1. Introduction
Melanoma is a malignant neoplasm arising from melanocytes. These are pigment cells located in the epidermis and within mucosal membranes of the upper airway, genitourinary tract, and gastrointestinal tract. The most common melanoma subtype, cutaneous melanoma, arises from melanocytes within the epidermis, whereas the significantly rarer mucosal melanomas arise from melanocytes in mucosal membranes. Traditionally, these pathologies have been seen to be similar and findings from one sub-type have often been extrapolated. Recent evidence suggests that although arising from a similar cell origin, melanomas are a highly heterogenous group and demonstrate significant differences in their underlying pathogenesis, immune microenvironment, genetic mutations, clinical behaviour and subsequent response to treatment. The more common cutaneous melanoma has seen improved survival over the past decade as a result of significant advances in the understanding of tumour biology and the development of new targeted and immune checkpoint inhibitors (ICIs). Our understanding of immune checkpoint pathways and the BRAF/CKIT genetic mutations which underpin the pathogenesis of many cancers has revolutionised cancer treatment. ICIs targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand (PD-L1) inhibit negative immune regulation, thereby enhancing anti-cancer immune activity. In contrast, partly due to their rarity, understanding of the distinct features of mucosal and vulvovaginal melanomas has progressed at a slower rate and the evidence used to guide treatment of these tumours is extremely limited, with only small retrospective studies and case series or largely extrapolated from cutaneous melanoma trials. This review synthesises current evidence to compare cutaneous melanoma, mucosal melanoma and particularly the subset of vulvovaginal melanoma as three distinct subtypes. The molecular and mutational changes, immune profile and how these changes translate to the efficacy of treatment and patient survival are explored to identify key areas for future progress.
2. Materials and Methods
Given the broad scope of this review—encompassing epidemiology, tumour biology, therapeutic options, and survival outcomes across three disease pathologies—a narrative design was chosen. A literature search was undertaken in MEDLINE (PubMed), Embase, and ClinicalTrials.gov to identify studies on cutaneous, mucosal, and vulvovaginal melanomas published up to 30 June 2025. The search strategy combined relevant keywords and Medical Subject Headings (MeSH) to perform several targeted literature reviews tailored to the specific focus of each component of the review. These keywords included: cutaneous melanoma, mucosal melanoma, vulvovaginal melanoma, BRAF mutation, NRAS mutation, KIT mutation, genetic mutation, tumour microenvironment, ICIs, PD-1 inhibitors, CTLA-4 inhibitors, targeted therapy, BRAF/MEK inhibitors, systemic therapy, adjuvant therapy, surgical management, recurrence, and survival outcomes. For example, keywords including ‘adjuvant treatment’ OR ‘immune checkpoint inhibitors’ OR ‘systemic treatment’ AND ‘vulvovaginal melanoma’ were used when performing the literature search for ICI use in vulvovaginal melanoma. This allowed for comprehensive and targeted identification and synthesis of studies, however, it also limits the ability to present a single flow of screened and excluded studies. Systematic reviews, randomised controlled trials, cohort and observational trials (both prospective and retrospective) were included. Case series and case reports were excluded. Due to logistical considerations, only English-language publications were included, which was a limitation of the study given the higher incidence of mucosal melanoma in Asian populations. Additionally, given the narrative nature of this review, a formal quality appraisal of each study was not undertaken. Additional relevant articles were identified by screening the reference lists of relevant studies. Meta-analysis of Observational Studies in Epidemiology—A Proposal for Reporting was used, where relevant, for included observational studies.
3. Discussion
3.1. Epidemiology, Presentation, Risk Factor
3.1.1. Cutaneous Melanoma
Cutaneous melanomas account for the majority of melanoma diagnoses (>90%) in white populations, with a steady rise in incidence worldwide since the 1950s. This has been attributed to greater exposure to ultraviolet radiation (UVR), both from natural and artificial sources [
1]. Reported global incidence of melanoma in 2020 was 3.4 per 100,000 [
2], with the highest incidence rates observed in Australia and New Zealand at 42 per 100,000 person-years in males and 31 per 100,000 person-years in females [
3]. Cutaneous melanoma arises from the progressive accumulation of genetic mutations, largely driven by the oncogenic effects of UVR, which disrupt normal cell proliferation, differentiation, and death. Additional interactions between inherited germline genetic modifiers and phenotypic risk factors (e.g., lighter skin tone, sun sensitivity, naevus count or type) also contribute. Accordingly, risk factors for cutaneous melanoma include UVR exposure and non-modifiable factors such as male sex, high naevus count, atypical naevi, first-degree relative with melanoma, previous melanoma, and immunosuppression [
1]. Melanoma tumours are classified into nine subtypes, a system originally developed by the World Health Organization (WHO), with each subtype characterised by distinct epidemiological, clinical, histopathological, and genomic features. The two most common forms of cutaneous melanoma are low cumulative sun damage melanoma (superficial spreading melanoma) and high cumulative sun damage melanoma (lentigo maligna melanoma). Superficial spreading melanoma usually arises in sun-exposed skin with minimal solar elastosis on the trunk or back of younger adults. In contrast, lentigo maligna melanoma typically occurs on the head and neck of older patients and is associated with marked solar elastosis and chronic sun exposure [
4]. Fortunately, with contributions from health education and awareness campaigns, cutaneous melanoma is predominantly diagnosed at early stages, with 77% of patients having localised disease at diagnosis [
5].
3.1.2. Mucosal Melanoma
Mucosal melanoma is a rare and aggressive subtype, accounting for less than 2% of all melanomas in white populations and up to 23% in Chinese populations [
6]. Unlike cutaneous melanoma, its incidence appears relatively stable. Mucosal melanomas can arise from any mucosal membrane and most commonly occur in the head and neck, anorectal, and vulvovaginal regions. They are more frequent in females, largely due to the occurrence of vulvovaginal melanomas, which represent the most common subtype in women, while head and neck tumours are the most common subtype in men [
7,
8]. Mucosal melanomas are usually diagnosed later in life, with a median age of 70 years [
8]. Their risk factors remain poorly understood. Unlike cutaneous melanoma, UVR does not appear to be a strong causative factor, and many sites in which mucosal melanoma develops are not routinely exposed to UVR [
9]. Mucosal melanoma is often diagnosed at a later stage, with two-thirds of patients presenting with regional, distant, or nodal metastasis at the time of diagnosis. This may be partly due to a lack of specific symptoms or signs and the wide variation in clinical presentation, depending on the site of origin, which can delay diagnosis. The sites are often more occult than those of cutaneous melanoma. These factors likely contribute to the poor prognosis, with overall five-year survival rates of only 25% despite optimal surgical resection [
10].
3.1.3. Vulvovaginal Melanoma
Vulvovaginal melanomas have traditionally been grouped with mucosal melanomas, but recent evidence examining their molecular and genetic characteristics shows that melanomas of the female genital tract differ from both other mucosal and cutaneous melanomas [
11]. Vulvovaginal melanomas are extremely rare, accounting for 1% of all malignant melanomas in women, 5.3% of vulval malignancies, and 5.5% of vaginal malignancies [
11]. The reported incidence is about 1.74 cases per million person-years [
12]. The median age at diagnosis is 68 years for vulval melanoma and 71 years for vaginal melanoma. Risk factors remain poorly understood but include advanced age and white ethnicity [
13]. Diagnosis often occurs at an advanced stage, with regional or distant metastatic disease present in 45.6% of vaginal melanoma patients and 31.6% of vulval melanoma patients. In both groups, about one-third also have nodal disease at diagnosis [
11]. Prognosis is worse compared with cutaneous melanoma, with reported five-year overall survival rates of 37% for vulval melanoma and 13–32% for vaginal melanoma [
14]. Women with vaginal melanomas appear to have the poorest prognosis, with survival outcomes consistently worse than those of vulval melanoma patients across all stages, and a reported median overall survival of 16 months [
11]. Refer to
Table 1.
