOp, frontal operculum; NBM, nucleus basalis of Meynert; OB, olfactory bulb; OFC, orbitofrontal cortex; OSA, obstructive sleep apnea; PFC, prefrontal cortex; RBD, REM sleep behavior disorder; RLS, restless leg syndrome; SC, spinal cord; SN, substantia nigra; VTA, ventral tegmental area. Figure created with BioRender.com, accessed on 5 February 2025.
We target a broad audience, including medical students, non-specialist physicians, and neuroscientists. We also discuss how emerging technologies—including multi-omic profiling, artificial intelligence, and network-based approaches—may refine patient stratification and therapeutic design. Reframing non-motor symptoms as core disease features, we call for a paradigm shift in PD research and clinical care, emphasizing the need for holistic, multidimensional models.
2. Autonomic Dysfunction in Parkinson’s Disease
Autonomic dysfunction is a core and early component of PD, affecting over 90% of patients across multiple physiological systems (see Table 1) [11]. These disturbances frequently precede motor onset stemming from widespread neurodegeneration extending beyond the basal ganglia. α-Synuclein aggregates accumulate in autonomic ganglia and peripheral nerves, disrupting sympathetic and parasympathetic regulation and impairing cardiovascular control, gastrointestinal motility, urinary function, and thermoregulation [12]. Despite its high prevalence and clinical impact, autonomic dysfunction remains underrecognized and poorly managed, often identified only when symptoms become disabling.

Table 1.
Autonomic disorders in Parkinson’s disease.
Cardiovascular manifestations, including orthostatic hypotension and arrhythmias, elevate fall and cardiovascular risk [13,14]. Gastrointestinal symptoms such as constipation, sialorrhea, and dysphagia frequently appear in the prodromal phase and reflect neurodegeneration in the enteric and parasympathetic systems [13,14]. Urinary dysfunction, anhidrosis, hyperhidrosis, and seborrhea further illustrate the diffuse nature of PD-related autonomic failure. Cutaneous and thermoregulatory disturbances, though commonly dismissed, may offer clinically useful early markers. Collectively, these features highlight the need for early detection and mechanistically informed interventions (Figure 1 and Figure 2) [15].
2.1. Sympathetic Autonomic Nervous System
Sympathetic dysfunction in PD typically emerges before motor onset and manifests clinically as orthostatic hypotension, impaired baroreflex sensitivity, and autonomic cardiovascular instability [16]. These features arise from combined central and peripheral neurodegeneration, including loss of preganglionic neurons and postganglionic sympathetic fibers [13]. α-Synuclein pathology in sympathetic ganglia, heart, adrenal tissue, and skin confirms that PD extends far beyond the central nervous system [17,18].
Autonomic structures derived from the neural crest—including the adrenal medulla, sympathetic chain, and spinal ganglia—show marked α-synuclein accumulation, reflecting the multisystem nature of PD [19]. Norepinephrine depletion, especially in cardiac sympathetic fibers, impairs vasoconstriction and contributes to orthostatic hypotension [20]. Pathology studies reveal the selective vulnerability of preganglionic neurons in the thoracic spinal cord and α-synuclein deposition in the celiac ganglion, which controls postganglionic output to visceral organs [10].
Although these mechanisms are increasingly well characterized, therapeutic strategies remain reactive and symptomatic. Clinicians rely on vasopressors and volume-expanding agents to control hypotension, but no disease-modifying approaches have targeted sympathetic neurodegeneration directly. Biomarkers of sympathetic failure, including MIBG cardiac imaging and skin α-synuclein detection, hold diagnostic promise, yet remain underutilized. Despite compelling evidence, sympathetic dysfunction remains peripheral to most PD frameworks, limiting progress in basic and translational domains.
Integrating assessments of autonomic dysfunction—such as 24 h ambulatory blood pressure monitoring, skin sympathetic response testing, and the SCOPA-AUT questionnaire—into the diagnostic and therapeutic frameworks of PD may be helpful for early detection and comprehensive management of non-motor symptoms [12,21,22,23]. These tools facilitate the identification of autonomic impairments that may precede motor symptoms, thereby enabling timely interventions that can improve patient outcomes.
2.2. Parasympathetic Autonomic Nervous System
Parasympathetic dysfunction contributes prominently to early gastrointestinal, urological, and cardiovascular symptoms in PD. Delayed gastric emptying, constipation, urinary retention, and chronotropic incompetence are among the earliest indicators of autonomic failure and often precede motor symptoms by several years [24].
The dorsal motor nucleus of the vagus (DMV)—a primary parasympathetic center—is among the first sites to exhibit α-synuclein pathology [9]. Its degeneration disrupts efferent parasympathetic output to the heart, gastrointestinal tract, and bladder, leading to multisystem dysfunction. DMV involvement supports the Braak staging model of caudorostral spread, though its diagnostic and therapeutic value remains underexplored.
Gastrointestinal symptoms arise from α-synuclein pathology in the DMV and enteric nervous system, reinforcing the gut–brain axis hypothesis. Recent studies implicate gut dysbiosis and increased intestinal permeability as potential accelerants of α-synuclein propagation and systemic inflammation. Cardiovascular parasympathetic failure impairs vagal modulation of heart rate and may contribute to arrhythmogenesis and sudden cardiac events [25].
Despite the breadth of symptoms, management remains limited to cholinergic or serotonergic agents and supportive therapies. Dysautonomia receives little attention in clinical trials, and parasympathetic pathology remains poorly integrated into PD treatment algorithms. A shift toward mechanistic interventions and biomarker-guided approaches is essential to move beyond symptom suppression toward precision therapeutics [26].
2.3. Sialorrhea and Dysphagia
Sialorrhea and dysphagia are among the most disabling non-motor symptoms of PD, significantly affecting quality of life and increasing morbidity (see Table 1). Sialorrhea affects up to 84% of patients and reflects impaired oral-phase swallowing rather than hypersalivation [27,28,29,30]. Orofacial bradykinesia, α-synuclein accumulation in brain stem nuclei, and cholinergic deficits contribute to reduced clearance of saliva. Dysphagia, affecting up to 87% of patients, progresses alongside motor symptoms and increases the risk of aspiration pneumonia—a leading cause of death in PD [31,32].
Neurodegeneration in the DMV and nucleus ambiguus impairs reflexive and voluntary swallowing [33,34,35,36]. Dopaminergic and cholinergic dysfunction further disrupts motor coordination during the oropharyngeal and esophageal phases. Although dopaminergic therapy may offer partial benefit, it often exacerbates incoordination. Botulinum toxin injections and anticholinergics can relieve sialorrhea, but risk worsening dysphagia. Swallowing rehabilitation, compensatory strategies, and dietary modifications remain central to care, yet long-term efficacy is limited. Multidisciplinary interventions involving speech therapists, neurologists, and nutritionists are essential.
Despite their impact, research into sialorrhea and dysphagia remains limited. These symptoms are not routinely assessed in clinical practice or trials. Targeting early brain stem involvement may offer opportunities for neuroprotective intervention, but such approaches remain unexplored. A more proactive diagnostic framework is required to prevent hospitalizations and complications.
2.4. Constipation
Constipation is one of the earliest and most common non-motor symptoms of PD, affecting up to 63% of patients and often emerging decades before motor signs (see Table 1) [37,38]. Early α-synuclein deposition in the enteric nervous system, DMV, and sacral parasympathetic nuclei underpins this symptom’s prominence in prodromal PD [39].
Disruption of cholinergic and serotonergic regulation leads to slowed colonic transit, incomplete evacuation, and dyssynergic defecation [40]. Altered gut microbiota and increased intestinal permeability contribute to systemic inflammation and may promote α-synuclein propagation via the gut–brain axis [41,42]. Small intestinal bacterial overgrowth (SIBO) and gut dysbiosis exacerbate gastrointestinal symptoms and may represent modifiable contributors to disease progression.
Despite its clinical relevance, constipation remains poorly managed. Most treatments—including laxatives, stool softeners, and fiber supplements—target symptoms rather than underlying neuropathology. Prokinetic agents show limited efficacy. A multidisciplinary approach incorporating neurologists and gastroenterologists is essential for optimal care. Constipation should be recognized not only as a quality-of-life issue but also as a potential driver of systemic neurodegeneration.
2.5. Neurogenic Sexual Dysfunction
Sexual dysfunction affects approximately 68% of men and 53% of women with PD [43]. Loss of libido, erectile dysfunction in men, and vaginal dryness in women are common manifestations. Neurodegeneration in the hypothalamus, amygdala, and spinal autonomic circuits contributes to disrupted sexual arousal, motivation, and performance (see Table 1) [44,45,46,47].
The mesolimbic dopaminergic pathway and hypothalamic structures, including the medial preoptic area and paraventricular nucleus, undergo early dysfunction in PD. Dopaminergic medications may paradoxically worsen symptoms by inducing impulse control disorders such as hypersexuality [46,48]. Deep brain stimulation of basal ganglia structures can alter sexual behavior, underscoring their regulatory role [49].
Despite the clear neurobiological basis, sexual dysfunction is underdiagnosed due to stigma and limited clinician inquiry. Treatment remains symptomatic, using PDE-5 inhibitors or hormone replacement, with limited mechanistic targeting. A multidisciplinary framework integrating neurology, psychiatry, and sexual health is critical for addressing this overlooked domain.
2.6. Urinary Symptoms
Urinary dysfunction is a pervasive, yet often overlooked non-motor symptom of PD, affecting approximately 61% of patients and profoundly impacting quality of life [50,51]. In more severe cases, lower urinary tract symptoms manifest as urgency, frequency, nocturia, incontinence, and urinary retention (see Table 1). These disturbances frequently appear in the premotor phase, reinforcing that PD is a multisystem disorder extending beyond the basal ganglia long before motor symptoms emerge [52]. As the disease progresses, urinary dysfunction correlates with increased motor disability and more extensive dopaminergic denervation, yet the underlying mechanisms remain only partially understood.
The pathophysiology of urinary symptoms in PD involves complex interactions between the central and peripheral nervous systems. The basal ganglia, through its dopaminergic D1-GABAergic direct pathway, plays a crucial role in suppressing involuntary bladder contractions, ensuring appropriate timing of voiding. Dysfunction in this circuit leads to detrusor overactivity, one of the most common urinary disturbances in PD, resulting in urgency and incontinence [52]. However, beyond dopaminergic deficits, neurodegeneration extends to brain regions directly involved in bladder control. The pontine micturition center, which coordinates voluntary voiding, exhibits α-synuclein pathology, as do preganglionic and postganglionic sympathetic neurons, sacral parasympathetic nuclei, and the pelvic plexus [51]. This widespread neurodegeneration disrupts the delicate balance between excitatory and inhibitory control of the bladder, leading to a spectrum of voiding dysfunctions that fluctuate with disease severity.
The presence of α-synuclein aggregates in the genitourinary tract further supports that PD involves peripheral autonomic structures not confined to the central nervous system [53,54]. These pathological changes suggest that bladder dysfunction is not simply a downstream consequence of motor impairment, but a fundamental feature of PD progression. Despite this, urinary symptoms remain underdiagnosed and undertreated, often attributed to ageing rather than being recognized as a direct consequence of neurodegeneration.
Current treatment strategies for urinary dysfunction in PD remain primarily symptomatic [55]. Anticholinergic agents and β3 adrenergic receptor agonists provide some relief by reducing detrusor overactivity, yet cognitive side effects and inconsistent efficacy often limit their effectiveness. Dopaminergic therapies, which are the mainstay of PD treatment, offer little to no improvement in urinary symptoms, highlighting the need for alternative therapeutic approaches that target non-dopaminergic pathways [55]. Non-pharmacological interventions, including pelvic floor therapy and behavioral modifications, are frequently recommended, yet their long-term impact on disease progression remains uncertain [56].
2.7. Cardiac Alterations
Autonomic cardiac dysfunction is a common but often underrecognized feature of PD, emerging early in the disease course and progressively worsening as neurodegeneration advances [57]. The imbalance between increased sympathetic activity and decreased parasympathetic regulation leads to significant cardiovascular abnormalities, including heart rate variability, arrhythmias, prolonged P–R and Q–T intervals, and in rare cases cardiomyopathy [58,59]. These alterations not only contribute to increased morbidity but also elevate the risk of sudden cardiac events, underscoring the systemic nature of PD.
The pathophysiology of cardiac dysfunction in PD is driven by widespread autonomic degeneration, characterized by selective loss of sympathetic nerve terminals. Immunohistochemical analyses for tyrosine hydroxylase (TH), a key enzyme in catecholamine synthesis, confirm marked sympathetic denervation of the heart. This loss has been further validated by imaging studies using (123I)-metaiodobenzylguanidine (123I-MIBG) and (18F)-fluorodopa, which demonstrate reduced cardiac norepinephrine uptake, indicative of sympathetic failure [60]. Post-mortem studies reveal α-synuclein aggregates within the epicardial sympathetic axon terminals, with axonal degeneration progressing in a distal-to-proximal manner—first affecting the heart before extending to prevertebral and paravertebral sympathetic ganglia [61].
This pattern of degeneration suggests that cardiac autonomic dysfunction in PD is not merely a secondary consequence of motor impairment or medication side effects, but rather a primary feature of disease pathology [26]. The involvement of the central and peripheral autonomic nervous systems reinforces that PD extends far beyond the dopaminergic system, disrupting regulatory networks that control cardiovascular homeostasis. Despite this, cardiac dysfunction remains underdiagnosed in routine clinical assessments, largely due to the overshadowing focus on motor symptoms.
Despite robust pathology, cardiac dysfunction remains underdiagnosed. Treatment is symptomatic, using beta-blockers or volume expanders [62,63]. No therapies target the underlying autonomic degeneration. Greater integration of cardiovascular assessments into PD care is needed.
2.8. Orthostatic Hypotension
Orthostatic hypotension (OH) is a debilitating and often underdiagnosed cardiovascular manifestation of PD, affecting approximately 30% of patients. Defined by a drop of more than 20 mmHg in systolic blood pressure or 10 mmHg in diastolic pressure within three minutes of standing, OH significantly impairs daily function, increasing the risk of dizziness, fatigue, syncope, falls, and injury [64,65]. Unlike primary hypotensive disorders, neurogenic OH in PD arises from a fundamental breakdown in autonomic regulation, reflecting widespread sympathetic denervation and baroreflex failure [66].
The underlying pathophysiology of OH in PD stems from impaired norepinephrine release, leading to deficient vasoconstriction and a subsequent failure to maintain blood pressure upon standing [65,67]. Studies indicate this dysfunction is primarily driven by sympathetic neural degeneration rather than baroreceptor failure, distinguishing Parkinson’s-related OH from other forms of dysautonomia [68,69]. The depletion of norepinephrine at both central and peripheral levels compromises vascular tone, exacerbating cerebral autoregulation deficits that further contribute to orthostatic intolerance. The insular cortex, a critical autonomic control region, also shows significant Lewy body accumulation in PD, correlating with the severity of OH and the overall progression of autonomic dysfunction [66].
Twenty-four-hour ambulatory blood pressure monitoring may be a valuable tool for detecting cardiovascular autonomic dysfunction, including abnormal circadian blood pressure patterns such as reverse dipping, which are associated with increased risk of adverse clinical events [21,70].
Despite its high prevalence and profound impact on patient well-being, OH remains inadequately managed in PD [71]. Symptomatic treatments include fludrocortisone, midodrine, droxidopa, and lifestyle modifications [72]. However, these interventions do not halt neurodegeneration. Targeted therapies remain lacking, underscoring the need for autonomic biomarkers in early disease.
