Secretan’s Syndrome of the Hand: Literature Review and Surgical Case Report of a Rarely Documented Condition
Abstract
1. Introduction
2. Materials and Methods of Literature Review
3. Review of the Literature
3.1. Epidemiology
3.2. Clinical Presentation
3.3. Imaging
3.4. Histology
3.5. Management
4. Detailed Case Description
4.1. Patient Information
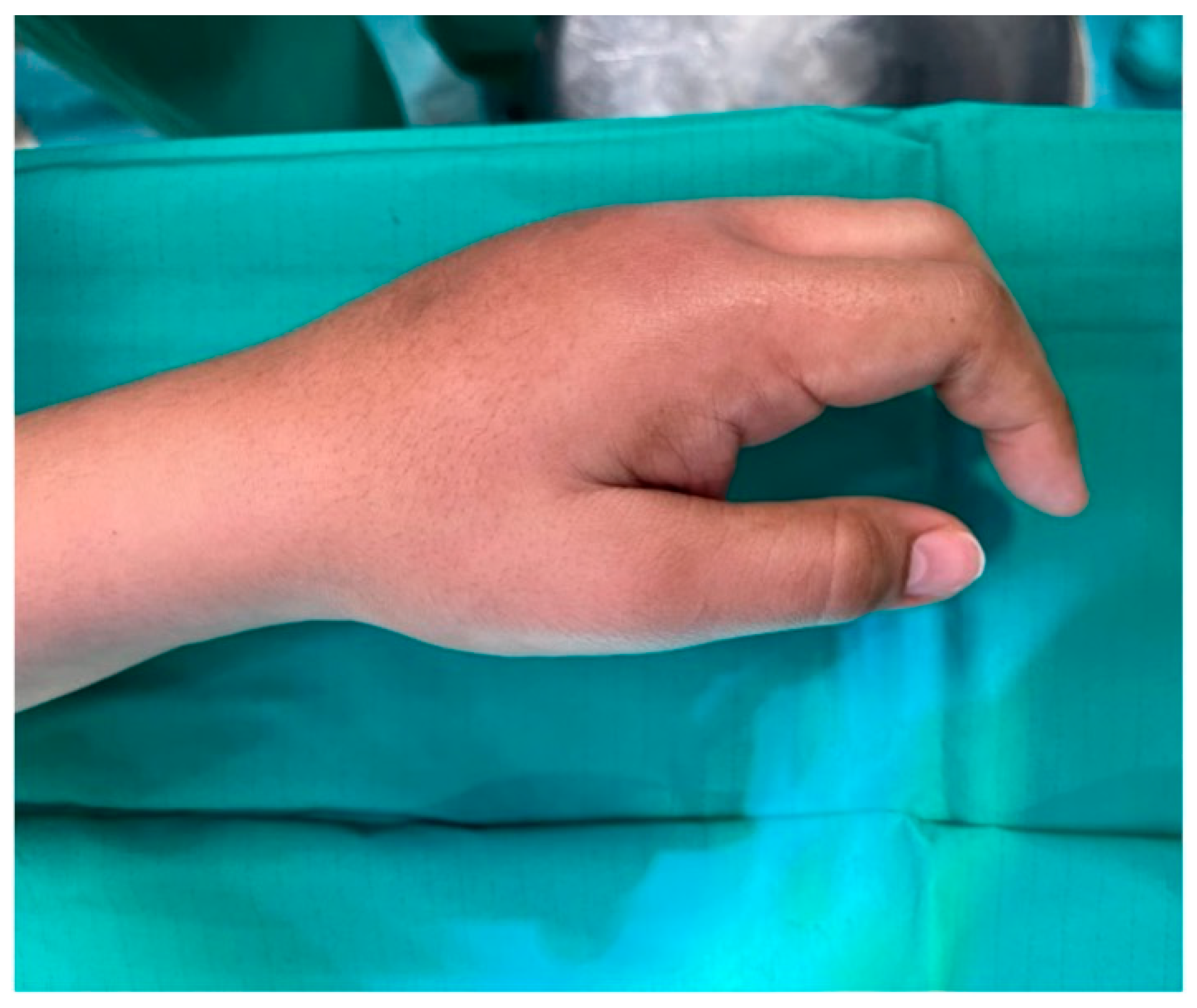
4.2. Clinical Findings
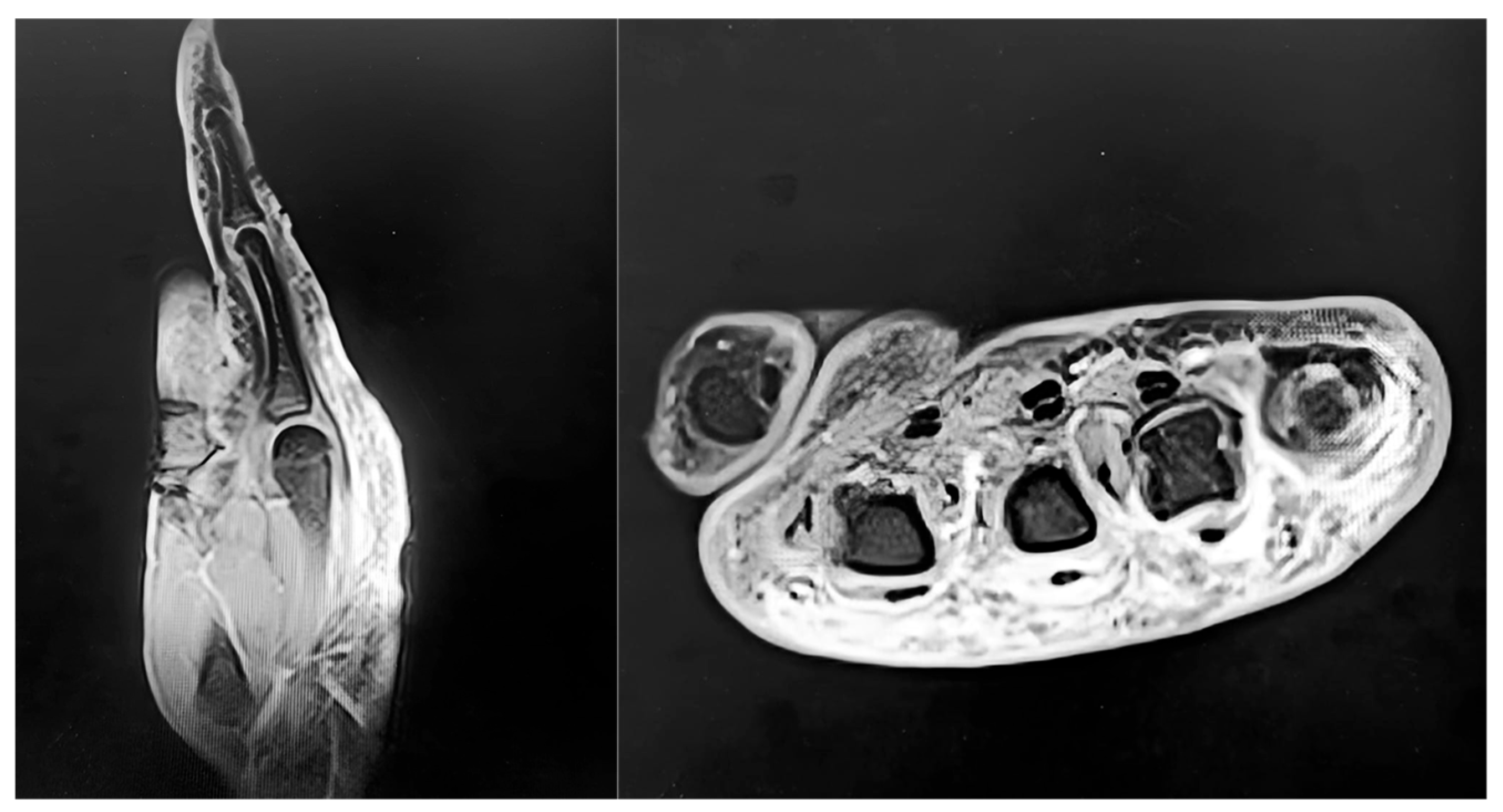
4.3. Diagnostic Assessment
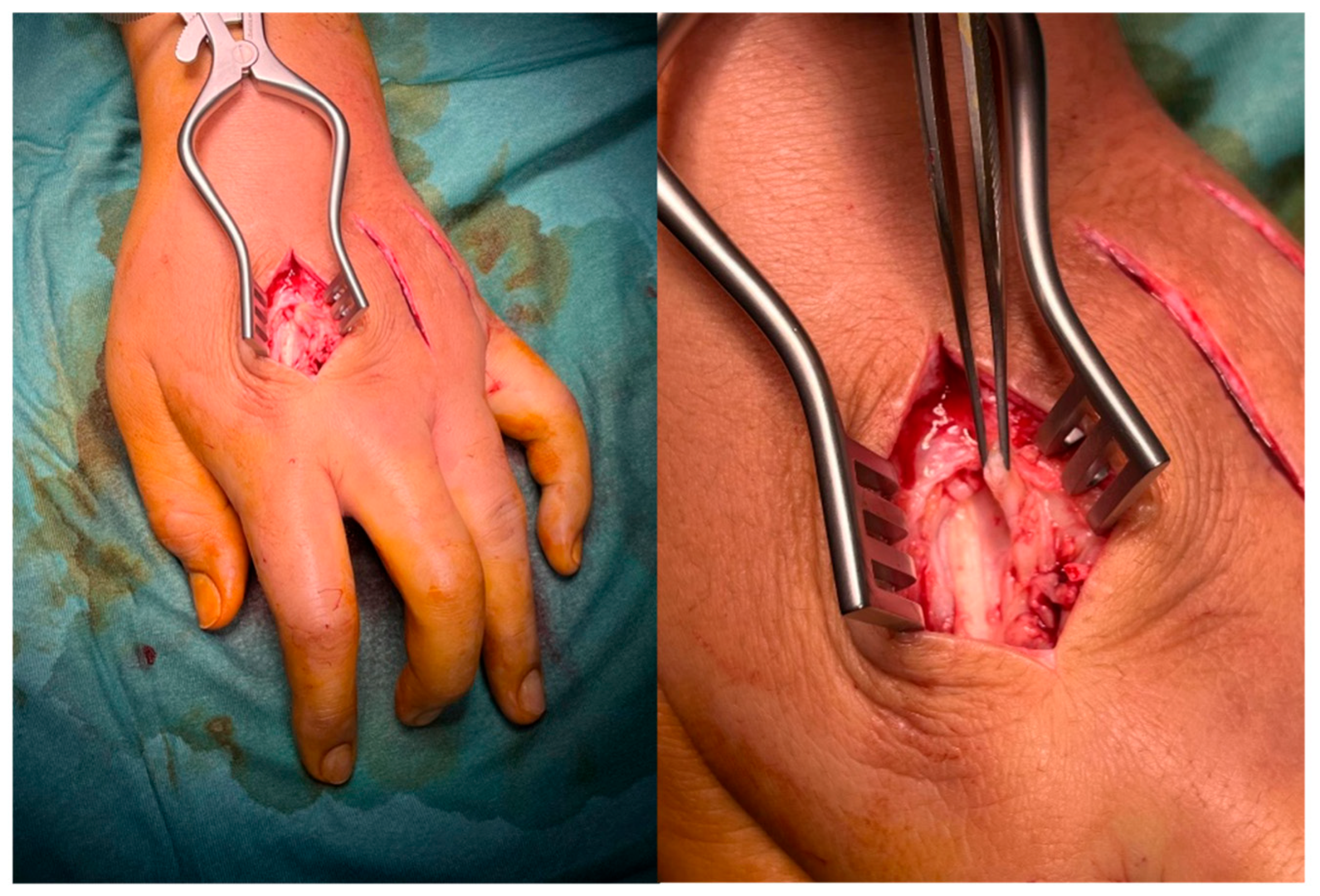
4.4. Therapeutic Intervention
4.5. Microbiological and Histological Findings
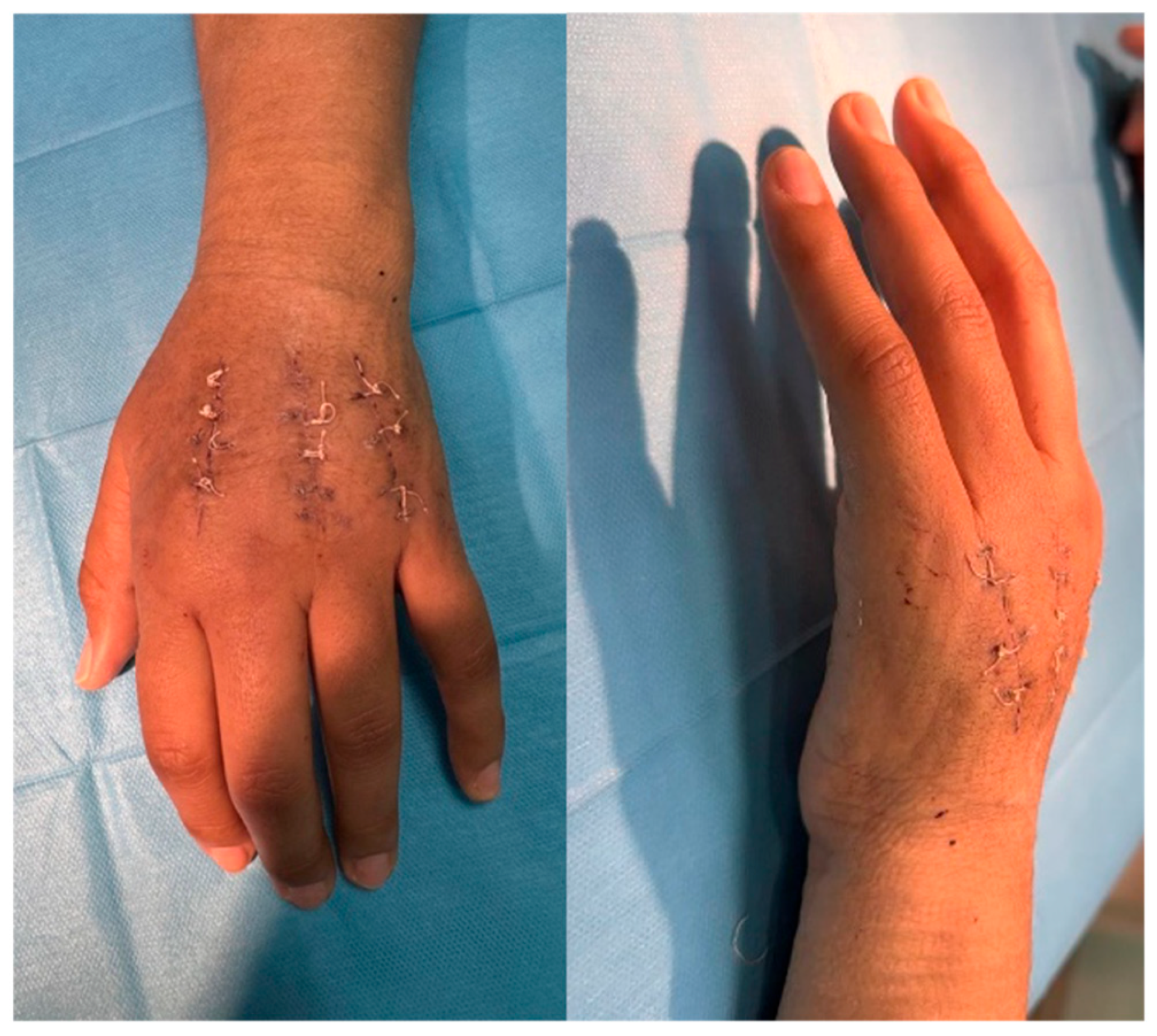
4.6. Follow-Up and Outcomes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRPS | Complex Regional Pain Syndrome |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| MCP | Metacarpophalangeal |
| MRI | Magnetic Resonance Imaging |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| VAS | Visual Analog Scale |
References
- Angelini, G.; Meneghini, C.L.; Vena, G.A. Secretan’s syndrome: An artefact oedema of the hand. Contact Dermat. 1982, 8, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Demircioğlu, D.; Öztürk Durmaz, E.; Sezer, E.; Şahin, S. Secretan syndrome: A fluctuating case of factitious lymphedema. Cutis 2021, 108, E23–E24. [Google Scholar] [CrossRef] [PubMed]