3.2. Biology and Immune Profile
3.2.1. Cutaneous Melanoma
The progression of melanoma results from interactions between environmental, genetic, and host factors. UVR induces DNA damage, with a higher number of mutations observed in cutaneous melanoma—particularly desmoplastic subtypes—compared with the low tumour mutational burden seen in mucosal melanomas. Somatic mutations also contribute to melanoma development and progression, with alterations in the mitogen-activated protein kinase (MAPK) signalling cascade representing a key pathogenic pathway present in about 90% of cutaneous melanomas [
15]. These mutations may occur in receptors (e.g.,
KITKIT), effectors (
BRAF,
NRAS), or inhibitors (
NF1,
RASA2,
CDKN2A,
SPRED1) of the pathway, leading to proliferation of transformed melanocytes, abnormal differentiation, and persistent survival with impaired apoptosis of tumour cells. The reported frequencies of mutations in cutaneous melanoma are 37–60% in
BRAF [
15,
16], 13–25% in
NRAS, 12% in
NF1, 6–7% in
MAP2K1, and 2–8% in
KIT [
4,
16]. Up to 78% of melanomas also carry a
TERT promoter mutation, which increases telomerase activity and allows cells to maintain telomeric elongation, resulting in prolonged survival and abnormal proliferation. This mutation is often seen with
BRAF and
NRAS mutations, as the increased telomerase expression stabilises the mutated
BRAF and
NRAS genome [
17]. Accordingly,
TERT positivity is associated with poorer patient survival. The tumour mechanisms that evade normal immune responses are increasingly understood in cutaneous melanoma and have provided meaningful targets for immunotherapy. Tumour-infiltrating lymphocytes, Natural Killer (NK) cells, and CD8+ T cells can initially control melanoma progression through antigen-specific cytotoxic mechanisms that destroy tumour cells. However, tumour cells promote an immunosuppressive and pro-tumorigenic environment through the release of interferons, interleukins, and colony-stimulating factors [
4]. In malignant cells, interferons drive excessive activation of the JAK1/2 and STAT kinase pathways, which stimulate the production of PD-L1 and PD-L2 ligands. These ligands are transported to the cell membrane and presented to T lymphocytes, which express PD-1checkpoints, thereby protecting the tumour cell from immune-mediated destruction [
13]. The tumour microenvironment also influences the response to immunotherapy, whereby immunologically ‘hot’ tumours display high tumour mutational burden, greater numbers of tumour-infiltrating lymphocytes, and tumour-secreted interferon, and are therefore more responsive to immunotherapy. Such features are commonly seen in cutaneous melanoma, underpinning the effectiveness of immunotherapy in these tumours [
4].
3.2.2. Mucosal Melanoma
Genetic alterations in mucosal melanoma are distinct from those in cutaneous melanoma. Compared with cutaneous melanoma, mucosal melanomas show lower rates of somatic mutations but greater genomic instability. Mutations in
BRAF and
NRAS are less common (up to 20% of patients) and, when present, usually involve non-canonical non-V600 mutations. Mutation frequencies also vary across specific anatomical sites, with head and neck melanomas more often showing mutations in
NRAS and
TERT promoters, and genitourinary or anorectal melanomas showing
KITKIT mutations [
18,
19].
Mucosal melanomas are less immunogenic and generally have a lower tumour mutational burden, particularly those arising in the anorectal and urogenital tracts compared with conjunctival or nasal sites [
16,
20]. This site-specific variation may partly reflect the mutagenic role of UVR, which influences the distinct mutational signatures of mucosal melanomas in facial sites compared with those in the lower body [
20]. The tumour immune microenvironment in mucosal melanomas also shows reduced immune cell infiltration and a weaker interferon-gamma signature compared with cutaneous melanoma—a difference that is especially marked in urogenital tumours compared with those of the head and neck [
21] (See
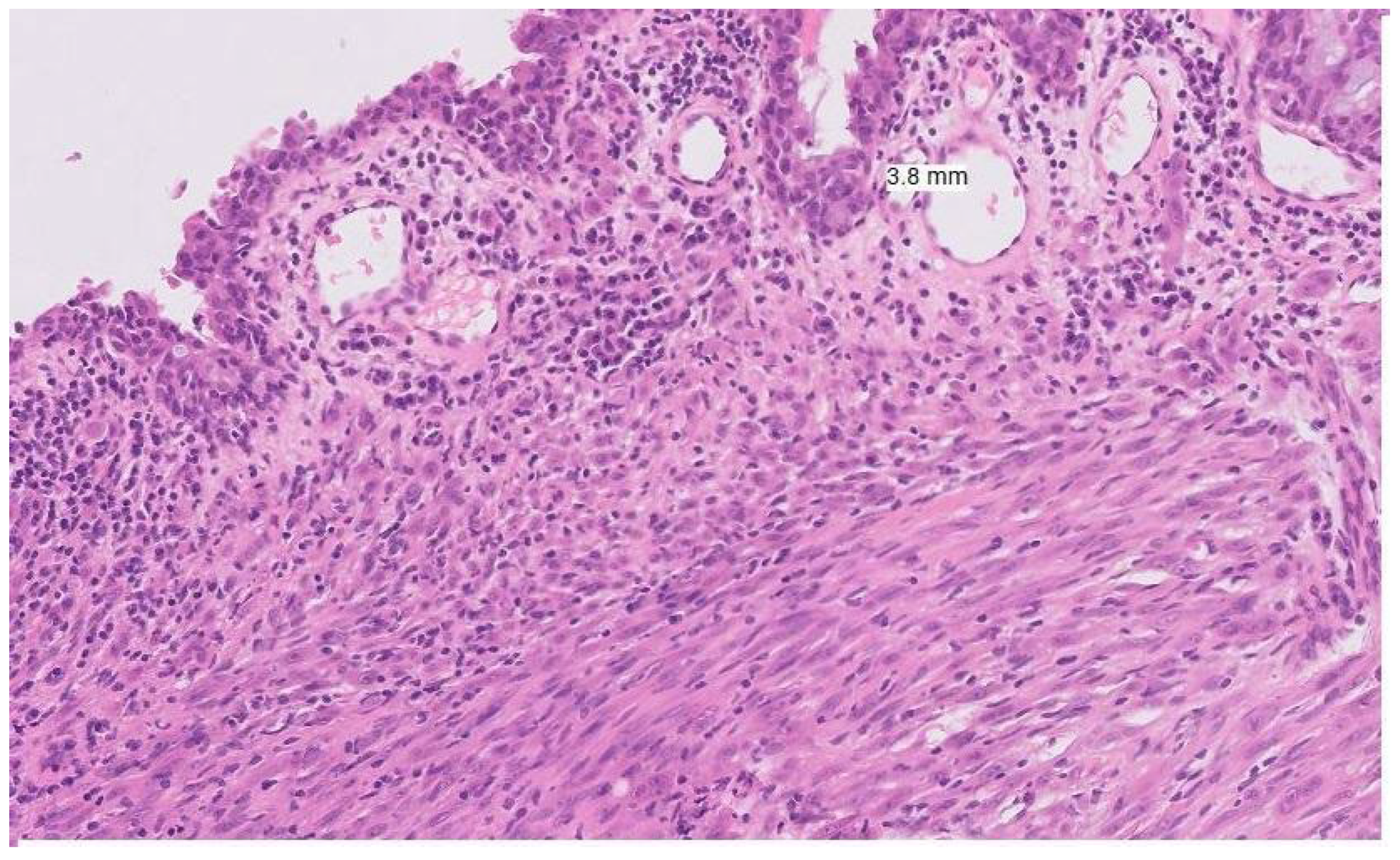
Figure 1). The above distinct genetic alterations and lower immunogenicity with low tumour mutational burden contribute to reduced efficacy of systemic therapies and ICIs. These factors combined with delayed and late diagnosis, significant heterogeneity within the mucosal melanoma group and challenges of surgical resection, contribute to the aggressive nature of this tumour group.
