A Geometric Morphometrics Approach for Predicting Olfactory Region Accessibility: Toward Personalized Nose-to-Brain Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Sample
2.3. Surface Imaging and Pre-Processing
2.4. Definition of the ROI and Landmarks Digitization
2.5. Shape Alignment and Principal Component Analysis (PCA)
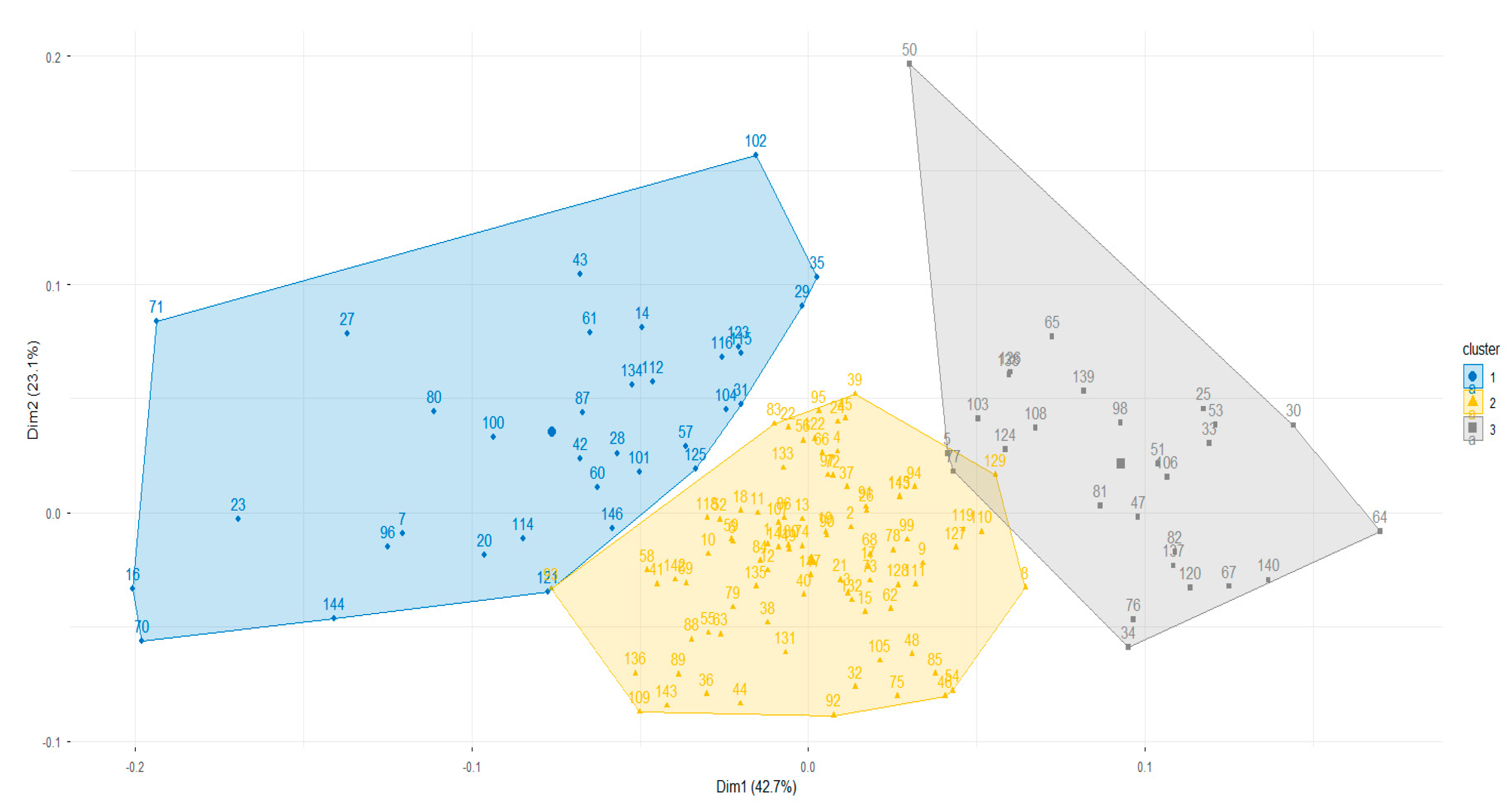
2.6. Cluster Identification
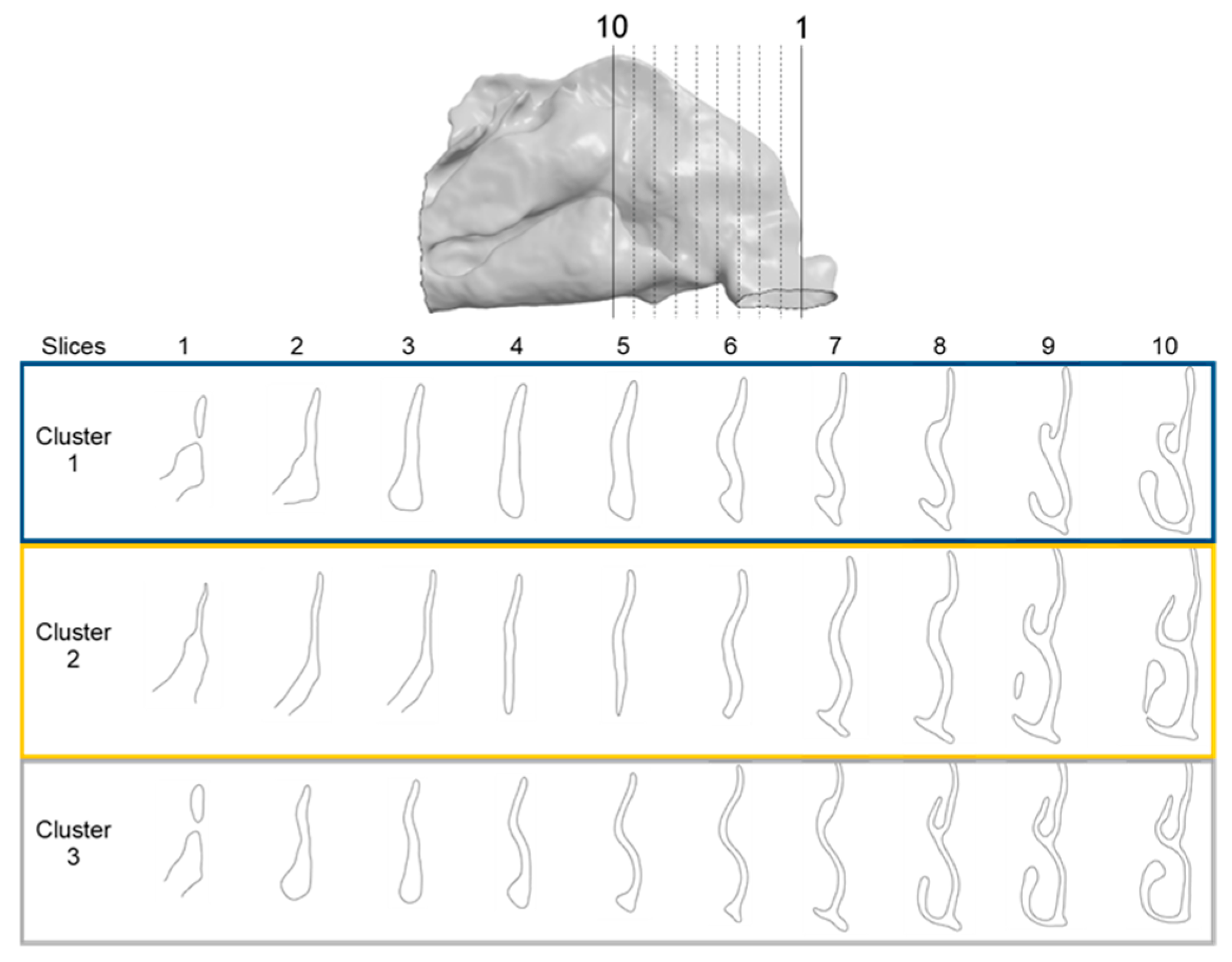
2.7. Cluster Characterization
2.8. Repeatability and Reproducibility Assessment
2.9. Bilateral Dimorphism Assessment
2.10. Optimal Sample Size
3. Results
4. Discussion
4.1. Summary of Key Findings
4.2. Methodological Considerations and Limitations
4.3. Clinical Implications and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Bilateral Dimorphism Assessment
Appendix A.1. Procrustes ANOVA Test on 151 Unilateral Nasal Cavities
| Df | SS | MS | Rsq | F | Z | p | |
|---|---|---|---|---|---|---|---|
| Laterality | 1 | 0.01061 | 0.010613 | 0.00417 | 0.6241 | −1.0364 | 0.844 |
| Residuals | 149 | 2.53374 | 0.017005 | 0.99583 | |||
| Total | 150 | 2.54435 |
Appendix A.2. Sensitivity Analysis Excluding Left Cavities of the Patients with Probe in the Right Side
| Df | SS | MS | Rsq | F | Z | p | |
|---|---|---|---|---|---|---|---|
| Laterality | 1 | 0.0086952 | 0.0035 | 0.506 | 0.0087 | −1.6843 | 0.957 |
| Residuals | 144 | 2.4746 | 0.0171848 | 0.9965 | |||
| Total | 145 | 2.4833 |
Appendix B. Inclusion of Principal Components
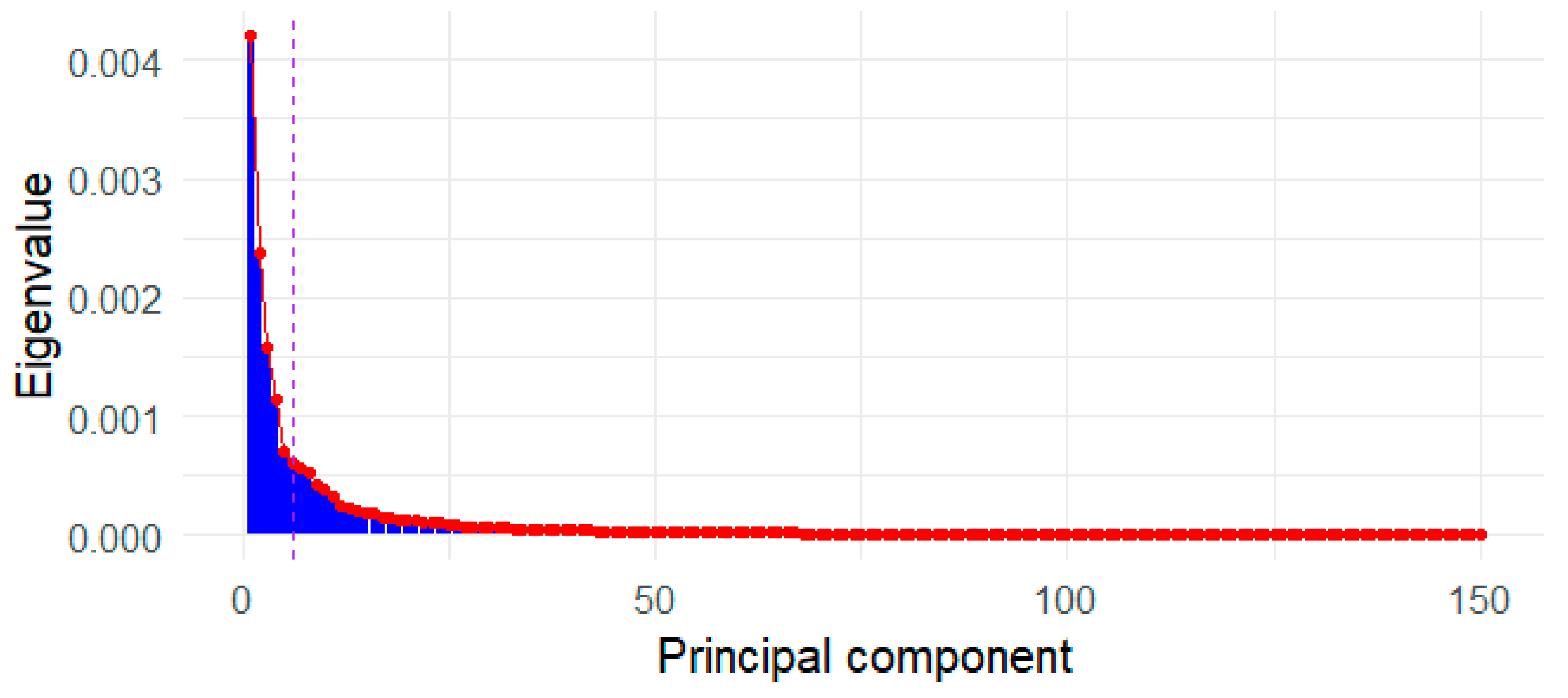
Appendix C. Sample Size Sufficiency for PCA Stability

Appendix D. Validation of the Optimal Number of Clusters
| Index | Optimal Number of Clusters Suggested | Index Value |
|---|---|---|
| KL | 5 | 17.7189 |
| CH | 2 | 43.3387 |
| Hartigan | 15 | 9.1558 |
| CCC | 15 | −2.8463 |
