Remimazolam for Procedural Sedation in Older Patients: A Systematic Review and Meta-Analysis with Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- (1)
- Patients (P): all older patients undergoing elective procedural sedation
- (2)
- Intervention (I): remimazolam bolus or continuous infusion
- (3)
- Comparison (C): other sedatives
- (4)
- Outcome measurements (O): The primary outcome of this systematic review and meta-analysis with a TSA was the rate of sedation success, which was defined as no rescue sedation with a sedative agent other than the assigned treatment to complete the entire endoscopic procedure. The incidence of hypoxemia (SpO2 < 90%) and hypotension (systolic blood pressure [SBP]) decreased by 20% or more compared to the baseline value or SBP ≤ 80 mmHg during the procedure. The secondary outcomes were the rates of bradycardia (≤50 beats/min or a decrease in heart rate of 20% or more from baseline), respiratory depression (respiratory rate < 8/min and/or SpO2 < 90%), time to loss of consciousness (LOC), recovery time (time from cessation of sedative agent to fully alert), injection pain, dizziness/headache, and postoperative nausea and vomiting (PONV).
- (5)
- Study design (SD): Full reports of RCTs were included. Observational studies, conference abstracts, posters, case reports, case series, comments, letters to the editor, reviews, and laboratory or animal studies were excluded.
2.2. Information Source and Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
2.6. Data Analysis
2.6.1. Conventional Meta-Analysis
2.6.2. TSA
2.6.3. Quality of the Evidence
3. Results
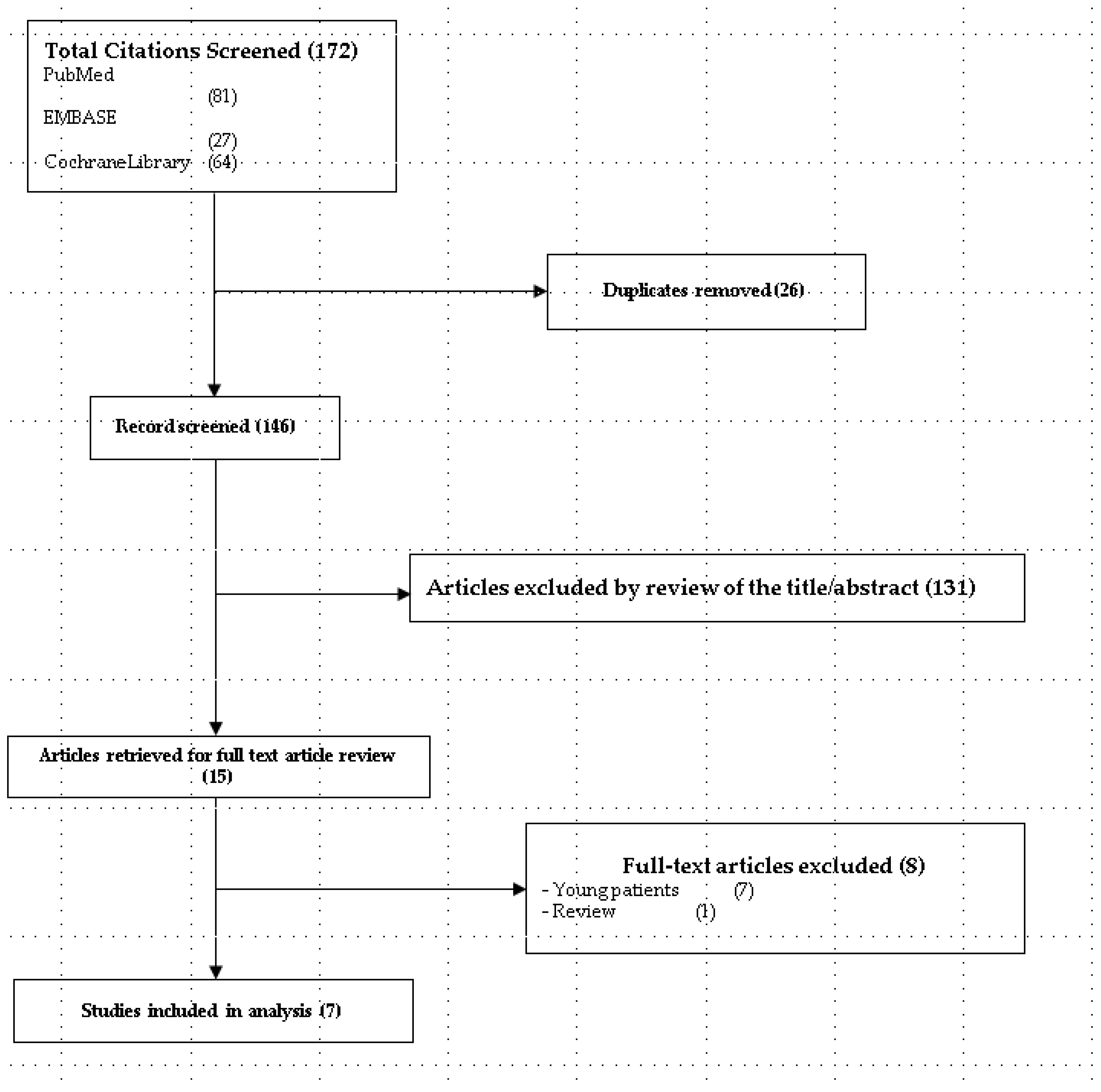
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Sedation Success Rate
3.5. Incidence of Hypoxemia
3.6. Incidence of Hypotension
3.7. Incidence of Bradycardia
3.8. Respiratory Depression
3.9. Time to LOC
3.10. Recovery Time
3.11. Injection Pain
3.12. Incidence of Dizziness/Headache
3.13. PONV
3.14. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stock, C.; Haug, U.; Brenner, H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: Review and analysis of recent trends. Gastrointest. Endosc. 2010, 71, 366–381.e362. [Google Scholar] [CrossRef]
- Zagari, R.M.; Eusebi, L.H.; Rabitti, S.; Cristoferi, L.; Vestito, A.; Pagano, N.; Bazzoli, F. Prevalence of upper gastrointestinal endoscopic findings in the community: A systematic review of studies in unselected samples of subjects. J. Gastroenterol. Hepatol. 2016, 31, 1527–1538. [Google Scholar] [CrossRef]