3.2.3. Vulvovaginal Melanoma
Emerging evidence highlights the distinct molecular profile and tumour microenvironment seen in vulvovaginal melanoma, with variation evident even between vulval and vaginal sites.
KITKIT mutations are more common in vulval melanomas (22–31% of cases) compared with vaginal melanomas (8%) [
22,
23]. Conversely,
NRAS mutations are uncommon in vulval melanomas (10.2%) and appear to have a comparatively higher incidence in vaginal melanomas [
22,
23].
BRAF mutations are significantly less common in vulvovaginal melanomas, and
TERT mutations may be entirely absent. Other frequently mutated genes include ATRX, SF3B1, B2M, NF1, and TP53 [
23]. In terms of immunogenicity, vulvovaginal melanomas demonstrate lower rates of PD-L1 positivity (18%) compared with cutaneous melanomas (29.5%) and overall reduced expression of immune checkpoint genes. They also have a lower tumour mutational burden, in contrast to 46.9% of cutaneous melanomas, which display a high tumour mutational burden [
23]. RNA deconvolution analysis of the tumour microenvironment further demonstrates reduced adaptive immune responses and decreased immunogenicity, with lower infiltration of immune-promoting macrophages, effector CD8+ T cells, and CD4+ T cells [
23]. These distinct mutational and immune characteristics highlight vulvovaginal melanoma as a unique subclass of mucosal melanoma and may help explain its differential responses to systemic melanoma therapies (See
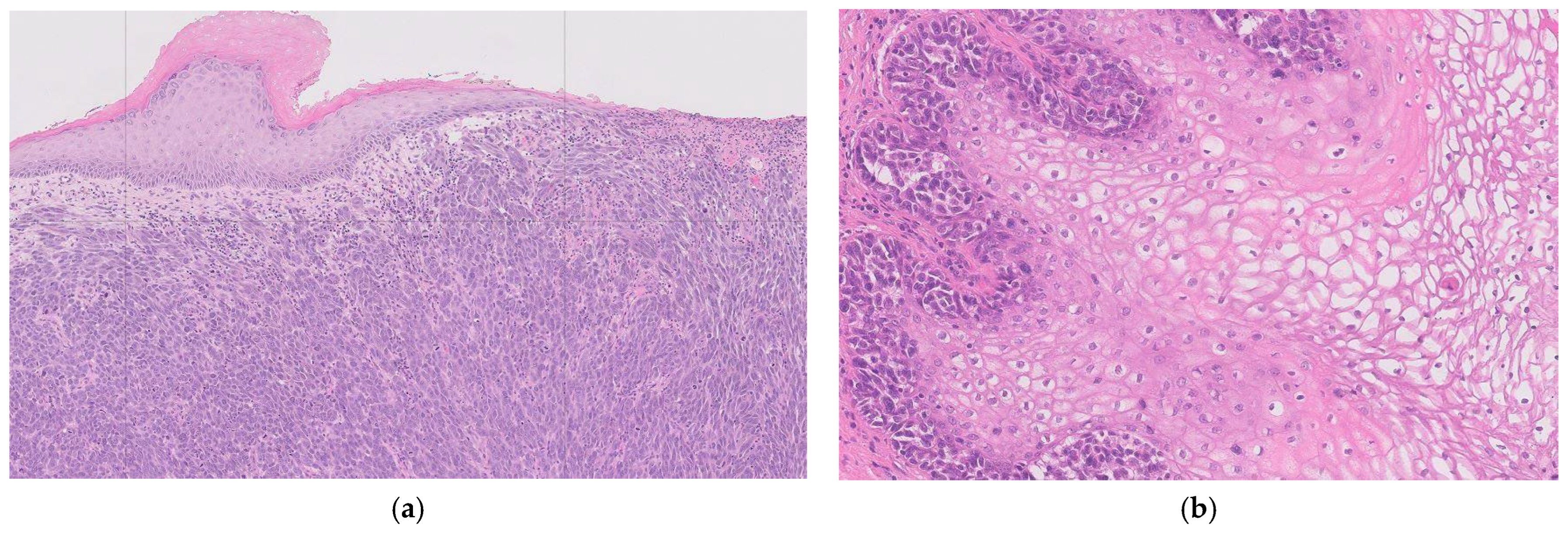
Figure 2).
3.3. Treatment
3.3.1. Surgery in Resectable Disease
In all melanoma types, when the disease is resectable, the standard of care is surgical resection of the primary lesion and, where possible, resectable metastatic disease. For cutaneous melanoma, this involves wide local excision at the primary site with a 1–2 cm radial surgical margin, depending on tumour thickness. Larger margins are not recommended by governing bodies in Europe, the United States, the United Kingdom, or Australia [
24]. Non-surgical approaches, including topical imiquimod and radiotherapy, have been shown to be inferior to surgical excision [
24]. As in cutaneous melanoma, surgery remains the mainstay of primary treatment for mucosal melanomas, including vulvovaginal subtypes however this conclusion is notably based only on retrospective observational studies as best level of evidence. This evidence suggests that primary surgery achieving negative margins provides the best survival outcomes [
25,
26]. Consensus guidelines and evidence to define the optimal surgical margin are lacking in vulvovaginal melanoma and therefore remain unclear. Notably, increased radicality of surgery has not been shown to improve survival outcomes compared with wide local excision with negative margins in retrospective analyses [
27,
28]. Vulvovaginal melanomas present unique surgical challenges due to their proximity to critical structures such as the urethra, bladder, anus, and rectum; thus, achieving negative margins alone may warrant more radical surgery or exenterative procedures [
29].
3.3.2. Lymph Node Assessment
Lymph node assessment is a key part of managing newly diagnosed cutaneous melanoma for selected patients, including those with T2 or higher tumours or high-risk T1 melanoma. In this group, sentinel lymph node assessment is now widely accepted for patients with clinically negative nodes and should be performed at the time of the initial excisional procedure. Historically, patients with a positive sentinel lymph node underwent completion lymph node dissection; however, this is no longer routinely recommended, as no survival benefit has been demonstrated, even in trials conducted before the introduction of more effective systemic therapies [
30]. In vulvovaginal melanoma, there is a paucity of high-quality evidence to guide decision-making regarding lymph node assessment and the optimal modality for achieving this. Traditionally, the standard treatment for vulval melanoma has been full inguinofemoral lymphadenectomy, but this is associated with significant morbidity, including wound complications, infection, and lymphoedema. Large population-based retrospective analyses have demonstrated that lymph node status is the strongest predictor of overall survival [
11], highlighting the importance of determining nodal disease in patients with vulvovaginal melanoma, particularly in the era of potentially effective adjuvant therapies.
In non-melanoma vulval cancer, sentinel node biopsy has replaced complete inguinofemoral lymphadenectomy for unifocal tumours smaller than 4 cm without suspicious nodal disease on imaging or examination [
31]. However, there is very limited evidence to guide similar practice in vulvovaginal melanoma. A recent systematic review identified six retrospective studies assessing the use of sentinel node biopsy in vulval melanoma across 48 patients using either radioactive or blue dye methods. Of 32 patients with negative sentinel nodes, only two experienced groin recurrence [
32]. These findings are limited by the small number of patients and the absence of prospective trials, meaning that sentinel node biopsy is not yet an established practice in vulvovaginal melanoma.