| Scott | 3 | 117.9024 |
| Marriot | 4 | 0.0002 |
| TrCovW | 3 | 0.0207 |
| TraceW | 3 | 0.0768 |
| Friedman | 4 | 2.0838 |
| Rubin | 5 | −0.0796 |
| Cindex | 14 | 0.219 |
| DB | 15 | 1.3075 |
| Silhouette | 3 | 0.2452 |
| Duda | 2 | 1.4595 |
| PseudoT2 | 2 | −38.0942 |
| Beale | 2 | −0.9695 |
| Ratkowsky | 5 | 0.2303 |
| Ball | 3 | 0.2651 |
| PtBiserial | 3 | 0.4999 |
| Frey | 1 | NA |
| McClain | 2 | 0.758 |
| Dunn | 15 | 0.1148 |
| Hubert | 0 | 0 |
| SDindex | 8 | 52.2909 |
| Dindex | 0 | 0 |
| SDbw | 14 | 0.3151 |
| Gap | 2 | 0.0153 |
| Gamma | 15 | 0.79 |
| Gplus | 15 | 84.5262 |
| Tau | 3 | 1769.315 |
References
- Holton, N.E.; Alsamawi, A.; Yokley, T.R.; Froehle, A.W. The Ontogeny of Nasal Shape: An Analysis of Sexual Dimorphism in a Longitudinal Sample. Am. J. Phys. Anthropol. 2016, 160, 52–61. [Google Scholar] [CrossRef]
- Russel, S.M.; Frank-Ito, D.O. Gender Differences in Nasal Anatomy and Function Among Caucasians. Facial Plast. Surg. Aesthet. Med. 2023, 25, 145–152. [Google Scholar] [CrossRef]
- Samoliński, B.K.; Grzanka, A.; Gotlib, T. Changes in Nasal Cavity Dimensions in Children and Adults by Gender and Age. Laryngoscope 2007, 117, 1429–1433. [Google Scholar] [CrossRef]
- Butaric, L.N.; Klocke, R.P. Nasal Variation in Relation to High-Altitude Adaptations among Tibetans and Andeans. Am. J. Hum. Biol. 2018, 30, e23104. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Frank-Ito, D.O. The Role of Normal Nasal Morphological Variations from Race and Gender Differences on Respiratory Physiology. Respir. Physiol. Neurobiol. 2022, 297, 103823. [Google Scholar] [CrossRef]
