Laterocervical Lymph Node Metastases in Papillary Thyroid Carcinoma: Predictive Factors for Recurrence and Oncological Outcome
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysys
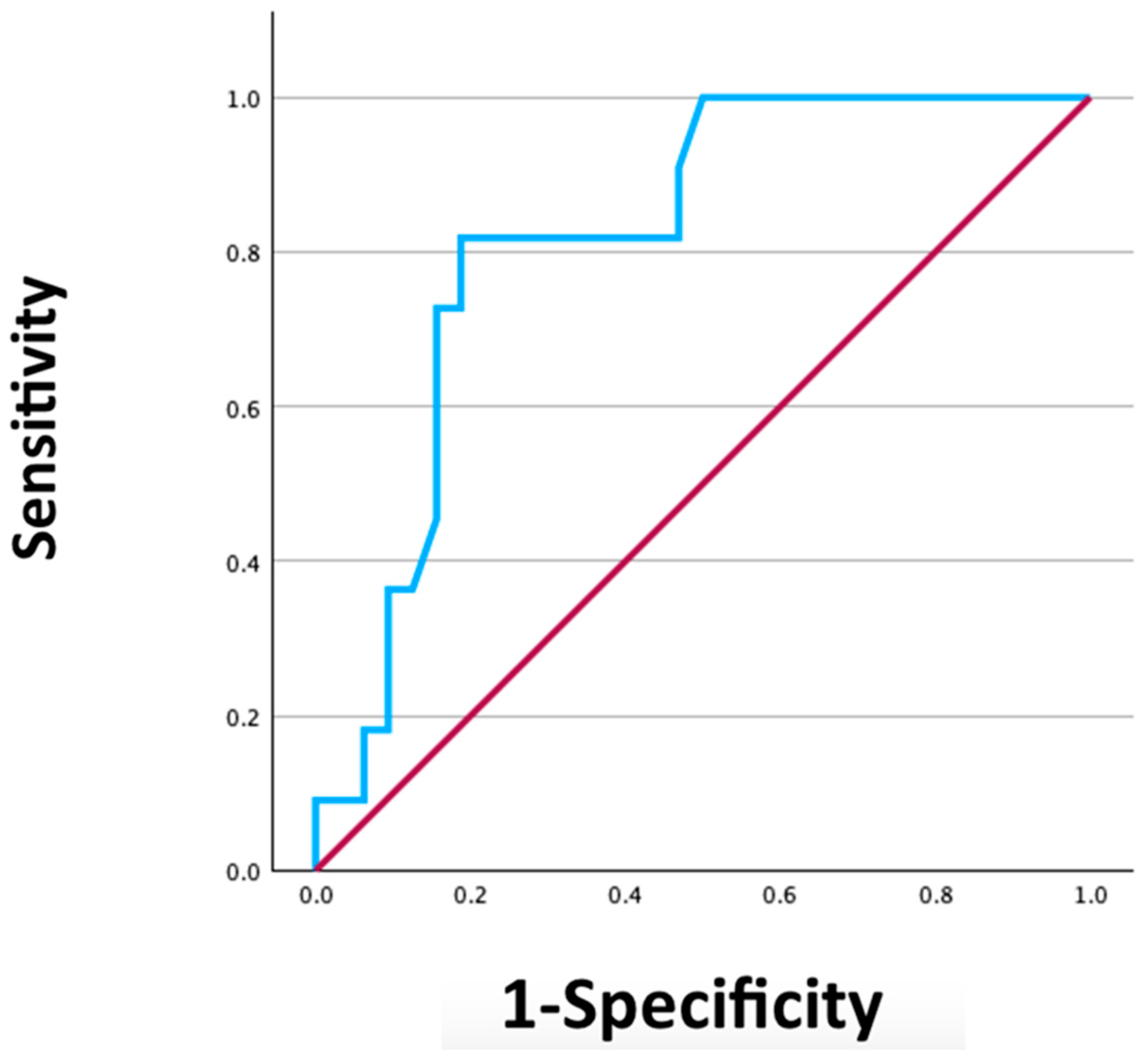
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Islami, F.; Torre, L.A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of gender inequity in thyroid cancer diagnosis: Differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Santini, F.; Corrado, A.; Materazzi, G.; Ulisse, S.; Miccoli, P.; Antonelli, A. Sorafenib and thyroid cancer. BioDrugs 2013, 27, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Aschebrook-Kilfoy, B.; Ward, M.H.; Sabra, M.M.; Devesa, S.S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011, 21, 125–134. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Primers 2015, 1, 15077. [Google Scholar] [CrossRef]
- Ganly, I.; Migliacci, J.C.; Aniss, A.; Sywak, M.; Eskander, A.E.; Freeman, J.L.; Campbell, M.J. Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid 2015, 25, 1106–1114. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, W.G.; Kim, T.H.; Kim, H.K.; Kim, B.H.; Yi, H.S.; Kim, E.S.; Kim, H.; Kim, Y.N.; Kim, E.H.; et al. Disease-specific mortality of differentiated thyroid cancer patients in Korea: A multicenter cohort study. Endocrinol. Metab. 2017, 32, 434–441. [Google Scholar] [CrossRef]
- Lee, C.W.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann. Surg. Oncol. 2015, 22, 117–124. [Google Scholar] [CrossRef]
- Park, S.; Kim, W.G.; Song, E.; Oh, H.S.; Kim, M.; Kwon, H.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Dynamic risk stratification for predicting recurrence in patients with differentiated thyroid cancer treated without radioactive iodine remnant ablation therapy. Thyroid 2016, 26, 64–71. [Google Scholar]
- Leboulleux, S.; Rubino, C.; Baudin, E.; Caillou, B.; Hartl, D.M.; Bidart, J.M.; Travagli, J.P.; Schlumberger, M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid. J. Clin. Endocrinol. Metab. 2005, 90, 5635–5641. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R. TNM classification of thyroid carcinoma. World J. Surg. 2007, 31, 879–887. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Nam, S.H.; Bae, M.R.; Roh, J.L.; Gong, G.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018, 87, 158–164. [Google Scholar] [CrossRef]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann. Thorac. Surg. 2012, 93, 1614–1620. [Google Scholar] [CrossRef]
- Yamashita, K.; Hosoda, K.; Ema, A.; Watanabe, M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur. J. Surg. Oncol. 2016, 42, 1253–1260. [Google Scholar] [CrossRef]
- Sjo, O.H.; Merok, M.A.; Svindland, A.; Nesbakken, A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis. Colon Rectum 2012, 55, 307–315. [Google Scholar] [CrossRef]