3.3.3. Adjuvant Treatment Using Immune Checkpoint Inhibitors
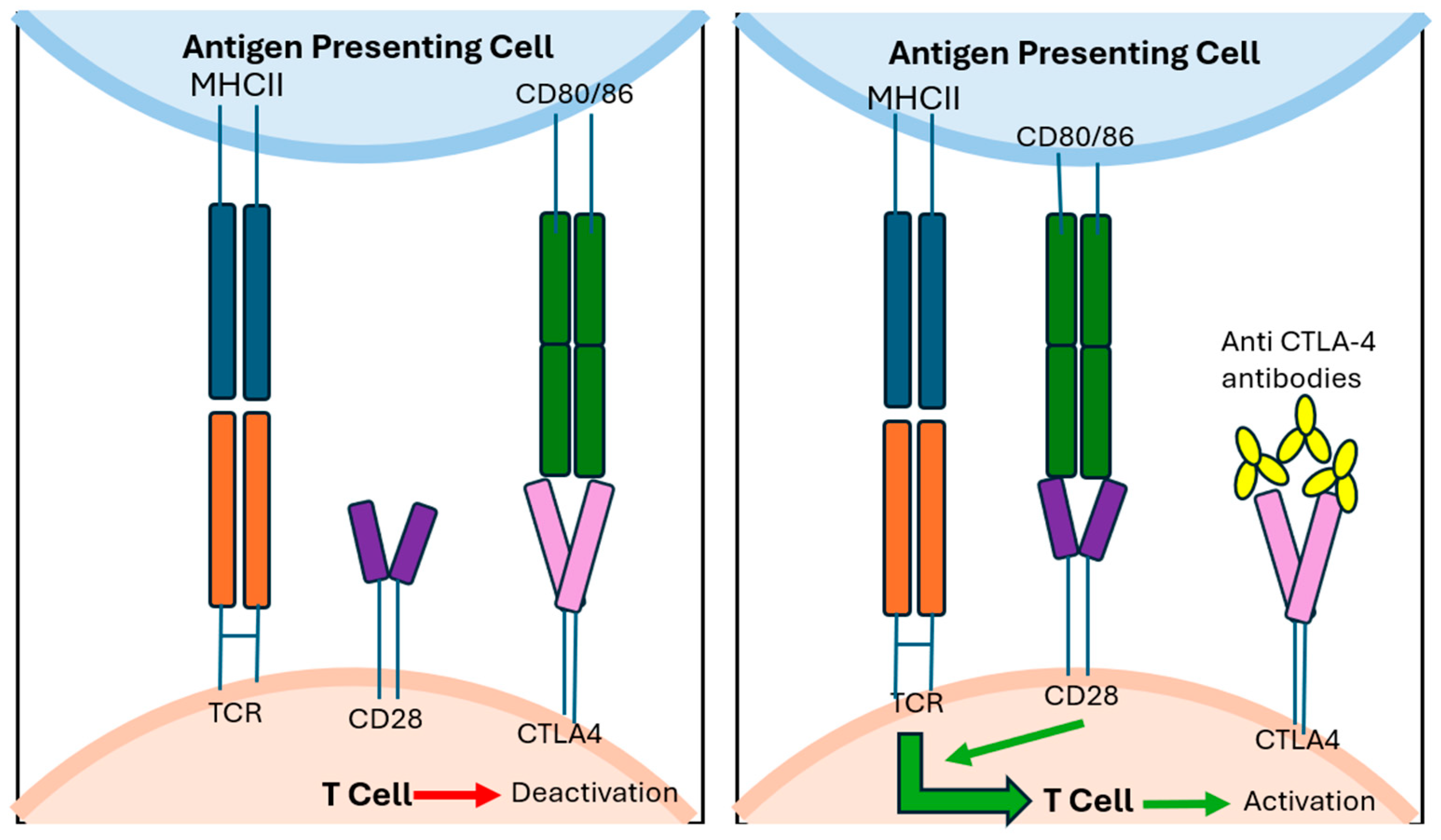
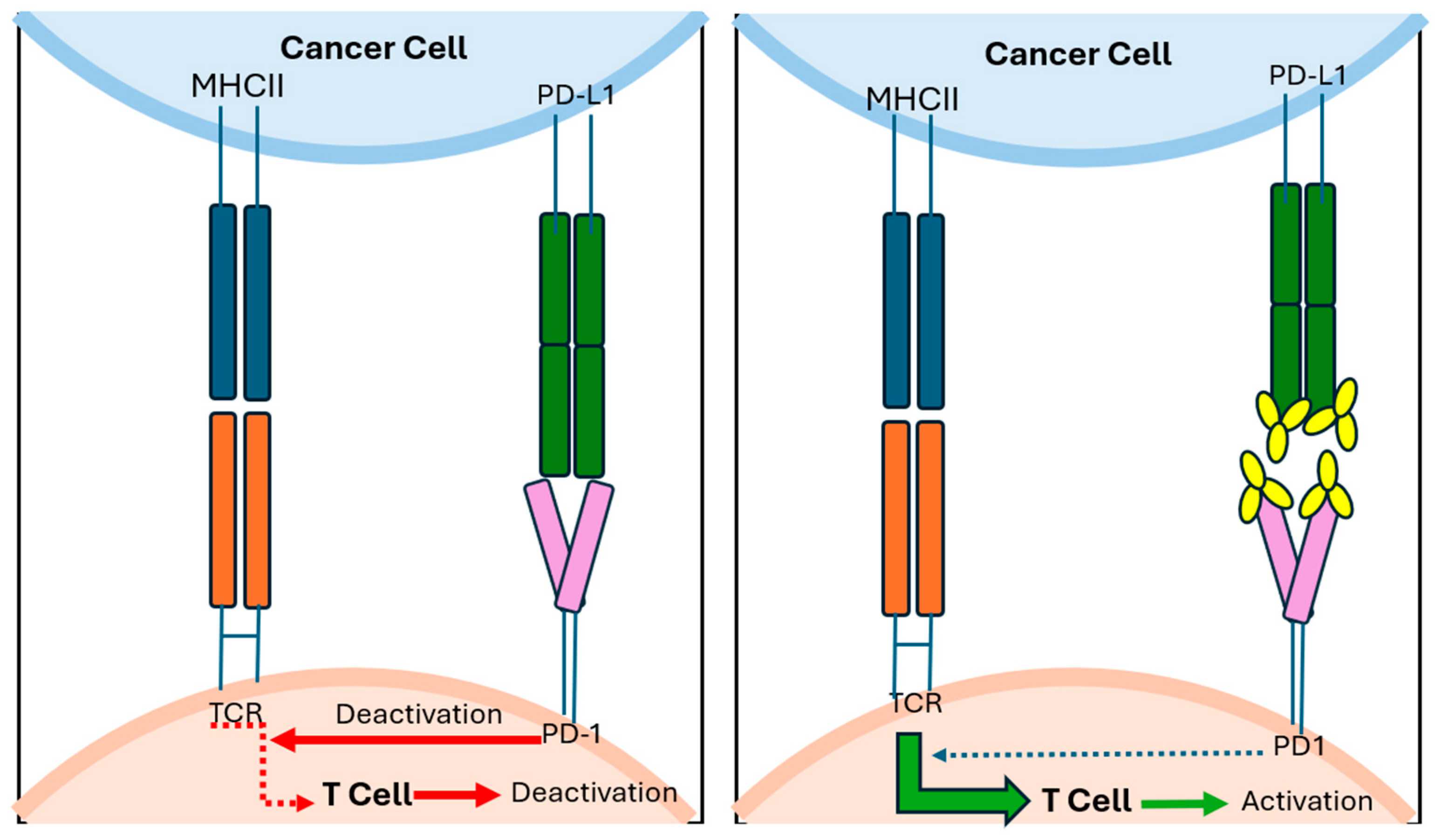
Despite optimal resection, recurrence is common across all melanoma subtypes. However, adjuvant therapy has been best established in cutaneous melanoma, more so than in mucosal and vulvovaginal melanomas. For patients with high-risk resectable cutaneous melanoma (stage IIB or higher), adjuvant treatment with checkpoint inhibitor immunotherapy (mechanisms demonstrated in
Figure 3 and
Figure 4) or
BRAF/
MEK targeted therapy is now the standard of care.
