Traditional Growth-Friendly Implants Result in Improved Health-Related Quality of Life in Cerebral Palsy Patients with Early-Onset Scoliosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HRQoL | Health-related quality of life |
| CP | Cerebral Palsy |
| EOS | Early-onset scoliosis |
| TGR | Traditional growing rods |
| VEPTR | Vertically expandable prosthetic titanium rib |
| MCGR | Magnetically controlled growing rod |
| EOSQ-24 | 24-item Early-Onset Scoliosis Questionnaire |
| GMFCS | Gross Motor Function Classification System |
| PSSG | Pediatric Spine Study Group |
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Thomson, J.D.; Banta, J.V. Scoliosis in cerebral palsy: An overview and recent results. J. Pediatr. Orthop. B 2001, 10, 6–9. [Google Scholar] [PubMed]
- El-Hawary, R.; Akbarnia, B.A. Early onset scoliosis—Time for consensus. Spine Deform. 2015, 3, 105–106. [Google Scholar] [CrossRef]
- Persson-Bunke, M.; Hagglund, G.; Lauge-Pedersen, H.; Wagner, P.; Westbom, L. Scoliosis in a total population of children with cerebral palsy. Spine 2012, 37, E708–E713. [Google Scholar] [CrossRef]
- Bohtz, C.; Meyer-Heim, A.; Min, K. Changes in health-related quality of life after spinal fusion and scoliosis correction in patients with cerebral palsy. J. Pediatr. Orthop. 2011, 31, 668–673. [Google Scholar] [CrossRef]
- DiFazio, R.L.; Miller, P.E.; Vessey, J.A.; Snyder, B.D. Health-related quality of life and care giver burden following spinal fusion in children with cerebral palsy. Spine 2017, 42, E733–E739. [Google Scholar] [CrossRef]
- Sewell, M.D.; Malagelada, F.; Wallace, C.; Gibson, A.; Noordeen, H.; Tucker, S.; Molloy, S.; Lehovsky, J. A Preliminary Study to Assess Whether Spinal Fusion for Scoliosis Improves Carer-assessed Quality of Life for Children with GMFCS Level IV or V Cerebral Palsy. J. Pediatr. Orthop. 2016, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Cloake, T.; Gardner, A. The management of scoliosis in children with cerebral palsy: A review. J. Spine Surg. 2016, 2, 299–309. [Google Scholar] [CrossRef]