- Iocca, O.; Copelli, C.; Campo, F.; Petruzzi, G.; Pellini, R.; Ramieri, G.; Di Maio, P. Lymph node ratio (LNR) and lymph node yield (LNY) in head and neck cancer: A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2024, 53, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Z.; Dong, L.; Zhang, L.; Li, X. The prognostic value of lymph node ratio for thyroid cancer: A meta-analysis. Front. Oncol. 2024, 14, 1333094. [Google Scholar] [CrossRef]
- Chatchomchuan, W.; Thewjitcharoen, Y.; Karndumri, K.; Porramatikul, S.; Krittiyawong, S.; Wanothayaroj, E.; Vongterapak, S.; Butadej, S.; Veerasomboonsin, V.; Kanchanapitak, A.; et al. Recurrence Factors and Characteristic Trends of Papillary Thyroid Cancer over Three Decades. Int. J. Endocrinol. 2021, 2021, 9989757. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Xiao, J.; Shao, C.; Zhang, Y.; Guo, Y.; Chen, X.; Li, J.; Chen, M.; Ma, J. Burden of Thyroid Cancer from 1990 to 2019 and Projections of Incidence and Mortality until 2039 in China: Findings from Global Burden of Disease Study. Front. Endocrinol. 2021, 12, 738213. [Google Scholar] [CrossRef]
- Yuksel, U.M.; Turanli, S.; Acar, Y.; Berberoglu, U. The prognostic factors for clinical N1b patients in thyroid papillary carcinoma. J. Cancer Res. Ther. 2019, 15, 681–685. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, P.; Zhu, L.; Tan, H.; Wei, B.; Li, N.; Tang, N.; Chang, S. Predicting lymph node metastasis in papillary thyroid carcinoma with Hashimoto’s thyroiditis using regression and network analysis. Sci. Rep. 2024, 14, 27585. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Zhang, H.; Zheng, K.; Wang, R.; Wang, G. Risk factors for lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. Front. Endocrinol. 2020, 11, 265. [Google Scholar] [CrossRef]
- Bertin, J.B.; Buffet, C.; Leenhardt, L.; Menegaux, F.; Chereau, N. Effect of skip metastasis to lateral neck lymph nodes on outcome of patients with papillary thyroid carcinoma. Langenbecks Arch. Surg. 2022, 407, 3025–3030. [Google Scholar] [CrossRef]
- Schneider, D.F.; Chen, H.; Sippel, R.S. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2013, 20, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, K.; Park, S.Y.; Choe, J.H.; Kim, J.H.; Kim, J.S.; Oh, Y.L.; Hahn, S.Y.; Shin, J.H.; Ahn, H.S.; et al. Refining the eighth edition AJCC TNM classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral Oncol. 2018, 78, 80–86. [Google Scholar] [CrossRef]
- Hei, H.; Luo, Z.; Zheng, C.; Gong, W.; Zhou, B.; Fang, J.; Qin, J. Lymph node ratio independently associated with postoperative thyroglobulin levels in papillary thyroid cancer. Oral Oncol. 2023, 146, 106563. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.W.; Son, S.H.; Hong, C.M.; Jeong, J.H.; Jeong, S.Y.; Ahn, B.C.; Lee, J. Prognostic value of lymph node uptake on pretreatment F-18 FDG PET/CT in patients with N1b papillary thyroid carcinoma. Endocr. Pract. 2019, 25, 787–793. [Google Scholar] [CrossRef]