2.9. Seborrhea and Seborrheic Dermatitis
Seborrhea and seborrheic dermatitis are common non-motor manifestations in PD, affecting between 52% and 59% of patients and frequently appearing in early disease stages [73]. Characterized by erythema, greasy scaling, and increased sebum production, these conditions reflect underlying autonomic dysregulation rather than primary dermatologic disease. Sebaceous gland activity is modulated by dopaminergic signaling, and degeneration of basal ganglia circuits disrupts this regulatory control [74].
Malassezia yeast colonization contributes to inflammation and barrier disruption; however, excessive sebum production and altered skin microbiota are likely consequences of neurodegenerative processes [75,76]. Histopathological studies reveal α-synuclein aggregates in dermal autonomic fibers and sebaceous glands, suggesting that cutaneous involvement is part of systemic PD pathology [76]. These changes offer a potential avenue for biomarker development. Indeed, skin biopsies detecting phosphorylated α-synuclein in peripheral nerves are under investigation as early diagnostic tools.
Despite their high prevalence, seborrheic manifestations are rarely integrated into PD assessment [74,77]. Treatment typically involves antifungals and corticosteroids, offering temporary symptom control without addressing the neurobiological substrate. Collaborative care between neurologists and dermatologists is needed to improve diagnosis and uncover therapeutic opportunities.
2.10. Anhidrosis/Hyperhidrosis
Sweating disturbances are frequently reported, but underexamined in PD. Hyperhidrosis (excessive sweating) typically affects the upper body, while anhidrosis (reduced sweating) is more common in the extremities, resulting in an asymmetric thermoregulatory profile [78,79]. These disturbances occur independently of motor symptom severity and reflect complex autonomic dysfunction.
Central mechanisms, including hypothalamic dysregulation and α2 adrenergic receptor dysfunction, contribute to abnormal sympathetic output. Peripherally, progressive pre- and postganglionic sympathetic innervation loss disrupts sudomotor function [80,81]. The resulting thermoregulatory imbalance exacerbates orthostatic symptoms, fatigue, and discomfort, impairing daily activities and sleep quality.
Treatment remains empirical. Anticholinergic agents and botulinum toxin injections can reduce hyperhidrosis, but risk side effects [82,83,84]. Anhidrosis lacks effective therapy. No interventions target the underlying autonomic neurodegeneration. These symptoms may serve as early markers of systemic dysfunction and merit further investigation.
2.11. Thermoregulatory Alterations
Thermoregulatory dysfunction is a clinically significant, yet underrecognized non-motor feature of PD. Symptoms include cold intolerance, paradoxical sweating, and episodes of hypothermia, which can occur even in normothermic environments [85,86]. Hypothermia (<35 °C) poses serious systemic risks, including arrhythmias and altered mental status.
The hypothalamus orchestrates thermoregulatory control through modulation of vasomotor tone, sweat gland activity, and metabolic rate. PD-related degeneration disrupts these mechanisms, impairing heat dissipation and conservation [85]. Sympathetic dysfunction reduces vasoconstrictive response and heat generation, while parasympathetic hyperactivity impairs recovery from cold exposure [80,87].
Sympathetic neurograms and heart rate variability studies confirm diminished autonomic responsiveness in PD. These impairments correlate with disease duration and severity, suggesting progressive autonomic decline. Despite the clinical relevance, no targeted therapies have addressed thermoregulatory dysfunction. Management focuses on behavioral adaptations and environmental controls.
Recognition of thermoregulatory abnormalities as intrinsic to PD pathology—not incidental complications—is essential for improving patient safety and quality of life. Greater mechanistic understanding and biomarker integration are needed to inform preventive and therapeutic strategies.
3. Sensory Alterations in Parkinson’s Disease
Sensory dysfunction is an increasingly recognized, but still underappreciated aspect of PD. These alterations—including hyposmia, ageusia, visual impairment, pain, and paresthesias—often precede motor onset and affect up to 90% of patients [88,89]. Their high prevalence and early appearance suggest a central role in PD pathophysiology (see Table 2). Despite this, most are underdiagnosed and undertreated, reflecting the persistent dominance of a motor-centric clinical model. Here, we examine the anatomical, molecular, and clinical underpinnings of PD-related sensory dysfunction, emphasizing their diagnostic, prognostic, and therapeutic relevance (Figure 1 and Figure 2).

Table 2.
Sensory disorders in Parkinson’s disease.
3.1. Hyposmia
Olfactory dysfunction is one of the most well-established non-motor symptoms of PD, first recognized over three decades ago as a frequent yet overlooked aspect of the disease [90,91,92]. Hyposmia, characterized by a diminished sense of smell, affects between 75% and 90% of patients and often precedes the onset of motor symptoms by several years. This strong temporal association makes olfactory impairment one of the most reliable early biomarkers of PD, with individuals experiencing hyposmia exhibiting a 3.84-fold increased risk of developing the disorder [93,94,95,96,97].
Hyposmia in PD is not merely an isolated sensory impairment, but rather a reflection of widespread neurodegeneration. The severity of olfactory dysfunction correlates with cognitive decline, depression, anxiety, and REM sleep behavior disorder, suggesting a shared pathophysiological mechanism underlying these non-motor symptoms [98,99,100,101]. The neuropathological hallmark of PD—Lewy pathology—originates in the olfactory bulb and spreads along neural pathways, leading to neuronal loss and reduced olfactory bulb volume. This process extends to limbic structures, including the amygdala and hippocampus, further implicating olfactory dysfunction as a harbinger of broader neurodegeneration [9,102,103].
Identifying α-synuclein aggregates in olfactory mucosal receptor neurons has raised the prospect of using nasal swabs as a potential diagnostic tool for PD (see Table 2). Although preliminary studies support the feasibility of this approach, additional research is needed to refine its sensitivity and specificity before it can be implemented in routine clinical practice [104,105]. Beyond its association with Lewy pathology, olfactory dysfunction in PD is also linked to disruptions in dopaminergic transmission. The nigrostriatal dopaminergic system, known for its role in motor function, also modulates sensory processing, including olfaction. Dopaminergic denervation correlates with the severity of hyposmia, with lower dopamine transporter activity in striatal regions corresponding to reduced olfactory performance [98,106]. Given that up to 80% of dopaminergic neurons are lost before the appearance of motor symptoms, olfactory dysfunction may provide a crucial window for early intervention [107].
Despite its predictive value, hyposmia lacks specificity and is rarely assessed in routine practice [108]. Standardized olfactory testing could enhance early diagnosis, but implementation remains limited. No established therapies exist to reverse olfactory decline. The absence of targeted interventions reflects a broader neglect of sensory symptoms in PD.
3.2. Ageusia
Ageusia, the loss of taste perception, is a frequently overlooked sensory deficit in PD, often dismissed as a secondary effect of olfactory dysfunction rather than a distinct neurological impairment (see Table 2). However, evidence suggests that ageusia results from independent neuroanatomical and molecular abnormalities, making it an essential but underrecognized component of the non-motor symptom spectrum. Prevalence estimates vary widely, ranging from 9% to 54%, depending on study methodology and disease stage [109,110,111,112]. Given its significant impact on appetite, nutrition, and overall quality of life, the failure to adequately assess and manage ageusia in PD reflects a broader neglect of sensory dysfunction in clinical practice.
Lewy pathology affects gustatory processing areas, including the orbitofrontal cortex, insula, and sensory integration hubs [109,113]. Cholinergic degeneration disrupts acetylcholine-dependent pathways, impairing taste perception and sensory integration [114,115]. Cognitive decline exacerbates these deficits by altering network connectivity across taste-related circuits.
At the molecular level, downregulation of taste receptor genes has been observed in PD models, suggesting that ageusia arises from combined neurodegenerative and genetic mechanisms [116]. Despite these findings, no diagnostic protocols or targeted treatments exist for ageusia in PD. Clinical oversight of this symptom reflects an outdated framework that minimizes sensory dysfunction.
3.3. Visual Disturbances
Visual impairment affects over 90% of patients with PD and ranges from reduced contrast sensitivity to hallucinations [117,118,119]. These deficits impact mobility, safety, and cognitive performance. Despite their prevalence, visual symptoms are rarely prioritized in PD care (see Table 2).
Visual dysfunction in PD arises from both cortical and retinal degeneration. Lewy pathology affects visual cortices and correlates with visuospatial and perceptual deficits [119,120]. Retinal dopaminergic amacrine cells degenerate early, impairing contrast sensitivity and color discrimination [121,122,123,124].
Retinal imaging via optical coherence tomography (OCT) reveals thinning of the retinal nerve fiber layer and microvascular alterations, correlating with disease severity [125,126,127]. These biomarkers may enable earlier diagnosis and disease monitoring, but clinical integration remains limited.
Therapeutic options are underdeveloped [128]. Dopaminergic therapy yields inconsistent benefits. Visual aids and rehabilitation strategies are underused. Diagnostic opportunities and symptom management will continue to lag without integrating visual assessment into PD protocols.
3.4. Pain
Pain is a highly prevalent, yet frequently underestimated non-motor symptom of PD, affecting between 20% and 98% of patients depending on study methodology and diagnostic criteria [129,130,131]. For many, pain is not merely a secondary complication, but a defining feature of the disease, often preceding the onset of motor symptoms by several years (see Table 2). The nature of pain in PD is heterogeneous, encompassing musculoskeletal pain linked to rigidity and postural abnormalities, dystonic pain arising from involuntary muscle contractions, and neuropathic or radicular pain indicative of peripheral nerve involvement [4,129]. Despite its impact on daily life, pain remains underdiagnosed mainly and poorly managed, highlighting the need for a more integrated approach to its assessment and treatment.
The pathophysiology of pain in PD is multifaceted, involving both central and peripheral mechanisms. Lewy pathology extends beyond the basal ganglia to regions directly involved in pain processing, including the spinal cord, amygdala, periaqueductal gray, and nucleus accumbens. Neurodegeneration in these areas alters nociceptive modulation, increasing pain sensitivity and disrupting the endogenous analgesic system [132]. Additionally, PD affects non-dopaminergic structures such as the subthalamic nucleus, anterior cingulate cortex, and insular cortex—regions implicated in pain perception and emotional processing—further amplifying the subjective experience of pain [133,134,135].
Dysregulation of pain-modulating neurotransmitters is another key contributor to heightened pain sensitivity in PD. Beyond dopamine, disruptions in norepinephrine and serotonin transmission impair descending pain inhibition, intensifying nociceptive signaling and altering the brain’s ability to regulate pain perception [135,136]. Furthermore, neuroinflammation is critical, as elevated levels of proinflammatory cytokines contribute to peripheral and central sensitization, exacerbating pain symptoms [137,138]. These overlapping mechanisms suggest that pain in PD is not merely a consequence of motor dysfunction, but a fundamental component of disease pathology, warranting targeted therapeutic strategies.
Despite its high prevalence, no definitive biomarkers have been identified to correlate pain severity with specific neuropathological changes in PD [139]. While some patients experience pain long before motor symptoms emerge, the mechanisms underlying this early manifestation remain poorly understood. Identifying reliable biomarkers and delineating the neurochemical pathways involved in Parkinson’s-related pain is critical for developing precision-targeted therapies.
Current treatment approaches remain largely symptomatic, relying on pharmacological and non-pharmacological interventions. However, conventional dopaminergic therapy provides inconsistent relief, underscoring the need for alternative strategies that address the non-dopaminergic contributions to pain processing. Neuromodulators and physical therapies offer partial relief, but lack disease-modifying potential. Recognizing pain as a primary neurodegenerative symptom is critical for targeted research and comprehensive care.
3.5. Paresthesias
Paresthesias—tingling, numbness, or burning sensations—affect approximately 40% of PD patients [140]. These symptoms are frequently misattributed to aging or comorbid neuropathy. However, α-synuclein pathology in cutaneous and peripheral nerves suggests a direct neurodegenerative basis (see Table 2) [141,142,143,144].
Small-fiber neuropathy, Schwann cell dysfunction, and autonomic denervation contribute to altered somatosensory signaling [145,146,147,148]. Loss of epidermal nerve fibers correlates with disease severity. Autonomic impairment alters pain thresholds and temperature perception, compounding paresthetic symptoms.
Despite their impact, paresthesias remain underdiagnosed. No standard diagnostic tools or therapeutic guidelines exist. Current treatment is symptomatic and often inadequate. Recognition of paresthesias as a core feature of PD could stimulate mechanistic research and improve patient outcomes.
4. Sleep-Related Disorders in Parkinson’s Disease
Sleep disturbances are among the most prevalent and disabling non-motor symptoms of PD, affecting between 60% and 98% of patients [149]. These disorders impair cognitive function, exacerbate mood instability, and worsen motor symptoms, creating a feedback loop that accelerates neurodegeneration. Despite their frequency, sleep-related symptoms remain underdiagnosed and undertreated, frequently overshadowed by the emphasis on motor impairment in clinical assessments (see Table 3).

Table 3.
Sleep-related disorders in Parkinson’s disease.
The spectrum of PD-related sleep disturbances is broad, encompassing insomnia, excessive daytime sleepiness, REM sleep behavior disorder (RBD), restless leg syndrome (RLS), obstructive sleep apnea (OSA), and nocturia. Many of these manifestations emerge during the prodromal phase, well before motor symptoms, positioning them as early biomarkers of neurodegeneration [149]. Their diagnostic value and impact on disease trajectory make sleep dysfunction an essential, yet overlooked component of PD pathophysiology (Figure 1 and Figure 2).
4.1. REM Sleep Behavior Disorder
RBD is characterized by the loss of muscle atonia during REM sleep, leading to dream enactment behaviors that may result in self-injury or harm to bed partners. It is one of the most specific prodromal markers of synucleinopathies. Longitudinal studies show that 80%–90% of patients with idiopathic RBD develop PD, dementia with Lewy bodies, or multiple system atrophy within 10–15 years [149].
The pathophysiology of RBD implicates early degeneration in the pontine tegmentum, including the sublaterodorsal nucleus, and magnocellular reticular formation. These brain stem circuits regulate REM atonia, and their dysfunction precedes motor decline. RBD also correlates with more rapid cognitive deterioration, hallucinations, and autonomic instability, supporting its role as a marker of a more malignant PD phenotype.
Despite its predictive value, RBD remains underdiagnosed. Polysomnography is required for definitive diagnosis, yet few centers implement routine screening. Clonazepam and melatonin offer partial symptomatic relief, but no disease-modifying interventions exist. Further research into RBD-associated neurodegeneration could improve early diagnosis and therapeutic targeting.
4.2. Insomnia and Sleep Fragmentation
Insomnia affects up to 80% of individuals with PD and contributes significantly to cognitive decline, fatigue, and emotional dysregulation [149,150,151]. Sleep-onset and maintenance difficulties arise from nocturnal motor symptoms, including rigidity, tremor, and dystonia, but also reflect intrinsic disruption of sleep-regulating circuits.
Neurodegeneration in serotonergic and noradrenergic brain stem nuclei impairs sleep–wake stability, while degeneration of the ventrolateral preoptic nucleus disrupts sleep-promoting mechanisms. Fragmented sleep architecture and reduced REM sleep duration are common, often compounded by anxiety and depression.
