Personalized Adipofascial Flap: A Game-Changer for Post-Traumatic Ulnar Nerve Neuropathy at the Wrist and Elbow
Abstract
1. Introduction
2. Materials and Methods
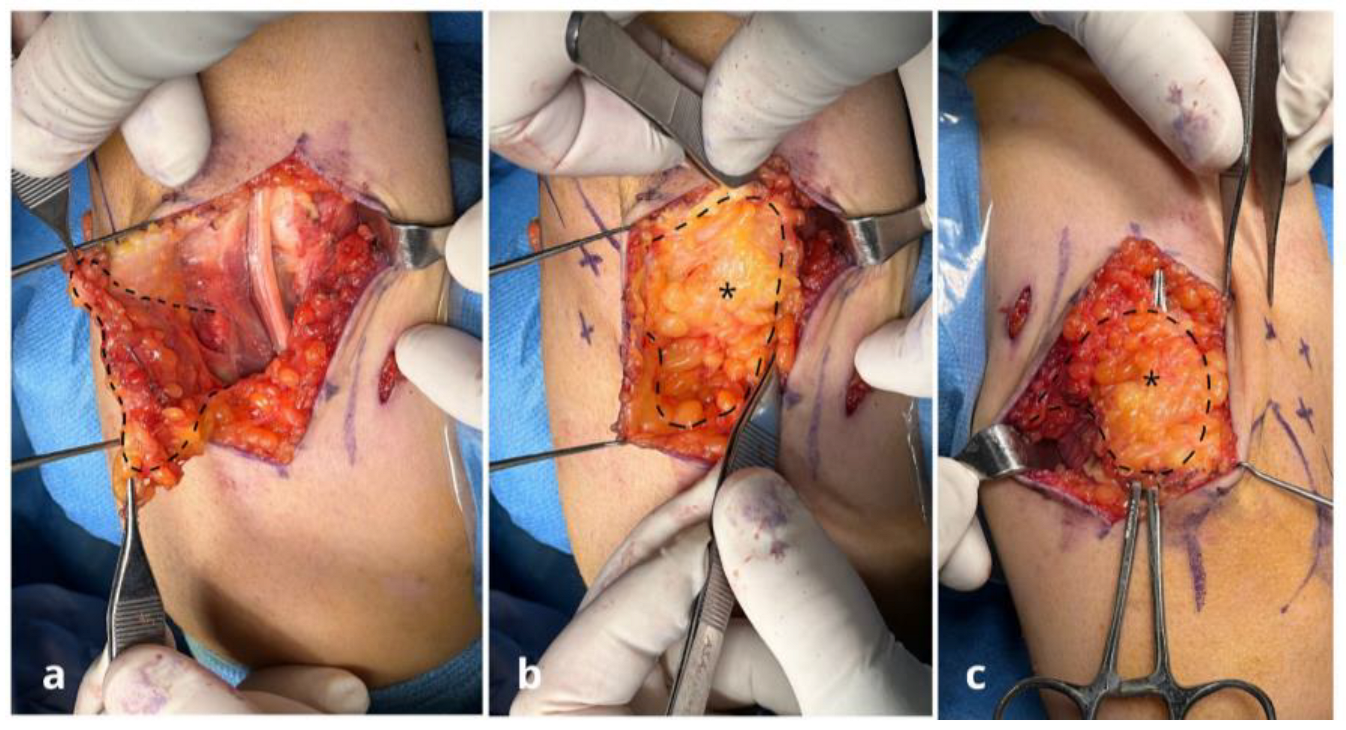
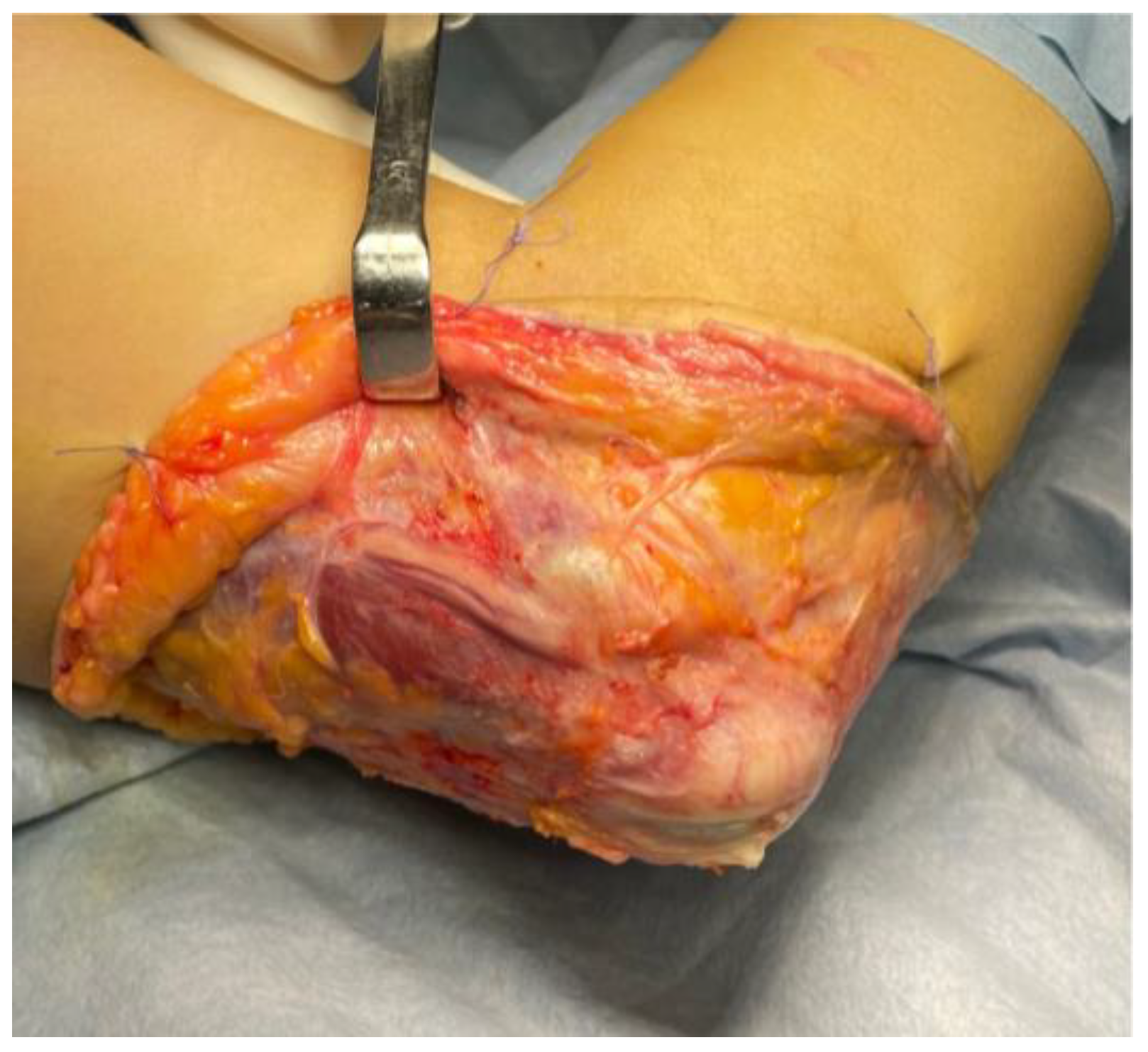
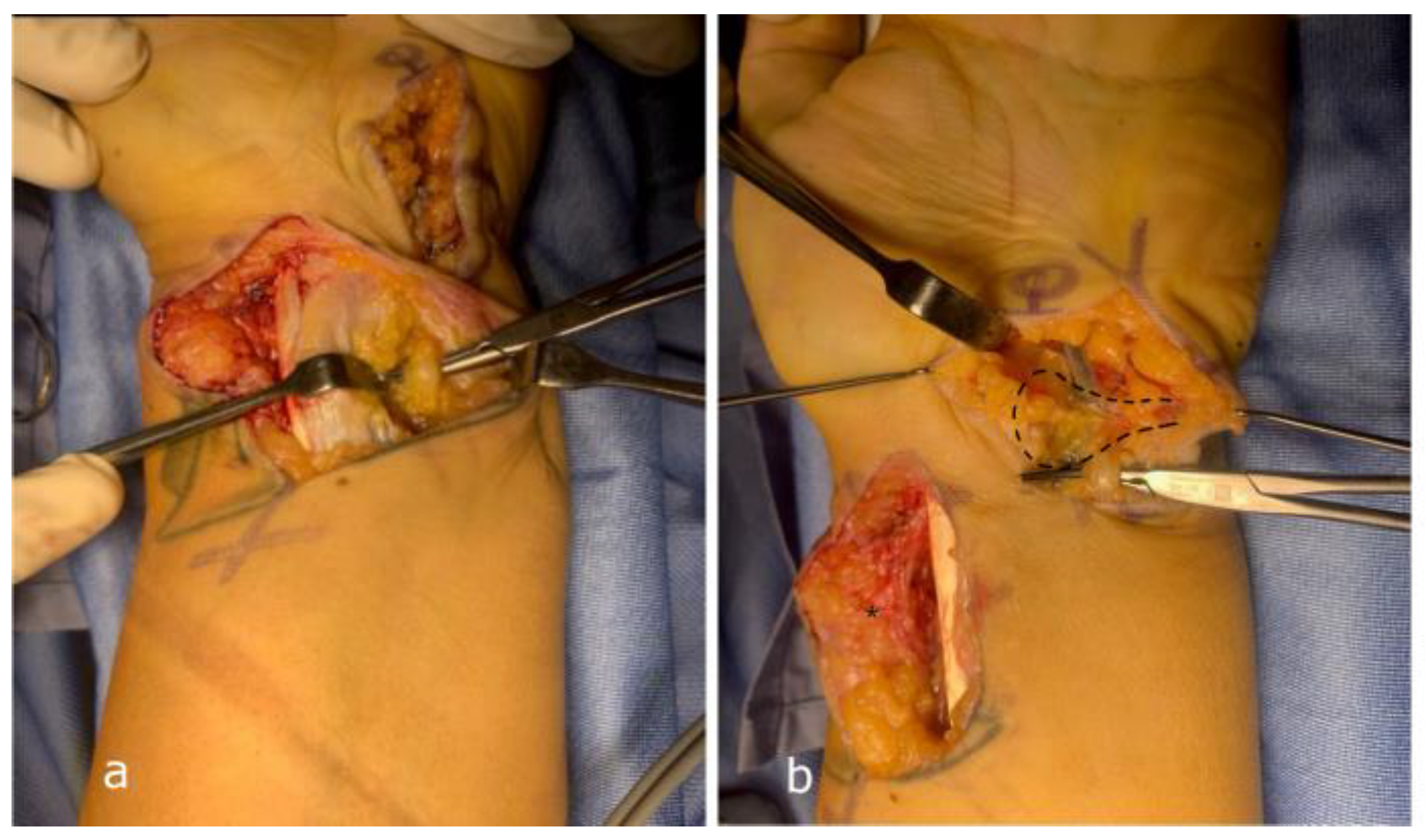
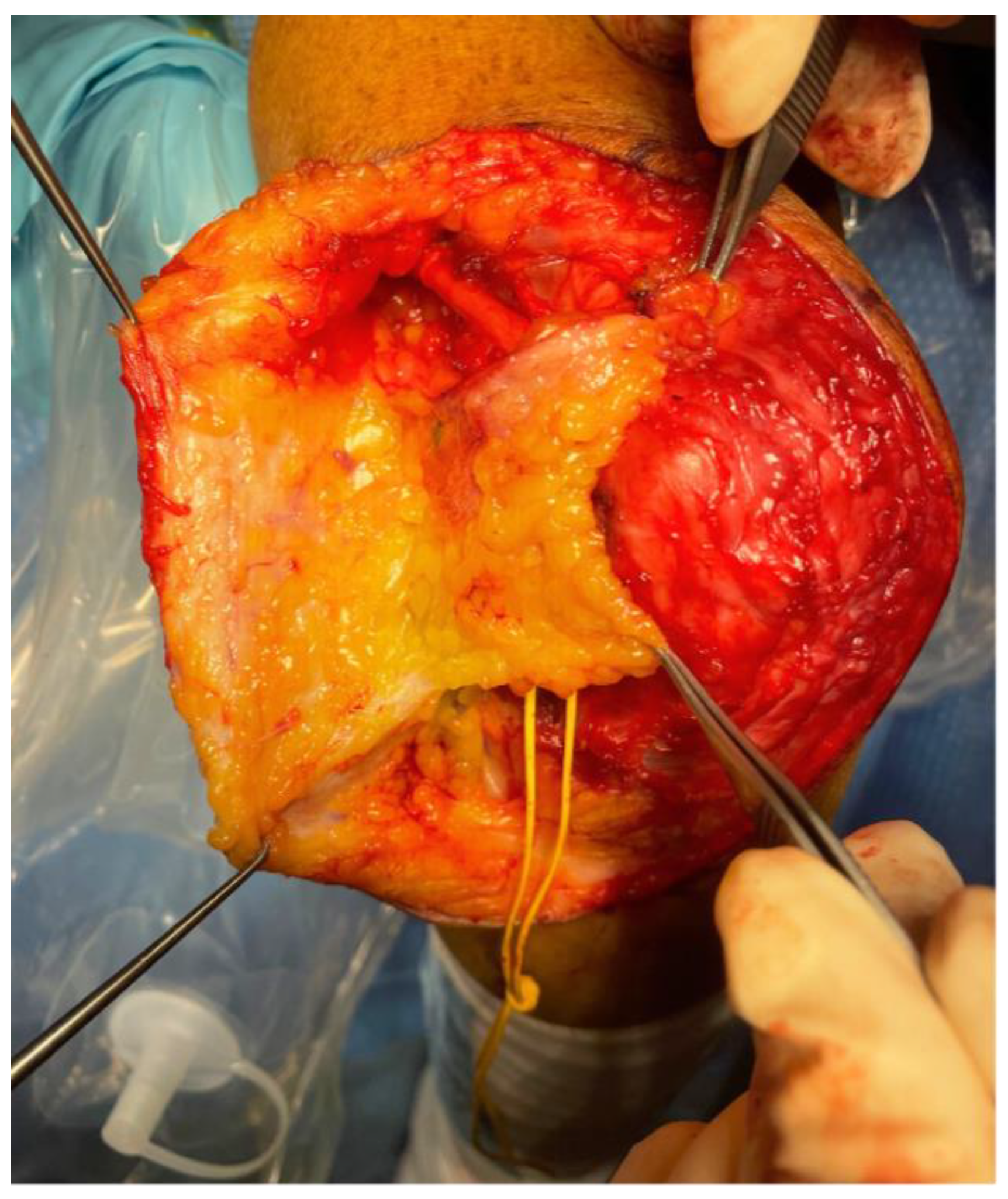
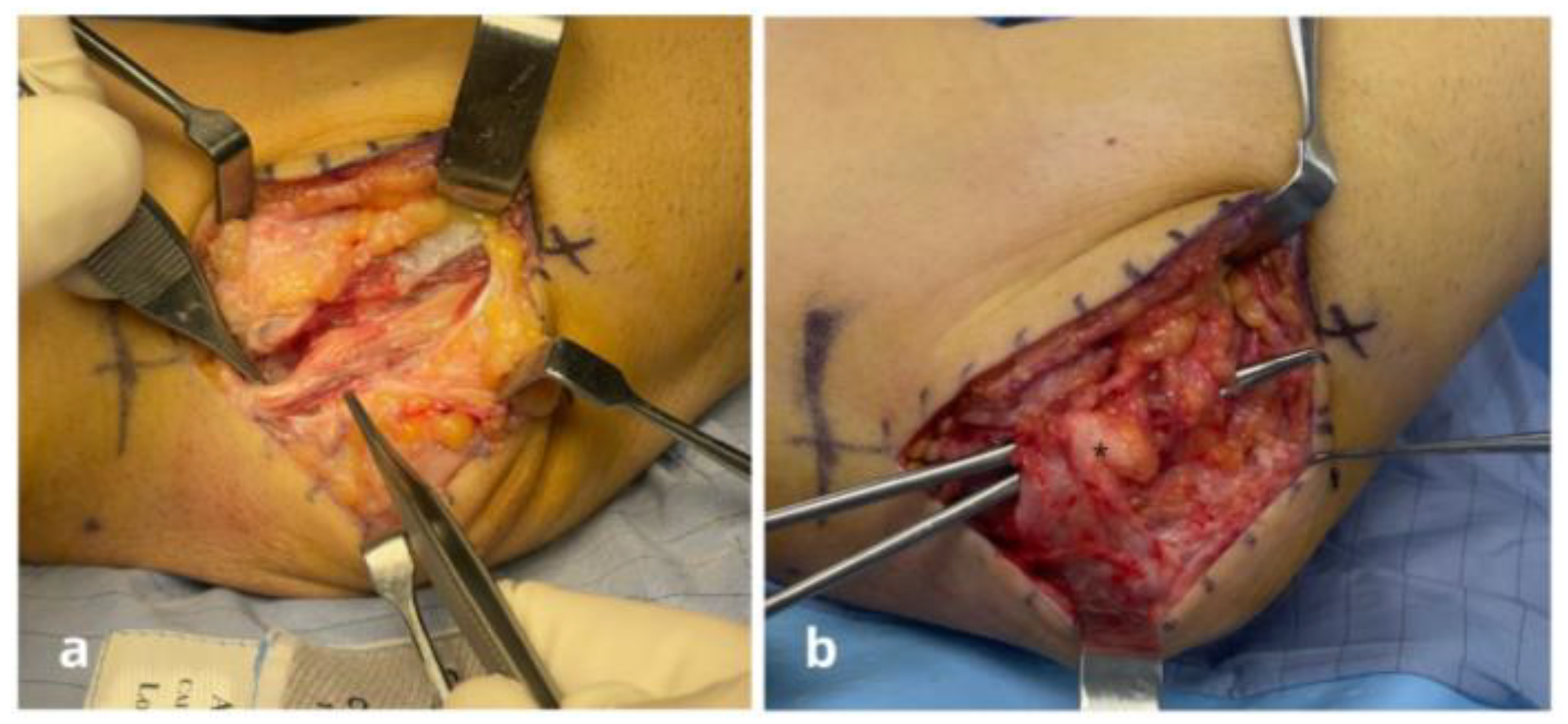
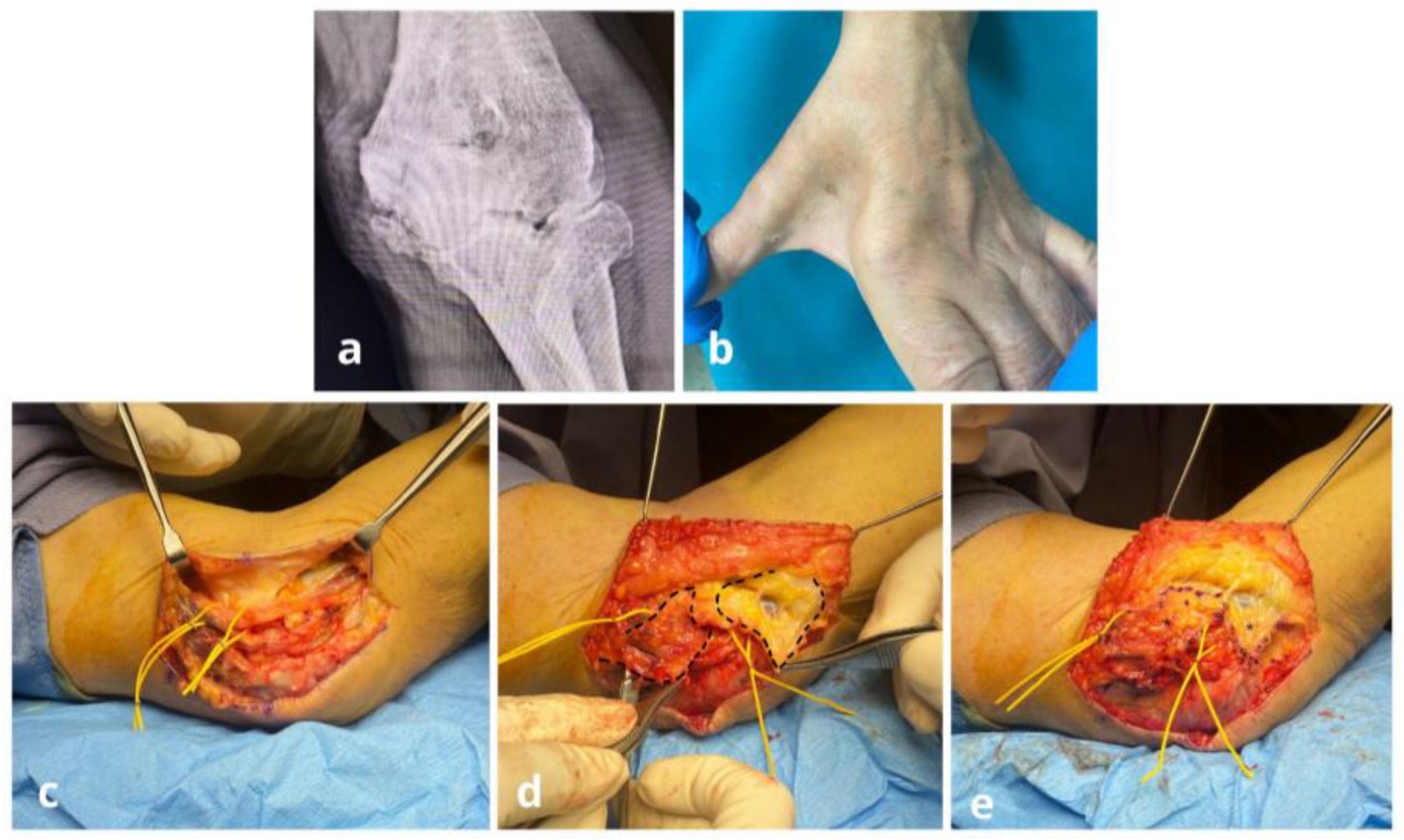
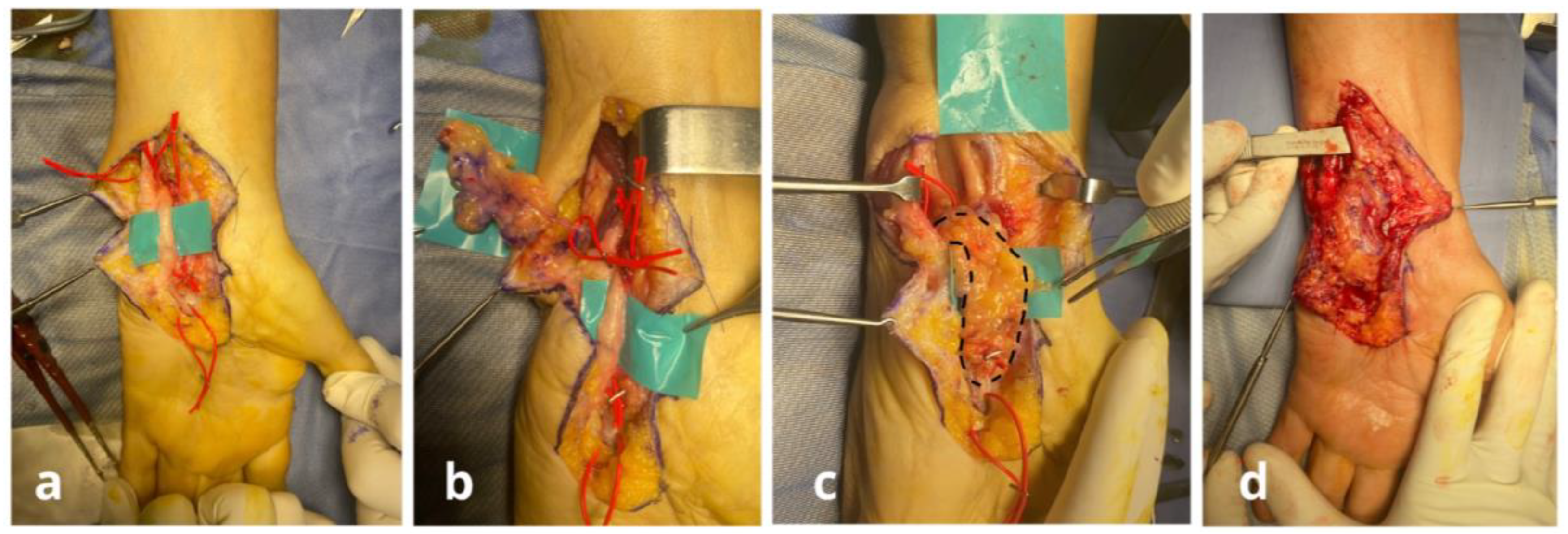
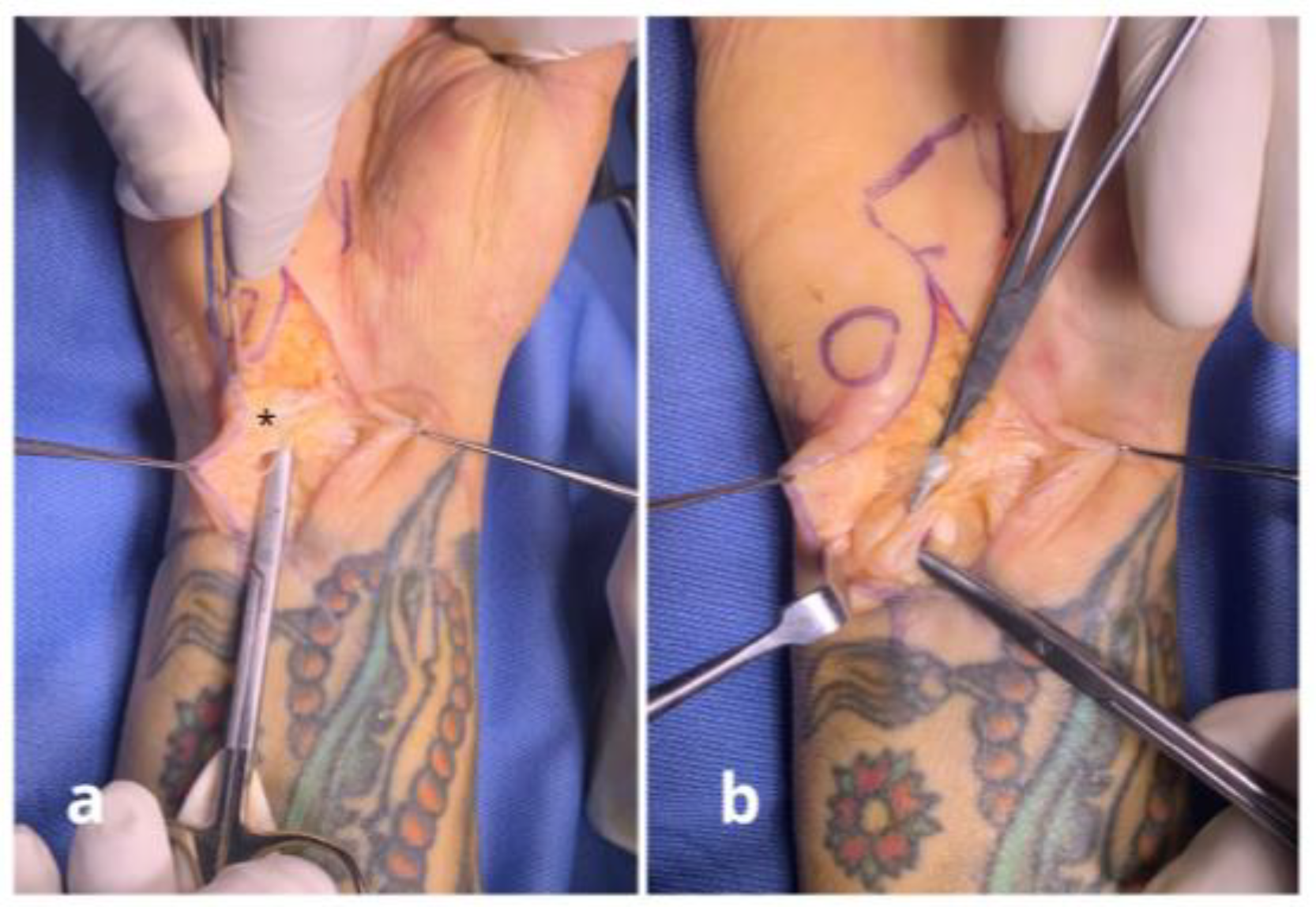
Surgical Techniques
3. Results
Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leffert, R.D. Anterior submuscular transposition of the ulnar nerves by the Learmonth technique. J. Hand Surg. 1982, 7, 147–155. [Google Scholar] [CrossRef]
- Nyman, E.; Dahlin, L.B. The Unpredictable ulnar nerve—Ulnar nerve entrapment from anatomical, pathophysiological, and biopsychosocial aspects. Diagnostics 2024, 14, 489. [Google Scholar] [CrossRef]
- Costa, J.Q.; Leite, M.J.; Relvas, M.; Vieira, P.; Negrão, P.; Vidinha, V. Ulnar-sided upper extremity traumatic wounds: What should we expect to find? J. Hand Surg. Asian Pac. 2023, 28, 435–440. [Google Scholar] [CrossRef]
- Afacan, M.Y.; Ozturk, B.; Guven, M.F. Ulnar Nerve Management in Distal Humerus Fracture Surgery: A Case of Developing Ulnar Neuropathy After Open Reduction and Internal Fixation. Cureus 2023, 15, e45477. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, K.; Tiel, R.L.; Murovic, J.A.; Kline, D.G. Surgical outcomes of 654 ulnar nerve lesions. J. Neurosurg. 2003, 98, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Ambade, R.; Shelke, A. From conservative measures to surgical interventions, treatment approaches for cubital tunnel syndrome: A comprehensive review. Cureus 2023, 15, e51262. [Google Scholar] [CrossRef] [PubMed]
- Hewson, D.W.; Kurien, T.; Hardman, J.G. Postoperative ulnar neuropathy: A systematic review of evidence with narrative synthesis. Br. J. Anaesth. 2023, 131, 135–149. [Google Scholar] [CrossRef]
