Does Intraoperative Navigation Improve K-Wire Positioning in Reverse Shoulder Arthroplasty?—A New Approach
Abstract
1. Introduction
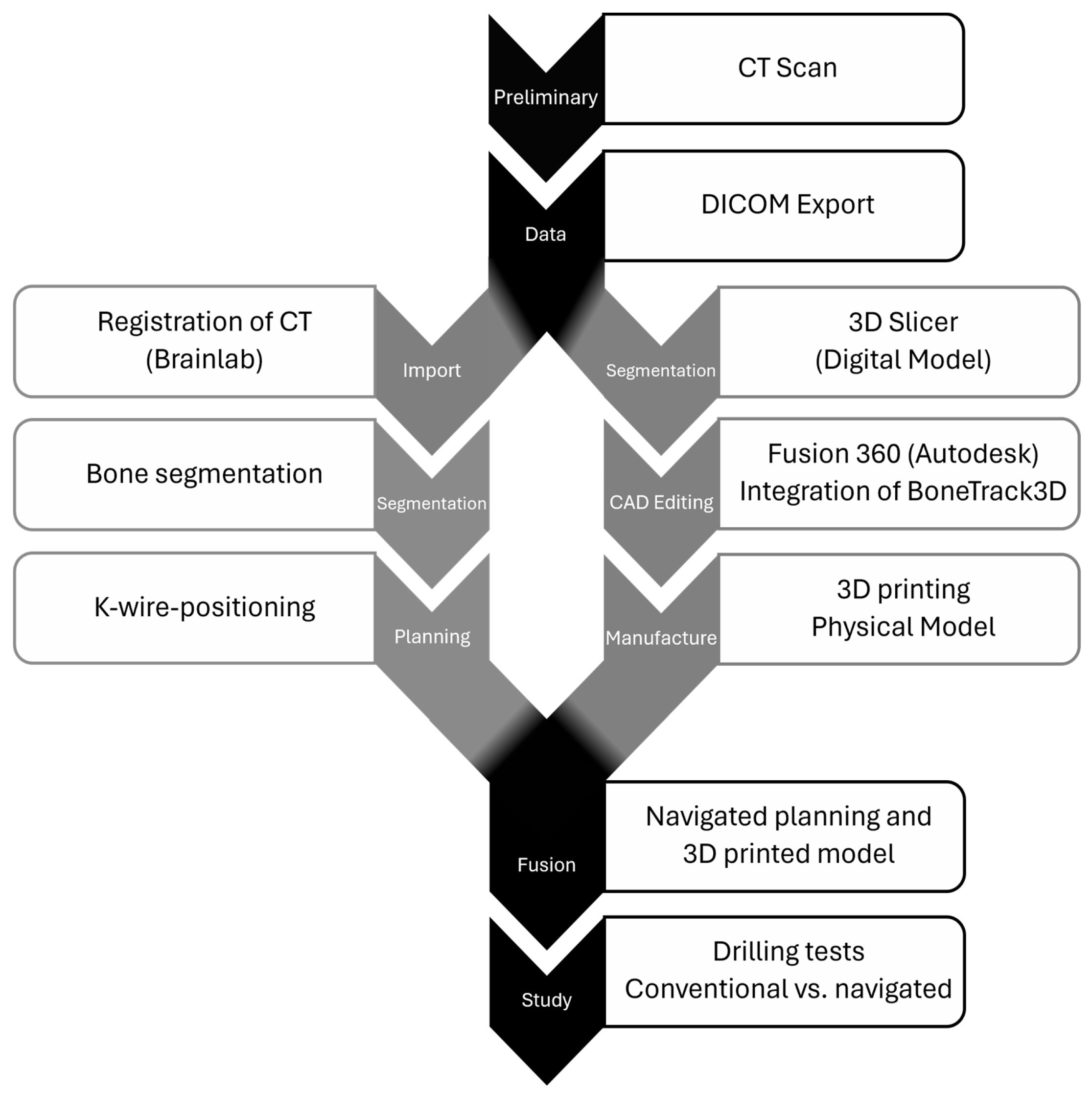
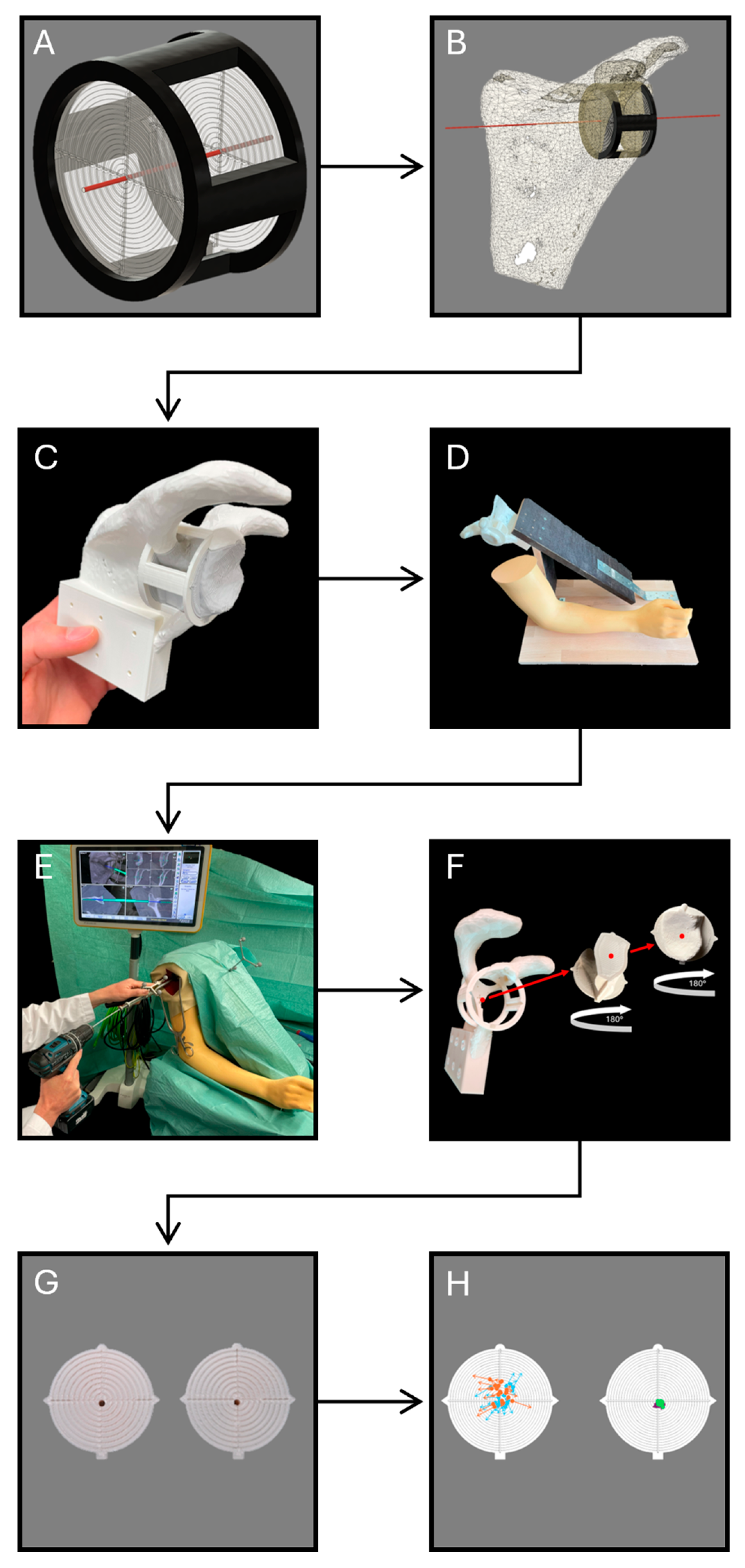
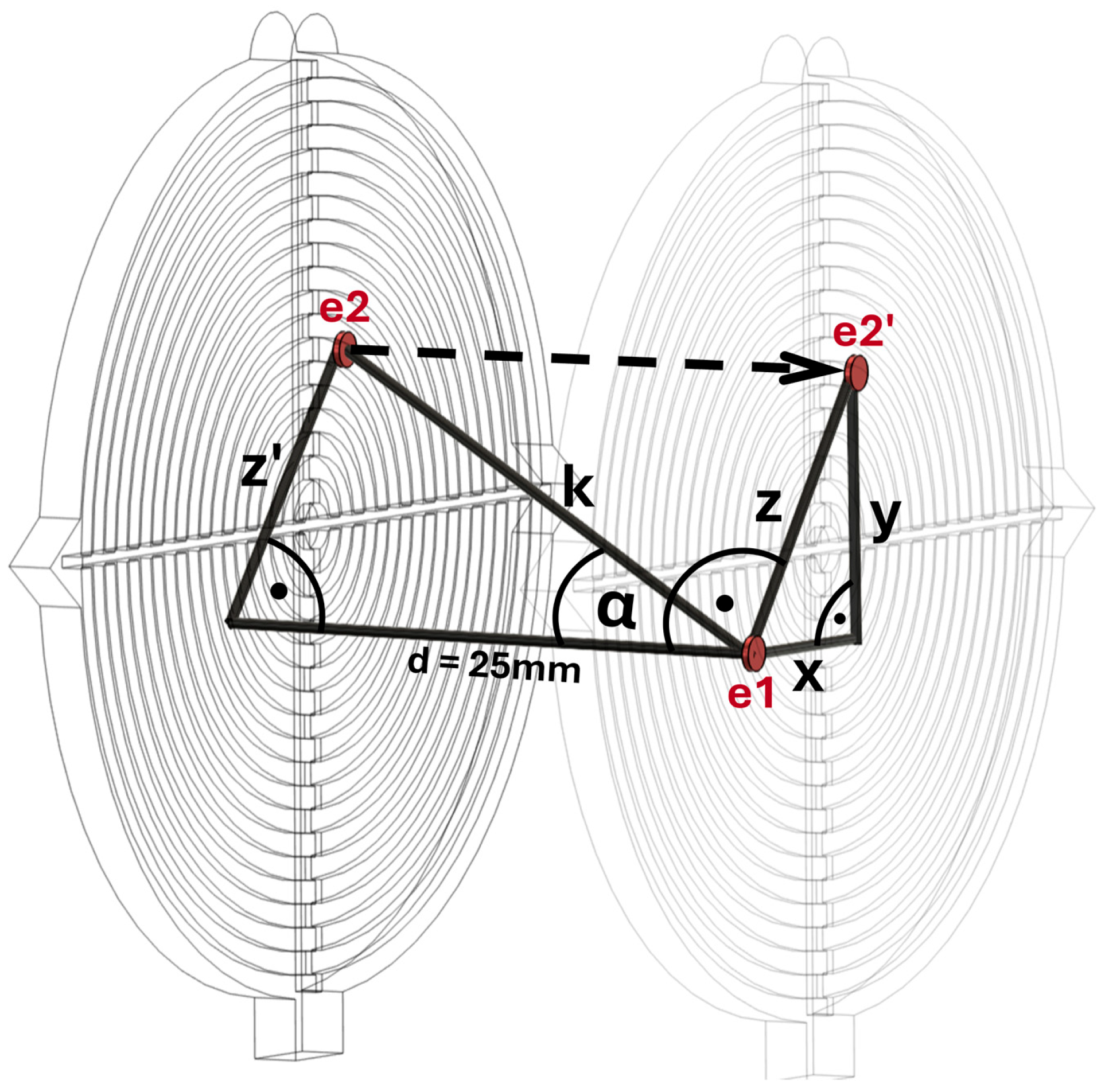
2. Materials and Methods
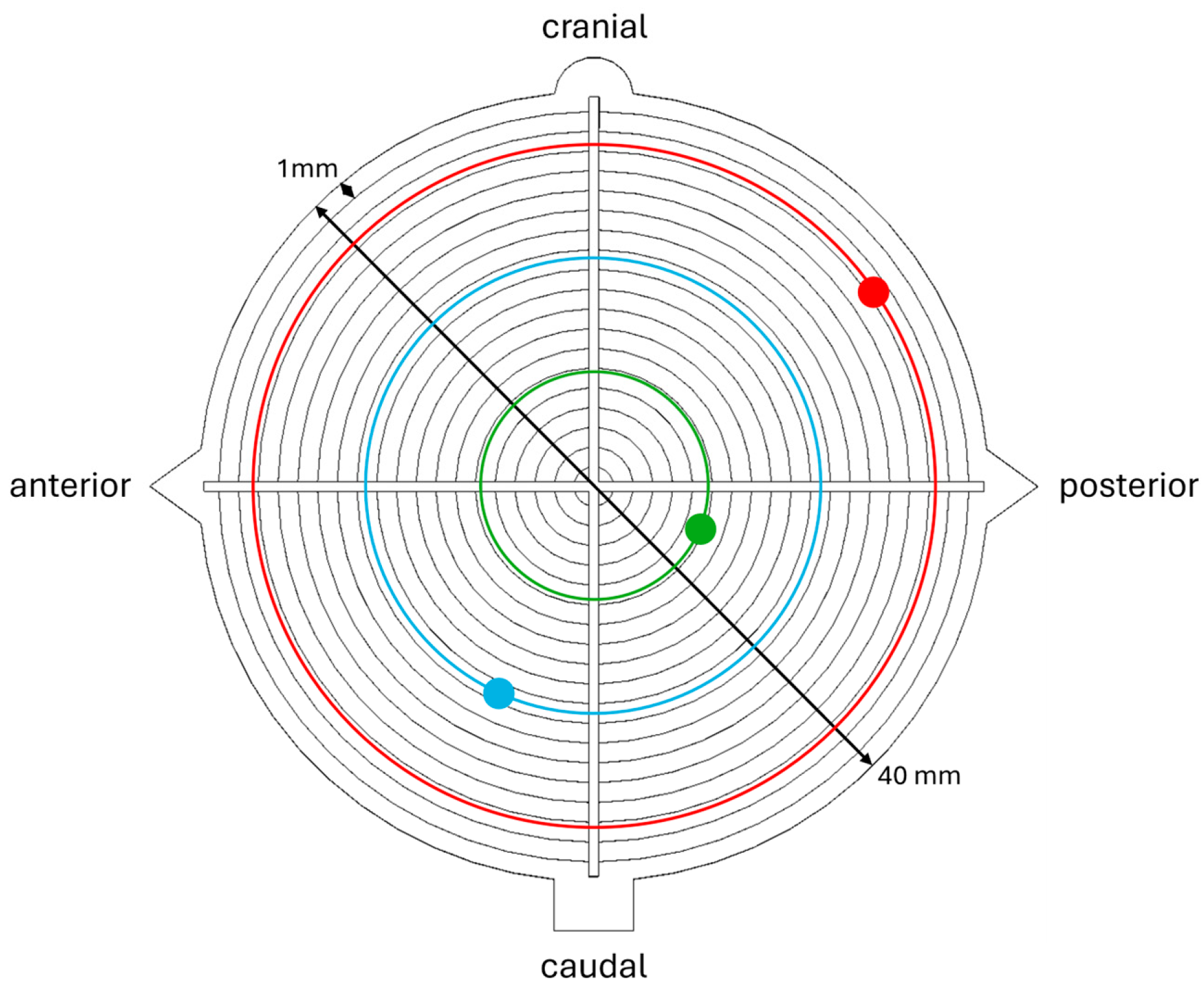
3. Results
3.1. Conventional vs. Navigated (C vs. N)
3.2. Inexperienced vs. Experienced (I vs. E)
3.2.1. Inexperienced Conventional vs. Experienced Conventional (IC vs. EC)
3.2.2. Inexperienced Navigated vs. Experienced Navigated (IN vs. EN)
3.2.3. Inexperienced Navigated vs. Experienced Conventional (IN vs. EC)
4. Discussion
4.1. Results of the Study
4.2. Strength and Limitations of the Model
4.3. Clinical Relevance
4.4. Further Research
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RSA | Reverse shoulder arthroplasty |
| CT | Computed tomography |
| DICOM | Digital Imaging and Communications in Medicine |
| STL | Standard tessellation language |
| CAD | Computer-aided design |
| PLA | Polylactic acid |
| PVA | Polyvinyl alcohol |
| C | Conventional |
| N | Navigated |
| I | Inexperienced |
| E | Experienced |
| IC | Inexperienced conventional |
| EC | Experienced conventional |
| IN | Inexperiencded navigated |
| EN | Experienced navigated |
| CI | Confidence interval |
| PRO | Patient-reported outcome |
References
- Jarrett, C.D.; Brown, B.T.; Schmidt, C.C. Reverse shoulder arthroplasty. Orthop. Clin. North Am. 2013, 44, 389–408. [Google Scholar]
- Stahl, D.; La Fuente, G.d. Reverse Total Shoulder Arthroplasty for a 4-Part Proximal Humerus Fracture. J. Orthop. Trauma 2016, 30 (Suppl. 2), S9–S10. [Google Scholar] [CrossRef]
- Boileau, P. Complications and revision of reverse total shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, S33–S43. [Google Scholar] [CrossRef]
