Validity and Reliability of the Singer Reflux Symptom Score (sRSS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of Singer Reflux Symptom Score
2.2. Subjects and Setting
2.3. Statistical Analyses
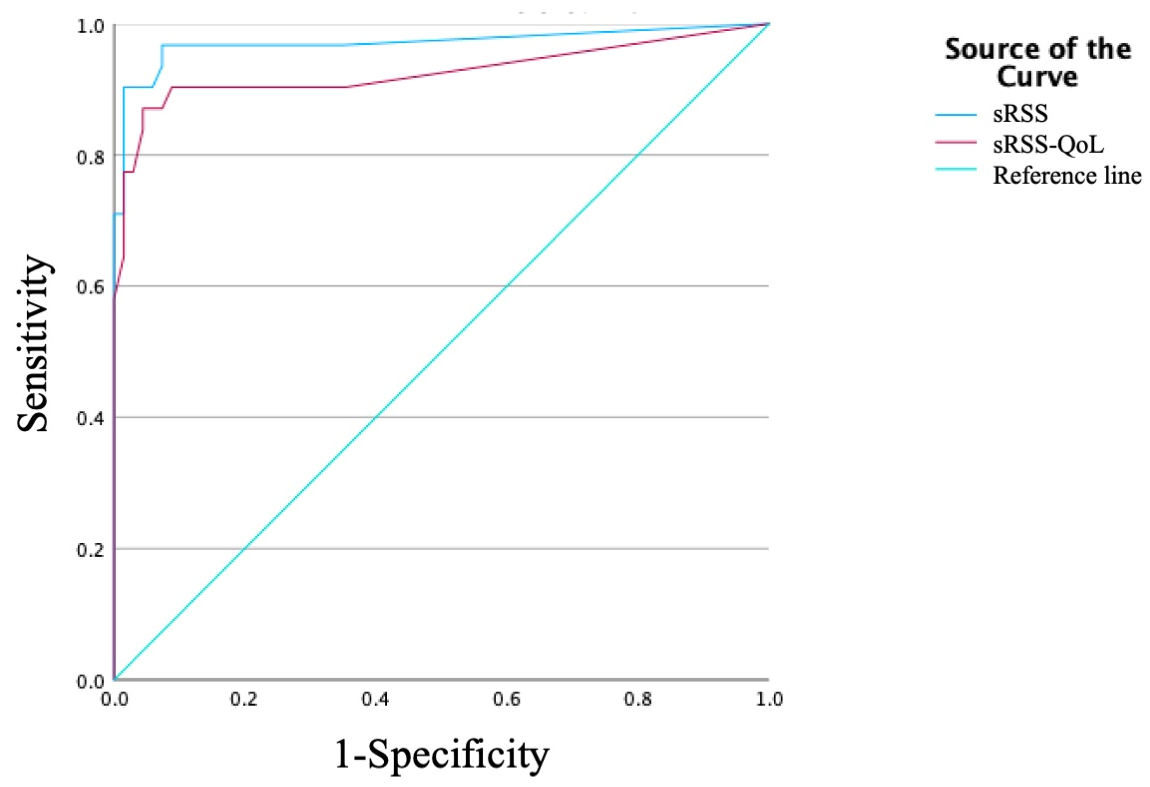
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPRD | Laryngopharyngeal reflux disease |
| sRSS | Singer Reflux Symptom Score |
| HEMII-pH | Hypopharyngeal–esophageal multichannel intraluminal impedance-pH testing |
| sVHI | Singing Voice Handicap Index |
References
- Lechien, J.R.; Vaezi, M.F.; Chan, W.W.; Allen, J.E.; Karkos, P.D.; Saussez, S.; Altman, K.W.; Amin, M.R.; Ayad, T.; Barillari, M.R.; et al. The Dubai Definition and Diagnostic Criteria of Laryngopharyngeal Reflux: The IFOS Consensus. Laryngoscope 2024, 134, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Briganti, G. Reflux Disease in Singers: A Systematic Review. J. Voice 2024, in press. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Harmegnies, B.; Finck, C.; Burns, J.A. Laryngopharyngeal Reflux and Voice Disorders: A Multifactorial Model of Etiology and Pathophysiology. J. Voice 2017, 31, 733–752. [Google Scholar] [CrossRef]
- Koufman, J.A.; Amin, M.R.; Panetti, M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol. Head Neck Surg. 2000, 123, 385–388. [Google Scholar] [CrossRef]
- Robotti, C.; Schindler, A.; Lechien, J.R.; Di Sabatino, A.; Capobianco, S.; Schindler, A.; Ottaviani, F.; Sims, H.S.; Bertino, G.; Benazzo, M.; et al. Prevalence of Laryngopharyngeal Reflux Symptoms, Dysphonia, and Vocal Tract Discomfort in Amateur Choir Singers. J. Voice 2023, 37, 932–944. [Google Scholar] [CrossRef]
- Cammarota, G.; Masala, G.; Cianci, R.; Palli, D.; Capaccio, P.; Schindler, A.; Cuoco, L.; Galli, J.; Ierardi, E.; Cannizzaro, O.; et al. Reflux symptoms in professional opera choristers. Gastroenterology 2007, 132, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Bobin, F.; Muls, V.; Thill, M.P.; Horoi, M.; Ostermann, K.; Huet, K.; Harmegnies, B.; Dequanter, D.; Dapri, G.; et al. Validity and reliability of the reflux symptom score. Laryngoscope 2020, 130, E98–E107. [Google Scholar] [CrossRef]