- Flores, L.P. Distal anterior interosseous nerve transfer to the deep ulnar nerve and end-to-side suture of the superficial ulnar nerve to the third common palmar digital nerve for treatment of high ulnar nerve injuries: Experience in five cases. Arq. Neuropsiquiatr. 2011, 69, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Dy, C.J.; Mackinnon, S.E. Ulnar neuropathy: Evaluation and management. Curr. Rev. Musculoskelet. Med. 2016, 9, 178–184. [Google Scholar] [CrossRef]
- Lan, C.Y.; Tien, H.Y.; Lin, Y.T.; Hsu, C.C.; Lin, C.H.; Chen, S.H. Prognosis of traumatic ulnar nerve injuries: A systematic review. Ann. Plast. Surg. 2019, 82, S45–S52. [Google Scholar] [CrossRef]
- Benoit, B.G.; Preston, D.N.; Atack, D.M.; Da Silva, V.F. Neurolysis combined with the application of a silastic envelope for ulnar nerve entrapment at the elbow. Neurosurgery 1987, 20, 594–598. [Google Scholar] [CrossRef]
- Kamat, A.S.; Jay, S.M.; Benoiton, L.A.; Correia, J.A.; Woon, K. Comparative outcomes of ulnar nerve transposition versus neurolysis in patients with entrapment neuropathy at the cubital tunnel: A 20-year analysis. Acta Neurochir. 2014, 156, 153–157. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.T.; Huang, S.Z.; Wang, W.S. Arthroscopic extra-articular ulnar nerve release in the setting of stiff elbow. Arthrosc. Tech. 2024, 13, 103062. [Google Scholar] [CrossRef]
- Tuaño, K.R.; Fisher, M.H.; Franzoni, D.V.; Iorio, M.L. Ulnar nerve compression at the elbow secondary to intramuscular lipoma of the flexor carpi ulnaris: A case report. JBJS Case Connect. 2023, 13, e23.00108. [Google Scholar] [CrossRef]
- Emamhadi, M.; Alijani, B.; Ghadarjani, S. Surgical outcome of ulnar nerve lesions: Not always disappointing. J. Neurol. Stroke 2015, 3, 00115. [Google Scholar] [CrossRef]
- Podsednik, A.; Cabrejo, R.; Rosen, J. Adipose tissue uses in peripheral nerve surgery. Int. J. Mol. Sci. 2022, 23, 644. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Marcovici, L.L.; Molayem, I.; Amendola, C.; Pagnotta, A. Management of the ulnar nerve using an adipofascial flap in elbow surgery. Musculoskelet. Surg. 2025. [Google Scholar] [CrossRef]
