A Systematic Review and Meta-Analysis Association Between Periodontitis and Age-Related Macular Degeneration: Potential for Personalized Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria of Studies for Review
2.3. Information Sources, Literature Search, and Study Selection
2.4. Data Collection and Risk of Bias Within Studies
2.5. Study Outcomes, Synthesis, and Risk of Bias Across Studies
3. Results
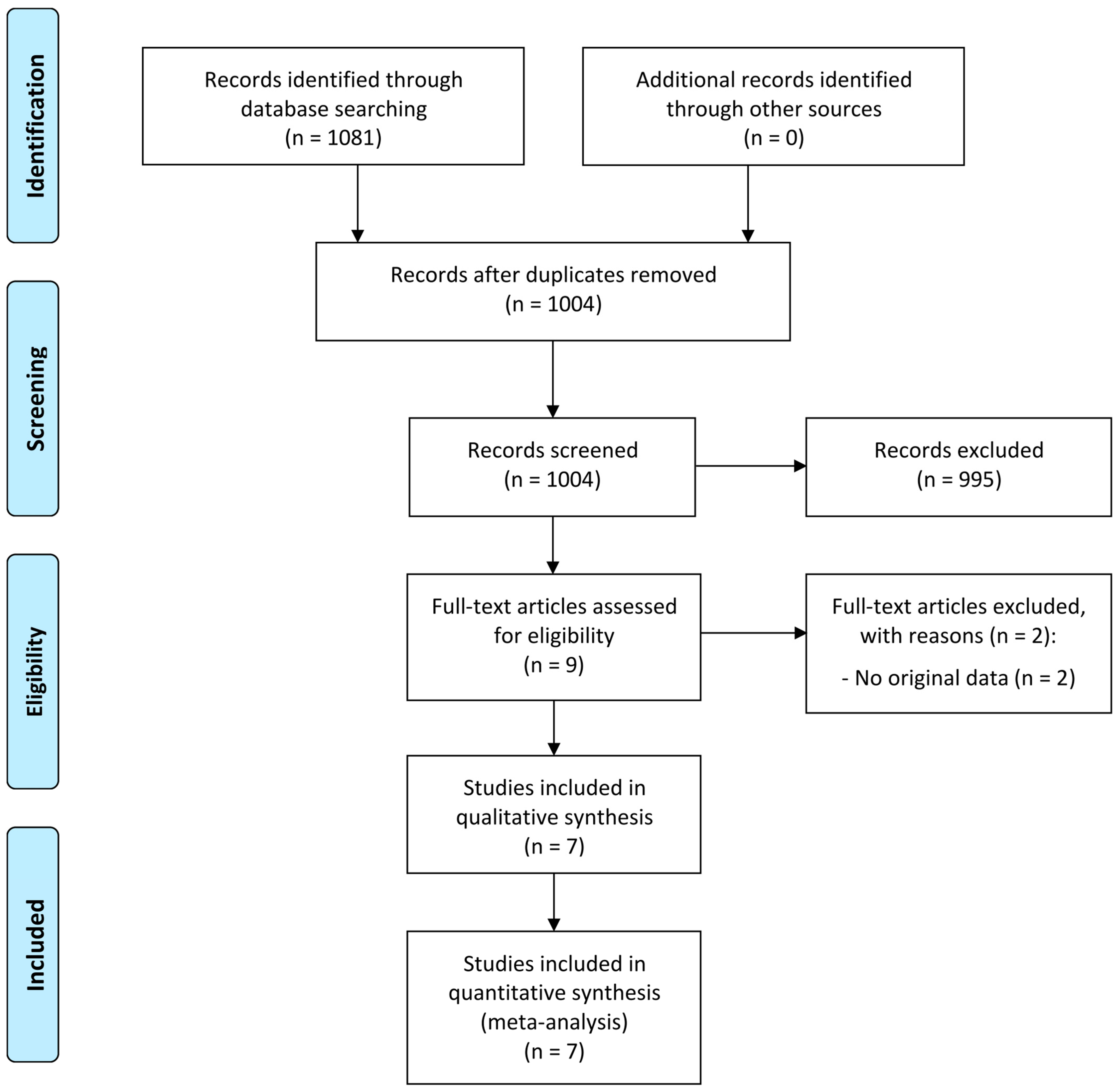
3.1. Study Selection
3.2. Study Characteristics and Populations
3.3. Results of Individual Studies
3.4. Risk of Bias Within Individual Studies
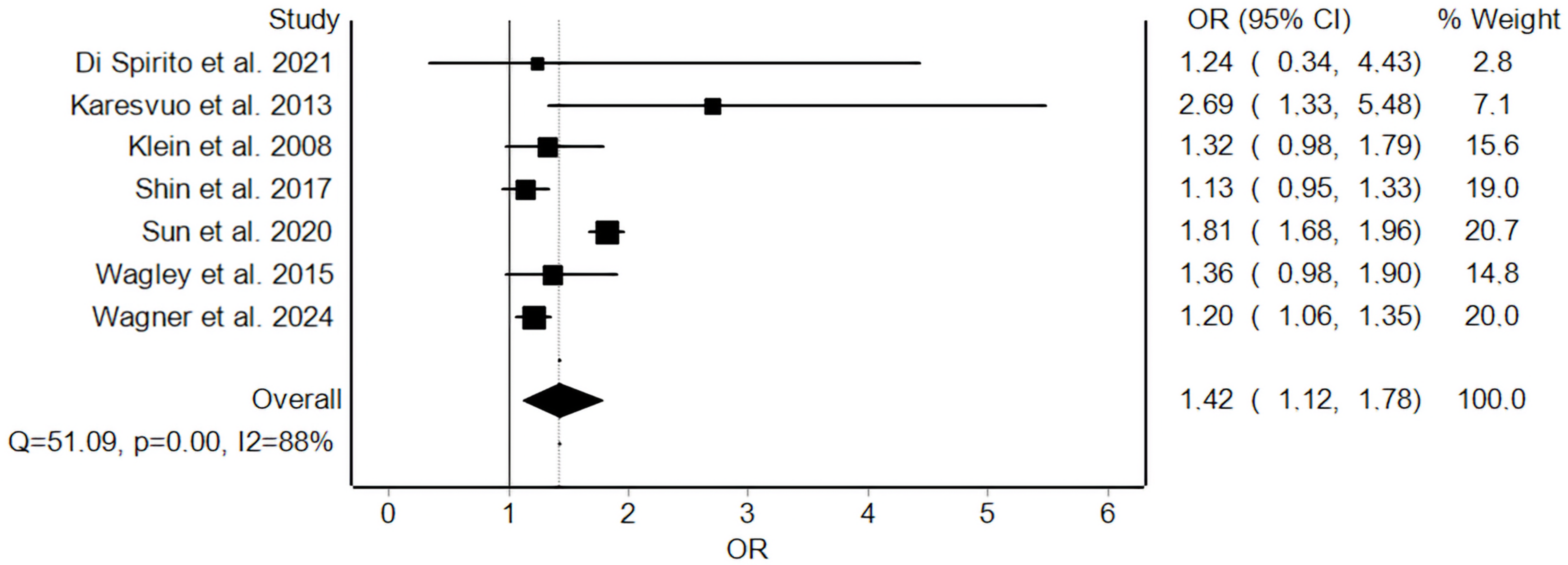
3.5. Meta-Analysis and Risk of Bias Across Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Selzman, C.H.; Cothren, C.; Sorensen, A.C.; Raeburn, C.D.; Harken, A.H. Diagnostic implications of C-reactive protein. Arch. Surg. 2003, 138, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N. C-reactive protein and coronary heart disease: Diagnostic and therapeutic implications for primary prevention. Cardiovasc. Toxicol. 2001, 1, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Moorthy, M.V.; Cook, N.R.; Rifai, N.; Lee, I.M.; Buring, J.E. Inflammation, Cholesterol, Lipoprotein(a), and 30-Year Cardiovascular Outcomes in Women. N. Engl. J. Med. 2024, 391, 2087–2097. [Google Scholar] [CrossRef]
- Tuomisto, K.; Jousilahti, P.; Sundvall, J.; Pajunen, P.; Salomaa, V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb. Haemost. 2006, 95, 511–518. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Zawistowski, M.; Fritsche, L.G.; Zhan, X.; Bragg-Gresham, J.; Branham, K.E.; Advani, J.; Othman, M.; Ratnapriya, R.; Teslovich, T.M.; et al. Whole genome sequencing of 4,787 individuals identifies gene-based rare variants in age-related macular degeneration. Hum. Mol. Genet. 2024, 33, 374–385. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-Related macular degeneration: Genetics and biology coming together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Subhi, Y.; Sørensen, T.L. Effect of aging and lifestyle on photoreceptors and retinal pigment epithelium: Cross-sectional study in a healthy danish population. Pathobiol. Aging Age-Relat. Dis. 2017, 7, 1398016. [Google Scholar] [CrossRef]
- Subhi, Y.; Forshaw, T.; Sørensen, T.L. Macular thickness and volume in the elderly: A systematic review. Ageing. Res. Rev. 2016, 29, 42–49. [Google Scholar] [CrossRef]
- Subhi, Y.; Nielsen, M.K.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. T-cell differentiation and CD56+ levels in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Aging 2017, 9, 2436–2452. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Systemic levels of C-reactive protein in patients with age-related macular degeneration: A systematic review with meta-analyses. Mech. Ageing Dev. 2020, 191, 111353. [Google Scholar] [CrossRef] [PubMed]
- Nahavandipour, A.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2020, 98, 434–444. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Wang, Y.; Kang, L.; Zhang, G.; Zhang, J.; Qin, B.; Yang, L.; Luo, J.; Li, P.; et al. Systemic inflammatory regulators and age-related macular degeneration: A bidirectional mendelian randomization study. Front. Genet. 2024, 15, 1391999. [Google Scholar] [CrossRef]
