Gene Therapy for Inherited Retinal Disease: Current Strategies, Personalized Medicine, and Future Implications—A Comprehensive Review
Abstract
1. Introduction
1.1. Gene Therapy for Eye Disease
1.2. Personalized Medicine
2. Advances in Retinal Gene Therapy
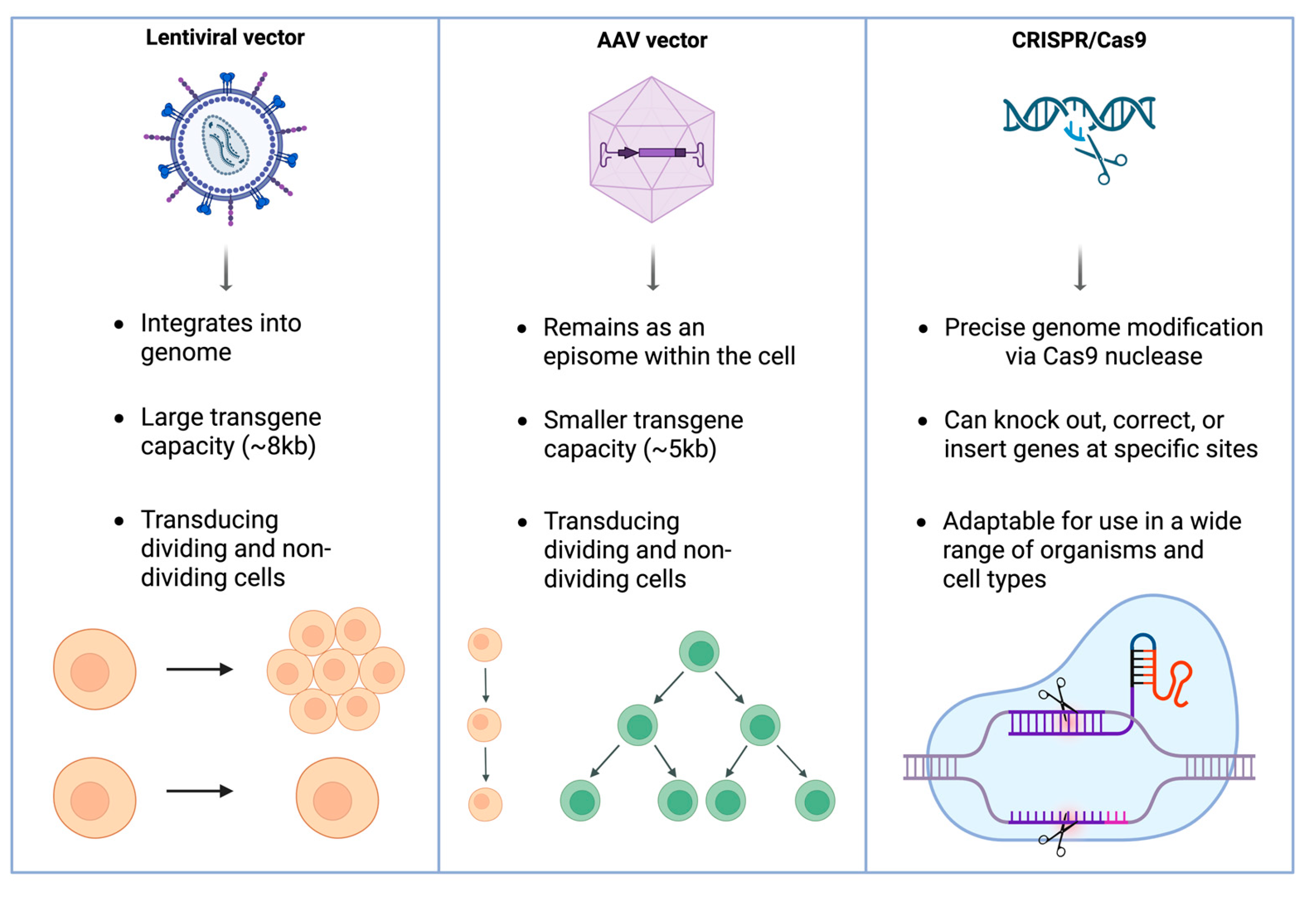
2.1. Overview of Gene Therapy Strategies
2.1.1. Adeno-Associated Virus Vectors
2.1.2. Lentiviral Vectors
2.1.3. CRISPR-Cas9
2.2. Key Clinical Trials and Approved Therapies
2.2.1. Voretigene Neparvovec-Rzyl (Luxturna)
2.2.2. X-Linked Retinoschisis
2.2.3. Stargardt Disease
2.2.4. Age-Related Macular Degeneration (AMD)
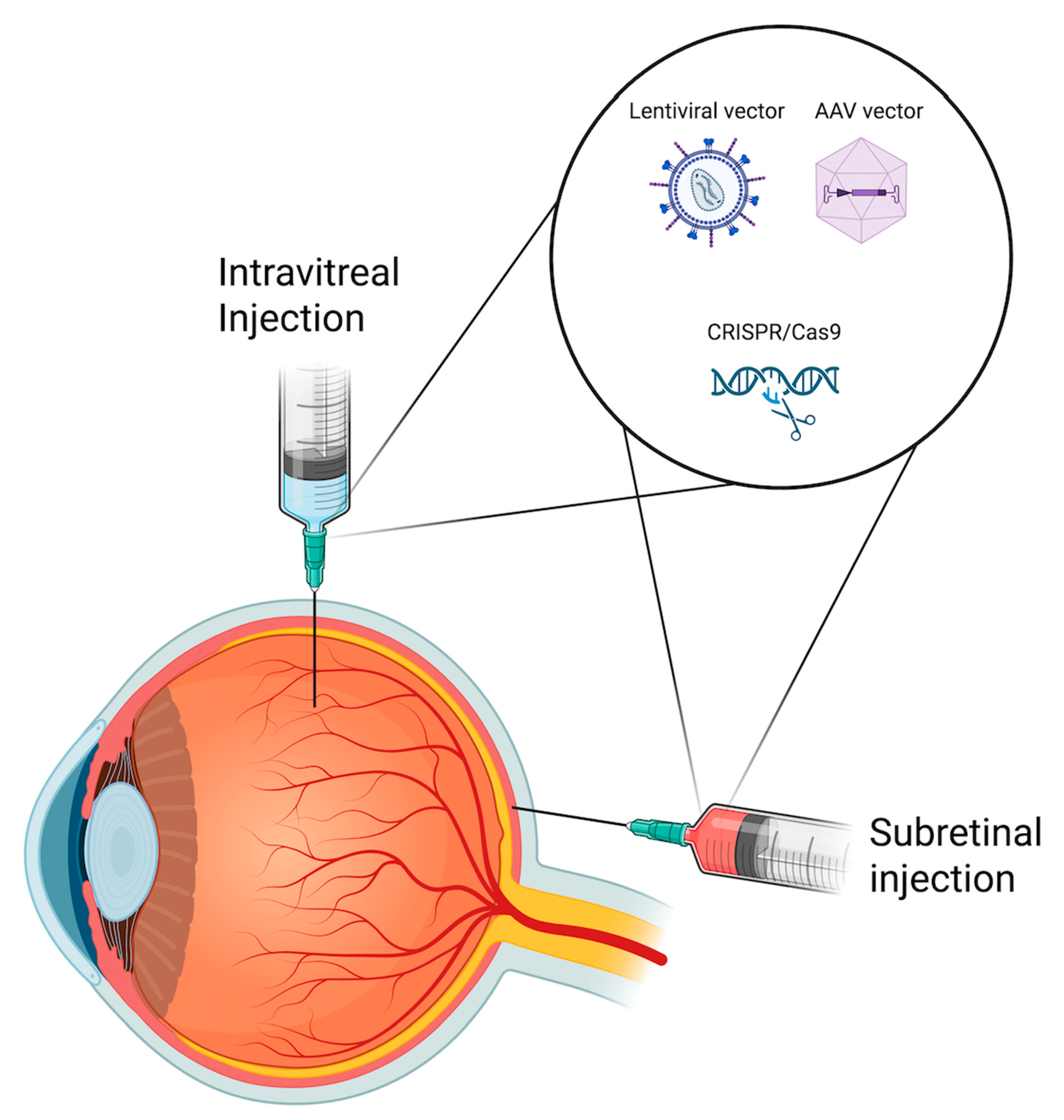
2.3. Challenges with Delivery Methods: Subretinal vs. Intravitreal
3. Personalised Medicine in Gene Therapy
3.1. Influence of Genetic Testing on Clinical Outcomes
3.2. Genetic Eligibility
3.3. Pharmacogenetics
4. Barriers to Widespread Adoption
4.1. Cost and Accessibility
4.2. Ethical and Regulatory Challenges
4.3. Scalability and Infrastructure
5. Future Directions
| Name | Description | Pros | Cons |
|---|---|---|---|
| Adeno-associated virus (AAV) vectors | Small, non-pathogenic viruses capable of delivering genetic material to both dividing and non-dividing cells. They deliver genetic material as an episome, reducing the risk of insertional mutagenesis [15,16]. | ||
| Lentiviral (LV) vectors | Derived from retroviruses and can infect both dividing and non-dividing cells. They integrate into the host genome, enabling stable, long-term expression [16,18]. |
| |
| Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9 | Genome-editing technology that allows for precise DNA modifications, including gene knockout, correction, or insertion [107,108]. |
| First Author | Gene Therapy Mechanism | Treatment Condition(s) | Sample Size (n) | Limitations | Success Rate |
|---|---|---|---|---|---|
| Russell et al. (2017) [6] | Gene augmentation therapy using AAV2-hRPE65v2 to restore RPE65 function in retinal cells [6]. | RPE65 mutation-associated IRDs, including LCA2 and RP | n = 31 | No participants under age 4; No data on patients whose visual acuity was better than specified in the protocol (visual acuity 20/60, visual field less than 20 degrees in any meridian) [6]. | Mean bilateral MLMT score improved by 1.8 light levels vs. 0.2 in controls at 1 year [6]. |
| Pennesi et al. (2022) [28] | Gene augmentation therapy using intravitreal delivery of rAAV2tYF-CB-hRS1 to enhance retinal transduction efficiency in XLRS patients [28]. | X-linked Retinoschisis (XLRS) | n = 27 | Ocular inflammation, chronic uveitis, retinal detachments [28,29] | No significant improvements in BCVA, VFs, or ERG [28]. |
| Cukras et al. (2018) [29] | Gene augmentation therapy using intravitreal injection of a self-complementary AAV8-RS1 vector to restore retinoschisin expression in XLRS patients [29]. | X-linked Retinoschisis (XLRS) | n = 9 | Ocular inflammation, chronic uveitis, retinal detachments [28,29] | BCVA remained within ±10 letters of baseline in all patients over 18 months; no statistically significant ERG changes observed [29]. Transient schisis cavity closure observed in 1 patient (11%); 4/9 patients (44.4%) had dose-dependent ocular inflammation that resolved with treatment [29]. |
| Parker et al. (2022) [33] | Gene augmentation therapy using an equine infectious anemia virus (EIAV) encoding ABCA4 gene delivered to RPE cells [33] | Stargardt Disease | n = 22 | RPE atrophy; Ocular hypertension; No clinically significant changes [33] | The treatment was not associated with any clinically meaningful improvements in visual function tests [33] |