- Narayanan, U.G.; Fehlings, D.; Weir, S.; Knights, S.; Kiran, S.; Campbell, K. Initial development and validation of the caregiver priorities and child health index of life with disabilities (CPCHILD). Dev. Med. Child. Neurol. 2006, 48, 804–812. [Google Scholar] [CrossRef]
- Doany, M.E.; Olgun, Z.D.; Kinikli, G.I.; Bekmez, S.; Kocyigit, A.; Demirkiran, G.; Karaagaoglu, A.E.; Yazici, M. Health-related quality of life in early-onset scoliosis patients treated surgically. Spine 2018, 43, 148–153. [Google Scholar] [CrossRef]
- Matsumoto, H.; Williams, B.; Park, H.Y.; Yoshimachi, J.Y.; Roye, B.D.; Roye, D.P., Jr.; Akbarnia, B.A.; Emans, J.; Skaggs, D.; Smith, J.T.; et al. The final 24-item early onset scoliosis questionnaires (EOSQ-24): Validity, reliability and responsiveness. J. Pediatr. Orthop. 2018, 38, 144–151. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Mundis, G.M. Magnetically controlled growing rods in early onset scoliosis: Indications, timing and treatment. Orthopäde 2019, 48, 477–485. [Google Scholar] [CrossRef]
- Jain, A.; Sullivan, B.T.; Shah, S.A.; Samdani, A.F.; Yaszay, B.; Marks, M.C.; Sponseller, P.D. Caregiver perceptions and health-related quality-of-life changes in cerebral palsy patients after spinal arthrodesis. Spine 2018, 43, 1052–1056. [Google Scholar] [CrossRef]
- Bauer, J.M.; Yorgova, P.; Neiss, G.; Rogers, K.; Sturm, P.F.; Sponseller, P.D.; Luhmann, S.; Pawelek, J.B.; Shah, S.A.; Growing Spine Study Group. Early onset scoliosis: Is there an improvement in quality of life with conversion from traditional growing rods to magnetically controlled growing rods? J. Peditr. Orthop. 2019, 39, e284–e288. [Google Scholar] [CrossRef]
