Computational Fluid Dynamics Approach for Direct Nose-to-Brain Drug Delivery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
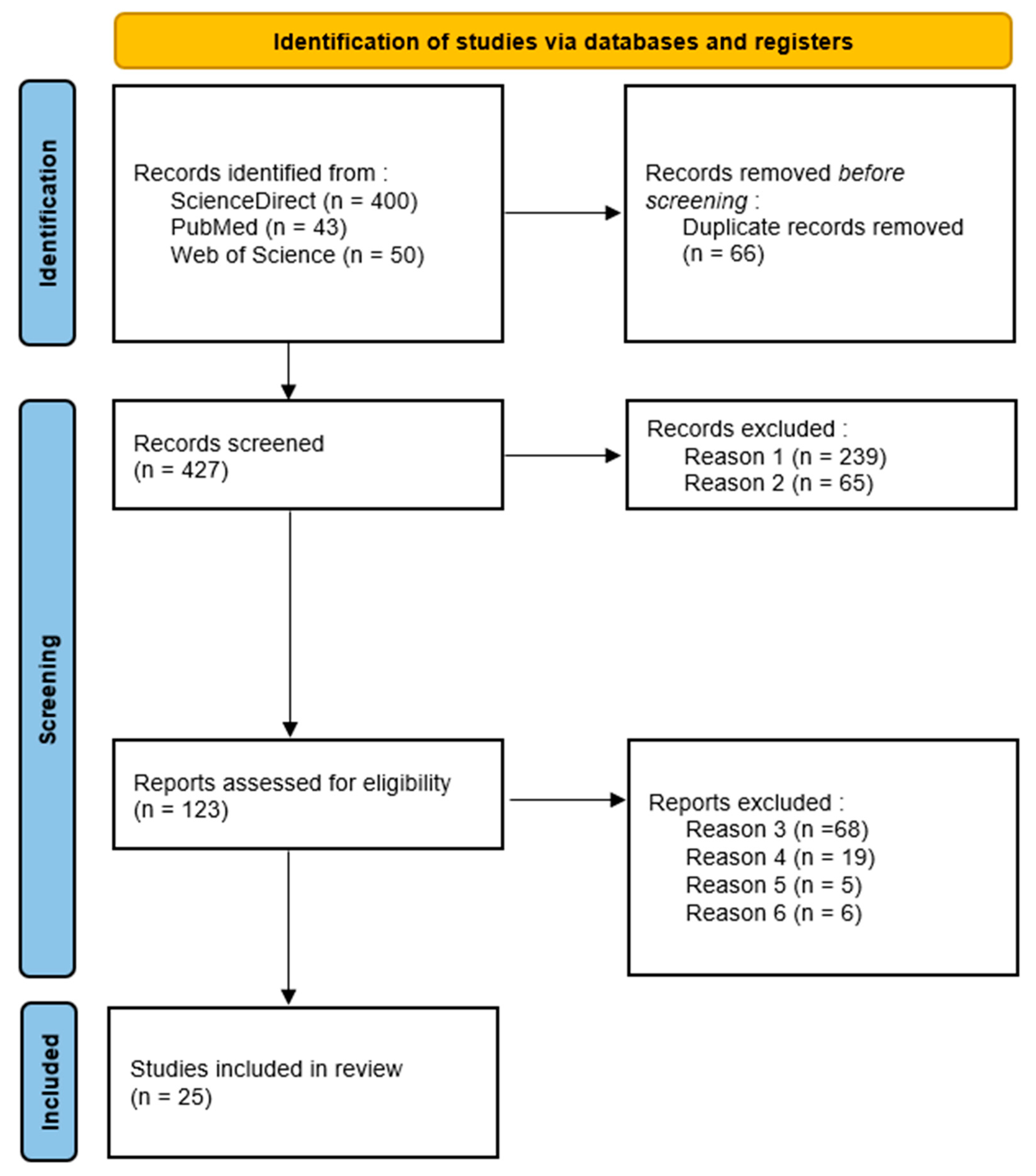
3.1. Study Selection
3.2. Descriptive Findings
3.3. Meta-Analysis
3.3.1. Patient-Dependent Parameters
- •
- Breathing flow rate
- •
- Breathing pattern
- •
- Head tilt position.