- Pagnotta, A.; Formica, V.M.; Marcovici, L.L.; Molayem, I.; Taglieri, E. A novel local adipofascial flap for the management of recalcitrant ulnar tunnel syndrome. Hand Surg. Rehabil. 2021, 40, 377–381. [Google Scholar] [CrossRef]
- Calotta, N.A.; Shores, J.T.; Tuffaha, S.H. Adipofascial Perforator Flaps for Peripheral Nerve Resurfacing after External Neurolysis. J. Hand Surg. Asian Pac. 2024, 29, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Danoff, J.R.; Lombardi, J.M.; Rosenwasser, M.P. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J. Hand Surg. 2014, 39, 552–555. [Google Scholar] [CrossRef]
- Verveld, C.J.; Danoff, J.R.; Lombardi, J.M.; Rosenwasser, M.P. Adipose flap versus fascial sling for anterior subcutaneous transposition of the ulnar nerve. Am. J. Orthop. 2016, 45, 89–94. [Google Scholar]
- Lo Torto, F.; Patanè, L.; Abbaticchio, D.; Pagnotta, A.; Ribuffo, D. Autologous Fat Grafting (AFG): A systematic review to evaluate oncological safety in breast cancer patients. J. Clin. Med. 2024, 13, 4369. [Google Scholar] [CrossRef]
- Lo Torto, F.; Turriziani, G.; Carella, S.; Pagnotta, A.; Ribuffo, D. Impact of the prepectoral breast reconstruction assessment score on expander-based reconstruction success. J. Clin. Med. 2024, 13, 6466. [Google Scholar] [CrossRef]
- Vaia, N.; Lo Torto, F.; Marcasciano, M.; Casella, D.; Cacace, C.; De Masi, C.; Ricci, F.; Ribuffo, D. From the “Fat Capsule” to the “Fat Belt”: Limiting protective lipofilling on irradiated expanders for breast reconstruction to selective key areas. Aesthet. Plast. Surg. 2018, 42, 986–994. [Google Scholar] [CrossRef]
- Adani, R.; Tos, P.; Tarallo, L.; Corain, M. Treatment of painful median nerve neuromas with radial and ulnar artery perforator adipofascial flaps. J. Hand Surg. 2014, 39, 721–727. [Google Scholar] [CrossRef]
- Strickland, J.W.; Idler, R.S.; Lourie, G.M.; Plancher, K.D. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J. Hand Surg. 1996, 21, 840–848. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R.D. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef]
- Kingham, P.J.; Reid, A.J.; Wiberg, M. Adipose-derived stem cells for nerve repair: Hype or reality? Cells Tissues Organs 2014, 200, 23–30. [Google Scholar] [CrossRef]