- Karkenny, A.J.; Mendelis, J.R.; Geller, D.S.; Gomez, J.A. The Role of Intraoperative Navigation in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e849–e858. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Gosheger, G.; Bockholt, S.; de Vaal, M.; Budny, T.; Tönnemann, M.; Pützler, J.; Bövingloh, A.S.; Rischen, R.; Hofbauer, V.; et al. Complex Bone Tumors of the Trunk-The Role of 3D Printing and Navigation in Tumor Orthopedics: A Case Series and Review of the Literature. J. Pers. Med. 2021, 11, 517. [Google Scholar] [CrossRef]
- Oberthür, S.; Sehmisch, S.; Weiser, L.; Viezens, L.; Stübig, T. Hat die Navigation in der Traumatologie noch einen Stellenwert? Orthopadie 2022, 51, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Alrajeb, R.; Zarti, M.; Shuia, Z.; Alzobi, O.; Ahmed, G.; Elmhiregh, A. Robotic-assisted versus conventional total knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Batailler, C.; White, N.; Ranaldi, F.M.; Neyret, P.; Servien, E.; Lustig, S. Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1232–1240. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Li, D.; Tian, Y.; Yuan, S.; Wang, L.; Liu, X. Safety and accuracy of cannulated pedicle screw placement in scoliosis surgery: A comparison of robotic-navigation, O-arm-based navigation, and freehand techniques. Eur. Spine J. 2023, 32, 3094–3104. [Google Scholar] [CrossRef]
- Morris, G.V.; Stevenson, J.D.; Evans, S.; Parry, M.C.; Jeys, L. Navigation in Musculoskeletal Oncology: An Overview. Indian J. Orthop. 2018, 52, 22–30. [Google Scholar] [CrossRef]
- Takao, M.; Hamada, H.; Sakai, T.; Sugano, N. Clinical Application of Navigation in the Surgical Treatment of a Pelvic Ring Injury and Acetabular Fracture. Adv. Exp. Med. Biol. 2018, 1093, 289–305. [Google Scholar] [CrossRef]
- Sebaaly, A.; Riouallon, G.; Zaraa, M.; Jouffroy, P. The added value of intraoperative CT scanner and screw navigation in displaced posterior wall acetabular fracture with articular impaction. Orthop. Traumatol. Surg. Res. 2016, 102, 947–950. [Google Scholar] [CrossRef]