- Abnousi, F.; Chou, L.B. Secretan’s disease of the foot: A case report and review. Foot Ankle Int. 2008, 29, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Whitney, T.M.; Jones, N.F. Magnetic resonance imaging findings in Secretan’s disease. J. Hand Surg. Am. 1995, 20, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L.; van Doninck, J.; de Smet, L. Secretan’s syndrome: Case report. Acta Chir. Belg. 2019, 119, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Tebbaa El Hassali, A.; Barrached, M.; Lachkar, A.; Abdeljaouad, N.; Yacoubi, H. Secretan’s syndrome: It is time to talk about it. Cureus 2024, 16, e62580. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.P. Secretan’s disease. Plast. Reconstr. Surg. 1977, 60, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Collet, S.; Forli, A.; Carpentier, P.H.; Laviolette, F.; Imbert, B.; Blaise, S. Secretan’s syndrome: Myth or pathomimia? J. Mal. Vasc. 2014, 39, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Redfern, A.B.; Curtis, R.M.; Wilgis, E.F.S. Experience with peritendinous fibrosis of the dorsum of the hand. J. Hand Surg. Am. 1982, 7, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Grobmyer, A.J., 3rd; Bruner, J.M.; Dragstedt, L.R., 2nd. Closed lymphangioplasty in Secretan’s disease. Arch. Surg. 1968, 97, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, R.K.; Barker, S.M. Factitious lymphoedema, Secretan’s syndrome. Dermatol. Clin. 1990, 8, 205. [Google Scholar] [CrossRef]