- Khan, A.H.; Pierce, C.O.; De Salvo, G.; Griffiths, H.; Nelson, M.; Cree, A.J.; Menon, G.; Lotery, A.J. The effect of systemic levels of TNF-alpha and complement pathway activity on outcomes of VEGF inhibition in neovascular AMD. Eye 2022, 36, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; George, S.; Rosner, B.; Rifai, N. Progression of age-related macular degeneration: Prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch. Ophthalmol. 2005, 123, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Subhi, Y.; Lykke Sørensen, T. New neovascular age-related macular degeneration is associated with systemic leucocyte activity. Acta. Ophthalmol. 2017, 95, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane. 2024. Available online: www.training.cochrane.org/handbook (accessed on 27 January 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid.-Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef]
- Karesvuo, P.; Gursoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Huumonen, S.; Vesti, E.; Könönen, E. Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2013, 84, 58–67. [Google Scholar] [CrossRef]
- Klein, R.; Knudtson, M.D.; Klein, B.E.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Cheng, C.Y.; Burke, G.L.; Saad, M.F.; Jacobs, D.R., Jr.; et al. Inflammation, complement factor h, and age-related macular degeneration: The Multi-ethnic Study of Atherosclerosis. Ophthalmology 2008, 115, 1742–1749. [Google Scholar] [PubMed]
- Shin, Y.U.; Lim, H.W.; Hong, E.H.; Kang, M.H.; Seong, M.; Nam, E.; Cho, H. The association between periodontal disease and age-related macular degeneration in the Korea National health and nutrition examination survey: A cross-sectional observational study. Medicine 2017, 96, e6418. [Google Scholar] [PubMed]
- Sun, K.T.; Hsia, N.Y.; Chen, S.C.; Lin, C.L.; Chen, I.A.; Wu, I.T.; Palanisamy, K.; Shen, T.C.; Li, C.Y. Risk of Age-Related Macular Degeneration in Patients with Periodontitis: A Nationwide Population-Based Cohort Study. Retina 2020, 40, 2312–2318. [Google Scholar]
- Wagley, S.; Marra, K.V.; Salhi, R.A.; Gautam, S.; Campo, R.; Veale, P.; Veale, J.; Arroyo, J.G. Periodontal Disease and Age-Related Macular Degeneration: Results From the National Health and Nutrition Examination Survey III. Retina 2015, 35, 982–988. [Google Scholar]
- Wagner, S.K.; Patel, P.J.; Huemer, J.; Khalid, H.; Stuart, K.V.; Chu, C.J.; Williamson, D.J.; Struyven, R.R.; Romero-Bascones, D.; Foster, P.J.; et al. Periodontitis and Outer Retinal Thickness: A Cross-Sectional Analysis of the United Kingdom Biobank Cohort. Ophthalmol. Sci. 2024, 4, 100472. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [PubMed]
- Wong, T.Y.; Lanzetta, P.; Bandello, F.; Eldem, B.; Navarro, R.; Lövestam-Adrian, M.; Loewenstein, A. Current Concepts and Modalities for Monitoring the Fellow Eye in Neovascular Age-Related Macular Degeneration: An Expert Panel Consensus. Retina 2020, 40, 599–611. [Google Scholar] [CrossRef]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology 2017, 124, 464–478. [Google Scholar] [PubMed]