3.3.2. Device Dependent Parameters
- •
- Monodispersed particle size
- •
- Injection velocity and spray cone angle
3.3.3. Patient–Device Interaction Parameters
- •
- Impaction parameter
- •
- Release position
- •
- Sagittal injection angle
4. Discussion
4.1. Summary of Key Findings
4.2. Interpretation in Context of Literature
4.3. Source of Heterogeneity
4.4. Potential Publication Bias
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Data | With Outlier Study | Without Outlier Study |
|---|---|---|
| Random-Effect Model | ||
| Estimate | 0.0607 | 0.00145 |
| SE | 0.0487 | 0.0331 |
| Z | 1.25 | 0.0440 |
| p | 0.212 | 0.965 |
| CI Lower Bound | −0.035 | −0.063 |
| CI Upper Bound | 0.156 | 0.066 |
| Heterogeneity Statistics | ||
| Tau | 0.105 | 0.050 |
| Tau2 | 0.0111 (SE = 0.0081) | 0.0025 (SE = 0.0036) |
| I2 | 60.93% | 27.69% |
| Q | 30.614 | 14.386 |
| p | <0.001 | 0.109 |
| Publication Bias assessment | ||
| Begg and Mazumdar Rank Correlation | p = 1.000 | p = 0.727 |
| Egger’s Regression | p = 0.085 | p = 0.227 |
References
- Chaib, F.; Brunier, A. Le Nombre de Personnes Atteintes de Démence Devrait Tripler au Cours des 30 Prochaines Années. Available online: https://www.who.int/fr/news/item/07-12-2017-dementia-number-of-people-affected-to-triple-in-next-30-years (accessed on 17 March 2023).
- Ahmad, J.; Haider, N.; Khan, M.A.; Md, S.; Alhakamy, N.A.; Ghoneim, M.M.; Alshehri, S.; Sarim Imam, S.; Ahmad, M.Z.; Mishra, A. Novel Therapeutic Interventions for Combating Parkinson’s Disease and Prospects of Nose-to-Brain Drug Delivery. Biochem. Pharmacol. 2022, 195, 114849. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Prabakaran, A.; Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-Brain Drug Delivery for the Treatment of Alzheimer’s Disease: Current Advancements and Challenges. Expert Opin. Drug Deliv. 2022, 19, 87–102. [Google Scholar] [CrossRef]
- Kakad, S.; Kshirsagar, S. Nose to Brain Delivery of Efavirenz Nanosuspension for Effective Neuro AIDS Therapy: In-Vitro, In-Vivo and Pharmacokinetic Assessment. Heliyon 2021, 7, e08368. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Golshahi, L. An in Vitro Evaluation of Importance of Airway Anatomy in Sub-Regional Nasal and Paranasal Drug Delivery with Nebulizers Using Three Different Anatomical Nasal Airway Replicas of 2-, 5- and 50-Year Old Human Subjects. Int. J. Pharm. 2019, 563, 426–436. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13, 1079. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Walenga, R.; Babiskin, A.; Golshahi, L.; Hindle, M.; Longest, W. Establishing Quantitative Relationships between Changes in Nasal Spray in Vitro Metrics and Drug Delivery to the Posterior Nasal Region. Int. J. Pharm. 2023, 635, 122718. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Si, X.A.; Zhou, Y. Nasal Dilation Effects on Olfactory Deposition in Unilateral and Bi-Directional Deliveries: In Vitro Tests and Numerical Modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Bahmanzadeh, H.; Abouali, O.; Faramarzi, M.; Ahmadi, G. Numerical Simulation of Airflow and Micro-Particle Deposition in Human Nasal Airway Pre- and Post-Virtual Sphenoidotomy Surgery. Comput. Biol. Med. 2015, 61, 8–18. [Google Scholar] [CrossRef]