- Birman, M.V.; Lee, D.H. Factitious disorders of the upper extremity. J. Am. Acad. Orthop. Surg. 2012, 20, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Billy, J.; Colletti, M.; Gazzoni, M.; Cescon, C.; Merletti, R. Applications of ultrasound elastography to hand and upper-limb soft tissues: Techniques and clinical value. J. Ultrasound Med. 2024, 43, 1121–1134. [Google Scholar] [CrossRef]
- Sahu, A.K.; Kataria, S.; Gandikota, G. Added value of high-resolution ultrasound and MRI in the evaluation of rheumatologic diseases. J. Ultrason. 2023, 23, e285–e298. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.; O’Neill, K.; Power, D.; Murphy, C.; Kelly, J.L. Rehabilitation of persistent poor hand function after trauma: Current strategies and future directions. J. Hand Ther. 2025; in press. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | No. of Cases | Site | Diagnostic Findings | Management | Outcome |
|---|---|---|---|---|---|
| Grobmyer et al., 1968 [10] | 1 | Hand | Indurated dorsal edema | Surgical (closed lymphangioplasty) | Persistent edema |
| Fleming, 1977 [7] | 1 | Hand | Indurated dorsal edema, fibrosis (histology) | Conservative | Chronic course with persistent symptoms |