- Yokley, T.R. Ecogeographic Variation in Human Nasal Passages. Am. J. Phys. Anthropol. 2009, 138, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Evteev, A.A.; Grosheva, A.N. Nasal Cavity and Maxillary Sinuses Form Variation among Modern Humans of Asian Descent. Am. J. Phys. Anthropol. 2019, 169, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.A.; Mattern, B.C.; Claes, P.; McEcoy, B.; Hughes, C.; Shriver, M.D. Investigating the Case of Human Nose Shape and Climate Adaptation. PLoS Genet. 2017, 13, e1006616. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Cheng, Y.-S.; Yeh, H.-C.; Guilmette, R.A.; Simpson, S.Q.; Yang, Y.-H.; Swift, D.L. In Vivo Measurements of Nasal Airway Dimensions and Ultrafine Aerosol Deposition in the Human Nasal and Oral Airways. J. Aerosol Sci. 1996, 27, 785–801. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Interindividual Variability in Nasal Filtration as a Function of Nasal Cavity Geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 139–156. [Google Scholar] [CrossRef]
- Chari, S.; Sridhar, K.; Kleinstreuer, C. Effects of Subject-Variability on Nasally Inhaled Drug Deposition, Uptake, and Clearance. J. Aerosol Sci. 2022, 165, 106021. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Tian, L.; Tu, J.; Corley, R.; Kuprat, A.P.; Dong, J. Investigation of Inter-Subject Variation in Ultrafine Particle Deposition across Human Nasal Airways: A Study Involving Children, Adults, and the Elderly. Sci. Total Environ. 2024, 955, 177028. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Prabakaran, A.; Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-Brain Drug Delivery for the Treatment of Alzheimer’s Disease: Current Advancements and Challenges. Expert. Opin. Drug Deliv. 2022, 19, 87–102. [Google Scholar] [CrossRef]
- Raghav, M.; Gupta, V.; Awasthi, R.; Singh, A.; Kulkarni, G.T. Nose-to-Brain Drug Delivery: Challenges and Progress towards Brain Targeting in the Treatment of Neurological Disorders. J. Drug Deliv. Sci. Technol. 2023, 86, 104756. [Google Scholar] [CrossRef]