- Hell, A.K.; Braunschweig, L.; Behrend, J.; Lorenz, H.M.; Tsaknakis, K.; von Deimling, U.; Mladenov, K. Health-related quality of life in early-onset-scoliosis patients treated with growth-friendly implants is influenced by etiology, complication rate and ambulatory ability. BMC Musculoskelet. Disord. 2019, 20, 588. [Google Scholar] [CrossRef]
- Whitaker, A.T.; Sharkey, M.; Diab, M. Spinal fusion for scoliosis in patients with globally involved cerebral palsy: An ethical assessment. J. Bone Jt. Surg. Am. 2015, 97, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Lenke, L.G.; Daubs, M.D.; Watanabe, K.; Bridwel, K.H.; Stobbs, G.; Hensley, M. Is spine deformity surgery in patients with spastic cerebral palsy truly beneficial?: A patient/parent evaluation. Spine 2009, 34, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.J.; Refakis, C.A.; Flynn, J.M.; Pahys, J.M.; Betz, R.R.; Bastrom, T.P.; Samdani, A.F.; Brusalis, C.M.; Sponseller, P.D.; Cahill, P.J. Surgeon and caregiver agreement on the goals and indications for scoliosis surgery in children with cerebral palsy. Spine Deform. 2019, 7, 304–311. [Google Scholar] [CrossRef]
- Angsupaisal, M.; Maathius, C.G.B.; Hadders-Algra, M. Adaptive seating systems in children with severe cerebral palsy across International Classification of Functioning, Disability and Health for Children and Youth version domains: A systematic review. Dev. Med. Child. Neurol. 2015, 57, 919–930. [Google Scholar] [CrossRef]
- Terjesen, T.; Lange, J.E.; Steen, H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev. Med. Child. Neurol. 2000, 42, 448–454. [Google Scholar] [CrossRef]
- Letts, M.; Rathbone, D.; Yamashita, T.; Nichol, B.; Keeler, A. Soft Boston orthosis in management of neuromuscular scoliosis: A preliminary report. J. Pediatr. Orthop. 1992, 12, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Murray, J.C.; Ganau, M.; Tan, Y.; Oshima, Y.; Tanaka, S. Does Posterior Scoliosis Correction Improve Respiratory Function in Adolescent Idiopathic Scoliosis? A Systematic Review and Meta-analysis. Glob. Spine J. 2019, 9, 866–873. [Google Scholar] [CrossRef]