- Chari, S.; Sridhar, K.; Walenga, R.; Kleinstreuer, C. Computational Analysis of a 3D Mucociliary Clearance Model Predicting Nasal Drug Uptake. J. Aerosol Sci. 2021, 155, 105757. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Schroeter, J.D.; Kimbell, J.S. Olfactory Deposition of Inhaled Nanoparticles in Humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Calmet, H.; Kleinstreuer, C.; Houzeaux, G.; Kolanjiyil, A.V.; Lehmkuhl, O.; Olivares, E.; Vázquez, M. Subject-Variability Effects on Micron Particle Deposition in Human Nasal Cavities. J. Aerosol Sci. 2018, 115, 12–28. [Google Scholar] [CrossRef]
- Hazeri, M.; Faramarzi, M.; Sadrizadeh, S.; Ahmadi, G.; Abouali, O. Regional Deposition of the Allergens and Micro-Aerosols in the Healthy Human Nasal Airways. J. Aerosol Sci. 2021, 152, 105700. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.L.; Oh, I.-S.; Hayes, T.L. Fixed- versus Random-Effects Models in Meta-Analysis: Model Properties and an Empirical Comparison of Differences in Results. Br. J. Math. Stat. Psychol. 2009, 62, 97–128. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J .Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Hunter, J.E.; Schmidt, F.L. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings; Sage Publications, Inc.: Thousand Oaks, CA, US, 1990; p. 592. ISBN 978-0-8039-3222-7. [Google Scholar]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 2000, 42, 65–68. [Google Scholar] [CrossRef]
- Aguinis, H.; Gottfredson, R.K.; Joo, H. Best-Practice Recommendations for Defining, Identifying, and Handling Outliers. Organ. Res. Methods 2013, 16, 270–301. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi, Version 2.3; Computer Software. 2022. Available online: https://www.jamovi.org (accessed on 17 March 2023).
- Calmet, H.; Dosimont, D.; Oks, D.; Houzeaux, G.; Almirall, B.V.; Inthavong, K. Machine Learning and Sensitivity Analysis for Predicting Nasal Drug Delivery for Targeted Deposition. Int. J. Pharm. 2023, 642, 123098. [Google Scholar] [CrossRef] [PubMed]
- Calmet, H.; Houzeaux, G.; Vázquez, M.; Eguzkitza, B.; Gambaruto, A.M.; Bates, A.J.; Doorly, D.J. Flow Features and Micro-Particle Deposition in a Human Respiratory System during Sniffing. J. Aerosol Sci. 2018, 123, 171–184. [Google Scholar] [CrossRef]
- Chiang, H.; Martin, H.L.; Sicard, R.M.; Frank-Ito, D.O. Olfactory Drug Delivery with Intranasal Sprays after Nasal Midvault Reconstruction. Int. J. Pharm. 2023, 644, 123341. [Google Scholar] [CrossRef]
- Dong, J.; Ma, J.; Shang, Y.; Inthavong, K.; Qiu, D.; Tu, J.; Frank-Ito, D. Detailed Nanoparticle Exposure Analysis among Human Nasal Cavities with Distinct Vestibule Phenotypes. J. Aerosol Sci. 2018, 121, 54–65. [Google Scholar] [CrossRef]
- Dong, J.; Shang, Y.; Inthavong, K.; Chan, H.-K.; Tu, J. Partitioning of Dispersed Nanoparticles in a Realistic Nasal Passage for Targeted Drug Delivery. Int. J. Pharm. 2018, 543, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, L.; Röhm, M.; Mavoungou, C.; Schindowski, K.; Schafmeister, A.; Simon, U. First Steps to Develop and Validate a CFPD Model in Order to Support the Design of Nose-to-Brain Delivered Biopharmaceuticals. Pharm. Res. 2016, 33, 1337–1350. [Google Scholar] [CrossRef]