- Calmet, H.; Kleinstreuer, C.; Houzeaux, G.; Kolanjiyil, A.V.; Lehmkuhl, O.; Olivares, E.; Vázquez, M. Subject-Variability Effects on Micron Particle Deposition in Human Nasal Cavities. J. Aerosol Sci. 2018, 115, 12–28. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, M.R.; Matida, E.A.; Kherani, S.; Marsan, J. Creation of a Standardized Geometry of the Human Nasal Cavity. J. Appl. Physiol. 2009, 106, 784–795. [Google Scholar] [CrossRef]
- Manniello, M.D.; Hosseini, S.; Alfaifi, A.; Esmaeili, A.R.; Kolanjiyil, A.V.; Walenga, R.; Babiskin, A.; Sandell, D.; Mohammadi, R.; Schuman, T.; et al. In Vitro Evaluation of Regional Nasal Drug Delivery Using Multiple Anatomical Nasal Replicas of Adult Human Subjects and Two Nasal Sprays. Int. J. Pharm. 2021, 593, 120103. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, A.; Hosseini, S.; Esmaeili, A.R.; Walenga, R.; Babiskin, A.; Schuman, T.; Longest, W.; Hindle, M.; Golshahi, L. Anatomically Realistic Nasal Replicas Capturing the Range of Nasal Spray Drug Delivery in Adults. Int. J. Pharm. 2022, 622, 121858. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.R.; Wilkins, J.V.; Hosseini, S.; Alfaifi, A.; Hejazi, M.; Hindle, M.; Longest, W.; Schuman, T.; Dhapare, S.; Kaviratna, A.; et al. In Vitro Evaluation of Intersubject Variability in Pediatric Intranasal Drug Delivery Using Nasal Spray Suspension Products. J. Aerosol Sci. 2024, 179, 106387. [Google Scholar] [CrossRef]
- Pasteur, M.; Arsouze, G.; Ilango, G.; Le Pennec, D.; Kulker, D.; Heyraud, A.; Cottier, J.-P.; Aussedat, C.; Heuzé-Vourc’h, N.; Hervé, V.; et al. Characterization of Anatomical Variations of the Nasal Cavity in a Subset of European Patients and Their Impact on Intranasal Drug Delivery. Int. J. Pharm. 2024, 667, 124851. [Google Scholar] [CrossRef]