| Busbee et al. (2021) [34] | Gene silencing anti-VEGF gene therapy using an AAV.7m8 vector to provide sustained anti-VEGF expression [34]. | Age-related macular degeneration | n = 30 | Ocular inflammation requiring steroid use; Unknown long-term efficacy [34] | 93% (high dose) and 67% (low dose) remained injection-free; BCVA was maintained (mean change: −2.5 to +0.2 letters) [34] CRT improved by 19.7 to 132.7 μm across Cohorts 1–3 [34] |
| Khanani et al. (2022) [35] | Anti-VEGF gene therapy using an AAV8 vector encoding a ranibizumab-like antibody fragment to provide long-term VEGF suppression [35]. | Age-related macular degeneration | n = 42 | Postoperative conjunctival hemorrhage; Post operative inflammation; Irritation and pain; Visual acuity reduction [35] | BCVA improved by +14 letters in Cohort 3 at 2 years; Cohorts 4 and 5 had changes of +1 and −1 letters at 1.5 years [35]. 67% showed retinal pigmentary changes; injection burden reduced by 58.3% to 81.2%; CRT change ranged from +2 to −93 µm [35]. |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, M.L.; Edwards, T.L.; O’Hare, F.; Hickey, D.G.; Wang, J.-H.; Liu, Z.; Ayton, L.N. Gene Therapy for Inherited Retinal Diseases: Progress and Possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef]
- Botto, C.; Rucli, M.; Tekinsoy, M.D.; Pulman, J.; Sahel, J.-A.; Dalkara, D. Early and Late Stage Gene Therapy Interventions for Inherited Retinal Degenerations. Prog. Retin. Eye Res. 2022, 86, 100975. [Google Scholar] [CrossRef] [PubMed]
- Willett, K.; Bennett, J. Immunology of AAV-Mediated Gene Transfer in the Eye. Front. Immunol. 2013, 4, 261. [Google Scholar] [CrossRef]
- Purdy, R.; John, M.; Bray, A.; Clare, A.J.; Copland, D.A.; Chan, Y.K.; Henderson, R.H.; Nerinckx, F.; Leroy, B.P.; Yang, P.; et al. Gene Therapy-Associated Uveitis (GTAU): Understanding and Mitigating the Adverse Immune Response in Retinal Gene Therapy. Prog. Retin. Eye Res. 2025, 106, 101354. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-Associated Virus as a Delivery Vector for Gene Therapy of Human Diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Askou, A.L.; Jakobsen, T.S.; Corydon, T.J. Retinal Gene Therapy: An Eye-Opener of the 21st Century. Gene Ther. 2021, 28, 209–216. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Invest. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Carrella, S.; Indrieri, A.; Franco, B.; Banfi, S. Mutation-Independent Therapies for Retinal Diseases: Focus on Gene-Based Approaches. Front. Neurosci. 2020, 14, 588234. [Google Scholar] [CrossRef]
- Ashley, E.A. The Precision Medicine Initiative: A New National Effort. JAMA 2015, 313, 2119. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Panikker, P.; Roy, S.; Ghosh, A.; Poornachandra, B.; Ghosh, A. Advancing Precision Medicines for Ocular Disorders: Diagnostic Genomics to Tailored Therapies. Front. Med. 2022, 9, 906482. [Google Scholar] [CrossRef]
- Fatima, M.; Pachauri, P.; Akram, W.; Parvez, M.; Ahmad, S.; Yahya, Z. Enhancing Retinal Disease Diagnosis through AI: Evaluating Performance, Ethical Considerations, and Clinical Implementation. Inform. Health 2024, 1, 57–69. [Google Scholar] [CrossRef]
- Liao, S.; Wang, L.; Wei, X. Pharmacogenetics and Pharmacogenomics in Glaucoma Therapeutics: The Way to Personalized Therapy. Chin. Med. J. 2023, 136, 2573–2575. [Google Scholar] [CrossRef]