- Kang, Y.; Guan, T.; Chen, X.; Zhang, Y. Patient-Reported Experience Measures in Adult Inpatient Settings: A Systematic Review. J. Nurs. Manag. 2024, 2024, 5166392. [Google Scholar] [CrossRef]
- Lechien, J.R.; Rodriguez Ruiz, A.; Dequanter, D.; Bobin, F.; Mouawad, F.; Muls, V.; Huet, K.; Harmegnies, B.; Remacle, S.; Finck, C.; et al. Validity and Reliability of the Reflux Sign Assessment. Ann. Otol. Rhinol. Laryngol. 2020, 129, 313–325. [Google Scholar] [CrossRef]
- Daniero, J.J. Decision Making in the Managment of Laryngopharyngeal Reflux Disease: Antacids, Alginates, and the Role of Diet. Otolaryngol. Clin. N. Am. 2025, 58, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Morsomme, D.; Gaspar, M.; Jamart, J.; Remacle, M.; Verduyckt, I. Voice handicap index adapted to the singing voice. Rev. Laryngol. Otol. Rhinol. 2007, 128, 305–314. [Google Scholar]
- Nacci, A.; Bastiani, L.; Barillari, M.R.; Martinelli, M.; Lechien, J.R.; Simoni, F.; Berrettini, S.; Fattori, B. Reflux Symptom Index (RSI) and Singing Voice Handicap Index (SVHI) in Singing Students: A Pilot Study. J. Voice 2022, 36, 288.e25–288.e34. [Google Scholar] [CrossRef]
- Nacci, A.; Capobianco, S.; Mazzoni, L.; Fattori, B.; Barillari, M.R.; Genovese, E.; Berrettini, S.; Bastiani, L. Development of a New Self-Assessment Tool for Laryngopharyngeal RefluxScreening in Singers (SVHI-12-LPR). Folia Phoniatr. Logop. 2023, 75, 284–294. [Google Scholar] [CrossRef]
- Campagnolo, A.M.; Benninger, M.S.; Priston, P.; Assunção, A. Dysphonia in Performers: Prevalence of Vocal Lesions and Voice Emergencies in a Private Otorhinolaryngology Practice. J. Voice 2023, 37, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Korn, G.P.; Azevedo, R.R.; Monteiro, J.C.; Pinheiro, D.S.; Park, S.W.; Biase, N.G. Difficulty producing high-pitched sounds in singing: Correlations with laryngostroboscopy and electromyographic findings. Braz. J. Otorhinolaryngol. 2020, 86, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Mattioni, J.; Sataloff, R.T. Endoscopic Findings in a Professional Singer with Frequent Throat Clearing. Ear Nose Throat J. 2019, 98, 128. [Google Scholar] [CrossRef]