- Kida, H.; Urita, A.; Momma, D.; Matsui, Y.; Endo, T.; Kawamura, D.; Taneichi, H.; Iwasaki, N. Implications of navigation system use for glenoid component placement in reverse shoulder arthroplasty. Sci. Rep. 2022, 12, 21190. [Google Scholar] [CrossRef]
- Troiano, E.; Masini, A.; Colasanti, G.B.; Drago, C.; Giannotti, S.; Mondanelli, N. 3D CT-Based Preoperative Planning and Intraoperative Navigation in Reverse Shoulder Arthroplasty: Early Clinical Outcomes. Medicina 2025, 61, 749. [Google Scholar] [CrossRef] [PubMed]
- Holzgrefe, R.E.; Hao, K.A.; Panther, E.J.; Schoch, B.S.; Roche, C.P.; King, J.J.; Wright, J.O.; Wright, T.W. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: A matched cohort study. J. Shoulder Elb. Surg. 2023, 32, 302–309. [Google Scholar] [CrossRef]
- Pérez-Sevilla, M.; Rivas-Navazo, F.; Latorre-Carmona, P.; Fernández-Zoppino, D. Protocol for Converting DICOM Files to STL Models Using 3D Slicer and Ultimaker Cura. J. Pers. Med. 2025, 15, 118. [Google Scholar] [CrossRef]
- Theopold, J.; Pieroh, P.; Scharge, M.L.; Marquaß, B.; Hohmann, T.; Josten, C.; Hepp, P. Improved accuracy of K-wire positioning into the glenoid vault by intraoperative 3D image intensifier-based navigation for the glenoid component in shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, 575–581. [Google Scholar] [CrossRef]
- Shapiro, T.A.; McGarry, M.H.; Gupta, R.; Lee, Y.S.; Lee, T.Q. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J. Shoulder Elb. Surg. 2007, 16, S90–S95. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Sheikh, R.; Atkinson, T.S.; Jacob, H.A.C.; Favre, P.; Gerber, C. Effects of glenoid component version on humeral head displacement and joint reaction forces: An experimental study. J. Shoulder Elb. Surg. 2006, 15, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Farron, A.; Terrier, A.; Büchler, P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J. Shoulder Elb. Surg. 2006, 15, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Boufadel, P.; Lopez, R.; Fares, M.Y.; Daher, M.; Dhytadak, D.; Gulotta, L.V.; Abboud, J.A. Intraoperative Navigation in Reverse Shoulder Arthroplasty: Advantages and Future Prospects. Clin. Orthop. Surg. 2024, 16, 679–687. [Google Scholar] [CrossRef]
- Sadoghi, P.; Vavken, J.; Leithner, A.; Vavken, P. Benefit of intraoperative navigation on glenoid component positioning during total shoulder arthroplasty. Arch. Orthop. Trauma Surg. 2015, 135, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.W.; Hayes, A.; Gibbons, R.; Mackie, K.E. Computer navigation of the glenoid component in reverse total shoulder arthroplasty: A clinical trial to evaluate the learning curve. J. Shoulder Elb. Surg. 2020, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gundle, K.R.; White, J.K.; Conrad, E.U.; Ching, R.P. Accuracy and Precision of a Surgical Navigation System: Effect of Camera and Patient Tracker Position and Number of Active Markers. Open Orthop. J. 2017, 11, 493–501. [Google Scholar] [CrossRef] [PubMed]
- van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med. Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef]
- Juergensen, L.; Rischen, R.; Hasselmann, J.; Toennemann, M.; Pollmanns, A.; Gosheger, G.; Schulze, M. Insights into geometric deviations of medical 3d-printing: A phantom study utilizing error propagation analysis. 3D Print. Med. 2024, 10, 38. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, T.; Wu, W.; Gan, W.; Wu, C.; Peng, S.; Huan, S.; Liu, N. A systematic review on the cost-effectiveness of the computer-assisted orthopedic system. Health Care Sci. 2022, 1, 173–185. [Google Scholar] [CrossRef]