- He, X.; Fu, Y.; Ma, L.; Yao, Y.; Ge, S.; Yang, Z.; Fan, X. AAV for Gene Therapy in Ocular Diseases: Progress and Prospects. Research 2023, 6, 0291. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Jiang, Z.; Dalby, P.A. Challenges in Scaling up AAV-Based Gene Therapy Manufacturing. Trends Biotechnol. 2023, 41, 1268–1281. [Google Scholar] [CrossRef]
- Arsenijevic, Y.; Berger, A.; Udry, F.; Kostic, C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics 2022, 14, 1605. [Google Scholar] [CrossRef]
- Miyoshi, H.; Blömer, U.; Takahashi, M.; Gage, F.H.; Verma, I.M. Development of a Self-Inactivating Lentivirus Vector. J. Virol. 1998, 72, 8150–8157. [Google Scholar] [CrossRef] [PubMed]
- Lohia, A.; Sahel, D.K.; Salman, M.; Singh, V.; Mariappan, I.; Mittal, A.; Chitkara, D. Delivery Strategies for CRISPR/Cas Genome Editing Tool for Retinal Dystrophies: Challenges and Opportunities. Asian J. Pharm. Sci. 2022, 17, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Burnight, E.R.; Wiley, L.A.; Drack, A.V.; Braun, T.A.; Anfinson, K.R.; Kaalberg, E.E.; Halder, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. CEP290 Gene Transfer Rescues Leber Congenital Amaurosis Cellular Phenotype. Gene Ther. 2014, 21, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Ragi, S.D.; Tsang, S.H. Therapy in Rhodopsin-Mediated Autosomal Dominant Retinitis Pigmentosa. Mol. Ther. 2020, 28, 2139–2149. [Google Scholar] [CrossRef]
- Gomez-Sosa, J.F.; Caviedes-Bucheli, J.; Díaz Barrera, L.E. Gene Expression of Vascular Endothelial Growth Factor A and Its Receptors in Dental Pulp of Immature and Mature Teeth. Eur. Endod. J. 2021, 6, 259–263. [Google Scholar] [CrossRef]
- Fischer, M.D.; Simonelli, F.; Sahni, J.; Holz, F.G.; Maier, R.; Fasser, C.; Suhner, A.; Stiehl, D.P.; Chen, B.; Audo, I.; et al. Real-World Safety and Effectiveness of Voretigene Neparvovec: Results up to 2 Years from the Prospective, Registry-Based PERCEIVE Study. Biomolecules 2024, 14, 122. [Google Scholar] [CrossRef]
- Gao, J.; Hussain, R.M.; Weng, C.Y. Voretigene Neparvovec in Retinal Diseases: A Review of the Current Clinical Evidence. Clin. Ophthalmol. 2020, 14, 3855–3869. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; Marshall, K.A.; et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-Rzyl in RPE65 Mutation–Associated Inherited Retinal Dystrophy. Ophthalmology 2019, 126, 1273–1285. [Google Scholar] [CrossRef]
- Ku, C.A.; Wei, L.W.; Sieving, P.A. X-Linked Retinoschisis. Cold Spring Harb. Perspect. Med. 2023, 13, a041288. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Yang, P.; Birch, D.G.; Weng, C.Y.; Moore, A.T.; Iannaccone, A.; Comander, J.I.; Jayasundera, T.; Chulay, J.; Chulay, J.; et al. Intravitreal Delivery of rAAV2tYF-CB-hRS1 Vector for Gene Augmentation Therapy in Patients with X-Linked Retinoschisis. Ophthalmol. Retin. 2022, 6, 1130–1144. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Waheed, N.; Laich, Y.; Yang, P.; Fujinami-Yokokawa, Y.; Higgins, J.J.; Lu, J.T.; Curtiss, D.; Clary, C.; Michaelides, M. Stargardt Macular Dystrophy and Therapeutic Approaches. Br. J. Ophthalmol. 2024, 108, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Tripathy, K.; Kaur, K. Stargardt Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Al-Khuzaei, S.; Broadgate, S.; Foster, C.R.; Shah, M.; Yu, J.; Downes, S.M.; Halford, S. An Overview of the Genetics of ABCA4 Retinopathies, an Evolving Story. Genes 2021, 12, 1241. [Google Scholar] [CrossRef]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Ségaut-Prévost, C.; et al. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients with ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1-5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef]