| Angelini et al., 1982 [1] | 1 | Hand | Non-pitting dorsal edema; fibrosis (histology) | Conservative | Partial resolution |
| Redfern et al., 1982 [9] | 4 | Hand | Peritendinous fibrosis | Surgical release | Functional improvement |
| Winkelmann & Barker, 1990 [11] | 2 | Hand | Chronic dorsal edema; suspected factitious lymphedema | Psychiatric + conservative | Resolution with follow-up |
| Whitney & Jones, 1995 [4] | 1 | Hand | Chronic dorsal edema; peritendinous fibrosis (MRI) | Conservative | Persistent symptoms with functional limitation |
| Abnousi & Chou, 2008 [3] | 1 | Foot | Dorsal edema, fibrotic band | Surgical excision | Symptom resolution |
| Birman & Lee, 2012 [12] | Review | Upper limb | Factitious disorders of the extremity | Psychiatric/varied | Highlights diagnostic overlap |
| Collet et al., 2014 [8] | 3 | Hand | Chronic dorsal edema, possible factitious cases | Conservative, psychiatric | Variable outcomes |
| Lemmens et al., 2019 [5] | 1 | Hand | Chronic dorsal edema; fibrosis (biopsy) | Conservative | Persistent symptoms |
| Demircioğlu et al., 2021 [2] | 1 | Hand | Fluctuating edema; suspected factitious etiology | Psychiatric + conservative | Symptom control |
| Tebbaa El Hassali et al., 2024 [6] | 1 | Hand | Chronic indurated dorsal edema | Conservative | Stable with follow-up |
| Timepoint | VAS (0–10) | QuickDASH (0–100) | Grip Strength (Jamar, % vs. Contralateral) |