- Park, Y.M.; Wang, S.G.; Shin, D.H.; Kim, I.J.; Son, S.M.; Lee, B.J. Lymph node status of lateral neck compartment in patients with N1b papillary thyroid carcinoma. Acta Otolaryngol. 2016, 136, 319–324. [Google Scholar] [CrossRef]
- Kang, I.K.; Kim, K.; Park, J.; Bae, J.S.; Kim, J.S. Central lymph node ratio predicts recurrence in patients with N1b papillary thyroid carcinoma. Cancers 2022, 14, 3677. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.G.; Kim, K.; Yim, S.H.; Ryu, H.; Lee, C.R.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; et al. Clinical value of lymph node ratio integration with the 8th edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci. Rep. 2019, 9, 13361. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, S.K.; Siraj, A.K.; Qadri, Z.; Ahmed, S.O.; DeVera, F.; Al-Sobhi, S.; Al-Dayel, F.; Al-Kuraya, K.S. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr. Connect. 2022, 11, e210518. [Google Scholar] [CrossRef] [PubMed]
| Total Thyroidectomy and Neck Dissection (n = 43) | Total Thyroidectomy (n = 43) | p-Value | ||
|---|---|---|---|---|
| Gender (%) | Female | 28 (32.6%) | 24 (27.9%) | 0.378 |
| Male | 15 (17.4%) | 19 (22.1%) | ||
| Average Age at Surgery (±SD) | 54.8 (±13.5) | 52.3 (±16.1) | 0.218 | |
| Smoking History (%) | Yes | 17 (20.2%) | 10 (11.9%) | 0.102 |
| No | 25 (29.8%) | 32 (38.1%) | ||
| Average BMI (±SD) | 27.6 (±5.9) | 27.7 (±5.8) | 0.197 | |
| Average TSH (±SD) µIU/mL | 2.4 (±1.7) | 2.7 (±1.9) | 0.337 | |
| Average fT3 (±SD) pg/mL | 3.5 (±1.1) | 3.4 (±1.2) | 0.459 | |
| Average fT4 (±SD) pg/mL | 15.5 (±8.8) | 16.7 (3.2) | 0.269 | |
| Average Days of Hospitalization (±SD) | 5.7 (±3.9) | 2.9 (±0.6) | <0.001 | |
| Tumor Focality (%) | Multifocal | 26 (30.6%) | 20 (23.5%) | 0.154 |
| Unifocal | 16 (18.8%) | 23 (27.1%) | ||
| Average Tumor Size (±SD) cm | 14.9 (±9.6) | 10.8 (±6.2) | 0.011 | |
| Margins (%) | Positive | 23 (26.7%) | 21 (24.4%) | 0.666 |
| Negative | 20 (23.3%) | 22 (25.6%) | ||
| Vascular Invasion (%) | Yes | 4 (4.7%) | 1 (1.2%) | 0.360 |
| No | 39 (45.3%) | 42 (48.8%) | ||
| Lymphatic Invasion (%) | Yes | 2 (2.3%) | 0 (0%) | 0.494 |
| No | 41 (47.7%) | 43 (50%) | ||
| Extrathyroid Extension (%) | Yes | 17 (19.8%) | 11 (12.8%) | 0.167 |
| No | 26 (30.2%) | 32 (37.2%) | ||
| I-131 (%) | Yes | 40 (46.5%) | 27 (31.4%) | <0.001 |
| No | 3 (3.5%) | 16 (18.6%) | ||
| Adjuvant Radiotherapy (%) | Yes | 5 (5.8%) | 0 (0%) | 0.055 |
| No | 38 (44.2%) | 43 (50%) | ||
| Recurrence (%) | Yes | 11 (12.8%) | 2 (2.3%) | 0.007 |
| No | 32 (37.2%) | 41 (47.7%) |
| Positive Lymph Node | Negative Lymph Node | p-Value | ||