- Giannopoulou, J.; Papadopoulou, E.; Bibas, A.; Papathanasiou, I. Validation of the Singing Voice Handicap Index in Greek Singers: Normal and Voice-Disordered Participants. J. Voice 2025, in press. [Google Scholar] [CrossRef]
- Nacci, A.; Barillari, M.R.; Capobianco, S.; Fattori, B.; Berrettini, S.; Tonacci, A.; Minichilli, F.; Bastiani, L. Adaptation and validation of the Italian Singing Voice Handicap Index-10 (SVHI-10-IT). Acta Otorhinolaryngol. Ital. 2023, 43, 114–122. [Google Scholar] [CrossRef]
- Okui, A.; Nimura, Y.; Komazawa, D.; Kanazawa, T.; Konomi, U.; Hirosaki, M.; Okano, M.; Nishida, M.; Watanabe, Y. Validation of the Japanese Version of the Singing Voice Handicap Index. J. Voice 2024, 38, 544.e15–544.e21. [Google Scholar] [CrossRef]
- Cohen, S.M.; Witsell, D.L.; Scearce, L.; Vess, G.; Banka, C. Treatment responsiveness of the Singing Voice Handicap Index. Laryngoscope 2008, 118, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Luo, Z.; Zhang, X.; Zhang, J.; Zhen, Z.; Ding, T. The influence of saliva pepsin concentration on subjective severity of seasonal allergic rhinitis. Acta Otolaryngol. 2025, 145, 329–333. [Google Scholar] [CrossRef]
- Kakaje, A.; Alhalabi, M.M.; Alyousbashi, A.; Ghareeb, A. Allergic rhinitis, asthma and laryngopharyngeal reflux disease: A cross-sectional study on their reciprocal relations. Sci. Rep. 2021, 11, 2870. [Google Scholar] [CrossRef]
- Eren, E.; Arslanoğlu, S.; Aktaş, A.; Kopar, A.; Ciğer, E.; Önal, K.; Katılmiş, H. Factors confusing the diagnosis of laryngopharyngeal reflux: The role of allergic rhinitis and inter-rater variability of laryngeal findings. Eur. Arch. Otorhinolaryngol. 