- Busbee, B.; Boyer, D.S.; Khanani, A.M.; Wykoff, C.C.; Pieramici, D.J.; Regillo, C.; Danzig, C.J.; Joondeph, B.C.; Major, J.; Hoang, C.; et al. Phase 1 Study of Intravitreal Gene Therapy with ADVM-022 for Neovascular AMD (OPTIC Trial). Invest. Ophthalmol. Vis. Sci. 2021, 62, 352. [Google Scholar]
- Khanani, A.M.; Thomas, M.J.; Aziz, A.A.; Weng, C.Y.; Danzig, C.J.; Yiu, G.; Kiss, S.; Waheed, N.K.; Kaiser, P.K. Review of Gene Therapies for Age-Related Macular Degeneration. Eye 2022, 36, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, C.; Amenabar Alonso, A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Siontas, O.; Ahn, S. Challenges in AAV-Based Retinal Gene Therapies and the Role of Magnetic Nanoparticle Platforms. J. Clin. Med. 2024, 13, 7385. [Google Scholar] [CrossRef]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 846782. [Google Scholar] [CrossRef]
- Xue, K.; Edwards, T.L.; Meenink, H.C.M.; Beelen, M.J.; Naus, G.J.L.; Simunovic, M.P.; de Smet, M.D.; MacLaren, R.E. Robot-Assisted Retinal Surgery: Overcoming Human Limitations. In Surgical Retina; Ohji, M., Ed.; Springer: Singapore, 2019; pp. 109–114. ISBN 978-981-13-6214-9. [Google Scholar]
- Ross, M.; Ofri, R. The Future of Retinal Gene Therapy: Evolving from Subretinal to Intravitreal Vector Delivery. Neural Regen. Res. 2021, 16, 1751–1759. [Google Scholar] [CrossRef]
- Chawla, H.; Tripathy, K.; Vohra, V. Retinal Dystrophies. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ellingford, J.M.; Hufnagel, R.B.; Arno, G. Phenotype and Genotype Correlations in Inherited Retinal Diseases: Population-Guided Variant Interpretation, Variable Expressivity and Incomplete Penetrance. Genes 2020, 11, 1274. [Google Scholar] [CrossRef]
- Malvasi, M.; Casillo, L.; Avogaro, F.; Abbouda, A.; Vingolo, E.M. Gene Therapy in Hereditary Retinal Dystrophies: The Usefulness of Diagnostic Tools in Candidate Patient Selections. Int. J. Mol. Sci. 2023, 24, 13756. [Google Scholar] [CrossRef]
- Petrukhin, K.; Koisti, M.J.; Bakall, B.; Li, W.; Xie, G.; Marknell, T.; Sandgren, O.; Forsman, K.; Holmgren, G.; Andreasson, S.; et al. Identification of the Gene Responsible for Best Macular Dystrophy. Nat. Genet. 1998, 19, 241–247. [Google Scholar] [CrossRef]
- Otto, E.A.; Loeys, B.; Khanna, H.; Hellemans, J.; Sudbrak, R.; Fan, S.; Muerb, U.; O’Toole, J.F.; Helou, J.; Attanasio, M.; et al. Nephrocystin-5, a Ciliary IQ Domain Protein, Is Mutated in Senior-Loken Syndrome and Interacts with RPGR and Calmodulin. Nat. Genet. 2005, 37, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ellingford, J.M.; Sergouniotis, P.I.; Lennon, R.; Bhaskar, S.; Williams, S.G.; Hillman, K.A.; O’Sullivan, J.; Hall, G.; Ramsden, S.C.; Lloyd, I.C.; et al. Pinpointing Clinical Diagnosis through Whole Exome Sequencing to Direct Patient Care: A Case of Senior-Loken Syndrome. Lancet 2015, 385, 1916. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, R.; Lantero, E.; Blanco-Kelly, F.; Avila-Fernandez, A.; Martin Merida, I.; del Pozo-Valero, M.; Perea-Romero, I.; Zurita, O.; Jiménez-Rolando, B.; Swafiri, S.T.; et al. RPE65-Related Retinal Dystrophy: Mutational and Phenotypic Spectrum in 45 Affected Patients. Exp. Eye Res. 2021, 212, 108761. [Google Scholar] [CrossRef]
- Bianco, L.; Arrigo, A.; Antropoli, A.; Manitto, M.P.; Martina, E.; Aragona, E.; Bandello, F.; Battaglia Parodi, M. Association Between Genotype and Phenotype Severity in ABCA4-Associated Retinopathy. JAMA Ophthalmol. 2023, 141, 826–833. [Google Scholar] [CrossRef]