Despite its burden, insomnia in PD is often addressed using sedative-hypnotics with limited benefit and potential adverse effects. Cognitive behavioral therapy for insomnia (CBT-I) and light therapy have demonstrated efficacy, but remain underused. Recognition of insomnia as a core manifestation of PD is essential for advancing treatment beyond symptom suppression.
4.3. Excessive Daytime Sleepiness
Excessive daytime sleepiness (EDS) occurs in up to 76% of PD patients and worsens with disease progression and dopaminergic therapy, particularly dopamine agonists [149]. EDS impairs attention, increases accident risk, and contributes to social withdrawal.
The etiology of EDS is multifactorial. Neurodegeneration in the locus coeruleus, tuberomammillary nucleus, and orexin-producing neurons in the lateral hypothalamus impairs wakefulness regulation. Medications exacerbate this dysfunction, and nocturnal sleep fragmentation compounds daytime fatigue.
Management of EDS remains limited to reducing sedating medications and promoting sleep hygiene. Modafinil and other wake-promoting agents offer partial benefits, but are inconsistently effective. Greater mechanistic understanding is needed to guide future interventions.
4.4. Restless Leg Syndrome and Obstructive Sleep Apnea
RLS is characterized by an urge to move the legs and uncomfortable sensations that worsen at night. It disrupts sleep quality and affects up to 20% of PD patients [149]. The pathogenesis remains unclear, but may involve dopaminergic and iron homeostasis abnormalities in the spinal cord and basal ganglia.
OSA affects approximately 20%–60% of individuals with PD and contributes to fragmented sleep, intermittent hypoxia, and oxidative stress. It has been linked to cognitive decline and may accelerate neurodegeneration. Polysomnographic evaluation and continuous positive airway pressure (CPAP) use remain underutilized.
4.5. Mechanisms and Implications for Disease Progression
Sleep-related disorders in PD arise from degeneration of the brain stem, hypothalamic, and cortical structures regulating sleep–wake transitions. Dopaminergic loss in the substantia nigra and ventral tegmental area destabilizes circadian and behavioral arousal. Early α-synuclein pathology in the raphe nuclei and locus coeruleus impairs serotonergic and noradrenergic tone, essential for REM and non-REM stability [9,10,149].
The flip-flop switch model highlights the role of cholinergic–monoaminergic interactions in sleep control, which are dysregulated in PD [149]. Additionally, the glymphatic system—responsible for clearing misfolded proteins during deep sleep—is compromised in PD, potentially promoting α-synuclein accumulation and disease progression [152]. Circadian dysfunction further exacerbates symptoms through impaired melatonin secretion, hypothalamic disruption, and retinal pathway degeneration [149].
4.6. Clinical Management and Future Directions
Current treatments for PD-related sleep disturbances are primarily symptomatic. Melatonin, sedatives, and dopaminergic agents offer partial relief, but often introduce new complications. Non-pharmacological approaches, including CBT-I, light therapy, and CPAP for OSA, are evidence-based, yet underutilized.
There is an urgent need to integrate sleep assessment into routine PD care. Personalized treatment strategies based on sleep phenotyping and early biomarker screening (e.g., RBD) could facilitate timely interventions and improve long-term outcomes. Targeting sleep dysfunction as a modifiable contributor to neurodegeneration could shift therapeutic goals from symptom management to disease modification.
Sleep disturbances are not merely downstream effects of PD, but integral components of its pathology. Addressing them requires a paradigm shift in research, clinical assessment, and care delivery—placing sleep at the forefront of PD management.
5. Neuropsychiatric Manifestations in Parkinson’s Disease
Neuropsychiatric symptoms are increasingly recognized as core components of PD, often emerging in the prodromal phase and exerting a profound influence on patient quality of life and disease progression [153]. Depression, anxiety, apathy, visual hallucinations, and phantosmia disrupt emotional regulation, cognitive stability, and social functioning, yet they remain underdiagnosed and inadequately treated (see Table 4). These symptoms are not reactive responses to chronic illness, but result from neurodegenerative changes in mesolimbic, serotonergic, cholinergic, and cortical circuits [154,155]. Recognizing neuropsychiatric dysfunction as integral to PD pathophysiology is essential for advancing timely diagnosis, improving patient outcomes, and developing mechanism-based interventions (Figure 1 and Figure 2).

Table 4.
Neuropsychiatric disorders in Parkinson’s disease.
5.1. Depression
Depression is one of the most common neuropsychiatric symptoms of PD, affecting between 35% and 45% of patients (see Table 4). It may present at any stage of the disease, frequently emerging before the onset of motor dysfunction and persisting throughout disease progression [156]. Unlike primary depressive disorders, Parkinson’s-related depression is often characterized by irritability, anhedonia, cognitive slowing, and profound apathy rather than overt sadness, complicating its recognition and differentiation from other non-motor symptoms. Sleep disturbances, fatigue, and psychomotor retardation further contribute to functional impairment, exacerbating the overall disease burden [156,157].
The underlying pathophysiology of Parkinson’s-related depression extends beyond dopamine depletion, implicating widespread neurodegeneration in key mood-regulating structures. Structural imaging studies reveal significant gray matter atrophy in the bilateral thalami and amygdalae, regions critical for emotional processing, while cortical gyrification deficits in the frontal and parietal lobes correlate with depressive severity [158]. Braak’s staging suggests that early degeneration of the mesolimbic dopaminergic system and noradrenergic neurons in the locus coeruleus and serotonergic neurons in the raphe nucleus underlies mood dysregulation in PD [9,10]. However, serotonin transporter reductions do not consistently correlate with depression severity, raising questions about additional mechanisms contributing to these symptoms [159]. Interestingly, increased SNCA gene expression, which encodes α-synuclein, has been observed in patients with Parkinson’s-related depression, suggesting a molecular link between neurodegeneration and mood disorders [160].
However, traditional antidepressants yield inconsistent results in PD. Dopaminergic agents such as pramipexole show promise, but are limited by behavioral side effects [161]. Despite their efficacy, cognitive behavioral therapy, transcranial stimulation, and structured exercise remain underused. A greater focus on tailored, mechanistically informed treatments is needed.
5.2. Apathy
Apathy, a profound lack of motivation that is independent of mood, consciousness, or cognition, is one of the most disabling non-motor symptoms of PD [162]. Affecting between 40% and 52% of patients, apathy often emerges early in the disease course with or without coexisting depression or anxiety, yet remains significantly underrecognized and undertreated [163,164]. It manifests in three domains—behavioral and cognitive inertia, emotional blunting, and diminished social interaction—each contributing to a progressive disengagement from daily activities and exacerbating functional decline (see Table 4). Apathy is diagnosed when these deficits persist for at least four weeks and impair at least two domains, making it a critical, yet frequently overlooked determinant of disease burden [165,166].
The impact of apathy extends far beyond motivation deficits, profoundly influencing cognition, emotional well-being, and survival. Patients with apathy exhibit more significant cognitive impairment, more severe depressive symptoms, and an increased risk of mortality compared to those without these symptoms. While early-onset apathy in PD is often associated with concurrent depression, its later emergence signals a heightened risk of cognitive decline and progression to dementia [167,168]. This evolution from a motivational deficit to a harbinger of cognitive deterioration highlights its potential role as a biomarker of disease progression.
Neuroimaging reveals disrupted connectivity in the mesocorticolimbic network, including the orbitofrontal cortex and nucleus accumbens [169,170,171]. Biomarker studies suggest oxidative stress, iron accumulation, and α-synuclein oligomers may exacerbate motivational dysfunction [172,173].
Standard dopaminergic therapies provide limited benefit, and few studies specifically target apathy [174]. Emerging evidence supports structured behavioral interventions and tailored neurostimulation protocols. Given its predictive value for cognitive decline, apathy warrants early recognition and integrated management.
5.3. Anxiety
Anxiety is a frequent and often debilitating non-motor symptom of PD characterized by excessive worry, an exaggerated perception of threat, and diminished coping mechanisms (see Table 4). Affecting approximately 31% of patients, anxiety in PD is not merely a secondary reaction to diagnosis, but an intrinsic feature of the disease, emerging as early as the prodromal phase and persisting throughout disease progression [175]. Unlike primary anxiety disorders, Parkinson’s-related anxiety exhibits a unique profile, with generalized anxiety disorder, social phobia, and panic disorder being the most prevalent manifestations [175]. The presence of anxiety accelerates disease progression, exacerbates motor dysfunction, interferes with treatment response, and increases overall mortality risk [176]. Despite its profound impact on patients’ quality of life, anxiety remains largely underdiagnosed and undertreated, often dismissed as a reactive component of living with a neurodegenerative disorder.
The emergence of anxiety in PD is closely tied to structural and functional alterations in key neural circuits. Neuroimaging studies reveal significant atrophy in the left amygdala, frontocingulate cortex, and parietal regions and heightened connectivity within the fear circuit and salience network [177]. These alterations disrupt emotional regulation and threat perception, likely contributing to the heightened anxiety response observed in PD. Furthermore, α-synuclein pathology extends beyond the dopaminergic system, with accumulating evidence linking α-synuclein deposits in the amygdala to anxiety symptoms. Elevated erythrocytic α-synuclein levels have been detected in Parkinson’s patients with anxiety, reinforcing its potential role in disease pathophysiology [178,179]. Beyond neurodegeneration, oxidative stress and systemic inflammation have also been implicated as key contributors to Parkinson’s-related anxiety, suggesting that peripheral immune dysfunction may play a previously underestimated role in neuropsychiatric symptoms [180].
Despite its impact, anxiety is underdiagnosed [181]. SSRIs and benzodiazepines have inconsistent efficacy and potential side effects. Behavioral therapies, including CBT and mindfulness, offer benefits, but are rarely implemented. Stratified approaches based on imaging and biomarker profiles could enhance diagnostic precision and treatment outcomes.
5.4. Visual Hallucinations
Visual hallucinations affect 27%–50% of individuals with PD and often indicate progression to cognitive impairment [182,183,184,185,186,187]. Minor hallucinations may precede structured, distressing perceptual disturbances. Their early emergence correlates with cognitive decline and reduced quality of life (see Table 4).
Cholinergic and GABAergic deficits in the ventral visual stream and visual cortex disrupt sensory gating [188,189]. Structural connectivity abnormalities involving the lateral geniculate nucleus, thalamus, and prefrontal cortex further impair perceptual integration [190,191,192,193].
While dose reduction of dopaminergic agents may reduce hallucinations, it risks worsening motor symptoms [194]. Cholinesterase inhibitors show partial benefit. Future research must prioritize predictive biomarkers and preventive strategies to reduce hallucination-related morbidity.
5.5. Phantosmia
Phantosmia, the perception of odors without an external source, is reported in up to 18% of patients with PD and may co-occur with other hallucinations [195,196,197,198]. Unlike hyposmia, phantosmia does not correlate with measured olfactory loss, suggesting a distinct pathophysiological basis (see Table 4).
Disruption of predictive coding networks in the olfactory bulb and anterior olfactory nucleus likely contributes to phantosmia [199]. No direct link to Lewy pathology has been established, complicating diagnostic efforts.
Management remains empirical. Further investigation is required to determine whether phantosmia represents a unique prodromal marker or reflects broader cortical sensory dysfunction. Standardized assessment tools and longitudinal studies are needed.
6. Cognitive Dysfunction in Parkinson’s Disease
Cognitive dysfunction is a pervasive non-motor feature of PD, with impairments in executive function, attention, and processing speed frequently emerging early in the disease course emerging early and progressively worsening as the disease advances (see Table 5) [200]. Approximately 27% of untreated patients already exhibit cognitive deficits, and prevalence approaches 93% in advanced stages [161,200]. These deficits reflect progressive dysfunction in frontostriatal and frontoparietal networks, beginning in the caudate nucleus and expanding to involve the mesocorticolimbic and thalamocortical circuits [201,202,203,204,205].

Table 5.
Cognitive disorders in Parkinson’s disease.
Cognitive symptoms disrupt daily functioning, interfere with motor control, and accelerate dependence and institutionalization [201,202,203,204,205,206,207,208]. Despite their clinical relevance, cognitive changes are often misattributed to aging, delaying diagnosis and intervention. Structured cognitive screening and early stratification of cognitive phenotypes remain underutilized in PD care (Figure 1 and Figure 2).
6.1. Inattention and Task-Switching Performance
Attentional deficits affect over 20% of drug-naïve PD patients, often preceding motor symptoms [209,210,211]. These impairments hinder goal-directed behavior, impair task switching, and reduce adaptive flexibility. Dopaminergic depletion in mesocortical and frontoparietal circuits underlies reduced top-down and bottom-up attentional control [212,213,214].
Neurophysiological studies reveal abnormal alpha and gamma-band oscillatory activity and disrupted dorsal attention network connectivity [215,216,217,218,219]. Lower-order attentional processes rely on corticostriatal dopamine, while higher-order attentional shifts depend on noradrenergic and serotonergic transmission, suggesting the need for multimodal therapies [220,221,222].
Treatment remains empirical. While dopamine replacement may partially improve attentional performance, patient variability and potential cognitive side effects limit its utility. Cognitive training and stimulant-based therapies are under investigation, but lack standardization. Accurate differentiation between attentional dysfunction and global cognitive decline is essential for early, targeted intervention.
6.2. Bradyphrenia
Bradyphrenia—slowing thought processes and reduced cognitive flexibility—is a hallmark of subcortical cognitive dysfunction in PD [201,223]. It contributes to diminished adaptability and poor decision-making, often without overt memory impairment. White matter changes and disrupted noradrenergic signaling have been implicated in its pathophysiology [201,203].
Cognitive assessments frequently detect slowed reaction times and mental inflexibility in patients without dementia, highlighting the need for sensitive diagnostic tools [224,225,226,227]. Computational modeling suggests that bradyphrenia parallels bradykinesia, with retained memory, but impaired strategy shifting and sensorimotor adaptation [225].
Levodopa shows inconsistent benefits and may worsen cognitive efficiency in some individuals [228]. Non-dopaminergic targets—particularly noradrenergic and cholinergic pathways—warrant further exploration. Improved characterization of bradyphrenia is essential to distinguish it from coexisting depression, apathy, or fatigue.
6.3. Dementia
PD dementia (PDD) affects 20%–40% of patients and develops in up to 80% over time [229]. Mild cognitive impairment in early PD is a strong predictor of dementia, especially in patients with older age, akinetic-rigid phenotype, hallucinations, or cardiovascular comorbidities [229,230].
PDD involves progressive dysfunction of cortico–striato–thalamo–cortical loops, manifesting as executive, visuospatial, and attentional impairments, often accompanied by hallucinations, delusions, and apathy [231,232,233]. While α-synuclein pathology predominates, co-pathologies such as tau, amyloid-β, and TDP-43 contribute to cognitive decline and variability in clinical phenotype [234,235].
Additional contributors include synaptic dysfunction, neuroinflammation, and ageing-related lesions [236,237]. Despite these insights, no approved treatments delay MCI conversion to dementia. Cholinesterase inhibitors provide modest symptomatic benefits, but precision-targeted therapies remain elusive. Future strategies must incorporate biomarker-driven phenotyping and predictive modeling to personalize interventions.
6.4. Impulse Control Disorders and Impulsive-Compulsive Behaviors
Impulse control disorders (ICDs) affect 6%–45% of PD patients, particularly those treated with dopamine agonists [238,239,240,241]. Early-onset PD, male sex, and premorbid addictive traits increase susceptibility. ICDs encompass behaviors such as pathological gambling, compulsive shopping, hypersexuality, and binge eating, leading to significant social and functional disruption [242,243,244].