- Charbel Issa, P.; De Silva, S.; Pfau, K.; Birtel, J. Differential Diagnosis of Age-Related Macular Degeneration. Klin. Monbl. Augenheilkd 2025, 242, 7–21. [Google Scholar] [PubMed]
- van Dijk, E.H.C.; Boon, C.J.F. Serous business: Delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog. Retin. Eye Res. 2021, 84, 100955. [Google Scholar]
- Khan, K.N.; Mahroo, O.A.; Khan, R.S.; Mohamed, M.D.; McKibbin, M.; Bird, A.; Michaelides, M.; Tufail, A.; Moore, A.T. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog. Retin. Eye Res. 2016, 53, 70–106. [Google Scholar] [PubMed]
- Borrelli, E.; Battista, M.; Gelormini, F.; Sacconi, R.; Querques, L.; Vella, G.; Viganò, C.; Bandello, F.; Querques, G. Rate of misdiagnosis and clinical usefulness of the correct diagnosis in exudative neovascular maculopathy secondary to AMD versus pachychoroid disease. Sci. Rep. 2020, 10, 20344. [Google Scholar]
- Heath Jeffery, R.C.; Chen, F.K. Macular neovascularization in inherited retinal diseases: A review. Surv. Ophthalmol. 2024, 69, 1–23. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar]
- Toledano, M.B.; Smith, R.B.; Brook, J.P.; Douglass, M.; Elliott, P. How to Establish and Follow Up a Large Prospective Cohort Study in the 21st Century--Lessons from UK COSMOS. PLoS ONE 2015, 10, e0131521. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, M.B.; Le, J.; Mitchell, P.; Gopinath, B.; Cerin, E.; Saksens, N.T.M.; Schick, T.; Hoyng, C.B.; Guymer, R.H.; Finger, R.P. Physical Activity and Age-related Macular Degeneration: A Systematic Literature Review and Meta-analysis. Am. J. Ophthalmol. 2017, 180, 29–38. [Google Scholar]
- van’t Klooster, C.C.; van der Graaf, Y.; Ridker, P.M.; Westerink, J.; Hjortnaes, J.; Sluijs, I.; Asselbergs, F.W.; Bots, M.L.; Kappelle, L.J.; Visseren, F.L.J.; et al. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis 2020, 301, 37–43. [Google Scholar] [PubMed]
- Javed, F.; Sculean, A.; Romanos, G.E. Association between age-related macular degeneration and periodontal and peri-implant diseases: A systematic review. Acta. Ophthalmol. 2021, 99, 351–356. [Google Scholar]
- Pockpa, Z.A.D.; Struillou, X.; Kone, D.; Mobio, G.S.; Soueidan, A.; Badran, Z. Periodontal Diseases and Age-Related Macular Degeneration: Is There a Link? A Review. Perm. J. 2019, 23, 18.260. [Google Scholar]
- Lv, X.; Li, W.; Fang, Z.; Xue, X.; Pan, C. Periodontal Disease and Age-Related Macular Degeneration: A Meta-Analysis of 112,240 Participants. BioMed Res. Int. 2020, 2020, 4753645. [Google Scholar]
| Reference | Country | Study Design | Population Description | Demographics |
|---|---|---|---|---|