- Molina-Ramírez, L.P.; Lenassi, E.; Ellingford, J.M.; Sergouniotis, P.I.; Ramsden, S.C.; Bruce, I.A.; Black, G.C.M. Establishing Genotype–Phenotype Correlation in USH2A-Related Disorders to Personalize Audiological Surveillance and Rehabilitation. Otol. Neurotol. 2020, 41, 431. [Google Scholar] [CrossRef]
- Lenassi, E.; Vincent, A.; Li, Z.; Saihan, Z.; Coffey, A.J.; Steele-Stallard, H.B.; Moore, A.T.; Steel, K.P.; Luxon, L.M.; Héon, E.; et al. A Detailed Clinical and Molecular Survey of Subjects with Nonsyndromic USH2A Retinopathy Reveals an Allelic Hierarchy of Disease-Causing Variants. Eur. J. Hum. Genet. 2015, 23, 1318–1327. [Google Scholar] [CrossRef]
- Burgess, R.; Millar, I.D.; Leroy, B.P.; Urquhart, J.E.; Fearon, I.M.; De Baere, E.; Brown, P.D.; Robson, A.G.; Wright, G.A.; Kestelyn, P.; et al. Biallelic Mutation of BEST1 Causes a Distinct Retinopathy in Humans. Am. J. Hum. Genet. 2008, 82, 19–31. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Poornachandra, B.; Verma, A.; Mehta, R.A.; Phalke, S.; Battu, R.; Ramprasad, V.L.; Peterson, A.S.; Ghosh, A.; Seshagiri, S. Next Generation Sequencing Identifies Novel Disease-Associated BEST1 Mutations in Bestrophinopathy Patients. Sci. Rep. 2018, 8, 10176. [Google Scholar] [CrossRef]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited Retinal Diseases: Linking Genes, Disease-Causing Variants, and Relevant Therapeutic Modalities. Prog. Retin. Eye Res. 2022, 89, 101029. [Google Scholar] [CrossRef]
- Meyers, K.J.; Liu, Z.; Millen, A.E.; Iyengar, S.K.; Blodi, B.A.; Johnson, E.; Snodderly, D.M.; Klein, M.L.; Gehrs, K.M.; Tinker, L.; et al. Joint Associations of Diet, Lifestyle, and Genes with Age-Related Macular Degeneration. Ophthalmology 2015, 122, 2286–2294. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Ciupińska, J.; Derwich, M.; Szczepanska, J.; Kaarniranta, K. A New Generation of Gene Therapies as the Future of Wet AMD Treatment. Int. J. Mol. Sci. 2024, 25, 2386. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.L.; Burr, A.; Pennesi, M. RPE65-Related Leber Congenital Amaurosis/Early-Onset Severe Retinal Dystrophy. In GeneReviews®; University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biol. Targets Ther. 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Yu, Z.; Coorens, T.H.H.; Uddin, M.M.; Ardlie, K.G.; Lennon, N.; Natarajan, P. Genetic Variation across and within Individuals. Nat. Rev. Genet. 2024, 25, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Grishin, D.; Wang, G.; Aach, J.; Zhang, C.-Z.; Chari, R.; Homsy, J.; Cai, X.; Zhao, Y.; Fan, J.-B.; et al. Targeted and Genome-Wide Sequencing Reveal Single Nucleotide Variations Impacting Specificity of Cas9 in Human Stem Cells. Nat. Commun. 2014, 5, 5507. [Google Scholar] [CrossRef]
- Scott, D.A.; Zhang, F. Implications of Human Genetic Variation in CRISPR-Based Therapeutic Genome Editing. Nat. Med. 2017, 23, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of Protein-Coding Genetic Variation in 60,706 Humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Durbin, R.M.; Altshuler, D.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Collins, F.S.; De La Vega, F.M.; Donnelly, P.; et al. A Map of Human Genome Variation from Population-Scale Sequencing. Nature 2010, 467, 1061–1073. [Google Scholar] [CrossRef]