- Farnoud, A.; Tofighian, H.; Baumann, I.; Ahookhosh, K.; Pourmehran, O.; Cui, X.; Heuveline, V.; Song, C.; Vreugde, S.; Wormald, P.-J.; et al. Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs. Pharmaceuticals 2023, 16, 81. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, L.; Li, T.; Li, C.; Zhang, Y.; Ren, H.; Zheng, Q.; Tong, Z.; Yu, A. A New Exhalation-Assisted Aerosol Delivery Method for Nasal Administration. Powder Technol. 2023, 427, 118708. [Google Scholar] [CrossRef]
- Hayati, H.; Feng, Y.; Chen, X.; Kolewe, E.; Fromen, C. Prediction of Transport, Deposition, and Resultant Immune Response of Nasal Spray Vaccine Droplets Using a CFPD-HCD Model in a 6-Year-Old Upper Airway Geometry to Potentially Prevent COVID-19. Exp. Comput. Multiph. Flow 2023, 5, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ma, R.; Wang, Y.; Lou, M.; Gong, M.; Wang, B.; Zheng, G.; Dong, J.; Zhang, Y. Quantitative Study of Artemisia Pollens Deposition in the Upper Airways of Children with Adenoidal Hypertrophy. J. Aerosol Sci. 2023, 172, 106191. [Google Scholar] [CrossRef]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. Regional Deposition of Nasal Sprays in Adults: A Wide Ranging Computational Study. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2968. [Google Scholar] [CrossRef]
- Li, B.; Feng, Y. In Silico Study to Enhance Delivery Efficiency of Charged Nanoscale Nasal Spray Aerosols to the Olfactory Region Using External Magnetic Fields. Bioengineering 2022, 9, 40. [Google Scholar] [CrossRef]
- Ren, H.X.; Zhang, L.X.; Guo, G.; Tong, Z.B.; Li, Z.Y.; Zhang, Y.; Yu, A.B. Numerical Simulation Investigation of Drug Deposition Process during Nasal Administration with Auxiliary Airflow. Powder Technol. 2023, 426, 118534. [Google Scholar] [CrossRef]
- Shen, Z.; Dong, J.; Milton-McGurk, L.; Cai, X.; Gholizadeh, H.; Chan, H.-K.; Lee, A.; Kourmatzis, A.; Cheng, S. Numerical Analysis of Airflow and Particle Deposition in Multi-Fidelity Designs of Nasal Replicas Following Nasal Administration. Comput. Methods Programs Biomed. 2023, 241, 107778. [Google Scholar] [CrossRef]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Modeling of Inertial Particle Transport and Deposition in Human Nasal Cavities with Wall Roughness. J. Aerosol Sci. 2007, 38, 398–419. [Google Scholar] [CrossRef]
- Shrestha, K.; Salati, H.; Fletcher, D.; Singh, N.; Inthavong, K. Effects of Head Tilt on Squeeze-Bottle Nasal Irrigation—A Computational Fluid Dynamics Study. J. Biomech. 2021, 123, 110490. [Google Scholar] [CrossRef] [PubMed]
- Si, X.A.; Xi, J.; Kim, J.; Zhou, Y.; Zhong, H. Modeling of Release Position and Ventilation Effects on Olfactory Aerosol Drug Delivery. Respir. Physiol. Neurobiol. 2013, 186, 22–32. [Google Scholar] [CrossRef]
- Si, X.A.; Sami, M.; Xi, J. Liquid Film Translocation Significantly Enhances Nasal Spray Delivery to Olfactory Region: A Numerical Simulation Study. Pharmaceutics 2021, 13, 903. [Google Scholar] [CrossRef]
- Tian, L.; Shang, Y.; Chen, R.; Bai, R.; Chen, C.; Inthavong, K.; Tu, J. Correlation of Regional Deposition Dosage for Inhaled Nanoparticles in Human and Rat Olfactory. Part. Fibre Toxicol. 2019, 16, 6. [Google Scholar] [CrossRef]
- Vachhani, S.; Kleinstreuer, C. Comparison of Micron- and Nano-Particle Transport in the Human Nasal Cavity with a Focus on the Olfactory Region. Comput. Biol. Med. 2021, 128, 104103. [Google Scholar] [CrossRef] [PubMed]