- Meng, Q.T.; Cao, C.; Liu, H.M.; Xia, Z.Y.; Li, W.; Tang, L.H.; Chen, R.; Jiang, M.; Wu, Y.; Leng, Y.; et al. Safety and efficacy of etomidate and propofol anesthesia in elderly patients undergoing gastroscopy: A double-blind randomized clinical study. Exp. Ther. Med. 2016, 12, 1515–1524. [Google Scholar] [CrossRef]
- Dossa, F.; Megetto, O.; Yakubu, M.; Zhang, D.D.Q.; Baxter, N.N. Sedation practices for routine gastrointestinal endoscopy: A systematic review of recommendations. BMC Gastroenterol. 2021, 21, 22. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Ko, J.S. Etomidate versus propofol sedation for electrical external cardioversion: A meta-analysis. Curr. Med. Res. Opin. 2018, 34, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Lee, J.J. Comparison of bolus versus continuous infusion of propofol for procedural sedation: A meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1935–1943. [Google Scholar] [CrossRef]
- Doenicke, A.; Lorenz, W.; Hoernecke, R.; Nebauer, A.E.; Mayer, M. Histamine release after injection of benzodiazepines and of etomidate. A problem associated with the solvent propylene glycol. Ann. Fr. Anesth. Reanim. 1993, 12, 166–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Choi, S.M.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Lee, J. Dexmedetomidine versus midazolam for sedation during endobronchial ultrasound-guided transbronchial needle aspiration: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 534–540. [Google Scholar] [CrossRef]
- Kim, K.M. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain Med. 2022, 17, 1158632. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, B.; Shi, F.; Zhang, Y.; Liu, L.; Sha, Y.; Zhao, T. The Efficacy and Safety of Remimazolam Tosilate versus Etomidate-Propofol in Elderly Outpatients Undergoing Colonoscopy: A Prospective, Randomized, Single-Blind, Non-Inferiority Trial. Drug Des. Dev. Ther. 2021, 15, 4675–4685. [Google Scholar] [CrossRef]
- Tan, Y.; Ouyang, W.; Tang, Y.; Fang, N.; Fang, C.; Quan, C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J. Gastroenterol. Hepatol. 2022, 37, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Qian, Y.; Zhang, X.; Han, S.; Shi, Q.; Xu, J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: A prospective, randomized and controlled study. BMC Anesthesiol. 