- McVean, G.A.; Altshuler, D.M.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. An Integrated Map of Genetic Variation from 1,092 Human Genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 Nuclease with Expanded Targeting Space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-Fidelity CRISPR–Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Pierce, E.A.; Aleman, T.S.; Jayasundera, K.T.; Ashimatey, B.S.; Kim, K.; Rashid, A.; Jaskolka, M.C.; Myers, R.L.; Lam, B.L.; Bailey, S.T.; et al. Gene Editing for CEP290-Associated Retinal Degeneration. N. Engl. J. Med. 2024, 390, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Spark’s Gene Therapy Price Tag: $850,000. Nat. Biotechnol. 2018, 36, 122. [CrossRef]
- FDA Approves Hereditary Blindness Gene Therapy. Nat. Biotechnol. 2018, 36, 6. [CrossRef] [PubMed]
- Johnson, S.; Buessing, M.; O’Connell, T.; Pitluck, S.; Ciulla, T.A. Cost-Effectiveness of Voretigene Neparvovec-Rzyl vs Standard Care for RPE65-Mediated Inherited Retinal Disease. JAMA Ophthalmol. 2019, 137, 1115–1123. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, P.Y.; Branham, K.; Schlegel, D.; Fahim, A.T.; Jayasundera, K.T.; Khan, N.; Besirli, C.G. Real-World Outcomes of Voretigene Neparvovec Treatment in Pediatric Patients with RPE65-Associated Leber Congenital Amaurosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1543–1550. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Macpherson, K.; Elston, L.; Myles, S.; Washington, J.; Sungum, N.; Briggs, M.; Newsome, P.N.; Calvert, M.J. Patient and Public Perspectives on Cell and Gene Therapies: A Systematic Review. Nat. Commun. 2020, 11, 6265. [Google Scholar] [CrossRef]
- King, W.D.; Wyatt, G.E.; Liu, H.; Williams, J.K.; DiNardo, A.D.; Mitsuyasu, R.T. Pilot Assessment of HIV Gene Therapy-Hematopoietic Stem Cell Clinical Trial Acceptability Among Minority Patients and Their Advisors. J. Natl. Med. Assoc. 2010, 102, 1123–1130. [Google Scholar] [CrossRef]
- Strong, H.; Mitchell, M.J.; Goldstein-Leever, A.; Shook, L.; Malik, P.; Crosby, L.E. Patient Perspectives on Gene Transfer Therapy for Sickle Cell Disease. Adv. Ther. 2017, 34, 2007–2021. [Google Scholar] [CrossRef]
- Peay, H.; Fischer, R.; Beaverson, K.; Morris, C.; Hesterlee, S.E.; Ricotti, V.; Martin, A.; Rensch, C.; Wand, H.; Mansfield, C.A.; et al. Parent and Adult Patient Attitudes About Gene Therapy as a Therapeutic Option for Duchenne Muscular Dystrophy. Value Health 2018, 21, S256. [Google Scholar] [CrossRef]
- Tanner, C.; Petersen, A.; Munsie, M. ‘No One Here’s Helping Me, What Do You Do?’: Addressing Patient Need for Support and Advice about Stem Cell Treatments. Regen. Med. 2017, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.J.; Kwon, B.K.; Lo, C.; Snyder, J.; Illes, J. Perspectives on Strategies and Challenges in the Conversation about Stem Cells for Spinal Cord Injury. Spinal Cord 2015, 53, 811–815. [Google Scholar] [CrossRef][Green Version]
- Kempf, L.; Goldsmith, J.C.; Temple, R. Challenges of Developing and Conducting Clinical Trials in Rare Disorders. Am. J. Med. Genet. Part A 2018, 176, 773–783. [Google Scholar] [CrossRef]
- Hanany, M.; Shalom, S.; Ben-Yosef, T.; Sharon, D. Comparison of Worldwide Disease Prevalence and Genetic Prevalence of Inherited Retinal Diseases and Variant Interpretation Considerations. Cold Spring Harb. Perspect. Med. 2024, 14, a041277. [Google Scholar] [CrossRef]
- Augustine, E.F.; Adams, H.R.; Mink, J.W. Clinical Trials in Rare Disease: Challenges and Opportunities. J. Child Neurol. 2013, 28, 1142–1150. [Google Scholar] [CrossRef]
- Belite Bio Finalizes Phase 3 Clinical Trial Plans for Advanced Dry AMD Treatment with Tinlarebant (LBS-008)|Belite Bio, Inc. Available online: https://investors.belitebio.com/news-releases/news-release-details/belite-bio-finalizes-phase-3-clinical-trial-plans-advanced-dry/ (accessed on 3 April 2025).