The pathophysiology involves mesolimbic dopaminergic dysregulation, with contributions from opioid and serotonergic circuits [245,246]. Functional imaging demonstrates enhanced reward sensitivity and impaired inhibitory control. Dopamine agonists potentiate impulsive decision-making and bias reward-based learning [247,248,249].
Reducing dopamine agonist dosage remains first-line management, but often compromises motor control. Cognitive behavioral therapy and novel approaches such as opioid antagonists and neuromodulation are under investigation. Biomarker-guided risk prediction and individualized treatment models will be essential for balancing motor and behavioral outcomes.
7. Emerging Technologies and the Future of Personalized Care
Despite decades of research, PD is still predominantly diagnosed based on motor symptoms that appear late in the disease course—by which time significant and irreversible neurodegeneration has occurred. This clinical inertia persists despite overwhelming evidence that non-motor symptoms, including REM sleep behavior disorder, constipation, hyposmia, depression, and anxiety, can precede motor onset for years or even decades. Nevertheless, these early warning signs remain largely ignored in diagnostic frameworks, risk stratification tools, and therapeutic trials. The continued neglect of these symptoms represents a missed opportunity and a critical failure of the field to evolve beyond outdated staging models and symptomatic care.
Emerging technologies—particularly multi-omic profiling, artificial intelligence (AI), and systems-level brain network modeling—potentially shift this paradigm fundamentally. Rather than treating PD as a single, dopaminergic disorder with a uniform trajectory, these tools support a more accurate characterization of disease heterogeneity, enable the identification of prodromal subtypes, and provide insight into mechanisms underlying non-motor burden.
Multi-omic approaches have begun to reveal molecular fingerprints associated with non-motor-dominant PD phenotypes, yet methodological inconsistency, small cohorts, and the absence of standardized biomarker validation pipelines hinder their translation into clinical practice. Despite these limitations, multi-omics provides a foundation for redefining disease subtypes at the molecular level—something urgently needed if personalized interventions are to be developed [250,251,252,253,254].
AI and machine learning offer unprecedented capacity to integrate large, multidimensional datasets, including longitudinal digital biomarkers from wearable sensors, sleep physiology, neuroimaging, and electronic health records. These algorithms have already successfully detected subtle, preclinical motor and non-motor changes. However, clinical deployment has been slow and regulatory frameworks have yet to catch up. Without rigorous validation and representative datasets, AI risks amplifying bias rather than resolving uncertainty [255,256,257].
Network neuroscience reframes PD as a disorder of dynamic, large-scale brain circuits rather than isolated nigral degeneration. Disrupted connectivity in frontostriatal, limbic, and brain stem networks has been linked to depression, apathy, autonomic failure, and cognitive decline—symptoms that drive disability, but are poorly captured by conventional motor scales. Although still in its infancy, this approach offers a systems-level lens to understand phenotypic variability and treatment response [258,259,260,261,262].
The promise of these technologies lies not in their novelty, but in their capacity to disrupt a model of care that has stagnated. Early identification of PD based on non-motor symptoms is not merely a research priority, but a clinical and ethical imperative. However, the field continues to design trials that exclude early-stage or non-motor-dominant patients, rely on scales developed decades ago, and treat emerging tools as experimental add-ons rather than diagnostic essentials.
To realize the potential of precision neurology, the next phase of PD research must reject uniformity, embrace heterogeneity, and prioritize the full spectrum of disease manifestations. Longitudinal, multicenter, multi-omic datasets integrated with digital phenotyping and network-level imaging must become the standard—not the exception. Only by moving beyond symptom palliation and toward mechanistically informed, stratified intervention can we hope to change the trajectory of PD.
8. Conclusions and Perspectives
Parkinson’s disease (PD) is a multisystem neurodegenerative disorder whose clinical expression extends far beyond its classical motor phenotype. The evidence synthesized throughout this review reinforces a transformative view of PD—one in which non-motor symptoms not only anticipate motor onset, but drive disease burden, accelerate disability, and profoundly affect the quality of life (see Figure 1 and Figure 2; Table 1, Table 2, Table 3, Table 4 and Table 5). These symptoms are neither incidental nor late-stage complications; they represent early and sustained disruptions in autonomic, cognitive, affective, and sensory networks. Despite this recognition, clinical frameworks and research priorities remain anchored in a dopamine-centric model that inadequately addresses the disease’s full complexity [2,3,4,5,6,27,129,131,229].
Non-motor symptoms—including REM sleep behavior disorder, constipation, hyposmia, depression, and cognitive dysfunction—often herald disease onset, yet they are frequently misdiagnosed or dismissed as age-related comorbidities. Their underrecognition impedes early diagnosis, limits timely intervention, and hinders therapeutic innovation. While the Braak staging hypothesis has provided a foundational framework for early pathology, inconsistencies in clinical correlation and α-synuclein topography across patient subtypes underscore the need to reassess this model in favor of a more integrative, systems-based approach [9,10].
The persistence of therapeutic stagnation—particularly in developing disease-modifying strategies—reflects a misalignment between pathophysiological insights and translational application. The neurocentric view of PD, centered on dopaminergic cell loss in the substantia nigra, has failed to account for parallel degeneration across serotonergic, cholinergic, noradrenergic, and glutamatergic systems. These circuits underlie many of the most debilitating non-motor symptoms, including cognitive impairment, mood disturbances, autonomic failure, and sleep dysfunction. Continued focus on dopamine alone cannot reverse or prevent the multisystem degeneration that characterizes PD.
Emerging technologies—such as multi-omic profiling, advanced neuroimaging, and digital biomarkers—offer the tools to redefine PD subtypes and identify at-risk individuals. However, these approaches require validation in large, longitudinal cohorts and integration into personalized clinical frameworks. Precision medicine in PD remains aspirational unless stratification efforts expand beyond motor phenotypes and incorporate non-motor domains from prodromal stages onward.
Cognitive impairment represents a defining, yet undertreated feature of PD. Mild executive deficits, often present at diagnosis, progress toward dementia in the majority of patients over time. The coexistence of α-synuclein pathology with Alzheimer’s-type tau and amyloid deposition in a subset of cases demands a broader conceptualization of PD-related cognitive decline. Current therapies, adapted from Alzheimer’s disease, yield only modest effects and ignore the dopaminergic and cholinergic interplay that shapes cognitive performance in PD. Redefining cognitive impairment as an early and mechanistically distinct manifestation of PD is essential to improve outcomes and delay dementia onset.
Similarly, the field has yet to address the clinical inertia surrounding sleep and autonomic dysfunction. Orthostatic hypotension, urinary symptoms, sialorrhea, constipation, and thermoregulatory instability remain poorly managed despite their high prevalence and early onset. These symptoms reflect central and peripheral autonomic failure, yet therapeutic strategies remain symptomatic, and their integration into routine care is limited. Sleep-related disorders, especially REM sleep behavior disorder, offer valuable diagnostic and prognostic insights, but are underutilized in practice and underrepresented in clinical trials.
Neuropsychiatric symptoms—including depression, anxiety, apathy, hallucinations, and impulse control disorders—emerge early, fluctuate with disease progression, and often coexist with cognitive decline. Their pathophysiology spans multiple neurotransmitter systems and neural circuits, yet pharmacological treatments remain extrapolated from non-neurological populations and rarely consider PD-specific mechanisms. Apathy in particular is frequently misdiagnosed and left untreated, despite its predictive value for cognitive deterioration. Addressing these symptoms requires dismantling artificial boundaries between neurology and psychiatry and adopting circuit-based models of behavioral dysfunction.
The gut–brain axis has opened promising avenues for rethinking PD origins. Enteric α-synuclein accumulation, altered microbiota composition, and increased intestinal permeability have been linked to early disease processes. However, the field has outpaced its evidence base, with many studies lacking mechanistic clarity or therapeutic validation. Intervening at the microbiome level remains an intriguing hypothesis, not a confirmed strategy. Future work must rigorously test whether peripheral interventions can alter central pathology.
The most pressing challenge in PD research is not a lack of hypotheses, but a reluctance to abandon outdated ones. The assumption that PD follows a singular pathological cascade, amenable to a universal intervention, has stymied therapeutic progress. The next generation of breakthroughs will arise not from refining dopaminergic therapies, but from reconceptualizing PD as a constellation of overlapping, subtype-specific syndromes. Embracing clinical and biological heterogeneity is not a limitation—it is the path forward.
This review does not seek to exhaustively catalogue every non-motor manifestation of PD. Instead, it offers a critical synthesis aimed at clinicians, researchers, and educators, advocating for reframing PD as a disorder of systems, not symptoms. To achieve meaningful advances, we must teach it as such, diagnose it accordingly, and treat it beyond its tremors. Non-motor symptoms are not ancillary—they are fundamental to the disease. The future of PD care depends on acknowledging this truth and building the diagnostic and therapeutic infrastructure to act on it.
Author Contributions
Conceptualization, O.A.-C.; methodology, O.A.-C.; investigation, O.A.-C., L.P.-Z., K.M.D.-M., M.M.V.-R., K.L.-A., A.S.-B., J.A.-G., I.P.-S., E.O.-R. and L.O.S.-R.; data curation, L.P.-Z. and O.A.-C.; writing—original draft preparation, O.A.-C., L.P.-Z., K.M.D.-M., M.M.V.-R., K.L.-A., A.S.-B., J.A.-G., I.P.-S., E.O.-R. and L.O.S.-R.; writing—review and editing, L.P.-Z., K.M.D.-M., M.M.V.-R., K.L.-A., A.S.-B., J.A.-G., I.P.-S., E.O.-R. and L.O.S.-R.; visualization, L.P.-Z., K.M.D.-M., M.M.V.-R., K.L.-A., A.S.-B., J.A.-G., I.P.-S., E.O.-R. and L.O.S.-R.; supervision, O.A.-C.; project administration, O.A.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank all those who have made us better—friends, foes, and those who said nothing. Even the sharpest criticism, when true, is a gift. Furthermore, it still teaches us where we stand when it is not. We are made stronger not only by praise but by fire. The manuscript’s clarity and readability were enhanced with the assistance of Grammarly.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| α-syn | α-synuclein |
| CBT | cognitive behavioral therapy |
| CPAP | continuous positive airway pressure |
| CSF | cerebrospinal fluid |
| CSP | cortical silent period |
| DBS | deep brain stimulation |
| DMV | dorsal motor nucleus of the vagus |
| DRT | dopamine replacement therapy |
| EDS | excessive daytime sleepiness |
| EEG | electroencephalogram |
| FOp | frontal insular operculum |
| ICD | impulse control disorder |
| MAO-B | monoamine oxidase B |
| NBM | nucleus basalis of Meynert |
| NPDs | neuropsychiatric disorders |
| NSAID | nonsteroidal anti-inflammatory drug |
| OB | olfactory bulb |
| OFC | orbitofrontal cortex |
| OH | orthostatic hypotension |
| OSA | obstructive sleep apnea |
| PDD | Parkinson’s disease dementia |
| PFC | prefrontal cortex |
| RBD | REM sleep behavior disorder |
| REM | rapid eye movement |
| RLS | restless leg syndrome |
| SC | spinal cord |
| SD | seborrheic dermatitis |
| SN | substantia nigra |
| SNRI | serotonin–norepinephrine reuptake inhibitor |
| SSRI | selective serotonin reuptake inhibitor |
| TCA | tricyclic antidepressant |
| TMS | transcranial magnetic stimulation |
| TNF-α | tumor necrosis factor alpha |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VTA | ventral tegmental area |
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Manuel Rojo-Abuin, J.; Rizos, A.; Rodriguez-Blazquez, C.; Trenkwalder, C.; Perkins, L.; Sauerbier, A.; Odin, P.; Antonini, A.; Chaudhuri, K.R. Distribution and impact on quality of life of the pain modalities assessed by the King’s Parkinson’s disease pain scale. NPJ Park.’s Dis. 2017, 3, 8. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Todorova, A.; Jenner, P.; Ray Chaudhuri, K. Non-motor Parkinson’s: Integral to motor Parkinson’s, yet often neglected. Pract. Neurol. 2014, 14, 310–322. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef]