2014, 271, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Eckley, C.A.; Tangerina, R. Using RSI and RFS scores to differentiate between reflux-related and other causes of chronic laryngitis. Braz. J. Otorhinolaryngol. 2023, 89, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Tarmizi, N.E.; Hamizan, A.W.; Ng, C.S.; Gendeh, H.S.; Guan, L.S.; Zahedi, F.D.; Baki, M.M.; Husain, S. The Nasal Endoscopic Features of Postnasal Drip: A Cross Sectional Study. Int. Arch. Otorhinolaryngol. 2023, 28, e95–e100. [Google Scholar] [CrossRef]
- Smallwood, D.; Ledford, D.; Kennedy, D.; Lockey, R. Postnasal Drip. J. Allergy Clin. Immunol. Pract. 2024, 12, 1472–1478. [Google Scholar] [CrossRef]
- Brown, H.J.; Kuhar, H.N.; Plitt, M.A.; Husain, I.; Batra, P.S.; Tajudeen, B.A. The Impact of Laryngopharyngeal Reflux on Patient-reported Measures of Chronic Rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2020, 129, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Aldajani, A.; Alhussain, F.; Mesallam, T.; AbaAlkhail, M.; Alojayri, R.; Bassam, H.; Alotaibi, O.; Alqahtani, M.; Alsaleh, S. Association Between Chronic Rhinosinusitis and Reflux Diseases in Adults: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2024, 38, 47–59. [Google Scholar] [CrossRef]
- Yeo, N.K.; Park, S.J.; An, T.H. Laryngopharyngeal reflux in chronic rhinosinusitis patients and the role of endoscopic sinus surgery. Auris Nasus Larynx 2022, 49, 663–669. [Google Scholar] [CrossRef]
- Anzić, S.A.; Turkalj, M.; Župan, A.; Labor, M.; Plavec, D.; Baudoin, T. Eight weeks of omeprazole 20 mg significantly reduces both laryngopharyngeal reflux and comorbid chronic rhinosinusitis signs and symptoms: Randomised, double-blind, placebo-controlled trial. Clin. Otolaryngol. 2018, 43, 496–501. [Google Scholar] [CrossRef]
- Kayalı Dinc, A.S.; Cayonu, M.; Sengezer, T.; Sahin, M.M. Smoking Cessation Improves the Symptoms and the Findings of Laryngeal Irritation. Ear Nose Throat J. 2020, 99, 124–127. [Google Scholar] [CrossRef]