Adjuvant anti-PD-1 therapy (nivolumab or pembrolizumab) has demonstrated a significant recurrence-free survival benefit over both ipilimumab (CTLA-4 inhibitor) and placebo in resected stage III–IV melanoma across multiple prospective, randomised phase III trials [
33,
34]. These trials also showed that nivolumab and pembrolizumab were more tolerable than ipilimumab. Further trials, such as CheckMate 915, investigated whether outcomes in stage IIIB–IV melanoma could be improved by adding a low-dose regimen of ipilimumab to nivolumab, compared with nivolumab monotherapy. No significant recurrence-free or overall survival benefit was observed at two years, but the combination group experienced higher rates of treatment-related adverse events [
35]. Thus, current evidence strongly supports a monotherapy approach with the ICIs nivolumab or pembrolizumab in the adjuvant treatment of advanced cutaneous melanoma. The effectiveness of immunotherapy in cutaneous melanoma reflects the tumour microenvironment and immunological changes that underpin its pathogenesis.
The efficacy of ICIs in the adjuvant setting is less well established in both mucosal and vulvovaginal melanoma, and there is currently no universally accepted standard for adjuvant treatment (included studies summarised in
Table 2). Limited evidence suggests that ICIs are less effective in mucosal melanoma than in cutaneous melanoma. The SALVO trial assessed a ‘flip dose’ regimen of ipilimumab and nivolumab for adjuvant treatment of mucosal melanomas. Participants had undergone R0/R1 resection with no prior systemic therapy and received ipilimumab (1 mg/kg every three weeks for four cycles) and nivolumab (3 mg/kg every three weeks for four cycles), followed by nivolumab 480 mg every four weeks for 11 cycles to complete one year of adjuvant therapy. This trial reported recurrence-free survival rates of 50% and 37% at one and two years, respectively, with overall survival rates of 87% and 68% at the same time points. Median recurrence-free survival was 10.3 months. The findings, however, were limited by the small sample size (
n = 35) [
36].
Lian et al. [
37] conducted a randomised trial comparing adjuvant high-dose interferon (HDI) with toripalimab (anti-PD-1) in patients with completely resected mucosal melanoma. Patients were randomised according to disease stage, tumour site, and PD-L1 expression status. One- and two-year relapse-free survival rates in the HDI and toripalimab arms were 52% versus 52.9% and 25.1% versus 30.5%, respectively. Median recurrence-free survival was 13.9 months in the HDI arm and 13.6 months in the toripalimab arm. No other prospective trials have been reported. However, in a subgroup analysis of 29 patients with mucosal melanoma included in the CheckMate 238 trial, recurrence-free survival was 54% with ipilimumab and 31% with nivolumab, suggesting a non-significant trend favouring ipilimumab in this cohort [
38,
39]. Across these prospective trials, relapse-free survival rates with immunotherapy are relatively consistent but notably lower than the 65% reported in the CheckMate 915 trial with ipilimumab/nivolumab regimens or monotherapy in cutaneous melanoma [
35,
38,
40].
Very limited data may, however, suggest that certain mucosal melanoma subgroups could derive greater benefit from anti-PD-1 ICIs. In the trial by Lian et al. [
37], 50% and 52.1% of patients in the HDI and toripalimab arms, respectively, were found to have PD-1-positive tumours. Among these patients, median recurrence-free survival was 11.1 months with HDI and 17.4 months with toripalimab, indicating a more pronounced benefit of toripalimab in PD-1-positive tumours. The trial was not powered to detect a statistically significant difference in this subgroup. Additionally, in a subgroup of patients with oral mucosal melanoma, retrospective data suggests significant improvement in two-year overall survival and progression-free survival with adjuvant anti-PD-1 combined with chemotherapy compared with chemotherapy alone, consistent with the higher tumour mutational burden and interferon expression seen in head and neck mucosal melanomas compared with other mucosal subgroups [
41].
In patients specifically with vulvovaginal melanoma, no prospective trials have assessed the use of immunotherapy in the adjuvant setting, and evidence is limited to retrospective analyses and case series. Wilhite et al. [
23] examined the molecular profile of 142 vulvovaginal and 3823 cutaneous melanomas and responses to ICIs. In line with previous evidence, they found a low tumour mutational burden and lower rates of PD-L1 expression in vulvovaginal melanomas compared with cutaneous melanomas, with a corresponding median overall survival of 17.5 months versus 37.9 months in the two groups, respectively, when treated with ICIs. Despite the poorer response, there may still be some benefit, although definitive conclusions are limited by the paucity of evidence. Albert et al. [
42] conducted a large retrospective analysis assessing treatment factors and prognosis in 1917 patients with vulval melanoma, of whom 95% underwent surgery and 190 received immunotherapy. In patients with distant metastatic disease, those treated with immunotherapy had a higher two-year overall survival than those who did not (33% versus 12%,
p = 0.054), although this did not reach statistical significance. This analysis was not stratified by immunotherapy type or disease stage.
Overall, in the context of adjuvant treatment, ICIs have repeatedly demonstrated in prospective phase III trials robust benefits in improving recurrence-free survival in cutaneous melanoma. Comparatively, current evidence in mucosal and particularly vulvovaginal melanoma is extremely limited and suggests a poorer response, likely reflecting their distinct underlying immune and biological characteristics.
3.3.4. Adjuvant Treatment Using Targeted Therapies
In cutaneous melanoma, improved understanding of the high frequency of
BRAF mutations has led to the development of targeted therapies with
BRAF and
MEK inhibitors. In patients with stage III melanoma with
BRAF V600E or V600K mutations, the phase III COMBI-AD trial demonstrated that adjuvant dabrafenib plus trametinib significantly improved relapse-free survival and overall survival compared with placebo at three and five years of follow-up [
43]. There are no prospective trials directly comparing dabrafenib plus trametinib with ICIs, and thus current guidelines recommend either treatment but note higher discontinuation rates in patients treated with dabrafenib plus trametinib compared with ICIs [
44].
Unlike cutaneous melanoma, mucosal melanoma shows
BRAF mutations less frequently (10–15%), and these are more commonly non-V600E/K mutations [
23], limiting the applicability of results from the COMBI-AD trial to this group. No studies have specifically evaluated the use of
BRAF/
MEK inhibitors in mucosal or vulvovaginal melanoma [
39]. A recent systematic review examined the use of
CKIT inhibitors for unresectable or metastatic mucosal, acral, or chronically sun-damaged melanoma and reported an objective response rate of 14% in patients with mucosal melanoma, with the highest response observed with imatinib (24%) [
45]. In a study by Jung et al., patients with mucosal melanoma and confirmed
KIT mutation demonstrated a response rate of 38% when treated with imatinib. The response rates of
KIT inhibitors seem to be enhanced in patients with
KIT-mutant mucosal tumours particularly in exons 11 and 13 with mutations of exon 17 associated with lower progression free survival in multi-variate analysis of prospective and retrospective trials [
46].
3.3.5. Adjuvant Treatment Using Chemotherapy
In cutaneous melanoma, adjuvant chemotherapy with alkylating agents was commonly used from the 1970s but achieved poor results, with objective response rates of only up to 15%. With the introduction of
BRAF/
MEK targeted therapies and ICIs, multiple systematic reviews have demonstrated significantly improved outcomes compared with conventional chemotherapy. As a result, chemotherapy is not recommended for the adjuvant treatment of high-risk cutaneous melanoma [
44,
47].
In mucosal melanoma, Lian et al. [
48] reported the only randomised prospective controlled trial of adjuvant systemic treatment following resection. The trial included patients who had undergone complete resection of stage II/III disease and were randomised to either high-dose interferon, cisplatin with temozolomide, or observation alone. At a median follow-up of 26.8 months, the median relapse-free survival was statistically significant between the groups: 5.4 months in the observation arm, 9.4 months in the interferon arm, and 20.8 months in the chemotherapy arm. Overall survival also demonstrated statistically significant differences, being 21.2, 40.4, and 48.7 months in the observation, interferon, and chemotherapy groups, respectively.
Extrapolating these findings is challenging, as they contradict current high-quality evidence in cutaneous melanoma, which demonstrates the superiority of immunotherapy over adjuvant chemotherapy, and the trial is limited by small numbers. Consequently, there is insufficient evidence to support a benefit of adjuvant chemotherapy in mucosal melanoma [
44].
Table 2.
Included studies assessing adjuvant treatment in mucosal and vulvovaginal melanoma. Mo—months. Yr—years.