| Di Spirito et al., 2021 [26] | Italy | Prospective case–control study | Cases were patients with AMD diagnosed at the local department of ophthalmology. Controls were individuals who sought routine oral examinations at the local department of dentistry. Controls were matched for age ±3 years and gender, and did not undergo eye examination. Individuals were not included in case of known advanced cataract or other ocular diseases, edentulism, oral and systemic infections, medication-related osteonecrosis of the jaws, periodontal treatment, antibiotic or corticosteroid therapy in the last 3 months. | Cases (n = 40) were aged mean 75.8 years. Controls (n = 40) were aged mean 71.2 years. Females constituted 62.5% of all participants (both cases and controls). |
| Karesvuo et al., 2013 [27] | Finland | Prospective cross-sectional study | A national survey study in Finland, which in a subsample, recruited 1799 individuals for oral health examination. Of these, 1751 individuals had data to determine whether or not there was AMD. Presence of AMD was based on self-reported answers to survey questions. | Individuals with AMD (n = 54) were aged mean 66.5 ± 13.4 years and 70% were females. Individuals without AMD (n = 1697) were aged mean 51.3 ± 12.7 years and 55% were females. Individuals with AMD had a higher incidence of diabetes and had a higher systolic blood pressure. |
| Klein et al., 2008 [28] | USA | Prospective cross-sectional study | The MESA study in 6 communities in the USA recruited 5887 individuals with gradable fundus images for the evaluation of retinal pathologies. A questionnaire was used to obtain information about past medical history, including periodontal disease. | Demographics specific for AMD or no AMD were not reported. The entire study population (n = 5887) had a mean age of 61.5 ± 10.0 years. Sex distribution was not reported. |
| Shin et al., 2017 [29] | South Korea | Prospective cross-sectional study | The KNHANES is a population-based cross-sectional survey in South Korea, which each year samples from the South Korean population for a health interview survey, nutritional survey, and health examinations, including ophthalmic and periodontal examinations. Ophthalmic examinations included fundus photography. Dental examinations were made by dentists. | Individuals with AMD (n = 901) were aged mean 64.8 ± 12.0 years and 62% were females. Individuals without AMD (n = 12,171) were aged mean 53.9 ± 22.1 years and 51% were females. Hypertension was more prevalent among individuals with AMD. |
| Sun et al., 2020 [30] | Taiwan | Retrospective case–control study | The Taiwan National Health Insurance Database is a compulsory system in the Taiwanese healthcare system, which registers all interactions. From this system, cases were defined as those with periodontitis, and controls were defined as those without periodontitis who were otherwise matched in terms of age and sex. Presence of periodontitis and AMD was based on diagnosis codes in the database. All were aged at least 50 years, and none had AMD at the time of index year. | A total of 83,322 individuals (41,661 with periodontitis and 41,661 without periodontitis) were followed for 15 years. Overall mean age was 60 years, and 51% were females. |