- Shuman, W.H.; Valliani, A.A.; Chapman, E.K.; Martini, M.L.; Neifert, S.N.; Baron, R.B.; Schupper, A.J.; Steinberger, J.M.; Caridi, J.M. Intraoperative Navigation in Spine Surgery: Effects on Complications and Reoperations. World Neurosurg. 2022, 160, e404–e411. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Nottmeier, E.W.; Pirris, S.M.; Deen, H.G.; Pichelmann, M.A. Intraoperative image-guided spinal navigation: Technical pitfalls and their avoidance. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef]
- Sielatycki, J.A.; Mitchell, K.; Leung, E.; Lehman, R.A. State of the art review of new technologies in spine deformity surgery-robotics and navigation. Spine Deform. 2022, 10, 5–17. [Google Scholar] [CrossRef]
- Thomas, A.; Pemmaraju, G.; Nagra, G.; Bassett, J.; Deshpande, S. Complications resulting from tracker pin-sites in computer navigated knee replacement surgery. Acta Orthop. Belg. 2015, 81, 708–712. [Google Scholar]
- Beldame, J.; Boisrenoult, P.; Beaufils, P. Pin track induced fractures around computer-assisted TKA. Orthop. Traumatol. Surg. Res. 2010, 96, 249–255. [Google Scholar] [CrossRef]
- Thomas, T.L.; Goh, G.S.; Nguyen, M.K.; Lonner, J.H. Pin-Related Complications in Computer Navigated and Robotic-Assisted Knee Arthroplasty: A Systematic Review. J. Arthroplast. 2022, 37, 2291–2307.e2. [Google Scholar] [CrossRef]
- Marescalchi, M.; El Motassime, A.; Andriollo, L.; Polizzi, A.; Niccoli, G.; Morea, V. Computer-Assisted Navigation in Shoulder Arthroplasty: A Narrative Review. J. Clin. Med. 2025, 14, 2763. [Google Scholar] [CrossRef]
- Can Kolac, U.; Paksoy, A.; Akgün, D. Three-dimensional planning, navigation, patient-specific instrumentation and mixed reality in shoulder arthroplasty: A digital orthopedic renaissance. EFORT Open Rev. 2024, 9, 517–527. [Google Scholar] [CrossRef]