- Kaufmann, H.; Goldstein, D.S. Autonomic dysfunction in Parkinson disease. Handb. Clin. Neurol. 2013, 117, 259–278. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Zhou, X.; Xiang, Y.; Zhu, L.; Qin, L.; Wang, Y.; Pan, H.; Zhao, Y.; Sun, Q.; et al. Characteristics of Autonomic Dysfunction in Parkinson’s Disease: A Large Chinese Multicenter Cohort Study. Front. Aging Neurosci. 2021, 13, 761044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Stewart, C.B.; Ledingham, D.; Foster, V.K.; Anderson, K.N.; Sathyanarayana, S.; Galley, D.; Pavese, N.; Pasquini, J. The longitudinal progression of autonomic dysfunction in Parkinson’s disease: A 7-year study. Front. Neurol. 2023, 14, 1155669. [Google Scholar] [CrossRef]
- Jain, S. Multi-organ autonomic dysfunction in Parkinson disease. Park. Relat. Disord. 2011, 17, 77–83. [Google Scholar] [CrossRef]
- Park, J.W.; Okamoto, L.E.; Kim, S.H.; Lee, C.N.; Park, K.W.; Baek, S.H.; Sung, J.H.; Jeon, N.; Koh, S.B.; Gamboa, A.; et al. Sympathetic dysfunction as an early indicator of autonomic involvement in Parkinson’s disease. Clin. Auton. Res. 2024, 34, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Chelimsky, T.C. Pure autonomic failure. Handb. Clin. Neurol. 2019, 161, 413–422. [Google Scholar] [CrossRef]
- Isonaka, R.; Sullivan, P.; Goldstein, D.S. Pathophysiological Significance of α-Synuclein in Sympathetic Nerves: In Vivo Observations. Neurology 2025, 104, e210215. [Google Scholar] [CrossRef]
- Wakabayashi, K. Where and how alpha-synuclein pathology spreads in Parkinson’s disease. Neuropathology 2020, 40, 415–425. [Google Scholar] [CrossRef]
- Fumimura, Y.; Ikemura, M.; Saito, Y.; Sengoku, R.; Kanemaru, K.; Sawabe, M.; Arai, T.; Ito, G.; Iwatsubo, T.; Fukayama, M.; et al. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in lewy body disease. J. Neuropathol. Exp. Neurol. 2007, 66, 354–362. [Google Scholar] [CrossRef]
- Shen, L.; Yang, X.; Lu, W.; Chen, W.; Ye, X.; Wu, D. 24-hour ambulatory blood pressure alterations in patients with Parkinson’s disease. Brain Behav. 2022, 12, e2428. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; Van Hilten, J.J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord. 2004, 19, 1306–1312. [Google Scholar] [CrossRef]
- Schestatsky, P.; Valls-Solé, J.; Ehlers, J.A.; Rieder, C.R.; Gomes, I. Hyperhidrosis in Parkinson’s disease. Mov. Disord. 2006, 21, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.C.; Cuenca-Bermejo, L.; Fernandez-Villalba, E.; Martin-Balbuena, S.; da Silva Fernandes, M.J.; Scorza, C.A.; Herrero, M.T. Heart Matters: Cardiac Dysfunction and Other Autonomic Changes in Parkinson’s Disease. Neuroscientist 2022, 28, 530–542. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakamura, T.; Hirayama, M.; Ueda, M.; Katsuno, M.; Sobue, G. Cardiac parasympathetic dysfunction in the early phase of Parkinson’s disease. J. Neurol. 2017, 264, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.; Kaufmann, H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov. Disord. 2018, 33, 372–390. [Google Scholar] [CrossRef]
- van Wamelen, D.J.; Leta, V.; Johnson, J.; Ocampo, C.L.; Podlewska, A.M.; Rukavina, K.; Rizos, A.; Martinez-Martin, P.; Chaudhuri, K.R. Drooling in Parkinson’s Disease: Prevalence and Progression from the Non-motor International Longitudinal Study. Dysphagia 2020, 35, 955–961. [Google Scholar] [CrossRef]
- Polychronis, S.; Nasios, G.; Dardiotis, E.; Messinis, L.; Pagano, G. Pathophysiology and Symptomatology of Drooling in Parkinson’s Disease. Healthcare 2022, 10, 516. [Google Scholar] [CrossRef]
- Isaacson, J.; Patel, S.; Torres-Yaghi, Y.; Pagán, F. Sialorrhea in Parkinson’s Disease. Toxins 2020, 12, 691. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ge, N.N.; Zhu, H.H.; Sha, Z.T.; Jiang, T.; Zhang, Y.D.; Tian, Y.Y. Dihydroergotoxine mesylate for the treatment of sialorrhea in Parkinson’s disease. Park. Relat. Disord. 2019, 58, 70–73. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Umemoto, G.; Furuya, H. Management of Dysphagia in Patients with Parkinson’s Disease and Related Disorders. Intern. Med. 2020, 59, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Labeit, B.; Berkovich, E.; Claus, I.; Roderigo, M.; Schwake, A.L.; Izgelov, D.; Mimrod, D.; Ahring, S.; Oelenberg, S.; Muhle, P.; et al. Dysphagia for medication in Parkinson’s disease. NPJ Park.’s Dis. 2022, 8, 156. [Google Scholar] [CrossRef]
- Cosentino, G.; Avenali, M.; Schindler, A.; Pizzorni, N.; Montomoli, C.; Abbruzzese, G.; Antonini, A.; Barbiera, F.; Benazzo, M.; Benarroch, E.E.; et al. A multinational consensus on dysphagia in Parkinson’s disease: Screening, diagnosis and prognostic value. J. Neurol. 2022, 269, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- López-Liria, R.; Parra-Egeda, J.; Vega-Ramírez, F.A.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Morales-Gázquez, M.J.; Rocamora-Pérez, P. Treatment of Dysphagia in Parkinson’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4104. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Huang, J.P.; Tan, Y.C.; Wang, T.T.; Zhang, H.; Qu, Y. The effectiveness and safety of botulinum toxin injections for the treatment of sialorrhea with Parkinson’s disease: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2023, 24, 52. [Google Scholar] [CrossRef]
- Safarpour, D.; Stover, N.; Shprecher, D.R.; Hamedani, A.G.; Pfeiffer, R.F.; Parkman, H.P.; Quigley, E.M.; Cloud, L.J. Consensus practice recommendations for management of gastrointestinal dysfunction in Parkinson disease. Park. Relat. Disord. 2024, 124, 106982. [Google Scholar] [CrossRef]
- Savica, R.; Boeve, B.F.; Mielke, M.M. When Do α-Synucleinopathies Start? An Epidemiological Timeline: A Review. JAMA Neurol. 2018, 75, 503–509. [Google Scholar] [CrossRef]
- Stocchi, F.; Torti, M. Constipation in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 811–826. [Google Scholar] [CrossRef]
- Yu, Q.J.; Yu, S.Y.; Zuo, L.J.; Lian, T.H.; Hu, Y.; Wang, R.D.; Piao, Y.S.; Guo, P.; Liu, L.; Jin, Z.; et al. Parkinson disease with constipation: Clinical features and relevant factors. Sci. Rep. 2018, 8, 567. [Google Scholar] [CrossRef]
- Frazzitta, G.; Ferrazzoli, D.; Folini, A.; Palamara, G.; Maestri, R. Severe Constipation in Parkinson’s Disease and in Parkinsonisms: Prevalence and Affecting Factors. Front. Neurol. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa Carrasco, A.J.; Timmermann, L.; Pedrosa, D.J. Management of constipation in patients with Parkinson’s disease. NPJ Park.’s Dis. 2018, 4, 6. [Google Scholar] [CrossRef]
- Raciti, L.; De Cola, M.C.; Ortelli, P.; Corallo, F.; Lo Buono, V.; Morini, E.; Quattrini, F.; Filoni, S.; Calabrò, R.S. Sexual Dysfunction in Parkinson Disease: A Multicenter Italian Cross-sectional Study on a Still Overlooked Problem. J. Sex. Med. 2020, 17, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Benigno, M.D.S.; Amaral Domingues, C.; Araujo Leite, M.A. Sexual Dysfunction in Parkinson’s Disease: A Systematic Review of the Arizona Sexual Experience Scale Sexual Dysfunction in Parkinson Disease: A Systematic Review of the Arizona Sexual Experience Scale. J. Geriatr. Psychiatry Neurol. 2023, 36, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Haktanır, D.; Yılmaz, S. Sexual Dysfunction and Related Factors in Patients with Parkinson’s Disease. J. Psychosoc. Nurs. Ment. Health Serv. 2023, 61, 45–55. [Google Scholar] [CrossRef]
- Codling, D.; Shaw, P.; David, A.S. Hypersexuality in Parkinson’s Disease: Systematic Review and Report of 7 New Cases. Mov. Disord. Clin. Pract. 2015, 2, 116–126. [Google Scholar] [CrossRef]
- Jost, W.H. Autonomic dysfunctions in idiopathic Parkinson’s disease. J. Neurol. 2003, 250 (Suppl. 1), I28–I30. [Google Scholar] [CrossRef]
- Robinson, B.W.; Mishkin, M. Penile erection evoked from forebrain structures in Macaca mulatta. Arch. Neurol. 1968, 19, 184–198. [Google Scholar] [CrossRef]
- Temel, Y.; Visser-Vandewalle, V.; Ackermans, L.; Beuls, E.A. Thalamus and penile erection. Int. J. Impot. Res. 2004, 16, 505–511. [Google Scholar] [CrossRef][Green Version]
- Pavy-Le Traon, A.; Cotterill, N.; Amarenco, G.; Duerr, S.; Kaufmann, H.; Lahrmann, H.; Tison, F.; Wenning, G.K.; Goetz, C.G.; Poewe, W.; et al. Clinical Rating Scales for Urinary Symptoms in Parkinson Disease: Critique and Recommendations. Mov. Disord. Clin. Pract. 2018, 5, 479–491. [Google Scholar] [CrossRef]
- Li, F.F.; Cui, Y.S.; Yan, R.; Cao, S.S.; Feng, T. Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 977572. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Bartolotta, T.V.; Cosentino, G.; Mastrilli, S.; Arnao, V.; Aridon, P.; Scurria, S.; Pavone, A.; Pavone, C.; D’Amelio, M. Urological dysfunctions in patients with Parkinson’s disease: Clues from clinical and non-invasive urological assessment. BMC Neurol. 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Nam, D.; Lee, J.Y.; Lee, M.; Kim, J.; Seol, W.; Son, I.; Ho, D.H. Detection and Assessment of α-Synuclein Oligomers in the Urine of Parkinson’s Disease Patients. J. Park’s Dis. 2020, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Cui, X.; Yoshimura, N.; Mao, W.; Xu, E.; Wang, Q.; Ou, T. Assessment and Management of Urinary Dysfunction in 187 Patients with Parkinson’s Disease. J. Park’s Dis. 2020, 10, 993–1001. [Google Scholar] [CrossRef]
- Yeo, L.; Singh, R.; Gundeti, M.; Barua, J.M.; Masood, J. Urinary tract dysfunction in Parkinson’s disease: A review. Int. Urol. Nephrol. 2012, 44, 415–424. [Google Scholar] [CrossRef]
- Li, S.T.; Dendi, R.; Holmes, C.; Goldstein, D.S. Progressive loss of cardiac sympathetic innervation in Parkinson’s disease. Ann. Neurol. 2002, 52, 220–223. [Google Scholar] [CrossRef]
- Cuenca-Bermejo, L.; Almela, P.; Navarro-Zaragoza, J.; Fernández Villalba, E.; González-Cuello, A.M.; Laorden, M.L.; Herrero, M.T. Cardiac Changes in Parkinson’s Disease: Lessons from Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 13488. [Google Scholar] [CrossRef]
- Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J. Clin. Neurosci. 2018, 53, 1–5. [Google Scholar] [CrossRef]
- Satoh, A.; Serita, T.; Seto, M.; Tomita, I.; Satoh, H.; Iwanaga, K.; Takashima, H.; Tsujihata, M. Loss of 123I-MIBG uptake by the heart in Parkinson’s disease: Assessment of cardiac sympathetic denervation and diagnostic value. J. Nucl. Med. 1999, 40, 371–375. [Google Scholar]
- Orimo, S.; Uchihara, T.; Nakamura, A.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008, 131, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Turrise, S.; MacLaughlin, E.J.; Chang, P.S.; Clair, W.K.; Lewis, E.F.; Forman, D.E.; Goodlin, S.J. Preventing and Managing Falls in Adults with Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e000108. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Autonomic Dysfunction in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1464–1479. [Google Scholar] [CrossRef]
- Lamotte, G.; Lenka, A. Orthostatic Hypotension in Parkinson Disease: What Is New? Neurol. Clin. Pract. 2022, 12, e112–e115. [Google Scholar] [CrossRef] [PubMed]
- Cutsforth-Gregory, J.K.; Low, P.A. Neurogenic Orthostatic Hypotension in Parkinson Disease: A Primer. Neurol. Ther. 2019, 8, 307–324. [Google Scholar] [CrossRef]
- Fathy, Y.Y.; Jonker, A.J.; Oudejans, E.; de Jong, F.J.J.; van Dam, A.W.; Rozemuller, A.J.M.; van De Berg, W.D.J. Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol. Appl. Neurobiol. 2019, 45, 262–277. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Q.; Xu, E.; Zeng, J.; Li, Q.; Mei, S.; Hua, Y. Impaired Cerebral Autoregulation in Parkinson’s Disease: An Orthostatic Hypotension Analysis. Front. Neurol. 2022, 13, 811698. [Google Scholar] [CrossRef]
- Goldstein, D.S. Dysautonomia in Parkinson’s disease: Neurocardiological abnormalities. Lancet Neurol. 2003, 2, 669–676. [Google Scholar] [CrossRef]
- Fujita, H.; Ogaki, K.; Shiina, T.; Sakuramoto, H.; Nozawa, N.; Suzuki, K. Impact of autonomic symptoms on the clinical course of Parkinson’s disease. Neurol. Sci. 2024, 45, 3799–3807. [Google Scholar] [CrossRef]
- Tulbă, D.; Cozma, L.; Bălănescu, P.; Buzea, A.; Băicuș, C.; Popescu, B.O. Blood Pressure Patterns in Patients with Parkinson’s Disease: A Systematic Review. J. Pers. Med. 2021, 11, 129. [Google Scholar] [CrossRef]
- Velseboer, D.C.; de Haan, R.J.; Wieling, W.; Goldstein, D.S.; de Bie, R.M. Prevalence of orthostatic hypotension in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2011, 17, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Sano, U. Finding the Balance: Review of Pharmacological Management of Orthostatic Hypotension in Patients with Parkinson’s Disease. J. Gerontol. Nurs. 2024, 50, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Tomic, S.; Kuric, I.; Kuric, T.G.; Popovic, Z.; Kragujevic, J.; Zubonja, T.M.; Rajkovaca, I.; Matosa, S. Seborrheic Dermatitis Is Related to Motor Symptoms in Parkinson’s Disease. J. Clin. Neurol. 2022, 18, 628–634. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Laurence, M.; Benito-León, J.; Calon, F. Malassezia and Parkinson’s Disease. Front. Neurol. 2019, 10, 758. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Clark, G.W.; Pope, S.M.; Jaboori, K.A. Diagnosis and treatment of seborrheic dermatitis. Am. Fam. Physician 2015, 91, 185–190. [Google Scholar]
- Schestatsky, P.; Ehlers, J.A.; Rieder, C.R.; Gomes, I. Evaluation of sympathetic skin response in Parkinson’s disease. Park. Relat. Disord. 2006, 12, 486–491. [Google Scholar] [CrossRef]
- Swinn, L.; Schrag, A.; Viswanathan, R.; Bloem, B.R.; Lees, A.; Quinn, N. Sweating dysfunction in Parkinson’s disease. Mov. Disord. 2003, 18, 1459–1463. [Google Scholar] [CrossRef]
- Greaney, J.L.; Alexander, L.M.; Kenney, W.L. Sympathetic control of reflex cutaneous vasoconstriction in human aging. J. Appl. Physiol. 2015, 119, 771–782. [Google Scholar] [CrossRef]