- Vachhani, S.; Kleinstreuer, C. Numerical Analysis of Enhanced Nano-Drug Delivery to the Olfactory Bulb. Aerosol Sci. Technol. 2021, 55, 1343–1358. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.E.; Zhang, Y.; Nevorski, D.; Wang, Z.; Zhou, Y. Visualization and Quantification of Nasal and Olfactory Deposition in a Sectional Adult Nasal Airway Cast. Pharm. Res. 2016, 33, 1527–1541. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Wang, Y.; Lou, M.; Ma, R.; Gong, M.; Dong, J.; Zheng, G.; Wang, B. Numerical Investigation of Nanoparticle Deposition in the Olfactory Region among Pediatric Nasal Airways with Adenoid Hypertrophy. Comput. Biol. Med. 2023, 167, 107587. [Google Scholar] [CrossRef]
- Inthavong, K.; Das, P.; Singh, N.; Sznitman, J. In Silico Approaches to Respiratory Nasal Flows: A Review. J. Biomech. 2019, 97, 109434. [Google Scholar] [CrossRef]

| Study ID | Source Selected | Sample Size | 3D Model | Mean Age of the Sample | Male Ratio of the Sample | Olfactory Surface (% of the Total Nasal Cavity) | Numerical Model Selected | Device Simulated | A | B | C | D | E | F | G | H | I | Maximum Olfactory Deposition (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CT | 1 | Realistic | 48 | 1 | NA | Lagrangian | Nasal spray | x * | x * | x * | x * | 1.60 | [29] | |||||
| 2 | CT | 1 | Realistic | 48 | 1 | NA | Lagrangian | Inhaled particles | x * | 2.5 | [30] | ||||||||
| 3 | 1 MRI + 2 CT | 3 | 2 realistic and 1 standardized | 44.43 | 1 | NA | Lagrangian | Inhaled particles | x * | x * | 1.33 | [17] | |||||||
| 4 | CT | 6 | Realistic and operated | NA | NA | NA | Euler– Lagrange | Nasal spray | x * | x * | x * | x * | 36.33 | [31] | |||||
| 5 | CT | 3 | Realistic | 62 | 1/3 | 3–5.9 | Lagrangian | Inhaled particles | x * | + * | 1.4 | [32] | |||||||
| 6 | CT | 1 | Realistic | 54 | 0 | NA | Lagrangian | Inhaled particles | x * | x | x | 3.1 | [33] | ||||||
| 7 | CT | 1 | Standardized | 45.3 | 13/30 | 8 | Euler– Lagrange | Vibrating mesh nebulizer | x * | x * | x * | + * | 8.5 | [34] | |||||
| 8 | CT | 1 | Realistic | 80 | NA | NA | Lagrangian | Inhaled particles | x * | x * | + * | 18.45 | [35] | ||||||
| 9 | CT | 1 | Realistic | 59 | 0 | NA | Lagrangian | EDM | x | x | x | 0 | [36] | ||||||
| 10 | CT | 1 | Realistic | 6 | 0 | 3.8 | Euler– Lagrange | Nasal spray | x | x | <6 | [37] | |||||||
| 11 S | CT | 30 | Realistic | 46.5 | 1/2 | 2.1–3.2 | Lagrangian | Inhaled particles | x * | x * | x * | 1.39 | [18] | ||||||
| 11 M | CT | 30 | Realistic | 46.5 | 1/2 | 4.3–6.4 | Lagrangian | Inhaled particles | x * | x * | x * | 5.94 | [18] | ||||||
| 11 L | CT | 30 | Realistic | 46.5 | 1/2 | 8.6–12.8 | Lagrangian | Inhaled particles | x * | x * | x * | 6.28 | [18] | ||||||
| 12 | CT | 12 | Realistic | 5 | 1/2 | NA | Lagrangian | Inhaled particles | x | 0.46 | [38] | ||||||||
| 13 | CT | 7 | Realistic | 60 | 5/7 | 2.8 | Lagrangian | Nasal spray | x | x | x | <25 | [39] | ||||||
| 14 | MRI | 1 | Realistic | 53 | 1 | NA | Euler– Lagrange | Nasal spray | x | x | x | x | <4 | [40] | |||||
| 15 | CT | 1 | Realistic | NA | NA | NA | Discrete phase model | Nasal spray | x | x | x | x | x | <1 | [41] | ||||