- Haidamous, G.; Lädermann, A.; Hartzler, R.U.; Parsons, B.O.; Lederman, E.S.; Tokish, J.M.; Denard, P.J. Radiographic parameters associated with excellent versus poor range of motion outcomes following reverse shoulder arthroplasty. Shoulder Elb. 2022, 14, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jennewine, B.R.; Brolin, T.J. Emerging Technologies in Shoulder Arthroplasty: Navigation, Mixed Reality, and Preoperative Planning. Orthop. Clin. North Am. 2023, 54, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.A.; Sutton, C.D.; Wright, T.W.; Schoch, B.S.; Wright, J.O.; Struk, A.M.; Haupt, E.T.; Leonor, T.; King, J.J. Influence of glenoid wear pattern on glenoid component placement accuracy in shoulder arthroplasty. JSES Int. 2022, 6, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.G.P.; Ikemoto, R.Y.; Wright, T. Navigation in Shoulder Arthroplasty Surgery. Rev. Bras. Ortop. 2022, 57, 540–545. [Google Scholar] [CrossRef]
- Sprowls, G.R.; Wilson, C.D.; Stewart, W.; Hammonds, K.A.P.; Baruch, N.H.; Ward, R.A.; Robin, B.N. Intraoperative navigation and preoperative templating software are associated with increased glenoid baseplate screw length and use of augmented baseplates in reverse total shoulder arthroplasty. JSES Int. 2021, 5, 102–108. [Google Scholar] [CrossRef]
- Hones, K.M.; King, J.J.; Schoch, B.S.; Struk, A.M.; Farmer, K.W.; Wright, T.W. The in vivo impact of computer navigation on screw number and length in reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, e629–e635. [Google Scholar] [CrossRef]
- Velasquez Garcia, A.; Abdo, G. Does computer-assisted navigation improve baseplate screw configuration in reverse shoulder arthroplasty? A systematic review and meta-analysis of comparative studies. J. Orthop. 2023, 36, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Codsi, M.J.; Iannotti, J.P. The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. J. Shoulder Elb. Surg. 2008, 17, 479–486. [Google Scholar] [CrossRef]