Table 2.
Included studies assessing adjuvant treatment in mucosal and vulvovaginal melanoma. Mo—months. Yr—years.
| Author | Trial Design | Patient Group | N | Intervention | Recurrence-Free Survival (95% CI) | Median RFS (95% CI) |
|---|
| Mucosal Melanoma |
| Kottschade et al. [36] | Single-arm, multicentre | Mucosal melanoma (R0/R1 resection) | 35 | Flip-dose ipilimumab + nivolumab, then maintenance nivolumab (1 year total) | 1 yr: 50% (31–66%); 2 yr: 37% (19–55%) | 10.3 mo (5.7–25.8) |
| Lian et al. [37] | Randomised controlled trial | Mucosal melanoma (R0/R1 resection) | 145 | High-dose interferon (HDI) vs. toripalimab for 1 year or until recurrence/toxicity/withdrawal | 1 yr: HDI 52% (38.6–63.8%); toripalimab 52.9% (40.3–63.9%)
2 yr: HDI 25.1% (14.8–36.8%); toripalimab 30.5% (19.7–41.9%) | HDI 13.9 mo (8.3–19.0); toripalimab 13.6 mo (8.3–19.0) |
| Wu et al. [41] | Single-centre, retrospective cohort | Oral mucosal melanoma (complete resection) | 193 | Dacarbazine/cisplatin + HDI vs. dacarbazine/cisplatin + ICI | — | Chemo 10.9 mo; HDI 43.6 mo; PD-1 53.6 mo |
| Lian et al. [48] | Randomised controlled trial | Stage II/III mucosal melanoma (complete resection) | 189 | Observation vs. HDI vs. temozolomide/cisplatin | — | Obs. 5.4 mo (4.2–6.6); HDI 9.4 mo (7.9–10.9); Tem/Cis 20.8 mo (17.9–23.7) |
| Vulvovaginal Melanoma |
| Wilhite et al. [23] | In vitro, retrospective cohort | Tissue samples (3965 total; 142 vulvovaginal) | 3965 | ICIs | — | Vulvovaginal: 17.5 mo; Cutaneous: 37.9 mo |
| Albert et al. [42] | Retrospective cohort | Vulval melanoma | 1917 | Immunotherapy (unspecified) | — | — |
3.3.6. Non-Resectable or Metastatic Disease
The introduction of targeted therapy and ICIs has dramatically improved outcomes for patients with advanced, unresectable cutaneous melanoma, achieving durable disease control and potentially a cure in approximately 50% of patients [
1,
4]. However, as in the adjuvant setting, the benefits of immunotherapy are less pronounced in mucosal, and particularly vulvovaginal, melanoma with only retrospective studies in the mucosal melanoma and vulvovaginal groups. For advanced and non-resectable
BRAF-mutant cutaneous melanoma, combination
BRAF/
MEK inhibitors produce high initial response rates and at least a partial objective response in most patients, with only mild toxicity. However, despite these high initial response rates, around 50% of patients develop resistance within one year and 80% within five years [
49,
50].
ICIs also demonstrate efficacy for patients with both
BRAF-mutant and wild-type advanced cutaneous melanoma. The anti-PD-1 antibodies pembrolizumab and nivolumab have shown superior efficacy to ipilimumab, with higher response rates and improved five-year progression-free and overall survival. Combination therapy with ipilimumab and nivolumab has demonstrated the highest response rates and five-year overall and progression-free survival, but is associated with more frequent toxicity [
51,
52].
Given the proven efficacy of both targeted therapy and ICIs, studies have been conducted to assess outcomes based on the sequence of their use. Randomised prospective trial data support the use of combination ICIs as the first-line treatment for advanced non-resectable cutaneous melanoma, showing improvements in overall survival and progression-free survival compared with patients treated initially with
BRAF/
MEK inhibitors [
53,
54]. The phase III DREAMseq trial randomised patients with advanced, non-resectable cutaneous melanoma to Arm A (nivolumab plus ipilimumab until progression, followed by dabrafenib plus trametinib) or Arm B (dabrafenib plus trametinib until progression, followed by nivolumab plus ipilimumab). Two-year overall survival was 71.8% for Arm A compared with 51.5% for Arm B. Objective response rates were 46% in Arm A and 43% in Arm B [
54].
For metastatic or unresectable mucosal melanoma, prospective evidence remains limited, with most studies comprising small observational cohorts or retrospective analyses (included studies summarised in
Table 3). Moya-Plana et al. [
55] conducted a single-centre prospective cohort study of patients with unresectable locally advanced and/or metastatic mucosal melanoma treated with either ipilimumab or pembrolizumab. Forty-four patients were enrolled, including 14 with vulvovaginal primaries. The objective response rate was 20% overall, 8% for ipilimumab, and 35% for pembrolizumab, with no significant difference observed according to tumour location. Two-year overall survival was 34% with ipilimumab and 44% with pembrolizumab, while median overall survival was 12 months and 16.2 months, respectively. A post hoc analysis of the KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 trials evaluated pembrolizumab in metastatic melanoma and reported an objective response rate of 19% in mucosal melanoma, compared with 33% in cutaneous melanoma, with a median overall survival of 11.3 months [
56].
As in cutaneous melanoma, patients with unresectable mucosal melanoma may benefit more significantly from combination therapy with ipilimumab and nivolumab than from checkpoint inhibitor monotherapy. In a pooled post hoc analysis of phase III trials, D’Angelo et al. [
57] assessed 86 patients with unresectable mucosal melanoma treated with nivolumab monotherapy and 35 treated with combined nivolumab and ipilimumab. Median progression-free survival was 3.0 months with nivolumab, 5.9 months with combination therapy, and 2.7 months with ipilimumab. Objective response rates were 23.3%, 37.1%, and 8.3% for nivolumab, combination therapy, and ipilimumab, respectively. This effect was more pronounced in patients with PD-L1 expression greater than 5%, with objective response rates of 53.3%, 60%, and 14.3% for nivolumab, combination therapy, and ipilimumab, respectively. The difference in objective response rates between patients with PD-L1 expression above and below 5% was greater in mucosal melanoma than in cutaneous melanoma.
Similar findings were reported in a large multicentre retrospective cohort study comparing anti-PD-1 monotherapy with combination immunotherapy in 545 patients with advanced, unresectable, or metastatic mucosal melanoma. Objective response rates were 29% with anti-PD-1 monotherapy and 31% with combination therapy. Median progression-free survival was 5 months and 4 months in the anti-PD-1 monotherapy and combination therapy groups, respectively. Median overall survival was 19 months and 21 months, and three-year overall survival rates were 33% and 30%, respectively, for anti-PD-1 monotherapy and combination therapy. Overall survival was initially numerically, but not statistically, higher with combination therapy; however, the survival curves crossed at the two-year mark [
58]. In the above two studies, no differences in objective response rates were observed across the primary sites of mucosal melanoma.
There are no prospective studies assessing the use of ICIs in vulvovaginal melanoma and studies looking specifically at the use of immunotherapy in vulvovaginal melanoma are limited to small, single centre retrospective cohort studies and case series. In a retrospective cohort study, data from 13 patients with unresectable or metastatic vulvovaginal melanoma were combined with a further 10 patients identified from previously published cases in the literature. The overall response rate to ICIs was 30.4%, with a median progression-free survival of 4.0 months and a median overall survival of 17 months. Ipilimumab was again demonstrated to be inferior to PD-1 monotherapy or combination immunotherapy [
59]. Another small retrospective case series reported similar response rates of 28.5% to immune checkpoint therapy, with patients receiving anti-PD-1 monotherapy achieving longer progression-free survival than those treated with anti-CTLA-4 therapy [
60]. These limited findings are consistent with response and survival rates reported in larger analyses of mucosal melanoma. Although there is a lack of prospective, randomised evidence and the available trials include patients with varied baseline characteristics, the evidence to date supports a possible role for immune checkpoint inhibitor therapy in the treatment of advanced, metastatic, unresectable melanoma. The benefit does not appear to be as marked as in cutaneous melanoma, likely due to underlying differences in tumour biology and immune profile. Patient counselling in a supported environment is recommended to facilitate informed, patient-centred decision-making. This should include discussion of potential serious side effects and the associated risk of reduced quality of life, alongside any potential improvements in outcome.