- Bookstein, F.L. Landmark Methods for Forms without Landmarks: Morphometrics of Group Differences in Outline Shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A Method for Quantifying Curves and Surfaces. Hystrix It. J. Mamm. 2013, 24, 103–109. [Google Scholar] [CrossRef]
- Agbolade, O.; Nazri, A.; Yaakob, R.; Ghani, A.A.; Cheah, Y.K. Morphometric Approach to 3D Soft-Tissue Craniofacial Analysis and Classification of Ethnicity, Sex, and Age. PLoS ONE 2020, 15, e0228402. [Google Scholar] [CrossRef]
- Perez, S.I.; Bernal, V.; Gonzalez, P.N. Differences between Sliding Semi-Landmark Methods in Geometric Morphometrics, with an Application to Human Craniofacial and Dental Variation. J. Anat. 2006, 208, 769–784. [Google Scholar] [CrossRef] [PubMed]
- The R Core Team. R: A Language and Environment for Statistical Computing, version 4.4.3; Core Team R: Vienna, Austria, 2025. [Google Scholar]
- Miolane, N.; Holmes, S.; Pennec, X. Template Shape Estimation: Correcting an Asymptotic Bias. SIAM J. Imaging Sci. 2017, 10, 808–844. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, Shape, and Form: Concepts of Allometry in Geometric Morphometrics. Dev. Genes. Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Zhang, C.; Porto, A.; Rolfe, S.; Kocatulum, A.; Maga, A.M. Automated Landmarking via Multiple Templates. PLoS ONE 2022, 17, e0278035. [Google Scholar] [CrossRef]
- Rohlf, F.J. Bias and Error in Estimates of Mean Shape in Geometric Morphometrics. J. Hum. Evol. 2003, 44, 665–683. [Google Scholar] [CrossRef]
- Eccles, R. Nasal Airflow in Health and Disease. Acta Otolaryngol. 2000, 120, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Mygind, N.; Dahl, R. Anatomy, Physiology and Function of the Nasal Cavities in Health and Disease. Adv. Drug Deliv. Rev. 1998, 29, 3–12. [Google Scholar] [CrossRef] [PubMed]