| Wagley et al., 2015 [31] | USA | Prospective cross-sectional study | The NHANES III is a national survey of health and nutrition in the USA, which includes evaluation of oral and retinal health. Using a mobile examination center, periodontal examinations were performed by a dentist, and retinal photographs were obtained for one randomly selected eye for retinal examination. | A total of 8208 individuals (940 with AMD and 7268 without AMD) were examined. Overall, 68% were older than 60 years, and 52% were females. |
| Wagner et al., 2024 [32] | UK | Prospective cross-sectional study | The UK Biobank is a cohort of more than 500,000 individuals in the UK. Participants were asked about dental problems experienced within the last year. A subset of these individuals additionally underwent a detailed ophthalmic evaluation that included retinal imaging. | A total of 36,897 individuals (1571 with severe periodontitis and 35,326 without severe periodontitis) were examined. Overall, mean age was 56 ± 8 years, and 54% were females. |
| Reference | Evaluation of Periodontitis | Evaluation of Age-Related Macular Degeneration |
|---|---|---|
| Di Spirito et al., 2021 [26] | Oral examination with a periodontal full-mouth charting and panoramic radiograph. Total number of teeth were recorded. Periodontal charting included assessment of CAL, PPD, GI, and PlI, all registered as six values around each tooth. Tooth mobility and class furcation were recorded. FMPS% and FMBS% were calculated. Panoramic X-rays were scored and assigned to RBL stages, and also to alveolar bone loss classes. Periodontitis case definition was performed for both cases and controls according to the 2017 classification of periodontal and peri-implant diseases and conditions. | Ophthalmic examination including BCVA, slit-lamp examination, IOP measurement, fundus examination, and SD-OCT. Ophthalmic examination was only performed on cases known to have AMD, and controls were not subject to ophthalmic examination. Cases were graded according to early AMD or late AMD with either neovascular AMD or geographic atrophy. |
| Karesvuo et al., 2013 [27] | Number of teeth was noted. Panoramic radiographs were taken. Alveolar bone loss was analyzed from the radiographs on mesial and distal surfaces of each tooth, in which a bone pocket was defined as a vertical deformity within the bone that extends from the alveolar bone crest apically at least to the middle third of the root. Saliva samples were analyzed for bacterial DNA. | The participants were subject to a home interview, which included the question: “Has a doctor diagnosed one of the following diseases: cataract, glaucoma, degenerative fundus changes, or other visual defect or injury?”. If the participant responded with the presence of degenerative fundus changes, this was accepted as a marker of AMD. |
| Klein et al., 2008 [28] | A detailed questionnaire was used to determine presence of medical comorbidities. One of these questions was: “Has a dentist ever told you that you had periodontitis or gum disease?” | All participants underwent 45-degree fundus photography centered on the fovea without pupillary dilation. Early AMD was defined by either the presence of any soft drusen or pigmentary abnormalities. Late AMD was defined by the presence of geographic atrophy, subretinal hemorrhage, visible subretinal neovascularization, or subretinal fibrous scar. |