4. Future Directions
This review highlights the significant paucity of evidence underpinning the diagnosis and management of mucosal and vulvovaginal melanomas which is reflected in the stagnation of survival outcomes. In cutaneous melanoma, ongoing studies are identifying new biomarkers to assist in the earlier diagnosis and assessment of metastatic potential which, if successful, will likely further broaden the gap between advances in cutaneous and mucosal melanoma subtypes. Emerging evidence suggests that profiling fatty acids and protein signatures in small extracellular vesicles may provide a liquid biopsy approach for earlier cutaneous melanoma detection and stage specific disease monitoring. Similar approaches are being investigated in breast and prostate cancers [
61].
There has been some progress made in identifying the key biological and genetic differences between cutaneous, mucosal and vulvovaginal melanoma, thus aiding our understanding of the poorer response and outcomes of these patients in response to current therapies. The key challenges limiting progress in mucosal and vulvovaginal melanomas currently include delayed diagnosis resulting in advanced disease, the lack of therapeutic gene and protein targets and the difficulty in designing robust studies given the rarity of these conditions. Future key areas of research must include more sensitive and specific diagnostic tools which facilitate earlier detection, as achieved in the context of cutaneous melanoma. Additionally, with acknowledgement of the distinct genetic and immunogenic features, identification of novel and more disease specific therapeutic targets will provide the first steps in formulation of more efficacious treatment options.
The largest overarching challenge in both mucosal and vulvovaginal melanoma is the rarity of these conditions, which limits potential recruitment for larger studies. Progress in this area will depend on collaborative efforts including the formation of international patient registries to aid in the identification of larger numbers of patients, data collection and international collaborative studies. Tissue banking also provides the possibility of in vitro or translation studies, which may be particularly helpful in further identification of future therapeutic targets. Additionally, with the move towards targeted therapy and innovative trial designs, such as basket trials with a novel approach of grouping tumours by molecular and genetic mutations rather than histopathological origin, may also provide meaningful therapeutic insights. This would be particularly useful in assessing the utility of future therapeutic strategies, given that large scale randomised controlled trials are not likely to be feasible for such a rare condition.
5. Conclusions
Compared with cutaneous melanoma, mucosal melanomas—and vulvovaginal melanomas in particular—remain poorly understood, with their rarity limiting the feasibility of large, high-powered studies. While advances in the understanding and treatment of cutaneous melanoma have translated into clear improvements in overall survival across all disease stages, knowledge of the pathogenesis, molecular mechanisms, and immune profile of vulvovaginal melanoma remains limited, with little evidence to support the benefit of surgery or ICIs. As a result, recurrence rates and overall survival outcomes in this population remain poor, with marked heterogeneity in treatment approaches. This review clearly compares and contrasts features of cutaneous melanoma, mucosal melanoma and vulvovaginal melanoma to reinforce the need to consider these tumour types as three distinct, albeit related pathologies. The synthesis of current evidence allows for a better understanding of these poorer outcomes and the underlying biological reasons for this as well as identifying key directions forward. A deeper understanding of the unique biology of mucosal and vulvovaginal melanomas is urgently needed to inform more effective therapeutic strategies.
Author Contributions
Conceptualisation, M.A.; methodology, D.C. and C.P.; validation, M.A. and C.P.; formal analysis, D.C., C.H., K.H., M.D., S.D., S.M., B.S. and M.A.; investigation, D.C., C.P., C.H., M.D., K.H., B.S. and M.A.; resources, D.C., C.P., C.H., K.H., M.D., S.D., S.M. and M.A.; data curation, D.C., C.P., C.H., K.H., M.D., S.D., S.M. and M.A.; writing—original draft preparation, D.C., C.P., C.H., K.H., M.D., B.S., S.D., S.M. and M.A.; writing—review and editing, D.C., C.P., C.H., K.H., M.D. and M.A.; visualisation; D.C. and M.A.; supervision, M.A.; project administration; D.C. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ICIs | immune checkpoint inhibitors |
| CTLA-4 | cytotoxic T-lymphocyte associated protein 4 |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| UVR | ultraviolet radiation |
| MAPK | mitogen-activated protein kinase |
| NK cells | natural killer cells |
| HDI | high-dose interferon |
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.-J.; Elcarte, E.; et al. Global Incidence, Mortality, Risk Factors and Trends of Melanoma: A Systematic Analysis of Registries. Am. J. Clin. Dermatol. 2023, 24, 965–975. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.; De Vries, E.; Whiteman, D.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495. [Google Scholar] [CrossRef]
- Tasdogan, A.; Sullivan, R.J.; Katalinic, A.; Lebbe, C.; Whitaker, D.; Puig, S.; Van De Poll-Franse, L.V.; Massi, D.; Schadendorf, D. Cutaneous melanoma. Nat. Rev. Dis. Primers 2025, 11, 23. [Google Scholar] [CrossRef]
- Okobi, O.E.; Abreo, E.; Sams, N.P.; Chukwuebuni, O.H.; Tweneboa Amoako, L.A.; Wiredu, B.; Uboh, E.E.; Ekechi, V.C.; Okafor, A.A.; Amoako, L.A. Trends in Melanoma Incidence, Prevalence, Stage at Diagnosis, and Survival: An Analysis of the United States Cancer Statistics (USCS) Database. Cureus 2024, 16, e70697. [Google Scholar] [CrossRef]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Carbó-Bagué, A.; Rubió-Casadevall, J.; Puigdemont, M.; Sanvisens, A.; Oliveras, G.; Coll, M.; Del Olmo, B.; Perez-Bueno, F.; Marcos-Gragera, R. Epidemiology and Molecular Profile of Mucosal Melanoma: A Population-Based Study in Southern Europe. Cancers 2022, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Clavero-Rovira, L.; Gómez-Tomás, Á.; Bassas-Freixas, P.; Bodet, D.; Ferrer, B.; Hernández-Losa, J.; Munoz-Couselo, E.; Perez-Benavente, A.; Garcia-Patos, V.; Ferrandiz-Pulido, C. Mucosal Melanoma Clinical Management and Prognostic Implications: A Retrospective Cohort Study. Cancers 2024, 16, 227. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Patrick, R.J.; Fenske, N.A.; Messina, J.L. Primary mucosal melanoma. J. Am. Acad. Dermatol. 2007, 56, 828–834. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I.; May, T.; Vicus, D.; Gien, L.T.; Laframboise, S. Malignant Melanoma of the Vulva and Vagina: A US Population-Based Study of 1863 Patients. Am. J. Clin. Dermatol. 2020, 21, 285–295. [Google Scholar] [CrossRef]
- Vyas, R.; Thompson, C.L.; Zargar, H.; Selph, J.; Gerstenblith, M.R. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J. Am. Acad. Dermatol. 2016, 75, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, A.; Kozłowski, M.; Lenkiewicz, S.; Kwiatkowski, S.; Cymbaluk-Płoska, A. Cutaneous Melanoma versus Vulvovaginal Melanoma—Risk Factors, Pathogenesis and Comparison of Immunotherapy Efficacy. Cancers 2022, 14, 5123. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, T.; Zhu, H.; Dong, J.; Fu, L. Primary malignant melanomas of the female lower genital tract: Clinicopathological characteristics and management. Am. J. Cancer Res. 2020, 10, 4017–4037. [Google Scholar]