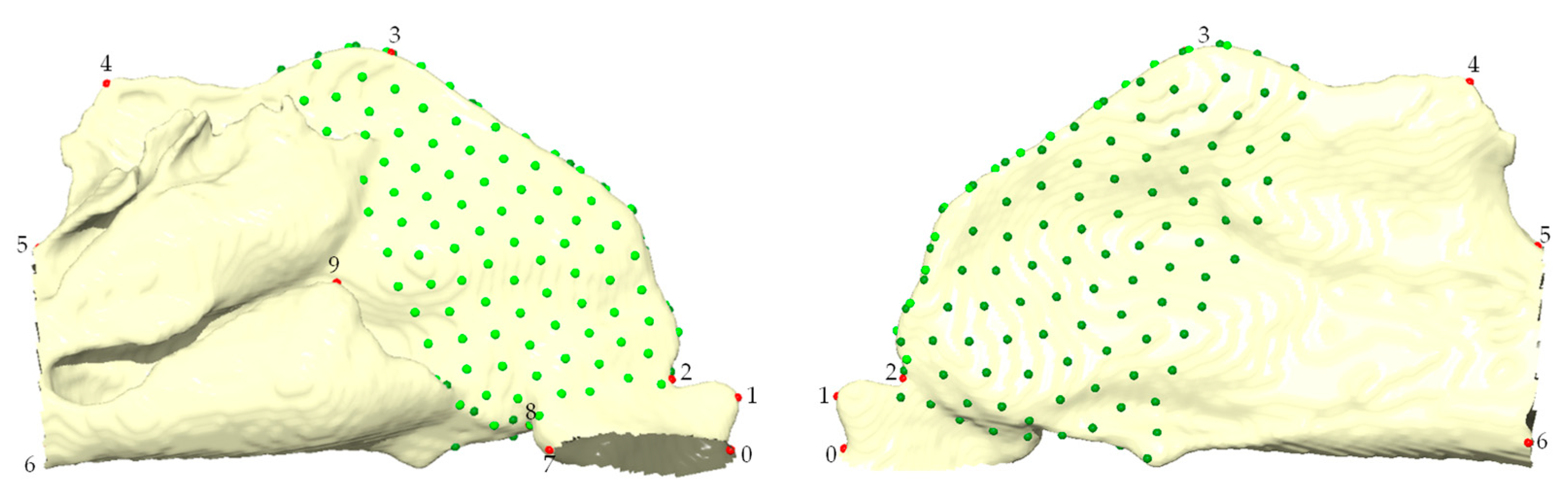
| Landmarks | Definition |
|---|---|
| 0 | Most anterior maximum at the angle between the nostril cutting plane and the front of the nasal cavity. |
| 1 | Most anterior maximum of the vestibule. |
| 2 | Highest point of the nasal valve, corresponding to the narrowest superior point between the vestibule and the nasal fossa. |
| 3 | Highest point of the nasal cavity, which is located at the front of the olfactory region. |
| 4 | Highest point of the nasal cavity, which is located at the back of the olfactory region. |
| 5 | Highest point of the choana, which is not aligned with the extension of the turbinate. |
| 6 | Lowest point of the nasal cavity, which is positioned closest to the nasal septum. |
| 7 | Most posterior maximum on the nostril cutting plane |
| 8 | Narrowest inferior point of the nasal valve |
| 9 | Highest anterior point of the inferior meatus |
| Landmarks | Repeatability | Reproducibility |
|---|---|---|
| Fixed | 0.934 | 0.877 |
| Sliding | 0.992 | 0.977 |
| Variable | Cluster 1 | Cluster 2 | Cluster 3 | p-Value |
|---|---|---|---|---|
| Number of unilateral cavities (N (%)) | 34 (22.5) | 89 (58.9) | 28 (18.5) | - |
| Side (Right:Left) | 17:17 | 44:45 | 12:16 | 0.812 a |
| Sex (Female:Male) | 15:19 | 47:41 | 19:8 | 0.119 a |
| Age (Mean ± standard deviation) | 51.7 ± 20.4 | 54.5 ± 20.1 | 53.2 ± 17.4 | 0.678 b |
| Axe | A = Cluster 2 B = Cluster 1 | A = Cluster 3 B = Cluster 1 | A = Cluster 3 B = Cluster 2 |
|---|---|---|---|
| X | 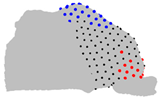 | 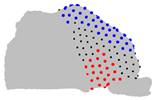 | 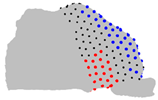 |
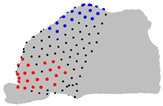 |  | 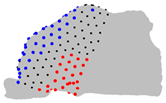 | |