- Dabby, R.; Djaldetti, R.; Shahmurov, M.; Treves, T.A.; Gabai, B.; Melamed, E.; Sadeh, M.; Avinoach, I. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J. Neural Transm. 2006, 113, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M. Sweating dysfunctions in Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. 7), vii42–vii47. [Google Scholar] [CrossRef]
- Benson, R.A.; Palin, R.; Holt, P.J.; Loftus, I.M. Diagnosis and management of hyperhidrosis. Br. Med. J. 2013, 347, f6800. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.A.; Low, P.A. Thermoregulation in Parkinson disease. Handb. Clin. Neurol. 2018, 157, 715–725. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Bavestrello Piccini, G.; Gri, N.; Nardone, A.; La Russa, R.; Saviano, A.; Piccioni, A.; Ricevuti, G.; Esposito, C. Hypothermia: Beyond the Narrative Review—The Point of View of Emergency Physicians and Medico-Legal Considerations. J. Pers. Med. 2023, 13, 1690. [Google Scholar] [CrossRef]
- Krämer, H.H.; Lautenschläger, G.; de Azevedo, M.; Doppler, K.; Schänzer, A.; Best, C.; Oertel, W.H.; Reuter, I.; Sommer, C.; Birklein, F. Reduced central sympathetic activity in Parkinson’s disease. Brain Behav. 2019, 9, e01463. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Estraneo, A.; Selim, M.M.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Lanzillo, B.; Santoro, L. Sensory deficit in Parkinson’s disease: Evidence of a cutaneous denervation. Brain 2008, 131, 1903–1911. [Google Scholar] [CrossRef]
- Haehner, A.; Hummel, T.; Reichmann, H. Olfactory loss in Parkinson’s disease. Park.’s Dis. 2011, 2011, 450939. [Google Scholar] [CrossRef]
- Ansari, K.A.; Johnson, A. Olfactory function in patients with Parkinson’s disease. J. Chronic Dis. 1975, 28, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Boesveldt, S.; Berendse, H.W.; Mackay-Sim, A.; Fleischmann, J.; Silburn, P.A.; Johnston, A.N.; Mellick, G.D.; Herting, B.; Reichmann, H.; et al. Prevalence of smell loss in Parkinson’s disease—A multicenter study. Park. Relat. Disord. 2009, 15, 490–494. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, E.J.; Zhang, W.; Lu, Y.; Liu, R.; Huang, X.; Ciesielski-Jones, A.J.; Justice, M.A.; Cousins, D.S.; Peddada, S. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl. Neurodegener. 2015, 4, 1. [Google Scholar] [CrossRef]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhou, C.; Li, J.; Chen, L.; Yang, X.; Li, F. Hyposmia as a Predictive Marker of Parkinson’s Disease: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 3753786. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Roos, D.S.; Twisk, J.W.R.; Raijmakers, P.; Doty, R.L.; Berendse, H.W. Hyposmia as a marker of (non-)motor disease severity in Parkinson’s disease. J. Neural Transm. 2019, 126, 1471–1478. [Google Scholar] [CrossRef]
- He, R.; Zhao, Y.; He, Y.; Zhou, Y.; Yang, J.; Zhou, X.; Zhu, L.; Zhou, X.; Liu, Z.; Xu, Q.; et al. Olfactory Dysfunction Predicts Disease Progression in Parkinson’s Disease: A Longitudinal Study. Front. Neurosci. 2020, 14, 569777. [Google Scholar] [CrossRef]
- Fang, T.C.; Chang, M.H.; Yang, C.P.; Chen, Y.H.; Lin, C.H. The Association of Olfactory Dysfunction with Depression, Cognition, and Disease Severity in Parkinson’s Disease. Front. Neurol. 2021, 12, 779712. [Google Scholar] [CrossRef]
- Fujita, H.; Shiina, T.; Sakuramoto, H.; Nozawa, N.; Ogaki, K.; Suzuki, K. Sleep and Autonomic Manifestations in Parkinson’s Disease Complicated with Probable Rapid Eye Movement Sleep Behavior Disorder. Front. Neurosci. 2022, 16, 874349. [Google Scholar] [CrossRef]
- Sengoku, R.; Matsushima, S.; Bono, K.; Sakuta, K.; Yamazaki, M.; Miyagawa, S.; Komatsu, T.; Mitsumura, H.; Kono, Y.; Kamiyama, T.; et al. Olfactory function combined with morphology distinguishes Parkinson’s disease. Park. Relat. Disord. 2015, 21, 771–777. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Kim, J.Y.; Park, J.H.; Lee, J.E.; Park, S.I.; Yim, Y. Clinical significance of MRI-measured olfactory bulb height as an imaging biomarker of idiopathic Parkinson’s disease. PLoS ONE 2024, 19, e0312728. [Google Scholar] [CrossRef]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef]
- Srivastava, A.; Wang, Q.; Orrù, C.D.; Fernandez, M.; Compta, Y.; Ghetti, B.; Zanusso, G.; Zou, W.Q.; Caughey, B.; Beauchemin, C.A.A. Enhanced quantitation of pathological α-synuclein in patient biospecimens by RT-QuIC seed amplification assays. PLoS Pathog. 2024, 20, e1012554. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Lee, S.; Jeong, S.H.; Ye, B.S.; Sohn, Y.H.; Yun, M.; Lee, P.H. Clinical and Dopamine Depletion Patterns in Hyposmia- and Dysautonomia-Dominant Parkinson’s Disease. J. Park.’s Dis. 2021, 11, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Zhao, X.; Nie, W.; Xu, X.; Liu, M.; Zhang, X. Olfactory dysfunction and its related molecular mechanisms in Parkinson’s disease. Neural Regen. Res. 2024, 19, 583–590. [Google Scholar] [CrossRef]
- Shah, M.; Deeb, J.; Fernando, M.; Noyce, A.; Visentin, E.; Findley, L.J.; Hawkes, C.H. Abnormality of taste and smell in Parkinson’s disease. Park. Relat. Disord. 2009, 15, 232–237. [Google Scholar] [CrossRef]
- Kashihara, K.; Hanaoka, A.; Imamura, T. Frequency and characteristics of taste impairment in patients with Parkinson’s disease: Results of a clinical interview. Intern. Med. 2011, 50, 2311–2315. [Google Scholar] [CrossRef]
- Cossu, G.; Melis, M.; Sarchioto, M.; Melis, M.; Melis, M.; Morelli, M.; Tomassini Barbarossa, I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov. Disord. 2018, 33, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Jagota, P.; Chotechuang, N.; Anan, C.; Kitjawijit, T.; Boonla, C.; Bhidayasiri, R. Umami and Other Taste Perceptions in Patients with Parkinson’s Disease. J. Mov. Disord. 2022, 15, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Oppo, V.; Melis, M.; Melis, M.; Tomassini Barbarossa, I.; Cossu, G. "Smelling and Tasting" Parkinson’s Disease: Using Senses to Improve the Knowledge of the Disease. Front. Aging Neurosci. 2020, 12, 43. [Google Scholar] [CrossRef]
- Crowley, S.J.; Kanel, P.; Roytman, S.; Bohnen, N.I.; Hampstead, B.M. Basal forebrain integrity, cholinergic innervation and cognition in idiopathic Parkinson’s disease. Brain 2024, 147, 1799–1808. [Google Scholar] [CrossRef]
- Mantovani, E.; Zanini, A.; Cecchini, M.P.; Tamburin, S. The Association Between Neurocognitive Disorders and Gustatory Dysfunction: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2024, 34, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Esparcia, P.; Schlüter, A.; Carmona, M.; Moreno, J.; Ansoleaga, B.; Torrejón-Escribano, B.; Gustincich, S.; Pujol, A.; Ferrer, I. Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: Novel putative chemoreceptors in the human brain. J. Neuropathol. Exp. Neurol. 2013, 72, 524–539. [Google Scholar] [CrossRef]
- van der Lijn, I.; de Haan, G.A.; Huizinga, F.; van der Feen, F.E.; Rutgers, A.W.F.; Stellingwerf, C.; van Laar, T.; Heutink, J. Self-Reported Visual Complaints in People with Parkinson’s Disease: A Systematic Review. J. Park’s Dis. 2022, 12, 785–806. [Google Scholar] [CrossRef]
- van der Lijn, I.; de Haan, G.A.; van der Feen, F.E.; Huizinga, F.; Stellingwerf, C.; van Laar, T.; Heutink, J. Prevalence and nature of self-reported visual complaints in people with Parkinson’s disease—Outcome of the Screening Visual Complaints questionnaire. PLoS ONE 2023, 18, e0283122. [Google Scholar] [CrossRef]
- Brown, T.; Kanel, P.; Griggs, A.; Carli, G.; Vangel, R.; Albin, R.L.; Bohnen, N.I. Regional cerebral cholinergic vesicular transporter correlates of visual contrast sensitivity in Parkinson’s disease: Implications for visual and cognitive function. Park. Relat. Disord. 2025, 131, 107229. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Beach, T.G.; Carew, J.; Serrano, G.; Adler, C.H.; Shill, H.A.; Sue, L.I.; Sabbagh, M.N.; Akiyama, H.; Cuenca, N. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci. Lett. 2014, 571, 34–38. [Google Scholar] [CrossRef]
- Ortuño-Lizarán, I.; Beach, T.G.; Serrano, G.E.; Walker, D.G.; Adler, C.H.; Cuenca, N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord. 2018, 33, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Lax, P.; Serrano, G.E.; Beach, T.G.; Adler, C.H.; Cuenca, N. Dopaminergic Retinal Cell Loss and Visual Dysfunction in Parkinson Disease. Ann. Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, J.; Zhou, L.; Liu, X.; Xiao, Z.; He, R.; Chu, H.; Tang, Y.; Liu, P.; Lu, X. Retinopathy in Parkinson’s disease: A potential biomarker for early diagnosis and clinical assessment. Neuroscience 2025, 565, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Eslami, F.; Ghiasian, M.; Mohamadrahimi, B.; Jiriaee, N.; Eslamighayour, A. Optical coherence tomography (OCT) findings in patients with Parkinson’s disease presenting to Farshchian Hospital (Sina) in 2019 compared to the normal population. J. Fr. Ophtalmol. 2025, 48, 104379. [Google Scholar] [CrossRef]
- Bilgin, Ş.; Uysal, H.A.; Bilgin, S.; Yaka, E.C.; Küsbeci, Ö.Y.; Şener, U. Assessment of Changes in Vascular Density in the Layers of the Eye in Patients with Parkinson’s Disease. Ann. Neurosci. 2024, 09727531241259841. [Google Scholar] [CrossRef]
- Erdem, M.; Soker, E.B.; Ozdogru, D.; Balal, M.; Ciloglu, E. Evaluation of retinal microvascular changes with OCT-A in Parkinson disease and essential tremor. Medicine 2024, 103, e40752. [Google Scholar] [CrossRef]
- Hamedani, A.G.; Abraham, D.S.; Maguire, M.G.; Willis, A.W. Visual Impairment Is More Common in Parkinson’s Disease and Is a Risk Factor for Poor Health Outcomes. Mov. Disord. 2020, 35, 1542–1549. [Google Scholar] [CrossRef]
- Mylius, V.; Perez Lloret, S.; Cury, R.G.; Teixeira, M.J.; Barbosa, V.R.; Barbosa, E.R.; Moreira, L.I.; Listik, C.; Fernandes, A.M.; de Lacerda Veiga, D.; et al. The Parkinson disease pain classification system: Results from an international mechanism-based classification approach. Pain 2021, 162, 1201–1210. [Google Scholar] [CrossRef]
- Nogueira, A.C.R.; Pereira, K.C.; Rodrigues, V.F.; Alves, D.P.A.; Marques, J.B.; Monteiro, E.R.; Jesus, I.R.T. Pain characterization in patients with Parkinson’s disease. Pain Pract. 2024, 24, 786–797. [Google Scholar] [CrossRef]
- Shalash, A.; Mohamed, S.R.; Badr, M.Y.; Elgamal, S.; Elaidy, S.A.; Elhamrawy, E.A.; Abdel-Tawab, H.; Elshebawy, H.; Abdelraheem, H.S.; Roushdy, T.; et al. Pain Characteristics of Parkinson’s Disease Using Validated Arabic Versions of the King’s Parkinson’s Disease Pain Scale and Questionnaire: A Multicenter Egyptian Study. J. Mov. Disord. 2024, 17, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, D.; Gambioli, F.; De Bartolo, M.I.; Mancinelli, R.; Biagioni, F.; Carotti, S.; Falato, E.; Leodori, G.; Puglisi-Allegra, S.; Vivacqua, G.; et al. Pain in Parkinson’s disease: A neuroanatomy-based approach. Brain Commun. 2024, 6, fcae210. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.T.; Lin, C.H. Pain in early-stage Parkinson’s disease: Implications from clinical features to pathophysiology mechanisms. J. Formos. Med. Assoc. 2017, 116, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.C.; Lin, C.H. An overview of pain in Parkinson’s disease. Clin. Park. Relat. Disord. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Cattaneo, C.; Jost, W.H. Pain in Parkinson’s Disease: Pathophysiology, Classification and Treatment. J. Integr. Neurosci. 2023, 22, 132. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Antonini, A.; Smania, N.; Tinazzi, M. Understanding and Treating Pain Syndromes in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 827–858. [Google Scholar] [CrossRef]
- Roversi, K.; Callai-Silva, N.; Roversi, K.; Griffith, M.; Boutopoulos, C.; Prediger, R.D.; Talbot, S. Neuro-Immunity and Gut Dysbiosis Drive Parkinson’s Disease-Induced Pain. Front. Immunol. 2021, 12, 759679. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Zhou, J.; Zou, H.; Ma, L.; Liu, C.; Xiao, Z.; Liu, X.; Tan, X.; Yu, T.; et al. Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J. Neuroinflam. 2022, 19, 123. [Google Scholar] [CrossRef]
- Thompson, T.; Gallop, K.; Correll, C.U.; Carvalho, A.F.; Veronese, N.; Wright, E.; Stubbs, B. Pain perception in Parkinson’s disease: A systematic review and meta-analysis of experimental studies. Ageing Res. Rev. 2017, 35, 74–86. [Google Scholar] [CrossRef]
- Corrà, M.F.; Vila-Chã, N.; Sardoeira, A.; Hansen, C.; Sousa, A.P.; Reis, I.; Sambayeta, F.; Damásio, J.; Calejo, M.; Schicketmueller, A.; et al. Peripheral neuropathy in Parkinson’s disease: Prevalence and functional impact on gait and balance. Brain 2023, 146, 225–236. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Garcia, J.; Wang, N.; Shih, L.C.; Freeman, R. The diagnostic discrimination of cutaneous α-synuclein deposition in Parkinson disease. Neurology 2016, 87, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Doppler, K.; Jentschke, H.M.; Schulmeyer, L.; Vadasz, D.; Janzen, A.; Luster, M.; Höffken, H.; Mayer, G.; Brumberg, J.; Booij, J.; et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 2017, 133, 535–545. [Google Scholar] [CrossRef] [PubMed]
- León-Bejarano, F.; Méndez, M.O.; Alba, A.; Rodríguez-Leyva, I.; González, F.J.; Rodríguez-Aranda, M.D.C.; Guevara, E.; Guirado-López, R.A.; Ramírez-Elías, M.G. Raman Spectroscopy Study of Skin Biopsies from Patients with Parkinson’s Disease: Trends in Alpha-Synuclein Aggregation from the Amide I Region. Appl. Spectrosc. 2022, 76, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, S.; Yang, J.; Zheng, H.; Yuan, Y.; Tang, M.; Wang, Y.; Liu, Y.; Xia, Z.; Luo, H.; et al. Detection of skin α-synuclein using RT-QuIC as a diagnostic biomarker for Parkinson’s disease in the Chinese population. Eur. J. Med. Res. 2024, 29, 114. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Sun, L.; Zhi, Y.; Ding, J.; Yuan, Y.S.; Shen, F.F.; Li, X.; Ji, P.; Wang, Z.; et al. Phosphorylated α-synuclein deposits in sural nerve deriving from Schwann cells: A biomarker for Parkinson’s disease. Park. Relat. Disord. 2019, 60, 57–63. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Manganelli, F.; Iodice, R.; Stancanelli, A.; Caporaso, G.; Saltalamacchia, A.; Califano, F.; Lanzillo, B.; Picillo, M.; et al. Loss of cutaneous large and small fibers in naive and l-dopa-treated PD patients. Neurology 2017, 89, 776–784. [Google Scholar] [CrossRef]