- Hayashi, A.; Maruyama, Y.; Saze, M.; Okada, E. Ulnar recurrent adipofascial flap for reconstruction of massive defects around the elbow and forearm. Br. J. Plast. Surg. 2004, 57, 632–637. [Google Scholar] [CrossRef]
- Asensio Ramos, S.; Sánchez-del Hoyo, R.; García Bernal, F.J. Pedicled adipofascial flaps for treatment and prophylaxis of neurodesis and neuromas in continuity of the upper extremity: A case series of 22 patients. J. Hand Microsurg. 2025, 17, 100320. [Google Scholar] [CrossRef]
- Tapp, M.; Wenzinger, E.; Tarabishy, S.; Ricci, J.; Herrera, F.A. The epidemiology of upper extremity nerve injuries and associated cost in the US Emergency Departments. Ann. Plast. Surg. 2019, 83, 676–680. [Google Scholar] [CrossRef]
- Saadat, S.; Eslami, V.; Rahimi-Movaghar, V. The incidence of peripheral nerve injury in trauma patients in Iran. Ulus. Travma Ve Acil Cerrahi Derg. 2011, 17, 539–544. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Youseffam, P.; Bagherzadeh, L.; Rayegani, S.M.; Bahrami, M.H.; Eliaspour, D. Electrodiagnostic findings in 441 patients with ulnar neuropathy—A retrospective study. Orthop. Res. Rev. 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Giannicola, G.; Sacchetti, F.M.; Greco, A.; Cinotti, G.; Postacchini, F. Management of complex elbow instability. Musculoskelet. Surg. 2010, 94 (Suppl. S1), S25–S36. [Google Scholar] [CrossRef] [PubMed]
- Giannicola, G.; Bullitta, G.; Rotini, R.; Murena, L.; Blonna, D.; Iapicca, M.; Restuccia, G.; Merolla, G.; Fontana, M.; Greco, A.; et al. Results of primary repair of distal triceps tendon ruptures in a general population: A multicentre study. Bone Jt. J. 2018, 100-B, 610–616. [Google Scholar] [CrossRef]
- Mondelli, M.; Giannini, F.; Ballerini, M.; Ginanneschi, F.; Martorelli, E. Incidence of ulnar neuropathy at the elbow in the province of Siena (Italy). J. Neurol. Sci. 2005, 234, 5–10. [Google Scholar] [CrossRef]