| Y | 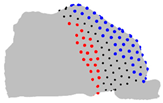 |  |  |
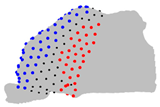 |  | 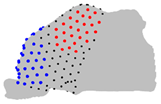 | |
| Z | 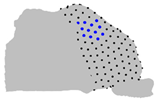 | 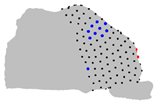 | 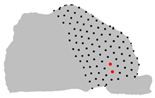 |
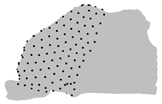 |  |  |
| Pair of Clusters | Number of Patients | % of Total Patients |
|---|---|---|
| 1-1 | 11 | 15.1% |
| 1-2 | 10 | 13.7% |
| 1-3 | 2 | 2.7% |
| 2-2 | 33 | 45.2% |
| 2-3 | 8 | 11.0% |
| 3-3 | 9 | 12.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnumurthy, P.; Radulesco, T.; Bouchet, G.; Regard, A.; Michel, J. A Geometric Morphometrics Approach for Predicting Olfactory Region Accessibility: Toward Personalized Nose-to-Brain Drug Delivery. J. Pers. Med. 2025, 15, 461. https://doi.org/10.3390/jpm15100461
Vishnumurthy P, Radulesco T, Bouchet G, Regard A, Michel J. A Geometric Morphometrics Approach for Predicting Olfactory Region Accessibility: Toward Personalized Nose-to-Brain Drug Delivery. Journal of Personalized Medicine. 2025; 15(10):461. https://doi.org/10.3390/jpm15100461
Chicago/Turabian StyleVishnumurthy, Priya, Thomas Radulesco, Gilles Bouchet, Alain Regard, and Justin Michel. 2025. "A Geometric Morphometrics Approach for Predicting Olfactory Region Accessibility: Toward Personalized Nose-to-Brain Drug Delivery" Journal of Personalized Medicine 15, no. 10: 461. https://doi.org/10.3390/jpm15100461
APA StyleVishnumurthy, P., Radulesco, T., Bouchet, G., Regard, A., & Michel, J. (2025). A Geometric Morphometrics Approach for Predicting Olfactory Region Accessibility: Toward Personalized Nose-to-Brain Drug Delivery. Journal of Personalized Medicine, 15(10), 461. https://doi.org/10.3390/jpm15100461