- Arenas-Miquelez, A.; Murphy, R.J.; Rosa, A.; Caironi, D.; Zumstein, M.A. Impact of humeral and glenoid component variations on range of motion in reverse geometry total shoulder arthroplasty: A standardized computer model study. J. Shoulder Elb. Surg. 2021, 30, 763–771. [Google Scholar] [CrossRef]
- Egol, K.A.; Phillips, D.; Vongbandith, T.; Szyld, D.; Strauss, E.J. Do orthopaedic fracture skills courses improve resident performance? Injury 2015, 46, 547–551. [Google Scholar] [CrossRef]
- Atesok, K.; Mabrey, J.D.; Jazrawi, L.M.; Egol, K.A. Surgical simulation in orthopaedic skills training. J. Am. Acad. Orthop. Surg. 2012, 20, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Larose, G.; Greene, A.T.; Jung, A.; Polakovic, S.V.; Davis, N.Z.; Zuckerman, J.D.; Virk, M.S. High intraoperative accuracy and low complication rate of computer-assisted navigation of the glenoid in total shoulder arthroplasty. J. Shoulder Elb. Surg. 2023, 32, S39–S45. [Google Scholar] [CrossRef] [PubMed]
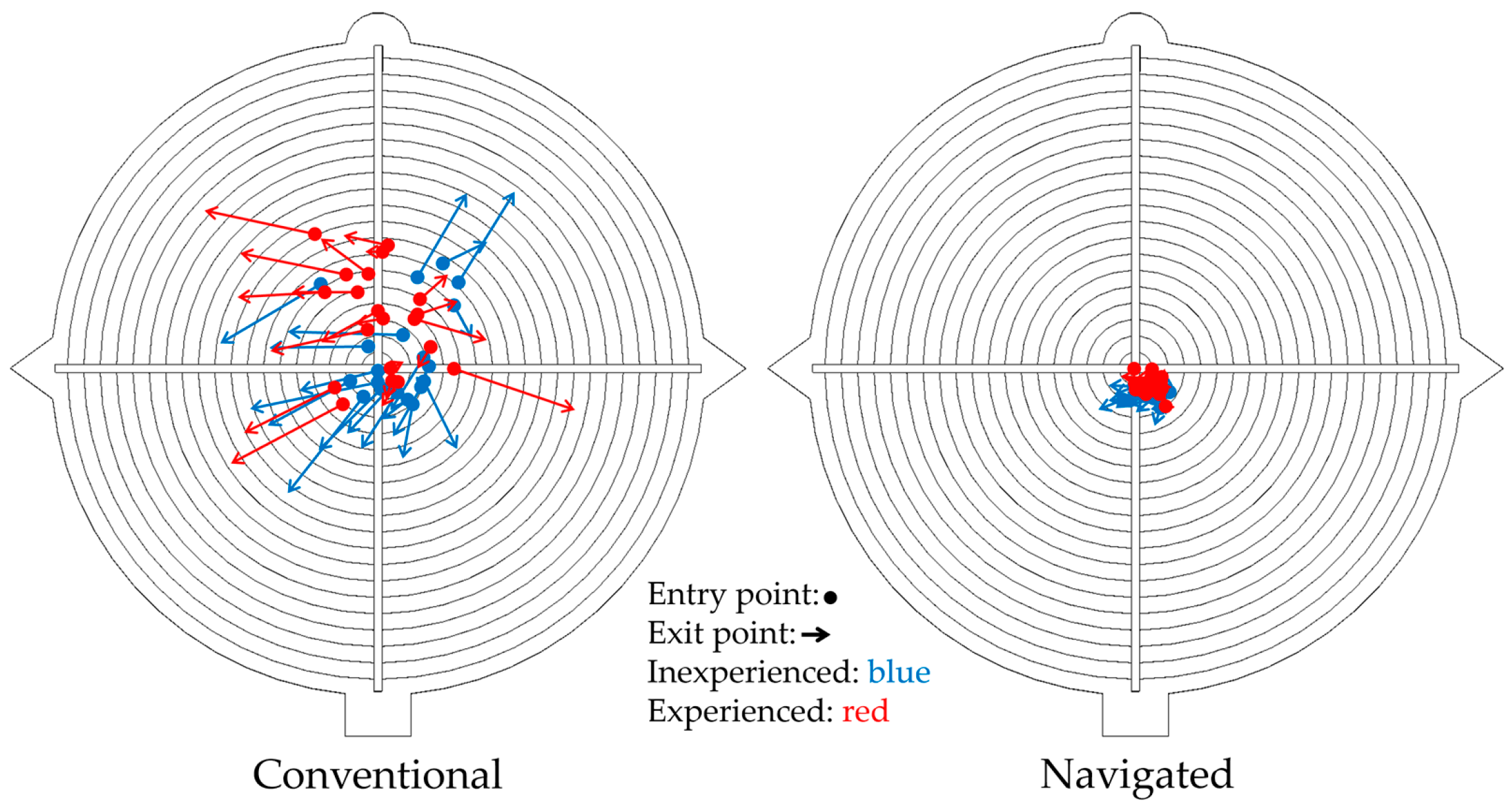
| Characteristics | Conventional 2 | Navigated 2 | p C vs. N [98.8% CI] |
|---|---|---|---|
| Entry point (mm) 1 | 3.0 (2.2, 4.9) | 1.6 (1.3, 1.8) | p < 0.001 [0.87, ∞] |
| Exit point (mm) 1 | 6.7 (5.3, 8.4) | 1.8 (1.4, 2.0) | p < 0.001 [3.7, ∞] |
| Drilling angle (°) | 8.9 (7.4, 12.3) | 2.6 (1.7, 3.4) | p < 0.001 [4.97, ∞] |
| Duration of the drilling (s) | 55.0 (37.8, 84.1) | 100.0 (80.4, 132.0) | p < 0.001 [−∞, −21.2] |
| Characteristics | IC 2 | EC 2 | p IC vs. EC [95% CI] |
|---|---|---|---|
| Entry point (mm) 1 | 2.5 (1.8, 3.8) | 4.3 (2.9, 5.6) | p = 0.218 [−3.14, 0.74] |
| Exit point (mm) 1 | 6.3 (5.3, 7.7) | 6.7 (5.9, 9.0) | p = 0.796 [−2.98, 2.3] |
| Drilling angle (°) | 10.6 (7.1, 13.5) | 8.8 (7.9, 10.5) | p = 0.353 [−2.1, 5.69] |
| Duration of the drilling (s) | 63.8 (40.2, 91.0) | 46.2 (29.8, 77.2) | p = 0.571 [−26.5, 41.0] |
| Characteristics | IN 2 | EN 2 | p IN vs. EN [95% CI] |