- Dede, O.; Motoyama, E.K.; Yang, C.I.; Mutich, R.L.; Walczak, S.A.; Bowles, A.J.; Deeney, V.F. Pulmonary and Radiographic Outcomes of VEPTR (Vertical Expandable Prosthetic Titanium Rib) Treatment in Early-Onset Scoliosis. J. Bone Jt. Surg. Am. 2014, 96, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Bettany-Saltikov, J.; Weiss, H.R.; Chockalingam, N.; Kandasamy, G.; Arnell, T. A comparison of patient-reported outcome measures following different treatment approaches for adolescents with severe idiopathic scoliosis: A systematic review. Asian Spine J. 2016, 10, 1170–1194. [Google Scholar] [CrossRef] [PubMed]
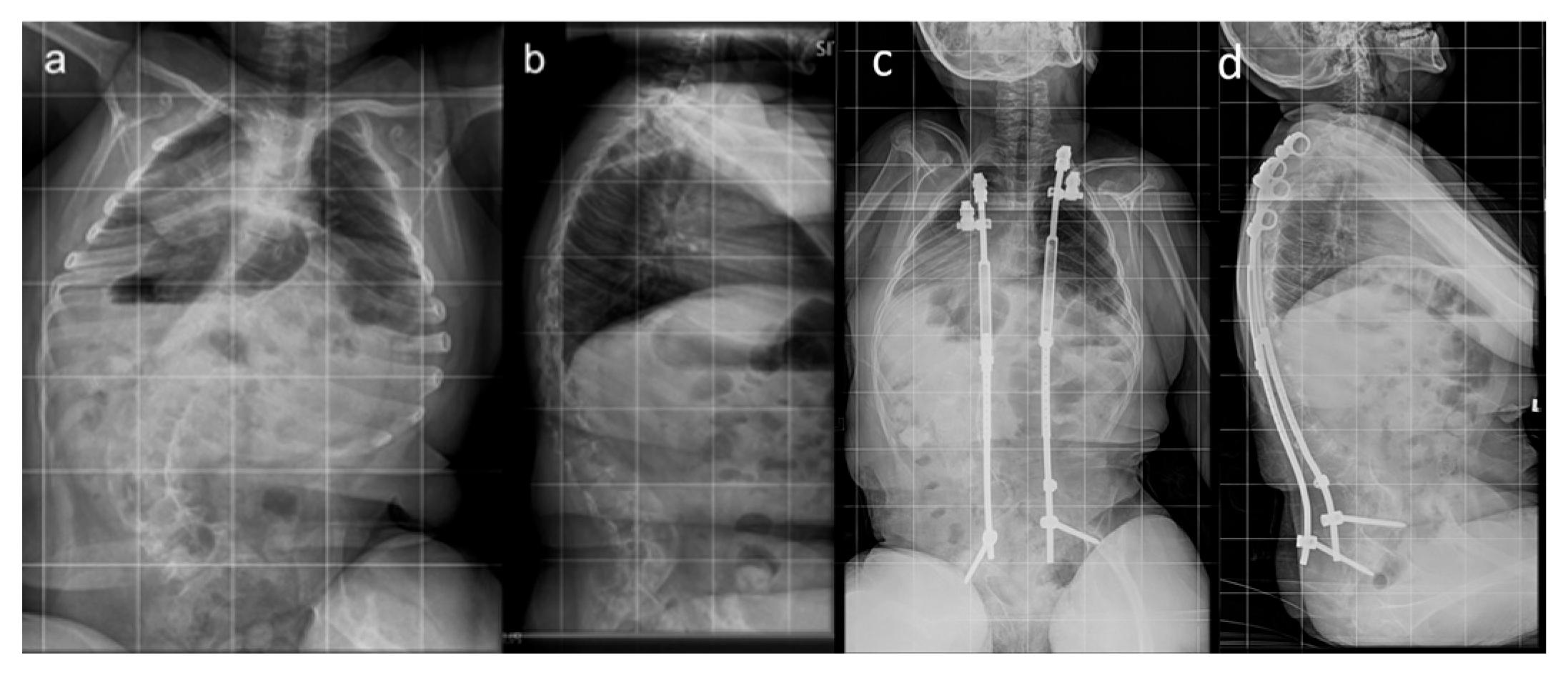
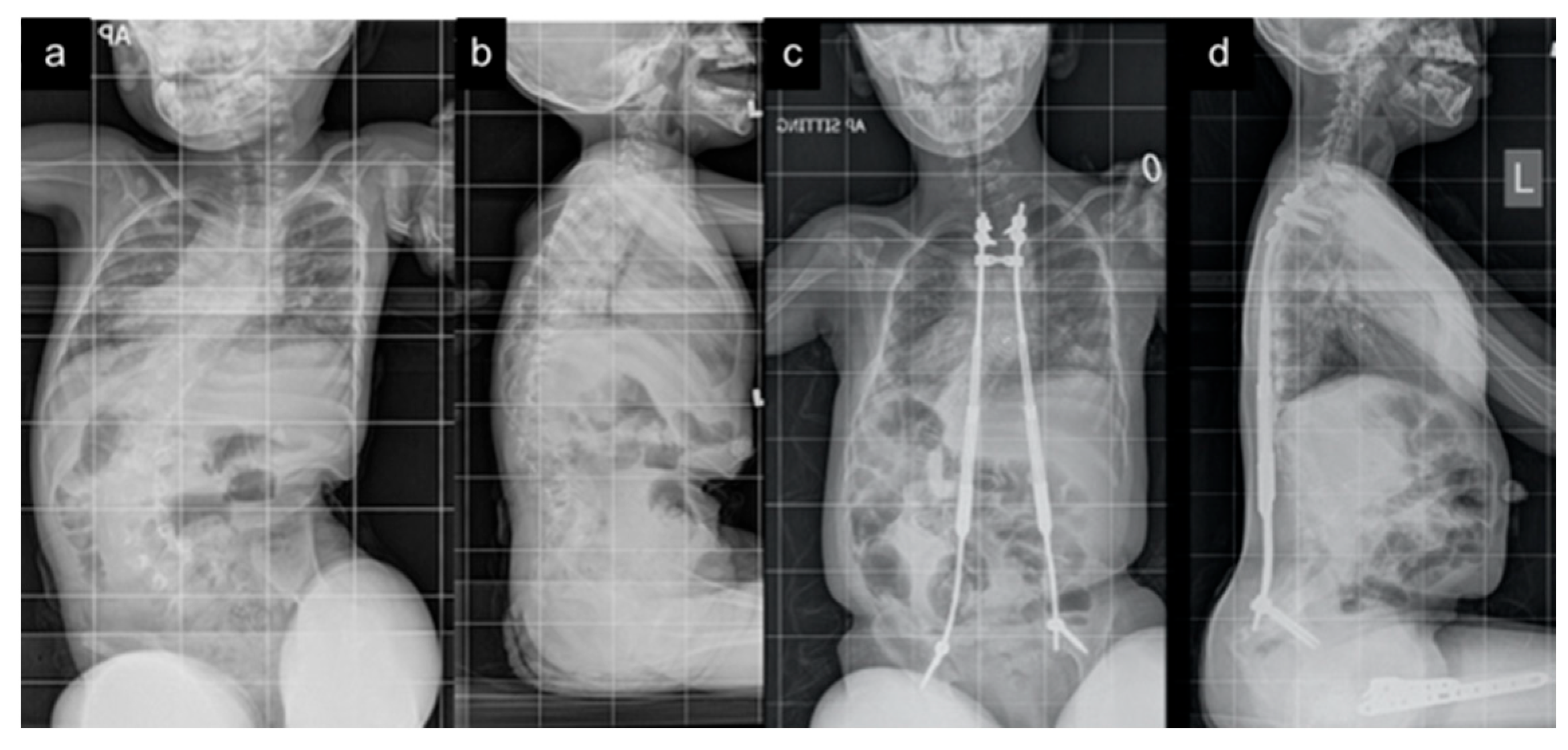
| All Patients (n = 24) | Traditional Implants (n = 9) | MCGR (n = 15) | |
|---|---|---|---|
| Growth-friendly implant type | 9 traditional/15 MCGRs | 5 VEPTR/4 TGRs | 15 MCGRs |
| Gender | 13 female/11 male | 3 female/6 male | 10 female/5 male |
| Prior treatment | 5 bracing/19 none | 3 bracing/6 none | 2 bracing/13 none |
| GMFCS level (I–V) | 21 V, 1 II, 1 I, 1 unknown | 9 V | 12 V, 1 II, 1 I, 1 unknown |
| Age at index surgery (years) | 7.4 (6.6–8.2) | 7.1 (5.8–8.4) | 7.7 (6.6–8.7) |
| Mean EOSQ-24 follow-up (years) | 3.5 (3.0–3.9) | 4.4 * (3.7–5.2) | 2.9 * (2.6–3.2) |
| Traditional Implants | MCGR | All | |||||
|---|---|---|---|---|---|---|---|
| Pre-Index | Follow-Up | Pre-Index | Follow-Up | Pre-Index | Follow-Up | p (All) | |
| Age (years) | 7.0 (5.7–8.3) n = 9 | 11.6 (9.4–13.8) n = 4 | 7.6 (6.5–8.6) n = 15 | 9.1 (7.4–10.9) n = 6 | 7.4 (6.6–8.1) n = 24 | 10.1 (8.6–11.6) n = 10 | 0.007 * |
| Primary curve (°) | 71.8 (59.4–84.1) n = 9 | 69.8 (37.3–102) n = 4 | 86.9 (76.9–96.9) n = 15 | 53.5 (39.1–67.8) n = 6 | 81.2 (73.1–89.4) n = 24 | 60.0 (44.6–75.4) n = 10 | 0.031 * |