- Arai, Y.; Okuyama, K.; Onishi, Y.; Schelfhout, J.; Tokita, S.; Kubo, T. Clinical characteristics and drug utilisation patterns in patients with chroniccough: A retrospective cohort study using a Japanese claims database. BMC Pulm. Med. 2022, 22, 429. [Google Scholar] [CrossRef]
- Lilly, G.L.; Carroll, T.; Pietsch, K.; Dhillon, V.; Bryson, P.C.; Akst, L.M. Refractory Chronic Cough: A State-of-the-Art Review for Otolaryngologists. Otolaryngol. Head Neck Surg. 2025, 172, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.A.; Gupta, M.R.; Lockey, R.F.; Bardin, P.G.; Baptist, A.P. Gastroesophageal reflux disease, laryngopharyngeal reflux, and vocal cord dysfunction/inducible laryngeal obstruction-overlapping conditions that affect asthma. J. Allergy Clin. Immunol. 2024, 154, 1369–1377. [Google Scholar] [CrossRef]
- Koshiyama, S.; Tanimura, K.; Ito, K.; Funayama, S.; Hira, D.; Komase, Y.; Sato, S. Gastroesophageal reflux-like symptoms are associated with hyposalivation and oropharyngeal problems in patients with asthma. Respir. Investig. 2021, 59, 114–119. [Google Scholar] [CrossRef]
- Lloyd, A.T.; Ruddy, B.H.; Silverman, E.; Lewis, V.M.; Lehman, J.J. Quantifying Laryngopharyngeal Reflux in Singers: Perceptual and Objective Findings. BioMed Res. Int. 2017, 2017, 3918214. [Google Scholar] [CrossRef] [PubMed]
- Myint, C.; Moore, J.E.; Hu, A.; Jaworek, A.J.; Sataloff, R.T. A Comparison of Initial and Subsequent Follow-Up Strobovideolaryngoscopic Examinations in Singers. J. Voice 2016, 30, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Butte, C.J.; Zhang, Y.; Song, H.; Jiang, J.J. Perturbation and nonlinear dynamic analysis of different singing styles. J. Voice 2009, 23, 647–652. [Google Scholar] [CrossRef]
- Bunch, M.; Chapman, J. Taxonomy of singers used as subjects in scientific research. J. Voice 2000, 14, 363–369. [Google Scholar] [CrossRef]
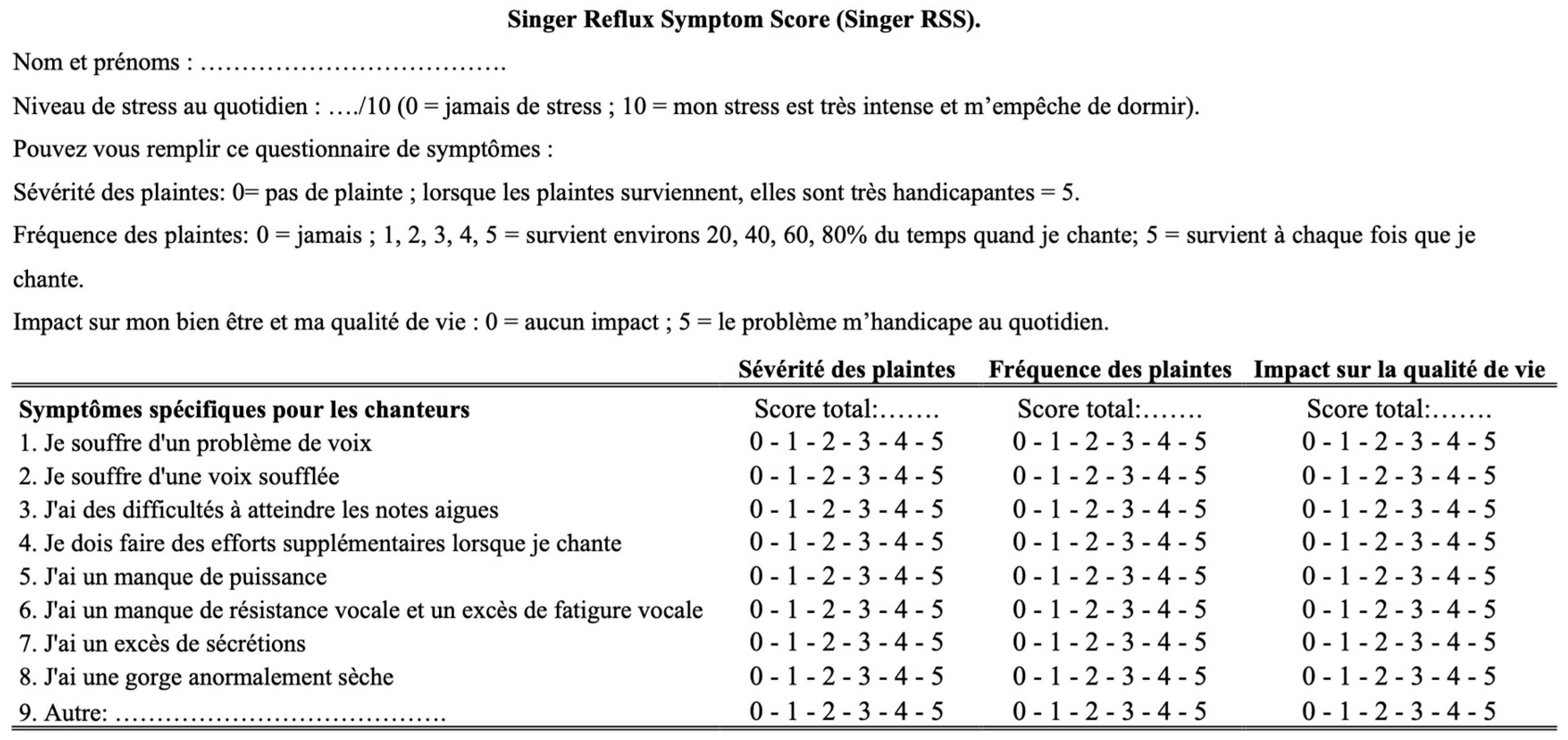
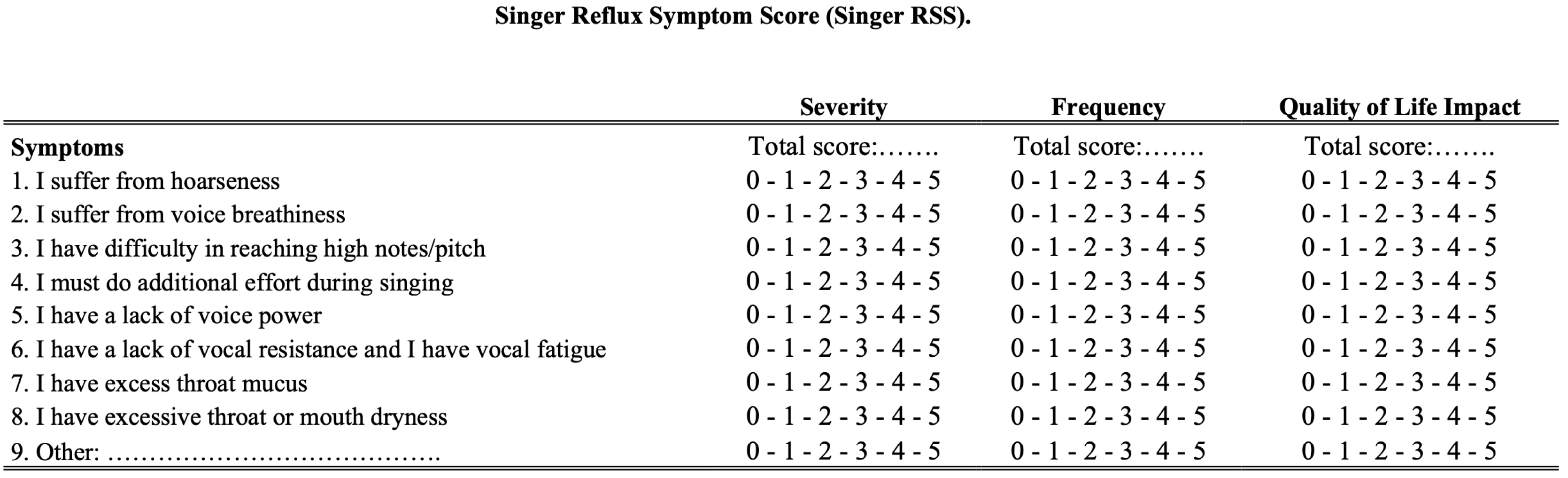
| Demographics | Patients (n = 33) |
|---|---|
| Mean age (SD) | 51.8 ± 17.2 |
| Body mass index (mean, SD) | 27.2 ± 8.0 |
| Gender (N, %) | |