- García-Cepeda, I.; Sanz-Peñas, A.-E.; de Blas-Sanz, I.; Simón-Pérez, C.; Frutos-Reoyo, E.-J.; Aguado-Maestro, I. Post-Surgical ulnar nerve neuropathy in distal humerus fractures: Comparison between in situ decompression and anterior subcutaneous transposition. J. Clin. Med. 2025, 14, 2490. [Google Scholar] [CrossRef]
- Shin, R.; Ring, D. The ulnar nerve in elbow trauma. J. Bone Jt. Surg. 2007, 89, 1108–1116. [Google Scholar] [CrossRef]
- Katayama, Y.; Senda, M.; Kaneda, D.; Hino, T.; Ikeda, Y.; Ozaki, T. O-3-14. Treatment of ulnar neuropathy after total elbow arthroplasty (TEA) and nerve conduction test. Clin. Neurophysiol. 2018, 129, e36. [Google Scholar] [CrossRef]
- Faccenda, C.; Dutto, E.; Bosco, F.; Lavia, A.D.; Battiston, B. Ulnar nerve management in complex elbow dislocations: A retrospective monocentric study. J. Pers. Med. 2024, 14, 1076. [Google Scholar] [CrossRef]
- Jewett, C.; Desai, M. Median and ulnar nerve injury at the elbow and wrist. In Peripheral Nerve Issues After Orthopedic Surgery; Springer International Publishing: Cham, Switzerland, 2022; pp. 167–185. [Google Scholar] [CrossRef]
- Greco, A.; Marcovici, L.L.; Muscatiello, A.L.; Pagnotta, A. Dry elbow arthroscopy: Positive pressure to improve tissue distension. Arthrosc. Tech. 2025, 14, 103436. [Google Scholar] [CrossRef] [PubMed]
- Trębacz, P.; Frymus, J.; Pawlik, M.; Barteczko, A.; Kurkowska, A.; Berczyńska, J.; Czopowicz, M. Risk of Ulnar Nerve Injury Following Caudo-Medial Arthroscopic Portal Creation in the Canine Elbow—A Cadaveric Study. Animals 2025, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.B.; Vitale, M.A.; Ahmad, C.S.; Ruchelsman, D.E. Ulnar Nerve complications after ulnar collateral ligament reconstruction of the elbow: A systematic review. Am. J. Sports Med. 2019, 47, 1263–1269. [Google Scholar] [CrossRef]
- Little, C.P.; Graham, A.J.; Carr, A.J. Total elbow arthroplasty: A systematic review of the literature in the English language until the end of 2003. J. Bone Jt. Surg. Br. 2005, 87, 437–444. [Google Scholar] [CrossRef]