|---|---|---|---|
| Entry point (mm) 1 | 1.7 (1.6, 1.8) | 1.4 (1.2, 1.7) | p = 0.151 [−0.12, 0.72] |
| Exit point (mm) 1 | 1.9 (1.8, 2.3) | 1.6 (1.2, 1.8) | p = 0.029 [0.06, 1.05] |
| Drilling angle (°) | 2.9 (2.1, 3.5) | 2.0 (1.3, 3.2) | p = 0.315 [−0.52, 1.73] |
| Duration of the drilling (s) | 130.0 (98.8, 146) | 86.8 (54.4, 101.0) | p = 0.017 [6.5, 77.5] |
| Characteristics | IN 2 | EC 2 | p IN vs. EC [95% CI] |
|---|---|---|---|
| Entry point (mm) 1 | 1.7 (1.6, 1.8) | 4.3 (2.9, 5.6) | p = 0.001 [−∞, −1.22] |
| Exit point (mm) 1 | 1.9 (1.8, 2.3) | 6.7 (5.9, 9.0) | p < 0.001 [−∞, −3.7] |
| Drilling angle (°) | 2.9 (2.1, 3.5) | 8.8 (7.9, 10.5) | p < 0.001 [−∞, −4.74] |
| Duration of the drilling (s) | 130.0 (98.8, 146) | 46.2 (29.8, 77.2) | p = 0.002 [41.5, ∞] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaszczyk, T.; Gosheger, G.; Wohlmuth, J.; Hofbauer, V. Does Intraoperative Navigation Improve K-Wire Positioning in Reverse Shoulder Arthroplasty?—A New Approach. J. Pers. Med. 2025, 15, 509. https://doi.org/10.3390/jpm15110509
Blaszczyk T, Gosheger G, Wohlmuth J, Hofbauer V. Does Intraoperative Navigation Improve K-Wire Positioning in Reverse Shoulder Arthroplasty?—A New Approach. Journal of Personalized Medicine. 2025; 15(11):509. https://doi.org/10.3390/jpm15110509
Chicago/Turabian StyleBlaszczyk, Timo, Georg Gosheger, Jonathan Wohlmuth, and Vincent Hofbauer. 2025. "Does Intraoperative Navigation Improve K-Wire Positioning in Reverse Shoulder Arthroplasty?—A New Approach" Journal of Personalized Medicine 15, no. 11: 509. https://doi.org/10.3390/jpm15110509
APA StyleBlaszczyk, T., Gosheger, G., Wohlmuth, J., & Hofbauer, V. (2025). Does Intraoperative Navigation Improve K-Wire Positioning in Reverse Shoulder Arthroplasty?—A New Approach. Journal of Personalized Medicine, 15(11), 509. https://doi.org/10.3390/jpm15110509