| Females | 17 (51.5) |
| Males | 16 (48.5) |
| Smoker (current Cig/d) and smoking history (Paq. Year) | 3 (9.1) |
| Cannabis consumption | 1 (3.0) |
| Alcohol (daily consumption) | 3 (9.1) |
| Alcohol (mean and SD for unit/day in consumers) | 0.2 ± 0.6 |
| Coffee/caffeine drink/day | 8 (24.2) |
| Singer types | |
| Soloist | 27 (81.8) |
| Chorister | 6 (18.2) |
| Voice range/classification | |
| Females | n = 17 |
| Soprano | 5 (15.2) |
| Mezzo-soprano | 9 (27.3) |
| Alto | 1 (3.0) |
| Several ranges | 2 (6.1) |
| Males | n = 16 |
| Tenor | 5 (15.2) |
| Baritone | 4 (12.1) |
| Bass | 1 (3.0) |
| Several ranges | 6 (18.2) |
| Singing habits | |
| Habit of performing with amplification (microphone) | 2 (6.1) |
| Warm up before singing | 15 (45.5) |
| Cool down after singing | 5 (15.2) |
| Involvement in professional singing activities | 8 (24.2) |
| Musical/Singing styles | |
| Classical | 12 (36.4) |
| Pop | 4 (12.1) |
| Rock/pop-rock | 3 (9.1) |
| Gospel | 2 (6.1) |
| Jazz | 1 (3.0) |
| Opera | 1 (3.0) |
| Modern | 3 (9.1) |
| Several styles | 7 (21.2) |
| Singer Reflux Symptom Score Outcomes | Prevalence (N, %) |
|---|---|
| Hoarseness | 31 (93.9) |
| Abnormal voice breathiness | 20 (60.6) |
| Difficulty to reach high pitch/notes | 32 (97.0) |
| Additional effort during singing | 31 (93.9) |
| Lack of voice power | 30 (90.9) |
| Vocal fatigue and lack of vocal resistance | 32 (97.0) |
| Excess throat mucus during singing | 29 (87.9) |
| Throat or mouth dryness during singing | 25 (75.8) |
| Singer Reflux Symptom Score Outcomes | Test–Retest r | p-Value |
|---|---|---|
| Hoarseness | 0.647 | 0.001 |
| Abnormal voice breathiness | 0.990 | 0.001 |
| Difficulty to reach high pitch/notes | 0.630 | 0.001 |
| Additional effort during singing | 0.679 | 0.001 |
| Lack of voice power | 0.729 | 0.001 |
| Vocal fatigue and lack of vocal resistance | 0.763 | 0.001 |
| Excess throat mucus during singing | 0.516 | 0.001 |
| Throat or mouth dryness during singing | 0.515 | 0.001 |