- Brito, A.C.N.L.; Santos, S.E.V.; Martins, W.A.; Queiroz, P.C.D.S.; Sougey, W.W.D.; Alves, P.K.N.; Ribeiro, K.L.; de Oliveira, M.D.L.; de Moraes, S.R.A. Efficacy of tubing technique with biomaterials compared to direct coaptation technique after peripheral neurotmesis in nerve healing and return to functionality in young adult rats: A systematic review protocol. Syst. Rev. 2020, 9, 118. [Google Scholar] [CrossRef]
- Podnar, S. No major nerve regeneration seems to occur during recovery of ulnar neuropathy at the elbow. J. Clin. Med. 2023, 12, 3906. [Google Scholar] [CrossRef]
- Kline, D.G. Timing for exploration of nerve lesions and evaluation of the neuroma-in-continuity. Clin. Orthop. Relat. Res. 1982, 163, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Thakker, A.; Sharma, S.C.; Hussain, N.M.; Devani, P.; Lahiri, A. Nerve wrapping for recurrent compression neuropathy: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 549–559. [Google Scholar] [CrossRef]
- Fones, L.; DePascal, M.; Ilyas, A.M. Use of Nerve Wraps in the Upper Extremity. SurgiColl 2024, 2, 1–7. [Google Scholar] [CrossRef]
- Sarris, I.K.; Sotereanos, D.G. Vein wrapping for recurrent median nerve compression. J. Hand Surg. 2004, 4, 189–194. [Google Scholar] [CrossRef]
- Leversedge, F.J.; Zoldos, J.; Nydick, J.; Kao, D.S.; Thayer, W.; MacKay, B.; McKee, D.; Hoyen, H.; Safa, B.; Buncke, G.M. A Multicenter Matched Cohort Study of Processed Nerve Allograft and Conduit in Digital Nerve Reconstruction. J. Hand Surg. 2020, 45, 1148–1156. [Google Scholar] [CrossRef]
- Cho, M.S.; Rinker, B.D.; Weber, R.V.; Chao, J.D.; Ingari, J.V.; Brooks, D.; Buncke, G.M. Functional Outcome Following Nerve Repair in the Upper Extremity Using Processed Nerve Allograft. J. Hand Surg. 2012, 37, 2340–2349. [Google Scholar] [CrossRef]
- DeVitis, R.; D’Orio, M.; Cannella, A.; Michel Gabriel, E.; Taccardo, G.; Marzella, L.; Cilli, V.; Sassara, G.M.; Passiatore, M. Short-Term outcomes of a novel fascio-aponeurotic flap technique for ulnar nerve instability at the elbow. Surgeries 2025, 6, 49. [Google Scholar] [CrossRef]