| Maximum kyphosis (°) | 51.4 (38.7–64.1) n = 7 | 45.5 (27.9–63.1) n = 4 | 52.8 (42.1–63.6) n = 13 | 45.6 (28.7–62.5) n = 5 | 52.4 (44.2–60.5) n = 20 | 45.6 (34.1–57.0) n = 9 | 0.356 |
| T1-S1 (cm) | 25.5 (22.1–29.0) n = 8 | 34.4 (31.8–37.1) n = 3 | 27.1 (25.2–28.9) n = 14 | 31.1 (28.9–33.4) n = 6 | 26.5 (24.8–28.2) n = 22 | 32.2 (30.3–34.2) n = 9 | <0.001 * |
| T1-T12 (cm) | 16.6 (14.9–18.3) n = 8 | 21.2 (19.0–23.4) n = 3 | 17.1 (16.0–18.3) n = 14 | 19.1 (17.6–20.6) n = 6 | 17.0 (16.0–17.9) n = 22 | 19.8 (18.4–21.1) n = 9 | 0.004 * |
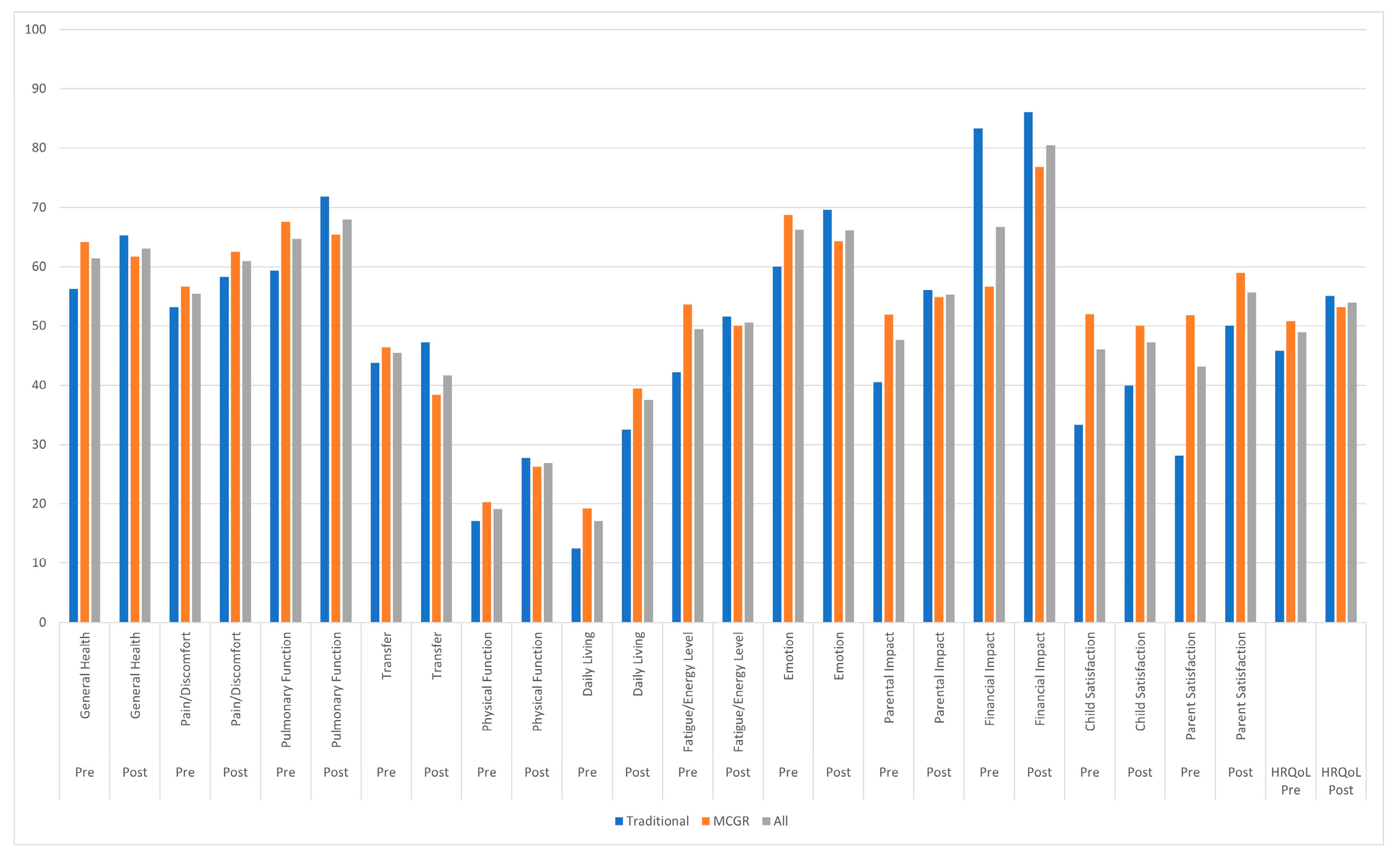
| Domain | Growth-Friendly Implant Type | Mean Absolute Difference (95% CI) from Pre-Index Surgery to Follow-Up | p (TI vs. MCGR) |
|---|---|---|---|
| General health | All | +2.7 (−7.1–12.6) | |
| TI | +12.5 (−8.7–33.7) | 0.236 | |
| MCGR | −2.5 (−12.1–7.1) | ||
| Pain/discomfort | All | +6.0 (−7.2–19.1) | |
| TI | +6.3 (−16.0–28.5) | 0.977 | |
| MCGR | +5.8 (−11.1–22.7) | ||
| Pulmonary function | All | −0.6 (−17.1–15.8) | |
| TI | +7.1 (−27.0–41.3) | 0.559 | |
| MCGR | −4.8 (−22.9–13.3) | ||
| Transfer | All | −6.8 (−24.5–10.9) | |
| TI | +3.1 (−29.5–35.8) | 0.445 | |
| MCGR | −12.5 (−33.5–8.5) | ||
| Physical function | All | +8.3 (−0.9–17.6) | |
| TI | +10.6 (−4.1–25.4) | 0.694 | |
| MCGR | +6.7 (−5.5–19.0) | ||
| Daily living | All | +23.3 (4.8–41.8) | |
| TI | +28.1 (−4.1–60.3) | 0.757 | |
| MCGR | +21.6 (−1.6–44.7) | ||
| Fatigue/energy level | All | −1.9 (−15.9–12.2) | |
| TI | +8.9 (−16.0–33.8) | 0.300 | |
| MCGR | −7.7 (−24.5–9.1) | ||
| Emotion | All | −0.8 (−17.3–15.6) | |
| TI | +34.4 (7.2–61.5) | 0.030 * | |
| MCGR | −13.6 (−27.8–0.6) | ||
| Parental impact | All | +7.7 (−2.6–18.1) | |
| TI | +15.5 (2.2–28.8) | 0.224 | |
| MCGR | +3.0 (−11.3–17.3) | ||
| Financial impact | All | +15.2 (3.4–27.0) | |
| TI | +2.8 (−10.0–15.5) | 0.070 | |
| MCGR | +23.2 (6.6–39.8) | ||
| Child satisfaction | All | +8.3 (−10.6–27.3) | |
| TI | +12.5 (−19.1–44.1) | 0.787 | |