|---|---|---|---|---|
| Gender (%) | Female | 19 (44.2%) | 9 (20.9%) | 0.127 |
| Male | 14 (32.6%) | 1 (2.3%) | ||
| Average Age at Surgery (±SD) | 53.9 (±14.1) | 57.7 (±11.5) | 0.204 | |
| Smoking History (%) | Yes | 15 (35.7%) | 2 (4.8%) | 0.162 |
| No | 17 (40.5%) | 8 (19%) | ||
| Average BMI (±SD) | 26.7 (±5.9) | 27.9 (±6) | 0.295 | |
| Average TSH (±SD) µIU/mL | 2.4 (±1.8) | 2.4 (±1.6) | 0.484 | |
| Average fT3 (±SD) pg/mL | 3.6 (±1.3) | 3.1 (±0.3) | 0.155 | |
| Average fT4 (±SD) pg/mL | 16.4 (±9.5) | 11.6 (±0.2) | 0.008 | |
| Thyroglobulin (%) | >5000 ng/mL | 10 (38.5%) | 1 (3.8%) | 0.614 |
| <5000 ng/mL | 12 (46.2%) | 3 (11.5%) | ||
| Tumor Focality (%) | Multifocal | 22 (52.4%) | 4 (9.5%) | 0.142 |
| Unifocal | 10 (23.8%) | 6 (14.3%) | ||
| Average Tumor Size (±SD) cm | 16.1 (±10.4) | 11(±4.7) | 0.019 |
| Recurrence (n = 11) | Not Recurrence (n = 32) | Total (n = 43) | p-Value | ||
|---|---|---|---|---|---|
| Gender (%) | Female | 7 (16.3%) | 21 (48.8%) | 28 (65.1%) | 1 |
| Male | 4 (9.3%) | 11 (25.6%) | 15 (34.9%) | ||
| Average Age at Surgery (±SD) | 52.91 (±17.8) | 55.50 (±11.9) | 54.20 years (±13.5) | 0.330 | |
| Smoking History (%) | Yes | 5 (11.9%) | 12 (28.6%) | 17 (40.5%) | 0.733 |
| No | 6 (14.3%) | 19 (45.2%) | 25 (59.5%) | ||
| Average BMI (±SD) | 28.9 (±5.9) | 27.2 (±5.9) | 27.6 (±5.9) | 0.198 | |
| Thyroid Cancer Family History (%) | Yes | 2 (4.8%) | 2 (4.8%) | 4 (9.5%) | 0.277 |
| No | 9 (21.4%) | 29 (69%) | 38 (90.5%) | ||
| Presence of Level VI Lymph Nodes (%) | Yes | 10 (23.8%) | 23 (54.8%) | 33 (78.6%) | 0.403 |
| No | 1 (2.4%) | 8 (19%) | 9 (21.4%) | ||
| Presence of Controlateral level II-V Lymph Nodes (%) | Yes | 4 (9.3%) | 2 (4.7%) | 6 (14%) | 0.029 |
| No | 7 (16.3%) | 30 (69.7%) | 37 (86%) | ||
| Average TSH (±SD) µIU/mL | 2.7 (±1.9) | 2.3 (±1.7) | 2.4 (±1.7) | 0.307 | |
| Average fT3 (±SD) pg/mL | 3.6 (±0.9) | 3.4 (±1.6) | 3.5 (±1.1) | 0.400 | |
| Average fT4 (±SD) pg/mL | 14.7 (±4.6) | 15.7 (±9.8) | 15.5 (±8.8) | 0.356 | |
| Average Anti-TPO (±SD) IU/mL | 59 (±95.9) | 67.9 (±121.4) | 65.8 (±113.1) | 0.442 | |
| Thyroglobulin (%) | >5000 ng/mL | 3 (11.5%) | 8 (30.8%) | 11 (42.3%) | 1 |
| <5000 ng/mL | 3 (11.5%) | 12 (46.2%) | 15 (57.7%) | ||
| Average Days of Hospitalization (±SD) | 7.9 (±5.5) | 5 (±2.9) | 5.7 (±3.9) | 0.060 | |
| Tumor Focality (%) | Multifocal | 8 (19%) | 18 (42.9%) | 26 (61.9%) | 0.270 |
| Unifocal | 2 (4.8%) | 14 (33.3%) | 16 (38.1%) | ||
| Average Tumor Size (±DS) cm | 22.1 (±11.5) | 12.6 (±7.7) | 14.9 (±9.6) | 0.015 | |
| Margins (%) | Positive | 8 (18.6%) | 15 (34.9%) | 23 (53.5%) | 0.138 |
| Negative | 3 (7%) | 17 (39.5%) | 20 (46.5%) | ||
| Vascular Invasion (%) | Yes | 3 (7%) | 1 (2.3%) | 4 (9.3%) | 0.045 |
| No | 8 (18.6%) | 31 (72.1%) | 39 (90.7%) | ||