- Caraban, B.M.; Aschie, M.; Deacu, M.; Cozaru, G.C.; Pundiche, M.B.; Orasanu, C.I.; Voda, R. A Narrative Review of Current Knowledge on Cutaneous Melanoma. Clin. Pract. 2024, 14, 214–241. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.; Kakavand, H.; Alexandrov, L.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Zhang, L.; Ma, L.; Jiang, K.; Yao, G.; Zhu, L.; Li, X. TERT Promoter Mutations and Telomerase in Melanoma. J. Oncol. 2022, 2022, 6300329. [Google Scholar] [CrossRef]
- Indini, A.; Roila, F.; Grossi, F.; Massi, D.; Mandalà, M. Molecular Profiling and Novel Therapeutic Strategies for Mucosal Melanoma: A Comprehensive Review. Int. J. Mol. Sci. 2021, 23, 147. [Google Scholar] [CrossRef]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.; Burke, H.; Dodds, T.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef]
- Vos, J.L.; Traets, J.J.H.; Qiao, X.; Seignette, I.M.; Peters, D.; Wouters, M.W.J.M.; Hoojiberg, E.; Broeks, A.; Van Der Wal, J.; Karakullukcu, M.B.; et al. Diversity of the immune microenvironment and response to checkpoint inhibitor immunotherapy in mucosal melanoma. JCI Insight 2024, 9, e179982. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Vulvar Melanoma: Molecular Characteristics, Diagnosis, Surgical Management, and Medical Treatment. Am. J. Clin. Dermatol. 2021, 22, 639–651. [Google Scholar] [CrossRef]
- Wilhite, A.M.; Wu, S.; Xiu, J.; Gibney, G.T.; Phung, T.; In, G.K.; Herzog, T.J.; Khabele, D.; Erikson, B.; Brown, J.; et al. A paradigm shift in understanding vulvovaginal melanoma as a distinct tumor type compared with cutaneous melanoma. Gynecol. Oncol. 2024, 188, 13–21. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment-Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Frumovitz, M.; Etchepareborda, M.; Sun, C.C.; Soliman, P.T.; Eifel, P.J.; Levenback, C.F.; Ramirez, P. Primary Malignant Melanoma of the Vagina. Obstet. Gynecol. 2010, 116, 1358–1365. [Google Scholar] [CrossRef]
- Sinasac, S.E.; Petrella, T.M.; Rouzbahman, M.; Sade, S.; Ghazarian, D.; Vicus, D. Melanoma of the Vulva and Vagina: Surgical Management and Outcomes Based on a Clinicopathologic Reviewof 68 Cases. J. Obstet. Gynaecol. Can. 2019, 41, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Lumiala, T.; Koljonen, V.; Ojala, K. Clinicopathologic features and surgical management in vulvovaginal melanoma—A retrospective single-center study. J. Plast. Reconstr. Aesthet. Surg. 2025, 100, 8–15. [Google Scholar] [CrossRef]
- Xia, L.; Han, D.; Yang, W.; Li, J.; Chuang, L.; Wu, X. Primary Malignant Melanoma of the Vagina. Int. J. Gynecol. Cancer 2014, 24, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Rodrigues, A.; Oonk, M.H.M.; Lorusso, D.; Slomovitz, B.; Leitão, M.M.; Baiocchi, G. Comprehensive management of vulvovaginal cancers. CA Cancer J. Clin. 2025, 75, 410–435. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.; Testori, A.; Beitsch, P.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer-Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Collarino, A.; Fuoco, V.; Garganese, G.; Pasciuto, T.; De Koster, E.J.; Florit, A.; Fragomeni, S.M.; Zagaria, L.; Fragano, L.; Martinelli, F.; et al. Lymphatic mapping and sentinel node biopsy in vulvar melanoma: The first multicenter study and systematic review. Gynecol. Oncol. 2023, 170, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Five-Year Analysis of Adjuvant Pembrolizumab or Placebo in Stage III Melanoma. NEJM Evid. 2022, 1, EVIDoa2200214. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant Therapy of Nivolumab Combined with Ipilimumab Versus Nivolumab Alone in Patients with Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915). J. Clin. Oncol. 2023, 41, 517–527. [Google Scholar] [CrossRef]
- Kottschade, L.A.; Pond, G.R.; Olszanski, A.J.; Zakharia, Y.; Domingo-Musibay, E.; Hauke, R.J.; Curti, B.; Schober, S.; Milhem, M.; Block, M.; et al. SALVO: Single-Arm Trial of Ipilimumab and Nivolumab as Adjuvant Therapy for Resected Mucosal Melanoma. Clin. Cancer Res. 2023, 29, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Si, L.; Chi, Z.H.; Sheng, X.N.; Kong, Y.; Wang, X.; Tian, H.; Li, K.; Mao, L.L.; Bai, X.; et al. Toripalimab (anti-PD-1) versus high-dose interferon-α2b as adjuvant therapy in resected mucosal melanoma: A phase II randomized trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1061–1070. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Tapia Rico, G.; Yong, C.H.; Herrera Gómez, R.G. Adjuvant systemic treatment for high-risk resected non-cutaneous melanomas: What is the evidence? Crit. Rev. Oncol. Hematol. 2021, 167, 103503. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, D.; Ren, G.; Guo, W. Chemotherapy in combination with anti-PD-1 agents as adjuvant therapy for high-risk oral mucosal melanoma. J. Cancer Res. Clin. Oncol. 2023, 149, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.; Lee, A.; Allbright, R.; Vijayakumar, S. Vulvar melanoma: An analysis of prognostic factors and treatment patterns. J. Gynecol. Oncol. 2020, 31, e66. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaxu, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4794–4820. [Google Scholar] [CrossRef] [PubMed]
- Steeb, T.; Wessely, A.; Petzold, A.; Kohl, C.; Erdmann, M.; Berking, C.; Heppt, M.V. c-KIT inhibitors for unresectable or metastatic mucosal, acral or chronically sun-damaged melanoma: A systematic review and one-arm meta-analysis. Eur. J. Cancer 2021, 157, 348–357. [Google Scholar] [CrossRef]
- Jung, S.; Armstrong, E.; Wei, A.Z.; Ye, F.; Lee, A.; Carlino, M.S.; Sullivan, R.J.; Carvajal, R.D.; Shoushtari, A.N.; Johnson, D.B. Clinical and genomic correlates of imatinib response in melanomas with KIT alterations. Br. J. Cancer. 2022, 127, 1726–1732. [Google Scholar] [CrossRef]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef]
- Lian, B.; Si, L.; Cui, C.; Chi, Z.; Sheng, X.; Mao, L.; Li, S.; Kong, Y.; Tang, B.; Guo, J. Phase II Randomized Trial Comparing High-Dose IFN-α2b with Temozolomide Plus Cisplatin as Systemic Adjuvant Therapy for Resected Mucosal Melanoma. Clin. Cancer Res. 2013, 19, 4488–4498. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schacter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Ribas, A.; Liszkay, G.; Maio, M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation-Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Moya-Plana, A.; Herrera Gómez, R.G.; Rossoni, C.; Dercle, L.; Ammari, S.; Girault, I.; Roy, S.; Scoazec, J.; Vagner, S.; Janot, F.; et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol. Immunother. 2019, 68, 1171–1178. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Namikawa, K.; Reijers, I.L.M.; Buchbinder, E.I.; Soon, J.A.; Zaremba, A.; Teterycz, P.; Mooradian, M.J.; Armstrong, E.; Nakamura, Y.; et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann. Oncol. 2022, 33, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, C.; Wohlmuth-Wieser, I.; Laframboise, S. Clinical Characteristics and Treatment Response with Checkpoint Inhibitors in Malignant Melanoma of the Vulva and Vagina. J. Low. Genit. Tract Dis. 2021, 25, 146–151. [Google Scholar] [CrossRef]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Lorusso, D.; Raspagliesi, F.; Del Vecchio, M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: A preliminary experience. J. Gynecol. Oncol. 2019, 30, e94. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Huber, V.; Camerini, S.; Casella, M.; Macone, A.; Bertuccini, L.; Iosi, F.; Moliterni, E.; Cecchetti, S.; Ruspantini, I.; et al. The Fatty Acid and Protein Profiles of Circulating CD81-Positive Small Extracellular Vesicles Are Associated with Disease Stage in Melanoma Patients. Cancers 2021, 13, 4157. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).