| MCGR | +6.8 (−17.1–30.7) | ||
| Parent satisfaction | All | +10.0 (−4.8–24.8) | |
| TI | +14.3 (−13.7–42.3) | 0.705 | |
| MCGR | +7.7 (−10.2–25.6) | ||
| Total score | All | +4.9 (−3.3–13.1) | |
| TI | +9.3 (−4.9–23.5) | 0.444 | |
| MCGR | +2.3 (−7.8–12.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckler, N.J.; Sun, M.; Al Nouri, M.; Howard, J.J.; Vaughan, M.; St. Hilaire, T.; Matsumoto, H.; Sponseller, P.D.; Smith, J.T.; Thompson, G.H.; et al. Traditional Growth-Friendly Implants Result in Improved Health-Related Quality of Life in Cerebral Palsy Patients with Early-Onset Scoliosis. J. Pers. Med. 2025, 15, 506. https://doi.org/10.3390/jpm15110506
Buckler NJ, Sun M, Al Nouri M, Howard JJ, Vaughan M, St. Hilaire T, Matsumoto H, Sponseller PD, Smith JT, Thompson GH, et al. Traditional Growth-Friendly Implants Result in Improved Health-Related Quality of Life in Cerebral Palsy Patients with Early-Onset Scoliosis. Journal of Personalized Medicine. 2025; 15(11):506. https://doi.org/10.3390/jpm15110506
Chicago/Turabian StyleBuckler, Nicholas J., Margaret Sun, Mason Al Nouri, Jason J. Howard, Majella Vaughan, Tricia St. Hilaire, Hiroko Matsumoto, Paul D. Sponseller, John T. Smith, George H. Thompson, and et al. 2025. "Traditional Growth-Friendly Implants Result in Improved Health-Related Quality of Life in Cerebral Palsy Patients with Early-Onset Scoliosis" Journal of Personalized Medicine 15, no. 11: 506. https://doi.org/10.3390/jpm15110506
APA StyleBuckler, N. J., Sun, M., Al Nouri, M., Howard, J. J., Vaughan, M., St. Hilaire, T., Matsumoto, H., Sponseller, P. D., Smith, J. T., Thompson, G. H., Pediatric Spine Study Group, & El-Hawary, R. (2025). Traditional Growth-Friendly Implants Result in Improved Health-Related Quality of Life in Cerebral Palsy Patients with Early-Onset Scoliosis. Journal of Personalized Medicine, 15(11), 506. https://doi.org/10.3390/jpm15110506