| Lymphatic Invasion (%) | Yes | 1 (2.3%) | 1 (2.3%) | 2 (4.7%) | 0.451 |
| No | 10 (23.3%) | 31 (72.1%) | 41 (95.3%) | ||
| Perineural Invasion (%) | Yes | 0 (0%) | 1 (2.3%) | 1 (2.3%) | 1 |
| No | 11 (25.6%) | 31 (72.1%) | 42 (97.7%) | ||
| Extrathyroid Extension (%) | Yes | 8 (18.6%) | 9 (20.9%) | 17 (39.5%) | 0.014 |
| No | 3 (7%) | 23 (53.5%) | 26 (60.5%) | ||
| Concomitant Thyroid Disorders (%) | Yes | 4 (9.3%) | 14 (32.6%) | 18 (41.9%) | 0.736 |
| No | 7 (16.3%) | 18 (41.8%) | 25 (58.1%) | ||
| Average Number Lymph Node Involved (±SD) (II–V affected side) | 14.1 (±15.9) | 2.9 (±4.2) | 5.8 (±9.9) | 0.022 | |
| Average LNR (±SD) (II–V affected side) | 0.35 (±0.21) | 0.14 (±0.18) | 0.19 (±0.20) | 0.004 | |
| Average Largest Lymph Node Size (±SD) (II–V affected side) | 34.3 (±25.8) | 20.4 (±11.6) | 25.2 (±18.2) | 0.060 | |
| Extranodal Extension (%) | Yes | 1 (2.4%) | 1 (2.4%) | 2 (4.8%) | 0.424 |
| No | 9 (21.4%) | 31 (73.8%) | 40 (95.2%) | ||
| I-131 (%) | Yes | 10 (23.3%) | 30 (69.7%) | 40 (93%) | 1 |
| No | 1 (2.3%) | 2 (4.7%) | 3 (7%) | ||
| Adjuvant Radiotherapy (%) | Yes | 4 (9.3%) | 1 (2.3%) | 5 (11.6%) | 0.011 |
| No | 7 (16.3%) | 31 (72.1%) | 38 (88.4%) | ||
| Adjuvant Chemotherapy (%) | Si | 1 (2.3%) | 0 (0%) | 1 (2.3%) | 0.256 |
| No | 10 (23.3%) | 32 (74.4%) | 42 (97.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorelli, A.; Manuelli, M.; Tringali, A.M.; Moretti, C.; Corazzi, V.; Geminiani, M.; Ciorba, A.; Stomeo, F.; Pelucchi, S.; Bianchini, C. Laterocervical Lymph Node Metastases in Papillary Thyroid Carcinoma: Predictive Factors for Recurrence and Oncological Outcome. J. Pers. Med. 2025, 15, 496. https://doi.org/10.3390/jpm15100496
Migliorelli A, Manuelli M, Tringali AM, Moretti C, Corazzi V, Geminiani M, Ciorba A, Stomeo F, Pelucchi S, Bianchini C. Laterocervical Lymph Node Metastases in Papillary Thyroid Carcinoma: Predictive Factors for Recurrence and Oncological Outcome. Journal of Personalized Medicine. 2025; 15(10):496. https://doi.org/10.3390/jpm15100496
Chicago/Turabian StyleMigliorelli, Andrea, Marianna Manuelli, Agnese Maria Tringali, Claudio Moretti, Virginia Corazzi, Matteo Geminiani, Andrea Ciorba, Francesco Stomeo, Stefano Pelucchi, and Chiara Bianchini. 2025. "Laterocervical Lymph Node Metastases in Papillary Thyroid Carcinoma: Predictive Factors for Recurrence and Oncological Outcome" Journal of Personalized Medicine 15, no. 10: 496. https://doi.org/10.3390/jpm15100496
APA StyleMigliorelli, A., Manuelli, M., Tringali, A. M., Moretti, C., Corazzi, V., Geminiani, M., Ciorba, A., Stomeo, F., Pelucchi, S., & Bianchini, C. (2025). Laterocervical Lymph Node Metastases in Papillary Thyroid Carcinoma: Predictive Factors for Recurrence and Oncological Outcome. Journal of Personalized Medicine, 15(10), 496. https://doi.org/10.3390/jpm15100496