- Vacchi, E.; Senese, C.; Chiaro, G.; Disanto, G.; Pinton, S.; Morandi, S.; Bertaina, I.; Bianco, G.; Staedler, C.; Galati, S.; et al. Alpha-synuclein oligomers and small nerve fiber pathology in skin are potential biomarkers of Parkinson’s disease. NPJ Park.’s Dis. 2021, 7, 119. [Google Scholar] [CrossRef]
- Melli, G.; Vacchi, E.; Biemmi, V.; Galati, S.; Staedler, C.; Ambrosini, R.; Kaelin-Lang, A. Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann. Clin. Transl. Neurol. 2018, 5, 1394–1407. [Google Scholar] [CrossRef]
- Arias-Carrion, O.; Ortega-Robles, E.; Ortuno-Sahagun, D.; Ramirez-Bermudez, J.; Hamid, A.; Shalash, A. Sleep-Related Disorders in Parkinson’s Disease: Mechanisms, Diagnosis, and Therapeutic Approaches. CNS Neurol. Disord. Drug Targets 2025, 24, 132–143. [Google Scholar] [CrossRef]
- Duan, X.; Liu, H.; Hu, X.; Yu, Q.; Kuang, G.; Liu, L.; Zhang, S.; Wang, X.; Li, J.; Yu, D.; et al. Insomnia in Parkinson’s Disease: Causes, Consequences, and Therapeutic Approaches. Mol. Neurobiol. 2025, 62, 2292–2313. [Google Scholar] [CrossRef]
- Stefani, A.; Högl, B. Sleep in Parkinson’s disease. Neuropsychopharmacology 2020, 45, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Liu, X.; Zu, J.; Zhang, W.; Zhou, S.; Zhu, J.; Zhang, T.; Cui, G.; Xu, C. Association between serum neurofilament light chain levels and sleep disorders in patients with Parkinson’s disease. Neurosci. Lett. 2023, 812, 137394. [Google Scholar] [CrossRef] [PubMed]
- Burchill, E.; Watson, C.J.; Fanshawe, J.B.; Badenoch, J.B.; Rengasamy, E.; Ghanem, D.A.; Holle, C.; Conti, I.; Sadeq, M.A.; Saini, A.; et al. The impact of psychiatric comorbidity on Parkinson’s disease outcomes: A systematic review and meta-analysis. Lancet Reg. Health Eur. 2024, 39, 100870. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, D.; Sousa, M.; Maradan-Gachet, M.E.; Debove, I.; Lhommée, E.; Krack, P. Psychiatric and cognitive symptoms of Parkinson’s disease: A life’s tale. Rev. Neurol. 2025, 181, 265–283. [Google Scholar] [CrossRef]
- Macías-García, P.; Rashid-López, R.; Cruz-Gómez, Á.J.; Lozano-Soto, E.; Sanmartino, F.; Espinosa-Rosso, R.; González-Rosa, J.J. Neuropsychiatric Symptoms in Clinically Defined Parkinson’s Disease: An Updated Review of Literature. Behav. Neurol. 2022, 2022, 1213393. [Google Scholar] [CrossRef]
- Cong, S.; Xiang, C.; Zhang, S.; Zhang, T.; Wang, H.; Cong, S. Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci. Biobehav. Rev. 2022, 141, 104749. [Google Scholar] [CrossRef]
- Qin, Y.; Li, J.; Quan, W.; Song, J.; Xu, J.; Chen, J. Risk of Parkinson’s disease and depression severity in different populations: A two-sample Mendelian randomization analysis. Brain Behav. 2024, 14, e3642. [Google Scholar] [CrossRef]
- Badenoch, J.B.; Paris, A.; Jacobs, B.M.; Noyce, A.J.; Marshall, C.R.; Waters, S. Neuroanatomical and prognostic associations of depression in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2024, 95, 966–973. [Google Scholar] [CrossRef]
- Pasquini, J.; Ceravolo, R.; Brooks, D.J.; Bonuccelli, U.; Pavese, N. Progressive loss of raphe nuclei serotonin transporter in early Parkinson’s disease: A longitudinal (123) I-FP-CIT SPECT study. Park. Relat. Disord. 2020, 77, 170–175. [Google Scholar] [CrossRef]
- Brazdis, R.M.; von Zimmermann, C.; Lenz, B.; Kornhuber, J.; Mühle, C. Peripheral Upregulation of Parkinson’s Disease-Associated Genes Encoding α-Synuclein, β-Glucocerebrosidase, and Ceramide Glucosyltransferase in Major Depression. Int. J. Mol. Sci. 2024, 25, 3219. [Google Scholar] [CrossRef]
- Sandoval-Rincon, M.; Saenz-Farret, M.; Miguel-Puga, A.; Micheli, F.; Arias-Carrion, O. Rational pharmacological approaches for cognitive dysfunction and depression in Parkinson’s disease. Front. Neurol. 2015, 6, 71. [Google Scholar] [CrossRef]
- den Brok, M.G.; van Dalen, J.W.; van Gool, W.A.; Moll van Charante, E.P.; de Bie, R.M.; Richard, E. Apathy in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2015, 30, 759–769. [Google Scholar] [CrossRef]
- Lee, S.; Song, E.; Zhu, M.; Appel-Cresswell, S.; McKeown, M.J. Apathy scores in Parkinson’s disease relate to EEG components in an incentivized motor task. Brain Commun. 2024, 6, fcae025. [Google Scholar] [CrossRef] [PubMed]
- Wee, N.; Kandiah, N.; Acharyya, S.; Chander, R.J.; Ng, A.; Au, W.L.; Tan, L.C. Baseline predictors of worsening apathy in Parkinson’s disease: A prospective longitudinal study. Park. Relat. Disord. 2016, 23, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.; MacAskill, M.; Pascoe, M.; Anderson, T.; Heron, C.L. Dimensions of apathy in Parkinson’s disease. Brain Behav. 2023, 13, e2862. [Google Scholar] [CrossRef]
- Robert, P.; Lanctôt, K.L.; Agüera-Ortiz, L.; Aalten, P.; Bremond, F.; Defrancesco, M.; Hanon, C.; David, R.; Dubois, B.; Dujardin, K.; et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry 2018, 54, 71–76. [Google Scholar] [CrossRef]
- Ou, R.; Lin, J.; Liu, K.; Jiang, Z.; Wei, Q.; Hou, Y.; Zhang, L.; Cao, B.; Zhao, B.; Song, W.; et al. Evolution of Apathy in Early Parkinson’s Disease: A 4-Years Prospective Cohort Study. Front. Aging Neurosci. 2020, 12, 620762. [Google Scholar] [CrossRef]
- Le Heron, C.; Horne, K.L.; MacAskill, M.R.; Livingstone, L.; Melzer, T.R.; Myall, D.; Pitcher, T.; Dalrymple-Alford, J.; Anderson, T.; Harrison, S. Cross-Sectional and Longitudinal Association of Clinical and Neurocognitive Factors with Apathy in Patients with Parkinson Disease. Neurology 2024, 102, e209301. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Cao, X.; Wang, L.; Gan, C.; Sun, H.; Shan, A.; Yuan, Y.; Zhang, K. Association between the functional connectivity of ventral tegmental area-prefrontal network and pure apathy in Parkinson’s disease: A cross-sectional study. Quant. Imaging Med. Surg. 2024, 14, 4735–4748. [Google Scholar] [CrossRef]
- Lucas-Jiménez, O.; Ojeda, N.; Peña, J.; Cabrera-Zubizarreta, A.; Díez-Cirarda, M.; Gómez-Esteban, J.C.; Gómez-Beldarrain, M.; Ibarretxe-Bilbao, N. Apathy and brain alterations in Parkinson’s disease: A multimodal imaging study. Ann. Clin. Transl. Neurol. 2018, 5, 803–814. [Google Scholar] [CrossRef]
- Martinez-Horta, S.; Sampedro, F.; Pagonabarraga, J.; Fernandez-Bobadilla, R.; Marin-Lahoz, J.; Riba, J.; Kulisevsky, J. Non-demented Parkinson’s disease patients with apathy show decreased grey matter volume in key executive and reward-related nodes. Brain Imaging Behav. 2017, 11, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, S.Y.; Zuo, L.J.; Cao, C.J.; Hu, Y.; Chen, Z.J.; Piao, Y.S.; Wang, Y.J.; Wang, X.M.; Chen, S.D.; et al. Excessive Iron and α-Synuclein Oligomer in Brain are Relevant to Pure Apathy in Parkinson Disease. J. Geriatr. Psychiatry Neurol. 2016, 29, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.P.; McDonald, K.R.; Allsop, D.; Diggle, P.J.; Leroi, I. Apathy as a behavioural marker of cognitive impairment in Parkinson’s disease: A longitudinal analysis. J. Neurol. 2020, 267, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Mele, B.; Van, S.; Holroyd-Leduc, J.; Ismail, Z.; Pringsheim, T.; Goodarzi, Z. Diagnosis, treatment and management of apathy in Parkinson’s disease: A scoping review. BMJ Open 2020, 10, e037632. [Google Scholar] [CrossRef]
- Broen, M.P.; Narayen, N.E.; Kuijf, M.L.; Dissanayaka, N.N.; Leentjens, A.F. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2016, 31, 1125–1133. [Google Scholar] [CrossRef]
- Jia, M.; Yang, S.; Li, S.; Chen, S.; Wu, L.; Li, J.; Wang, H.; Wang, C.; Liu, Q.; Wu, K. Early identification of Parkinson’s disease with anxiety based on combined clinical and MRI features. Front. Aging Neurosci. 2024, 16, 1414855. [Google Scholar] [CrossRef]
- Carey, G.; Lopes, R.; Viard, R.; Betrouni, N.; Kuchcinski, G.; Devignes, Q.; Defebvre, L.; Leentjens, A.F.G.; Dujardin, K. Anxiety in Parkinson’s disease is associated with changes in the brain fear circuit. Park. Relat. Disord. 2020, 80, 89–97. [Google Scholar] [CrossRef]
- Torres, E.R.S.; Stanojlovic, M.; Zelikowsky, M.; Bonsberger, J.; Hean, S.; Mulligan, C.; Baldauf, L.; Fleming, S.; Masliah, E.; Chesselet, M.F.; et al. Alpha-synuclein pathology, microgliosis, and parvalbumin neuron loss in the amygdala associated with enhanced fear in the Thy1-aSyn model of Parkinson’s disease. Neurobiol. Dis. 2021, 158, 105478. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, G.; Li, Y.; Arkin, E.; Zheng, Y.; Feng, T. Erythrocytic α-Synuclein Species for Parkinson’s Disease Diagnosis and the Correlations with Clinical Characteristics. Front. Aging Neurosci. 2022, 14, 827493. [Google Scholar] [CrossRef]
- Lian, T.; Zhang, W.; Li, D.; Guo, P.; He, M.; Zhang, Y.; Li, J.; Guan, H.; Zhang, W.; Luo, D.; et al. Parkinson’s disease with anxiety: Clinical characteristics and their correlation with oxidative stress, inflammation, and pathological proteins. BMC Geriatr. 2024, 24, 433. [Google Scholar] [CrossRef]
- Shi, Y.; Dobkin, R.; Weintraub, D.; Cho, H.R.; Caspell-Garcia, C.; Bock, M.; Brown, E.; Aarsland, D.; Dahodwala, N. Association of Baseline Depression and Anxiety with Longitudinal Health Outcomes in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2024, 11, 1103–1112. [Google Scholar] [CrossRef]
- Yao, N.; Shek-Kwan Chang, R.; Cheung, C.; Pang, S.; Lau, K.K.; Suckling, J.; Rowe, J.B.; Yu, K.; Ka-Fung Mak, H.; Chua, S.E.; et al. The default mode network is disrupted in Parkinson’s disease with visual hallucinations. Hum. Brain Mapp. 2014, 35, 5658–5666. [Google Scholar] [CrossRef]
- Clegg, B.J.; Duncan, G.W.; Khoo, T.K.; Barker, R.A.; Burn, D.J.; Yarnall, A.J.; Lawson, R.A. Categorising Visual Hallucinations in Early Parkinson’s Disease. J. Park’s Dis. 2018, 8, 447–453. [Google Scholar] [CrossRef]
- Fénelon, G.; Mahieux, F.; Huon, R.; Ziégler, M. Hallucinations in Parkinson’s disease: Prevalence, phenomenology and risk factors. Brain 2000, 123 Pt 4, 733–745. [Google Scholar] [CrossRef]
- Murphy, N.; Killen, A.; Gupta, R.K.; Graziadio, S.; Rochester, L.; Firbank, M.; Baker, M.R.; Allan, C.; Collerton, D.; Taylor, J.P.; et al. Exploring Bottom-Up Visual Processing and Visual Hallucinations in Parkinson’s Disease with Dementia. Front. Neurol. 2020, 11, 579113. [Google Scholar] [CrossRef]
- Ignatavicius, A.; Matar, E.; Lewis, S.J.G. Visual hallucinations in Parkinson’s disease: Spotlight on central cholinergic dysfunction. Brain 2025, 148, 376–393. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, B.; Lu, L.; Lan, W.; Pan, Y.; Zhang, L.; Dong, J.; Wang, M.; Zhang, L. Prevalence and risk factors for visual hallucinations in Chinese patients with Parkinson’s disease. J. Neurol. Sci. 2017, 372, 471–476. [Google Scholar] [CrossRef]
- d’Angremont, E.; van der Zee, S.; Slingerland, S.; Slomp, A.C.; de Vries, E.F.J.; van Laar, T.; Sommer, I.E. Cholinergic deficiency in Parkinson’s disease patients with visual hallucinations. Brain 2024, 147, 3370–3378. [Google Scholar] [CrossRef]
- Firbank, M.J.; Parikh, J.; Murphy, N.; Killen, A.; Allan, C.L.; Collerton, D.; Blamire, A.M.; Taylor, J.P. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 2018, 91, e675–e685. [Google Scholar] [CrossRef]
- Thomas, G.E.C.; Zeidman, P.; Sultana, T.; Zarkali, A.; Razi, A.; Weil, R.S. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 2023, 5, fcac329. [Google Scholar] [CrossRef]
- Zarkali, A.; McColgan, P.; Leyland, L.A.; Lees, A.J.; Weil, R.S. Longitudinal thalamic white and grey matter changes associated with visual hallucinations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, M.E.; Christian, L.M.; Moran, L.B.; Graeber, M.B.; Pearce, R.K.; Gentleman, S.M. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Park. Relat. Disord. 2009, 15, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ikeda, T.; Ishida, C.; Komai, K.; Yamada, M. Delusions and visual hallucinations in a patient with Parkinson’s disease with dementia showing pronounced Lewy body pathology in the nucleus basalis of Meynert. Neuropathology 2019, 39, 319–323. [Google Scholar] [CrossRef]
- Williams, D.R.; Warren, J.D.; Lees, A.J. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2008, 79, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Gillette, B.; Reid, J.A.; Shermetaro, C. Phantosmia. In StatPearls; StatPearls Publishing: Treasure Island, Finland, 2024. [Google Scholar]
- Solla, P.; Masala, C.; Pinna, I.; Ercoli, T.; Loy, F.; Orofino, G.; Fadda, L.; Defazio, G. Frequency and Determinants of Olfactory Hallucinations in Parkinson’s Disease Patients. Brain Sci. 2021, 11, 841. [Google Scholar] [CrossRef]
- Bannier, S.; Berdagué, J.L.; Rieu, I.; de Chazeron, I.; Marques, A.; Derost, P.; Ulla, M.; Llorca, P.M.; Durif, F. Prevalence and phenomenology of olfactory hallucinations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1019–1021. [Google Scholar] [CrossRef]
- Landis, B.N.; Burkhard, P.R. Phantosmias and Parkinson disease. Arch. Neurol. 2008, 65, 1237–1239. [Google Scholar] [CrossRef]
- Ercoli, T.; Bagella, C.F.; Frau, C.; Ruiu, E.; Othmani, S.; Gusinu, G.; Masala, C.; Sechi, L.A.; Solla, P.; Defazio, G. Phantosmia in Parkinson’s Disease: A Systematic Review of the Phenomenology of Olfactory Hallucinations. Neurol. Int. 2023, 16, 20–32. [Google Scholar] [CrossRef]
- Wallace, E.R.; Segerstrom, S.C.; van Horne, C.G.; Schmitt, F.A.; Koehl, L.M. Meta-Analysis of Cognition in Parkinson’s Disease Mild Cognitive Impairment and Dementia Progression. Neuropsychol. Rev. 2022, 32, 149–160. [Google Scholar] [CrossRef]