| 16 | MRI | 3 | Realistic and modification | 28 | 1 | NA | Discrete phase model | Inhaled particles | x | <5 | [42] | ||||||||
| 17 | MRI | 1 | Realistic | 53 | 1 | NA | Euler– Lagrange | Inhaled particles | x * | x | x * | x * | 30.8 | [43] | |||||
| 18 | CT | 1 | Realistic | 25 | 0 | NA | VOF | Squeeze-bottle nasal irrigation | x | - | [44] | ||||||||
| 19 | MRI | 1 | Realistic | 53 | 1 | 8 | Lagrangian | Deep or vestibular intubation | x * | x * | x | + * | 1.09 | [45] | |||||
| 20 | MRI | 1 | Realistic | 53 | 1 | 8 | Lagrangian | Nasal spray | x | x | x | x | x | <6 | [46] | ||||
| 21 | CT | 1 | Realistic | 48 | 1 | 10.5 | Lagrangian | Inhaled particles | x * | x * | + * | 3.02 | [47] | ||||||
| 22 | MRI | 1 | Realistic | 53 | 1 | NA | Lagrangian | Inhaled particles | x * | x * | + * | 3.97 | [48] | ||||||
| 23 | MRI | 1 | Realistic | 53 | 1 | NA | Lagrangian | Inhaled particles | x * | x * | x | + * | 53.21 | [49] | |||||
| 24 | MRI | 1 | Realistic | 53 | 1 | 8 | Lagrangian | Inhaled particles | x | x | <2 | [50] | |||||||
| 25 | CT | 8 | Realistic and virtual surgery | 4 | 3/4 | 10 | Lagrangian | Inhaled particles | x | x | x | 2.78 | [51] |
| Study ID | Flow Rate | Breathing Pattern | Head Position | Particle Size | Best Particle Size (µm) | Injection Velocity | Spray Cone Angle | Impaction Parameter | Sagittal Insertion Angle | Release Position | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Null | Null | Null | Null | [29] | ||||||
| 2 | Non-linear | 10 | [30] | ||||||||
| 3 | Inconclusive | NC | Direct | [17] | |||||||
| 4 | Direct | Null | Inverse | Null | [31] | ||||||
| 5 | Inverse | <0.007 | [32] | ||||||||
| 6 | Non-linear | Non-linear | 0.001 | MSR | [33] | ||||||
| 7 | Direct | Null | Between 0.001 and 0.007 | Direct | [34] | ||||||
| 8 | Direct | Non-linear | <0.02 and >10 | [35] | |||||||
| 9 | Inverse | Inconclusive | Inconclusive | NC | [36] | ||||||
| 10 | Null | Inverse | [37] | ||||||||
| 11 | Direct | Non-linear | 20 | Inverse | [18] | ||||||
| 12 | Inverse | [38] | |||||||||
| 13 | Non-linear | Between 20 and 30 | Inverse | MSR | [39] | ||||||
| 14 | Null | NC | Null | Null | MSR | [40] | |||||
| 15 | Non-linear | Non-linear | 25 | Inverse | Direct or inverse depending on the airflow rate | MSR | [41] | ||||
| 16 | Direct | [42] | |||||||||
| 17 | Non-linear | Direct | 10 | Non-linear | [43] | ||||||
| 18 | 45° backward head tilt position enhance olfactory deposition | [44] | |||||||||
| 19 | Higher olfactory deposition rate during inhalation. Lowest deposition rate during breath holding. | Inverse | < 30 | MSR | [45] | ||||||
| 20 | Vertex-to-floor position is supposed to increase olfactory deposition | Non-linear | 60 | Inverse | Direct | IR | [46] | ||||
| 21 | Direct | Non-linear | 0.002 | [47] | |||||||
| 22 | Non-linear | Non-linear | Between 0.01 and 0.1 and between 10 and 20 | [48] | |||||||
| 23 | Direct | Inverse | 0.001 | MSR | [49] | ||||||
| 24 | Inconclusive | NC | Inverse | [50] | |||||||
| 25 | Inverse | Higher deposition rate during inhalation and lower deposition rate during exhalation | Inverse | 0.001 | [51] |