| Singer Reflux Symptom total Score | 0.750 | 0.001 |
| Baseline Evaluations | Post-Treatment (3 Months) | ||||||
|---|---|---|---|---|---|---|---|
| Singer Reflux Symptom Score Outcomes | Patients | Controls | Z | p-Value | Patients | Z | p-Value |
| Hoarseness | 14.9 ± 8.2 | 0.4 ± 3.0 | −8.60 | 0.001 | 4.2 ± 5.6 | −2.24 | 0.025 |
| Abnormal voice breathiness | 6.8 ± 8.1 | 0.1 ± 0.5 | −6.46 | 0.001 | 1.6 ± 2.6 | −1.86 | 0.063 |
| Difficulty to reach high pitch/notes | 15.9 ± 7.8 | 0.1 ± 3.3 | −8.16 | 0.001 | 7.1 ± 9.2 | −2.19 | 0.028 |
| Additional effort during singing | 13.9 ± 8.3 | 0.8 ± 3.3 | −7.69 | 0.001 | 5.1 ± 7.1 | −2.24 | 0.025 |
| Lack of voice power | 12.9 ± 9.2 | 0.7 ± 3.4 | −7.99 | 0.001 | 3.7 ± 5.9 | −2.52 | 0.012 |
| Vocal fatigue and lack of vocal resistance | 14.4 ± 8.2 | 0.7 ± 3.4 | −8.34 | 0.001 | 5.7 ± 7.6 | −2.20 | 0.028 |
| Excess throat mucus during singing | 11.4 ± 9.2 | 0.6 ± 2.7 | −8.32 | 0.001 | 3.8 ± 5.5 | −2.37 | 0.018 |
| Throat or mouth dryness during singing | 6.5 ± 7.5 | 0.1 ± 0.2 | −6.72 | 0.001 | 1.5 ± 2.9 | −2.03 | 0.042 |
| Total Singer Reflux Symptom Score | 96.6 ± 45.0 | 3.7 ± 11.0 | −7.87 | 0.001 | 30.1 ± 37.4 | −2.93 | 0.003 |
| SRSS—Quality-of-life | 21.1 ± 10.9 | 1.4 ± 3.1 | −7.17 | 0.001 | 9.0 ± 9.4 | −2.19 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechien, J.R. Validity and Reliability of the Singer Reflux Symptom Score (sRSS). J. Pers. Med. 2025, 15, 348. https://doi.org/10.3390/jpm15080348
Lechien JR. Validity and Reliability of the Singer Reflux Symptom Score (sRSS). Journal of Personalized Medicine. 2025; 15(8):348. https://doi.org/10.3390/jpm15080348
Chicago/Turabian StyleLechien, Jérôme R. 2025. "Validity and Reliability of the Singer Reflux Symptom Score (sRSS)" Journal of Personalized Medicine 15, no. 8: 348. https://doi.org/10.3390/jpm15080348
APA StyleLechien, J. R. (2025). Validity and Reliability of the Singer Reflux Symptom Score (sRSS). Journal of Personalized Medicine, 15(8), 348. https://doi.org/10.3390/jpm15080348