| Shin et al., 2017 [29] | The World Health Organization CPI was used to assess periodontal conditions and defined periodontal disease as a CPI ≥ 3. Periodontal tissues of permanent index teeth in each area were evaluated and included in the examination of bleeding upon the application of 20 g of pressure using a CPI probe, the presence of dental plaque, and the presence of periodontal pockets with measurable depths. The CPI scored on a score of 0 to 4 based on findings. A score of 0–2 points was defined as absence of periodontal disease, 3 points was defined as mild periodontal disease, and 4 points was defined as severe periodontal disease. | All participants underwent 45-degree fundus photography centered on the fovea without pupillary dilation. Early AMD was defined as presence of drusen and/or pigment abnormalities. Late AMD was defined as the presence of neovascularization or geographic atrophy. In this study, the authors categorized their data into either any AMD (both early or late AMD) or no AMD. |
| Sun et al., 2020 [30] | Presence of ICD-9-CM diagnosis codes 523.3 and 523.4. | Not outlined in detail. However, the authors distinguish between nonexudative and exudative AMD. |
| Wagley et al., 2015 [31] | Buccal and mesial-buccal aspects of each tooth from one randomly assigned upper quadrant and one randomly assigned lower quadrant were scored for loss of attachment. Level of periodontal attachment was reported in mm and calculated by measuring the distance from the cementoenamel junction to the bottom of the sulcus. Periodontal disease was defined as >10% sites with >3 mm of loss of attachment. | All participants underwent 45-degree fundus photography centered between the optic nerve head and the macula. Photographs were obtained without pupillary dilation. Early AMD was defined as presence of drusen and/or pigment abnormalities. Late AMD was defined as the presence of geographic atrophy, subretinal hemorrhage, subretinal fibrous scar, or serous subretinal detachment. In this study, the authors categorized their data into either any AMD (both early or late AMD) or no AMD. |
| Wagner et al., 2024 [32] | Questionnaire-based evaluation. Individuals reporting painful gums or loose teeth were considered as having very severe periodontitis. A sensitivity analysis was made by only including those with self-reported loose teeth. | All participants underwent retinal imaging using Topcon 3D-OCT, which included 45-degree fundus photography and a 6.0 × 6.0 mm OCT scan of the macula. OCTs were segmented automatically using proprietary Topcon software, and the thickness of the RPL and RPE-BM were used as a pseudo measure of AMD. |
| Reference | Variables Adjusted |
|---|---|
| Di Spirito et al., 2021 [26] | No adjustment for co-variates in the analyses, but the study attempted to recruit cases and controls that were homogenous in relation to BMI, blood pressure, hypertension, and total cholesterol (methodologically unclear). |