| Study ID | Flow Rate | Monodispersed Particle Size | Injection Velocity | Spray Cone Angle | Sagittal Injection Angle | Impaction Parameter | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | r | p | Event | r | p | Event | r | p | Event | r | p | Event | r | p | Event | r | p | ||
| 1 | 384 | −0.098 | 0.056 | 384 | −0.003 | 0.961 | 384 | 0.202 *** | <0.001 | 384 | −0.1 ** | 0.008 | [29] | ||||||
| 2 | 12 | 0.508 | 0.092 | [30] | |||||||||||||||
| 3 | 21 | 0.232 | 0.312 | 21 | 0.232 | 0.312 | [17] | ||||||||||||
| 4 | 12 | 0.563 | 0.056 | 12 | −0.631 * | 0.028 | [31] | ||||||||||||
| 5 | 18 | −0.879 *** | <0.001 | 18 | −0.879 *** | 0.001 | [32] | ||||||||||||
| 6 | 3 | 0.866 | 0.333 | NA | NA | NA | [33] | ||||||||||||
| 7 | 15 | 0.638 * | 0.011 | 10 | −0.067 | 0.865 | 15 | 0.638 * | 0.011 | 10 | 0.286 | 0.301 | [34] | ||||||
| 8 | 45 | −0.001 | 0.994 | 45 | 0.02 | 0.897 | 45 | 0.023 | 0.883 | [35] | |||||||||
| 9 | NA | NA | NA | NA | NA | NA | [36] | ||||||||||||
| 10 | NA | NA | NA | NA | NA | NA | [37] | ||||||||||||
| 11 | 400 | −0.02 | 0.696 | 400 | −0.449 *** | <0.001 | 400 | −0.422 *** | 0.001 | [18] | |||||||||
| 11 | 400 | −0.006 | 0.912 | 400 | −0.412 *** | <0.001 | 400 | −0.393 *** | 0.001 | [18] | |||||||||
| 11 | 400 | 0.041 | 0.409 | 400 | −0.387 *** | <0.001 | 400 | −0.354 *** | 0.001 | [18] | |||||||||
| 12 | NA | NA | NA | [38] | |||||||||||||||
| 13 | NA | NA | NA | NA | NA | NA | [39] | ||||||||||||
| 14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | [40] | |||||||||
| 15 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | [41] | ||||||
| 16 | NA | NA | NA | [42] | |||||||||||||||
| 17 | 6 | −0.293 | 0.573 | 6 | 0.239 | 0.648 | 6 | 0.143 | 0.803 | [43] | |||||||||
| 18 | [44] | ||||||||||||||||||
| 19 | 12 | 0.145 | 0.653 | 10 | 0.104 | 0.774 | [45] | ||||||||||||
| 20 | NA | NA | NA | NA | NA | NA | NA | NA | NA | [46] | |||||||||
| 21 | 12 | −0.043 | 0.894 | 12 | −0.946 *** | <0.001 | 12 | −0.909 *** | 0.001 | [47] | |||||||||
| 22 | 32 | 0.233 | 0.199 | 32 | −0.143 | 0.434 | 32 | −0.115 | 0.53 | [48] | |||||||||
| 23 | 16 | −0.012 | 0.964 | 16 | −0.897 *** | <0.001 | 16 | −0.874 *** | 0.001 | [49] | |||||||||
| 24 | NA | NA | NA | NA | NA | NA | [50] | ||||||||||||
| 25 | NA | NA | NA | NA | NA | NA | [51] | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnumurthy, P.; Radulesco, T.; Bouchet, G.; Regard, A.; Michel, J. Computational Fluid Dynamics Approach for Direct Nose-to-Brain Drug Delivery: A Systematic Review and Meta-Analysis. J. Pers. Med. 2025, 15, 447. https://doi.org/10.3390/jpm15100447
Vishnumurthy P, Radulesco T, Bouchet G, Regard A, Michel J. Computational Fluid Dynamics Approach for Direct Nose-to-Brain Drug Delivery: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2025; 15(10):447. https://doi.org/10.3390/jpm15100447
Chicago/Turabian StyleVishnumurthy, Priya, Thomas Radulesco, Gilles Bouchet, Alain Regard, and Justin Michel. 2025. "Computational Fluid Dynamics Approach for Direct Nose-to-Brain Drug Delivery: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 15, no. 10: 447. https://doi.org/10.3390/jpm15100447
APA StyleVishnumurthy, P., Radulesco, T., Bouchet, G., Regard, A., & Michel, J. (2025). Computational Fluid Dynamics Approach for Direct Nose-to-Brain Drug Delivery: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 15(10), 447. https://doi.org/10.3390/jpm15100447