| Pt | Site | Diagnosis | Surgical Procedure | Previous Surgery | Pre-Op NRS | Pre-Op QDASH | Post-Op NRS | Post-Op QDASH | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Wrist | Postsurgical ulnar nerve neuroma | Nerve decompression + neurolysis + freestyle adipofascial flap | Wrist laceration with involvement of the ulnar nerve (open wound) | 10 | 90.9 | 6 | 22.7 | |
| 2 | Elbow | Post-surgical ulnar nerve pseudo-palsy following total elbow arthroplasty | Nerve decompression + neurolysis + Pagnotta’s adipofascial flap | Oncology elbow resection and total elbow arthroplasty (TEA) | 8 | 72.7 | 5 | 25.0 | |
| 3 | Elbow | Post-surgical elbow stiffness and ulnar nerve pseudo-palsy | Hardware removal + arthrolysis + ulnar nerve anterior transposition + freestyle anterior adipofascial flap | Distal humerus open reduction and internal fixation (ORIF) | 9 | 68.2 | 3 | 11.4 | |
| 4 | Wrist | Post-surgical ulnar nerve pseudo-palsy and complex regional pain syndrome (CRPS) | Nerve decompression + neurolysis + freestyle adipofascial flap | Neurorrhaphy and FCU repair (open wound) | 9 | 70.5 | 2 | 13.6 | |
| 5 | Wrist | Post-surgical ulnar nerve neuroma and complex regional pain syndrome following distal radius fracture treated with osteosynthesis and wrist CRPS | Nerve decompression + neurolysis + adipofascial flap based on dorsal ulnar artery (DUA) | Distal radius open reduction and internal fixation (ORIF) | 9 | 81.8 | 2 | 13.6 | |
| 6 | Wrist | Post-surgical ulnar nerve palsy and wrist CRPS | Nerve decompression + neurolysis + adipofascial flap based on dorsal ulnar artery (DUA) | Neurorrhaphy and FCU repair (open wound) | 10 | 70.5 | 3 | 22.7 | |
| 7 | Elbow | Ulnar nerve pseudo-palsy in multiple hereditary exostoses, | Ulnar nerve decompression + anterior transposition + double adipofascial flap | None | 8 | 72.7 | 4 | 15.9 | |
| 8 | Elbow | Post-surgical ulnar nerve neuropathy in post-traumatic osteoarthritis | Ulnar nerve decompression + anterior transposition + free style anterior adipofascial flap + (TEA) | Open debridement and in situ ulnar nerve neurolysis | 9 | 59.1 | 5 | 22.7 | |
| 9 | Elbow | Post-traumatic ulnar nerve neuropathy and elbow stiffness following elbow dislocation | Nerve decompression + neurolysis + Pagnotta’s adipofascial flap | None | 9 | 75.0 | 5 | 15.9 | |