- Lanni, K.E.; Ross, J.M.; Higginson, C.I.; Dressler, E.M.; Sigvardt, K.A.; Zhang, L.; Malhado-Chang, N.; Disbrow, E.A. Perceived and performance-based executive dysfunction in Parkinson’s disease. J. Clin. Exp. Neuropsychol. 2014, 36, 342–355. [Google Scholar] [CrossRef]
- Dirnberger, G.; Frith, C.D.; Jahanshahi, M. Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage 2005, 25, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Mayeux, R.; Côté, L. Reaction time and vigilance in Parkinson’s disease. Possible role of altered norepinephrine metabolism. Arch. Neurol. 1984, 41, 1086–1089. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive impairment in Parkinson’s disease: The dual syndrome hypothesis. Neurodegener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef]
- Salazar, R.D.; Ren, X.; Ellis, T.D.; Toraif, N.; Barthelemy, O.J.; Neargarder, S.; Cronin-Golomb, A. Dual tasking in Parkinson’s disease: Cognitive consequences while walking. Neuropsychology 2017, 31, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Proud, E.; Morris, M.E.; Bilney, B.; Miller, K.J.; Nijkrake, M.J.; Munneke, M.M.; McGinley, J.L. Effects of dual-task interference on dexterity performance in people with mild to moderately severe Parkinson’s disease: An observational analysis. J. Hand Ther. 2025, 38, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.R.; Uc, E.Y. Real-life consequences of cognitive dysfunction in Parkinson’s disease. Prog. Brain Res. 2022, 269, 113–136. [Google Scholar] [CrossRef]
- Föcker, J.; Cole, D.; Beer, A.L.; Bavelier, D. Neural bases of enhanced attentional control: Lessons from action video game players. Brain Behav. 2018, 8, e01019. [Google Scholar] [CrossRef]
- Arabacı, G.; Parris, B.A. Inattention and task switching performance: The role of predictability, working memory load and goal neglect. Psychol. Res. 2020, 84, 2090–2110. [Google Scholar] [CrossRef]
- Li, S.; Ou, R.; Yuan, X.; Liu, H.; Hou, Y.; Wei, Q.; Song, W.; Cao, B.; Chen, Y.; Shang, H. Executive dysfunctions and behavioral changes in early drug-naïve patients with Parkinson’s disease. J. Affect. Disord. 2019, 243, 525–530. [Google Scholar] [CrossRef]
- Pont-Sunyer, C.; Hotter, A.; Gaig, C.; Seppi, K.; Compta, Y.; Katzenschlager, R.; Mas, N.; Hofeneder, D.; Brücke, T.; Bayés, A.; et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov. Disord. 2015, 30, 229–237. [Google Scholar] [CrossRef]
- Melugin, P.R.; Nolan, S.O.; Kandov, E.; Ferrara, C.F.; Farahbakhsh, Z.Z.; Siciliano, C.A. Medial prefrontal dopamine dynamics reflect allocation of selective attention. bioRxiv 2024. [Google Scholar] [CrossRef]
- Arias-Carrion, O.; Poppel, E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol. Exp. 2007, 67, 481–488. [Google Scholar] [CrossRef]
- Woodward, T.S.; Bub, D.N.; Hunter, M.A. Task switching deficits associated with Parkinson’s disease reflect depleted attentional resources. Neuropsychologia 2002, 40, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Yousaf, J.; Ahmad, H. Frontal-subcortical defects correlate with task switching deficits in Parkinson`s disease. Neurosciences 2017, 22, 224–227. [Google Scholar] [CrossRef]
- Bin Yoo, H.; Concha, E.O.; De Ridder, D.; Pickut, B.A.; Vanneste, S. The Functional Alterations in Top-Down Attention Streams of Parkinson’s disease Measured by EEG. Sci. Rep. 2018, 8, 10609. [Google Scholar] [CrossRef]
- Katsuki, F.; Constantinidis, C. Bottom-up and top-down attention: Different processes and overlapping neural systems. Neuroscientist 2014, 20, 509–521. [Google Scholar] [CrossRef]
- Tommasi, G.; Fiorio, M.; Yelnik, J.; Krack, P.; Sala, F.; Schmitt, E.; Fraix, V.; Bertolasi, L.; Le Bas, J.F.; Ricciardi, G.K.; et al. Disentangling the Role of Cortico-Basal Ganglia Loops in Top-Down and Bottom-Up Visual Attention: An Investigation of Attention Deficits in Parkinson Disease. J. Cogn. Neurosci. 2015, 27, 1215–1237. [Google Scholar] [CrossRef] [PubMed]
- Bocquillon, P.; Bourriez, J.L.; Palmero-Soler, E.; Destée, A.; Defebvre, L.; Derambure, P.; Dujardin, K. Role of basal ganglia circuits in resisting interference by distracters: A swLORETA study. PLoS ONE 2012, 7, e34239. [Google Scholar] [CrossRef]
- Wulaer, B.; Kunisawa, K.; Tanabe, M.; Yanagawa, A.; Saito, K.; Mouri, A.; Nabeshima, T. Pharmacological blockade of dopamine D1- or D2-receptor in the prefrontal cortex induces attentional impairment in the object-based attention test through different neuronal circuits in mice. Mol. Brain 2021, 14, 43. [Google Scholar] [CrossRef]
- Fischer, M.; Moscovitch, M.; Alain, C. A systematic review and meta-analysis of memory-guided attention: Frontal and parietal activation suggests involvement of fronto-parietal networks. Wiley Interdiscip. Rev. Cogn. Sci. 2021, 12, e1546. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Revisiting the effects of Parkinson’s disease and frontal lobe lesions on task switching: The role of rule reconfiguration. J. Neuropsychol. 2014, 8, 53–74. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y.; Sano, M.; Cote, L.; Williams, J.B. Clinical and biochemical correlates of bradyphrenia in Parkinson’s disease. Neurology 1987, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Peavy, G.M. Mild cognitive deficits in Parkinson disease: Where there is bradykinesia, there is bradyphrenia. Neurology 2010, 75, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Steinke, A.; Lange, F.; Seer, C.; Hendel, M.K.; Kopp, B. Computational Modeling for Neuropsychological Assessment of Bradyphrenia in Parkinson’s Disease. J. Clin. Med. 2020, 9, 1158. [Google Scholar] [CrossRef]
- Letanneux, A.; Velay, J.L.; Viallet, F.; Pinto, S. Altered Inhibitory Mechanisms in Parkinson’s Disease: Evidence from Lexical Decision and Simple Reaction Time Tasks. Front. Hum. Neurosci. 2021, 15, 624026. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.M.; Cummings, J.L. Frontal-subcortical dementias. Neurologist 2008, 14, 100–107. [Google Scholar] [CrossRef]
- Wang, W.; Baker, K.; Umamahesan, C.; Gilmour, S.; Charlett, A.; Taylor, D.; Young, A.H.; Dobbs, R.J.; Dobbs, S.M. Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background. J. Clin. Med. 2023, 12, 6499. [Google Scholar] [CrossRef]
- Gibson, L.L.; Weintraub, D.; Lemmen, R.; Perera, G.; Chaudhuri, K.R.; Svenningsson, P.; Aarsland, D. Risk of Dementia in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2024, 39, 1697–1709. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707; quiz 1837. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathobiology of Cognitive Impairment in Parkinson Disease: Challenges and Outlooks. Int. J. Mol. Sci. 2023, 25, 498. [Google Scholar] [CrossRef]
- Åström, D.O.; Simonsen, J.; Raket, L.L.; Sgarbi, S.; Hellsten, J.; Hagell, P.; Norlin, J.M.; Kellerborg, K.; Martinez-Martin, P.; Odin, P. High risk of developing dementia in Parkinson’s disease: A Swedish registry-based study. Sci. Rep. 2022, 12, 16759. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Drozdova, A.; Wang, W.; Thomas, M. The impact of dementia development concurrent with Parkinson’s disease: A new perspective. CNS Neurol. Disord. Drug Targets 2014, 13, 1160–1168. [Google Scholar] [CrossRef]
- Caballol, N.; Martí, M.J.; Tolosa, E. Cognitive dysfunction and dementia in Parkinson disease. Mov. Disord. 2007, 22 (Suppl. 17), S358–S366. [Google Scholar] [CrossRef]
- Emre, M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003, 2, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, A.; Hampshire, A.; Barker, R.A.; Owen, A.M. Normal aging and Parkinson’s disease are associated with the functional decline of distinct frontal-striatal circuits. Cortex 2017, 93, 178–192. [Google Scholar] [CrossRef]
- Novikov, N.I.; Brazhnik, E.S.; Kitchigina, V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochemistry 2023, 88, 1890–1904. [Google Scholar] [CrossRef]
- Schreiber, L.; Odlaug, B.L.; Grant, J.E. Impulse control disorders: Updated review of clinical characteristics and pharmacological management. Front. Psychiatry 2011, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Theis, H.; Prange, S.; Bischof, G.N.; Hoenig, M.C.; Tittgemeyer, M.; Timmermann, L.; Fink, G.R.; Drzezga, A.; Eggers, C.; van Eimeren, T. Impulsive-compulsive behaviour in early Parkinson’s disease is determined by apathy and dopamine receptor D3 polymorphism. NPJ Park.’s Dis. 2023, 9, 154. [Google Scholar] [CrossRef]
- Jellinger, K.A. Behavioral disorders in Parkinson disease: Current view. J. Neural Transm. 2025, 132, 169–201. [Google Scholar] [CrossRef]
- Turcano, P.; Jacobson, J.; Ghoniem, K.; Mullan, A.; Camerucci, E.; Stang, C.; Piat, C.; Bower, J.H.; Savica, R. Impulse control disorders and use of dopamine agonists in early onset Parkinson’s disease. Front. Neurol. 2024, 15, 1404904. [Google Scholar] [CrossRef]
- Poletti, M.; Logi, C.; Lucetti, C.; Del Dotto, P.; Baldacci, F.; Vergallo, A.; Ulivi, M.; Del Sarto, S.; Rossi, G.; Ceravolo, R.; et al. A single-center, cross-sectional prevalence study of impulse control disorders in Parkinson disease: Association with dopaminergic drugs. J. Clin. Psychopharmacol. 2013, 33, 691–694. [Google Scholar] [CrossRef]
- Joutsa, J.; Martikainen, K.; Vahlberg, T.; Voon, V.; Kaasinen, V. Impulse control disorders and depression in Finnish patients with Parkinson’s disease. Park. Relat. Disord. 2012, 18, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Violante, M.; González-Latapi, P.; Cervantes-Arriaga, A.; Camacho-Ordoñez, A.; Weintraub, D. Impulse control and related disorders in Mexican Parkinson’s disease patients. Park. Relat. Disord. 2014, 20, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Claassen, D.O. Impulse Control and Related Disorders in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 679–717. [Google Scholar] [CrossRef] [PubMed]
- Wolters, E.; van der Werf, Y.D.; van den Heuvel, O.A. Parkinson’s disease-related disorders in the impulsive-compulsive spectrum. J. Neurol. 2008, 255 (Suppl. 5), 48–56. [Google Scholar] [CrossRef]
- Voon, V.; Mehta, A.R.; Hallett, M. Impulse control disorders in Parkinson’s disease: Recent advances. Curr. Opin. Neurol. 2011, 24, 324–330. [Google Scholar] [CrossRef]
- Napier, T.C.; Corvol, J.C.; Grace, A.A.; Roitman, J.D.; Rowe, J.; Voon, V.; Strafella, A.P. Linking neuroscience with modern concepts of impulse control disorders in Parkinson’s disease. Mov. Disord. 2015, 30, 141–149. [Google Scholar] [CrossRef]
- Wolfschlag, M.; Cedergren Weber, G.; Weintraub, D.; Odin, P.; Håkansson, A. Impulse control disorders in Parkinson’s disease: A national Swedish registry study on high-risk treatments and vulnerable patient groups. J. Neurol. Neurosurg. Psychiatry 2024, 96, 265–271. [Google Scholar] [CrossRef]
- Luo, Y.; Xiang, Y.; Liu, J.; Hu, Y.; Guo, J. A Multi-omics Framework Based on Machine Learning as a Predictor of Cognitive Impairment Progression in Early Parkinson’s Disease. Neurol. Ther. 2025, 14, 643–658. [Google Scholar] [CrossRef]
- Tng, T.J.W.; Wong, B.W.Y.; Sim, E.H.Y.; Tan, E.K.; Goh, W.W.B.; Lim, K.-L. Systematic analysis of multi-omics data reveals component-specific blood-based biomarkers for Parkinson’s disease. Transl. Med. Commun. 2024, 9, 12. [Google Scholar] [CrossRef]
- Lejeune, F.-X.; Ichou, F.; Camenen, E.; Colsch, B.; Mauger, F.; Peltier, C.; Moszer, I.; Gilson, E.; Pierre-Jean, M.; Floch, E.L.; et al. A Multimodal Omics Exploration of the Motor and Non-Motor Symptoms of Parkinson’s Disease. Int. J. Transl. Med. 2022, 2, 97–112. [Google Scholar] [CrossRef]
- Hu, M.; Skjærbæk, C.; Borghammer, P. Approaches to Early Parkinson’s Disease Subtyping. J. Park’s Dis. 2024, 14, S297–S306. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, C.; Termine, A.; Caltagirone, C. Transcriptomics profiling of Parkinson’s disease progression subtypes reveals distinctive patterns of gene expression. J. Cent. Nerv. Syst. Dis. 2025, 17, 11795735241286821. [Google Scholar] [CrossRef]
- Serag, I.; Azzam, A.Y.; Hassan, A.K.; Diab, R.A.; Diab, M.; Hefnawy, M.T.; Ali, M.A.; Negida, A. Multimodal diagnostic tools and advanced data models for detection of prodromal Parkinson’s disease: A scoping review. BMC Med. Imaging 2025, 25, 103. [Google Scholar] [CrossRef]
- Rabie, H.; Akhloufi, M.A. A review of machine learning and deep learning for Parkinson’s disease detection. Discov. Artif. Intell. 2025, 5, 24. [Google Scholar] [CrossRef]
- Malaguti, M.C.; Gios, L.; Jurman, G. The third wheel or the game changer? How AI could team up with neurologists in Parkinson’s care. Park. Relat. Disord. 2025, 134, 107797. [Google Scholar] [CrossRef]
- Cerasa, A.; Novellino, F.; Quattrone, A. Connectivity Changes in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2016, 16, 91. [Google Scholar] [CrossRef]
- Caligiore, D.; Helmich, R.C.; Hallett, M.; Moustafa, A.A.; Timmermann, L.; Toni, I.; Baldassarre, G. Parkinson’s disease as a system-level disorder. NPJ Park.’s Dis. 2016, 2, 16025. [Google Scholar] [CrossRef]
- Liao, H.; Cai, S.; Shen, Q.; Fan, J.; Wang, T.; Zi, Y.; Mao, Z.; Situ, W.; Liu, J.; Zou, T.; et al. Networks Are Associated with Depression in Patients with Parkinson’s Disease: A Resting-State Imaging Study. Front. Neurosci. 2020, 14, 573538. [Google Scholar] [CrossRef]
- Permezel, F.; Alty, J.; Harding, I.H.; Thyagarajan, D. Brain Networks Involved in Sensory Perception in Parkinson’s Disease: A Scoping Review. Brain Sci. 2023, 13, 1552. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Yu, J.; Li, R.; Liao, W.; Chen, Q.; Xing, H.; Lu, F.; Hu, X.; Chen, H.; et al. Amygdala-centered fusional connections characterized nonmotor symptoms in Parkinson’s disease. Cereb. Cortex 2025, 35, bhaf002. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).