- Fu, Q.; Polanco, A.; Lee, Y.S.; Yoon, S. Critical Challenges and Advances in Recombinant Adeno-Associated Virus (rAAV) Biomanufacturing. Biotechnol. Bioeng. 2023, 120, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.T.; Park, D.; Canova, C.T.; Sangerman, J.; Srinivasan, P.; Ou, R.W.; Barone, P.W.; Neufeld, C.; Wolfrum, J.M.; Springs, S.L.; et al. Perfusion-Based Production of rAAV via an Intensified Transient Transfection Process. Biotechnol. Bioeng. 2025, 122, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lv, Y.-L.; Wang, X.-T.; Yuan, L.-H.; Zhao, K.; Du, Z.-M.; Xiao, X. Production of Recombinant Adeno-Associated Virus Through High-Cell-Density Transfection of HEK293 Cells Based on Fed-Perfusion Culture. Hum. Gene Ther. 2025, 36, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Emerson, S.G.; Punt, J.; Goff, W.D. Decreased Naïve T-Cell Production Leading to Cytokine Storm as Cause of Increased COVID-19 Severity with Comorbidities. Aging Dis. 2020, 11, 742–745. [Google Scholar] [CrossRef]
- Zhao, H.; Lee, K.-J.; Daris, M.; Lin, Y.; Wolfe, T.; Sheng, J.; Plewa, C.; Wang, S.; Meisen, W.H. Creation of a High-Yield AAV Vector Production Platform in Suspension Cells Using a Design-of-Experiment Approach. Mol. Ther. Methods Clin. Dev. 2020, 18, 312–320. [Google Scholar] [CrossRef]
- Baxter, M.F.; Borchert, G.A. Gene Therapy for Achromatopsia. Int. J. Mol. Sci. 2024, 25, 9739. [Google Scholar] [CrossRef]
- Understanding Glaucoma: Symptoms, Causes, Diagnosis, Treatment. Available online: https://www.aao.org/eye-health/diseases/what-is-glaucoma (accessed on 3 April 2025).
- Sharma, R.; Grover, A. Myocilin-Associated Glaucoma: A Historical Perspective and Recent Research Progress. Mol. Vis. 2021, 27, 480. [Google Scholar]
- Wang, R.; Wiggs, J.L. Common and Rare Genetic Risk Factors for Glaucoma. Cold Spring Harb. Perspect. Med. 2014, 4, a017244. [Google Scholar] [CrossRef]
- Crawford, A.; Souzeau, E.; Agar, A.; Ridge, B.; Dubowsky, A.; Burdon, K.P.; Craig, J.E. Identification of a Novel MYOC Mutation, p.(Trp373*), in a Family with Open Angle Glaucoma. Gene 2014, 545, 271–275. [Google Scholar] [CrossRef]
- Craig, J.E.; Baird, P.N.; Healey, D.L.; McNaught, A.I.; McCartney, P.J.; Rait, J.L.; Dickinson, J.L.; Roe, L.; Fingert, J.H.; Stone, E.M.; et al. Evidence for Genetic Heterogeneity within Eight Glaucoma Families, with the GLC1A Gln368STOP Mutation Being an Important Phenotypic Modifier. Ophthalmology 2001, 108, 1607–1620. [Google Scholar] [CrossRef]
- Zhuo, Y.-H.; Wei, Y.-T.; Bai, Y.-J.; Duan, S.; Lin, M.-K.; Saragovi, H.U.; Ge, J. Pro370Leu MYOC Gene Mutation in a Large Chinese Family with Juvenile-Onset Open Angle Glaucoma: Correlation Between Genotype and Phenotype. Mol. Vis. 2008, 14, 1533–1539. [Google Scholar] [PubMed]
- Anton, N.; Geamănu, A.; Iancu, R.; Pîrvulescu, R.A.; Popa-Cherecheanu, A.; Barac, R.I.; Bandol, G.; Bogdănici, C.M. A Mini-Review on Gene Therapy in Glaucoma and Future Directions. Int. J. Mol. Sci. 2024, 25, 11019. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER Stress via a Chemical Chaperone Prevents Disease Phenotypes in a Mouse Model of Primary Open Angle Glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9–Based Treatment of Myocilin-Associated Glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef]
- Patil, S.V.; Kaipa, B.R.; Ranshing, S.; Sundaresan, Y.; Millar, J.C.; Nagarajan, B.; Kiehlbauch, C.; Zhang, Q.; Jain, A.; Searby, C.C.; et al. Lentiviral Mediated Delivery of CRISPR-Cas9 Reduces Intraocular Pressure in a Mouse Model of Myocilin Glaucoma. Sci. Rep. 2024, 14, 6958. [Google Scholar] [CrossRef]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune Responses to Retinal Gene Therapy Using Adeno-Associated Viral Vectors—Implications for Treatment Success and Safety. Prog. Retin. Eye Res. 2021, 83, 100915. [Google Scholar] [CrossRef]