| Karesvuo et al., 2013 [27] | Age, diabetic status, smoking, systolic blood pressure, and the carriage of salivary bacteria. |
| Klein et al., 2008 [28] | Age, sex, race/ethnicity, and study site. |
| Shin et al., 2017 [29] | Age, sex, education level, household income, smoking, hypertension, CVD, anemia, hepatitis B infection, serum HDL level, BMI, serum ferritin level, and WBC count. |
| Sun et al., 2020 [30] | Age, sex, hypertension, diabetes, hyperlipidemia, asthma/COPD, CLD, and CKD. |
| Wagley et al., 2015 [31] | Age, sex, race, education, PIR, smoking status, BMI, hypertension, CVD, and CRP |
| Wagner et al., 2024 [32] | Age, sex, ethnicity, socioeconomic status, diabetes, hypertension, alcohol drinker status, smoking status, refractive error, and previous cataract surgery. |
| Reference | Defines Source | Eligibility Criteria | Time Period | Consecutive Recruitment | Quality Assurance | Explains Exclusions |
|---|---|---|---|---|---|---|
| Di Spirito et al., 2021 [26] | Yes | Yes | No | No | No | Not relevant |
| Karesvuo et al., 2013 [27] | Yes | Yes | Yes | Yes | No | Yes |
| Klein et al., 2008 [28] | Yes | Yes | Yes | Yes | No | Yes |
| Shin et al., 2017 [29] | Yes | Yes | Yes | Yes | Yes | Yes |
| Sun et al., 2020 [30] | Yes | Yes | Yes | Yes | No | Not relevant |
| Wagley et al., 2015 [31] | Yes | Yes | Yes | Yes | Yes | Yes |
| Wagner et al., 2024 [32] | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boberg-Ans, S.; Arnold-Vangsted, F.; Scheel-Bech, A.B.; Boberg-Ans, L.C.; Arnold-Vangsted, A.; Jakobsen, C.; Stokbro, K.; Subhi, Y. A Systematic Review and Meta-Analysis Association Between Periodontitis and Age-Related Macular Degeneration: Potential for Personalized Approach. J. Pers. Med. 2025, 15, 145. https://doi.org/10.3390/jpm15040145
Boberg-Ans S, Arnold-Vangsted F, Scheel-Bech AB, Boberg-Ans LC, Arnold-Vangsted A, Jakobsen C, Stokbro K, Subhi Y. A Systematic Review and Meta-Analysis Association Between Periodontitis and Age-Related Macular Degeneration: Potential for Personalized Approach. Journal of Personalized Medicine. 2025; 15(4):145. https://doi.org/10.3390/jpm15040145
Chicago/Turabian StyleBoberg-Ans, Sophie, Frederikke Arnold-Vangsted, Anna Bonde Scheel-Bech, Lars Christian Boberg-Ans, Andreas Arnold-Vangsted, Christian Jakobsen, Kasper Stokbro, and Yousif Subhi. 2025. "A Systematic Review and Meta-Analysis Association Between Periodontitis and Age-Related Macular Degeneration: Potential for Personalized Approach" Journal of Personalized Medicine 15, no. 4: 145. https://doi.org/10.3390/jpm15040145
APA StyleBoberg-Ans, S., Arnold-Vangsted, F., Scheel-Bech, A. B., Boberg-Ans, L. C., Arnold-Vangsted, A., Jakobsen, C., Stokbro, K., & Subhi, Y. (2025). A Systematic Review and Meta-Analysis Association Between Periodontitis and Age-Related Macular Degeneration: Potential for Personalized Approach. Journal of Personalized Medicine, 15(4), 145. https://doi.org/10.3390/jpm15040145