| 10 | Elbow | Post-surgical ulnar nerve neuropathy | Hardware removal + arthrolysis + ulnar nerve anterior transposition + freestyle anterior adipofascial flap | Distal humerus ORIF | 9 | 75.0 | 6 | 52.3 | |
| 11 | Elbow | Post-traumatic ulnar nerve neuropathy and elbow stiffness following radial head fracture dislocation | Arthroscopic arthrolysis + nerve decompression + Pagnotta’s adipofascial flap | None | 7 | 77.3 | 4 | 9.1 | |
| 12 | Wrist | Post-traumatic ulnar nerve neuropathy following distal radius fracture malunion with fragment impinging on the ulnar nerve | Ulnar bony fragment removal + distal radius osteotomy + ulnar nerve neurolysis + freestyle adipofascial flap | None | 8 | 81.8 | 2 | 11.4 | 15 |
| 13 | Wrist | Post-surgical ulnar nerve neuropathy following K-Wires penetration | Distal radius osteotomy + ulnar nerve neurolysis + freestyle adipofascial flap | Distal radius osteosynthesis with plate and K-wires | 9 | 79.5 | 3 | 9.1 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, A.; Bizzarri, M.; Marcovici, L.L.; Pagnotta, A. Personalized Adipofascial Flap: A Game-Changer for Post-Traumatic Ulnar Nerve Neuropathy at the Wrist and Elbow. J. Pers. Med. 2025, 15, 521. https://doi.org/10.3390/jpm15110521
Greco A, Bizzarri M, Marcovici LL, Pagnotta A. Personalized Adipofascial Flap: A Game-Changer for Post-Traumatic Ulnar Nerve Neuropathy at the Wrist and Elbow. Journal of Personalized Medicine. 2025; 15(11):521. https://doi.org/10.3390/jpm15110521
Chicago/Turabian StyleGreco, Alessandro, Martina Bizzarri, Lucian Lior Marcovici, and Alessia Pagnotta. 2025. "Personalized Adipofascial Flap: A Game-Changer for Post-Traumatic Ulnar Nerve Neuropathy at the Wrist and Elbow" Journal of Personalized Medicine 15, no. 11: 521. https://doi.org/10.3390/jpm15110521
APA StyleGreco, A., Bizzarri, M., Marcovici, L. L., & Pagnotta, A. (2025). Personalized Adipofascial Flap: A Game-Changer for Post-Traumatic Ulnar Nerve Neuropathy at the Wrist and Elbow. Journal of Personalized Medicine, 15(11), 521. https://doi.org/10.3390/jpm15110521