- Agbandje-McKenna, M.; Kleinschmidt, J. AAV Capsid Structure and Cell Interactions. In Adeno-Associated Virus: Methods and Protocols; Snyder, R.O., Moullier, P., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 47–92. ISBN 978-1-61779-370-7. [Google Scholar]
- Davidson, B.A.; Miranda, A.X.; Reed, S.C.; Bergman, R.E.; Kemp, J.D.J.; Reddy, A.P.; Pantone, M.V.; Fox, E.K.; Dorand, R.D.; Hurley, P.J.; et al. An in Vitro CRISPR Screen of Cell-Free DNA Identifies Apoptosis as the Primary Mediator of Cell-Free DNA Release. Commun. Biol. 2024, 7, 441. [Google Scholar] [CrossRef]
- Martino, R.A.; Wang, Q.; Xu, H.; Hu, G.; Bell, P.; Arroyo, E.J.; Sims, J.J.; Wilson, J.M. Vector Affinity and Receptor Distribution Define Tissue-Specific Targeting in an Engineered AAV Capsid. J. Virol. 2023, 97, e0017423. [Google Scholar] [CrossRef]
- Guo, J.; Lin, L.F.; Oraskovich, S.V.; de Jesús, J.A.R.; Listgarten, J.; Schaffer, D.V. Computationally Guided AAV Engineering for Enhanced Gene Delivery. Trends Biochem. Sci. 2024, 49, 457–469. [Google Scholar] [CrossRef]
- Marques, A.D.; Kummer, M.; Kondratov, O.; Banerjee, A.; Moskalenko, O.; Zolotukhin, S. Applying Machine Learning to Predict Viral Assembly for Adeno-Associated Virus Capsid Libraries. Mol. Ther. Methods Clin. Dev. 2021, 20, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Brookes, D.H.; Busia, A.; Carneiro, A.; Fannjiang, C.; Popova, G.; Shin, D.; Donohue, K.C.; Lin, L.F.; Miller, Z.M.; et al. Optimal Trade-off Control in Machine Learning–Based Library Design, with Application to Adeno-Associated Virus (AAV) for Gene Therapy. Sci. Adv. 2024, 10, eadj3786. [Google Scholar] [CrossRef]
- Becker, J.; Fakhiri, J.; Grimm, D. Fantastic AAV Gene Therapy Vectors and How to Find Them—Random Diversification, Rational Design and Machine Learning. Pathogens 2022, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; O’Doherty, U. Clinical Use of Lentiviral Vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Badran, A.H.; Liu, D.R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 2017, 168, 20–36. [Google Scholar] [CrossRef]
- Ayanoğlu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical Issues in Genome Editing by CRISPR-Cas9 Technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.-A.; Marazova, K.; Audo, I. Clinical Characteristics and Current Therapies for Inherited Retinal Degenerations. Cold Spring Harb. Perspect. Med. 2015, 5, a017111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, F.R.; Dhivagaran, T.; Li, B.; Ashamalla, M.; Tao, B.K.; Balas, M.; Pereira, A.; Yan, P.; Arjmand, P. Gene Therapy for Inherited Retinal Disease: Current Strategies, Personalized Medicine, and Future Implications—A Comprehensive Review. J. Pers. Med. 2025, 15, 619. https://doi.org/10.3390/jpm15120619
Butt FR, Dhivagaran T, Li B, Ashamalla M, Tao BK, Balas M, Pereira A, Yan P, Arjmand P. Gene Therapy for Inherited Retinal Disease: Current Strategies, Personalized Medicine, and Future Implications—A Comprehensive Review. Journal of Personalized Medicine. 2025; 15(12):619. https://doi.org/10.3390/jpm15120619
Chicago/Turabian StyleButt, Fahad R., Thanansayan Dhivagaran, Boaz Li, Mark Ashamalla, Brendan K. Tao, Michael Balas, Austin Pereira, Peng Yan, and Parnian Arjmand. 2025. "Gene Therapy for Inherited Retinal Disease: Current Strategies, Personalized Medicine, and Future Implications—A Comprehensive Review" Journal of Personalized Medicine 15, no. 12: 619. https://doi.org/10.3390/jpm15120619
APA StyleButt, F. R., Dhivagaran, T., Li, B., Ashamalla, M., Tao, B. K., Balas, M., Pereira, A., Yan, P., & Arjmand, P. (2025). Gene Therapy for Inherited Retinal Disease: Current Strategies, Personalized Medicine, and Future Implications—A Comprehensive Review. Journal of Personalized Medicine, 15(12), 619. https://doi.org/10.3390/jpm15120619