|---|---|---|---|
| Preoperative | 7 | 65 | 40% |
| Early post-op (1 month) | 5 | 50 | 50% |
| 3-Month Follow-up | 4 | 35 | 65% |
| 6-Month Follow-up | 2 | 20 | 75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruciani, A.; Gerace, E.; Vavalle, G.; Di Dio, E.; Pietramala, S.; Rocchi, L. Secretan’s Syndrome of the Hand: Literature Review and Surgical Case Report of a Rarely Documented Condition. J. Pers. Med. 2025, 15, 586. https://doi.org/10.3390/jpm15120586
Cruciani A, Gerace E, Vavalle G, Di Dio E, Pietramala S, Rocchi L. Secretan’s Syndrome of the Hand: Literature Review and Surgical Case Report of a Rarely Documented Condition. Journal of Personalized Medicine. 2025; 15(12):586. https://doi.org/10.3390/jpm15120586
Chicago/Turabian StyleCruciani, Andrea, Emanuele Gerace, Gianmarco Vavalle, Elisa Di Dio, Silvia Pietramala, and Lorenzo Rocchi. 2025. "Secretan’s Syndrome of the Hand: Literature Review and Surgical Case Report of a Rarely Documented Condition" Journal of Personalized Medicine 15, no. 12: 586. https://doi.org/10.3390/jpm15120586
APA StyleCruciani, A., Gerace, E., Vavalle, G., Di Dio, E., Pietramala, S., & Rocchi, L. (2025). Secretan’s Syndrome of the Hand: Literature Review and Surgical Case Report of a Rarely Documented Condition. Journal of Personalized Medicine, 15(12), 586. https://doi.org/10.3390/jpm15120586