2022, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wei, S.; Ling, W.; Wei, Y.; Ran, X.; Huang, H.; Wang, M.; Wei, N.; Liao, Y.; Qin, Z.; et al. Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J. Clin. Pharm. Ther. 2022, 47, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jiang, K.; Shi, W.; Xiao, S.; Zhang, S.; Zhang, Y.; Zhou, Y.; Tan, C.; Tan, S.; Zou, X. Effect of Remimazolam Tosilate on Respiratory Depression in Elderly Patients Undergoing Gastroscopy: A Multicentered, Prospective, and Randomized Study. Drug Des. Dev. Ther. 2022, 16, 4151–4159. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Wang, Y.; Li, K.; Peng, T.; Fang, N.; Pasunooti, K.K.; Jun, S.; Yang, X.; Wu, J. Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing gastrointestinal endoscopy: A bi-center, prospective, randomized controlled study. Front. Pharmacol. 2023, 14, 1131391. [Google Scholar] [CrossRef]
- Ye, E.; Wu, K.; Ye, H.; Zhang, W.; Chu, L.; Zhang, K.; Xie, G.; Jin, Y.; Fang, X. Comparison of 95% effective dose of remimazolam besylate and propofol for gastroscopy sedation on older patients: A single-centre randomized controlled trial. Br. J. Clin. Pharmacol. 2023, 89, 3401–3410. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Choi, G.J.; Kang, H. Introduction to Umbrella Reviews as a Useful Evidence-Based Practice. J. Lipid Atheroscler. 2023, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Borkett, K.M.; Riff, D.S.; Schwartz, H.I.; Winkle, P.J.; Pambianco, D.J.; Lees, J.P.; Wilhelm-Ogunbiyi, K. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth. Analg. 2015, 120, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bhandari, R.; Desta, T.; DeMicco, M.P.; Schaeffer, C.; Etzkorn, K.; Barish, C.F.; Pruitt, R.; Cash, B.D.; Quirk, D.; et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 2018, 88, 427–437.e426. [Google Scholar] [CrossRef] [PubMed]
- Pastis, N.J.; Yarmus, L.B.; Schippers, F.; Ostroff, R.; Chen, A.; Akulian, J.; Wahidi, M.; Shojaee, S.; Tanner, N.T.; Callahan, S.P.; et al. Safety and Efficacy of Remimazolam Compared With Placebo and Midazolam for Moderate Sedation During Bronchoscopy. Chest 2019, 155, 137–146. [Google Scholar] [CrossRef]
- Chen, S.H.; Yuan, T.M.; Zhang, J.; Bai, H.; Tian, M.; Pan, C.X.; Bao, H.G.; Jin, X.J.; Ji, F.H.; Zhong, T.D.; et al. Remimazolam tosilate in upper gastrointestinal endoscopy: A multicenter, randomized, non-inferiority, phase III trial. J. Gastroenterol. Hepatol. 2021, 36, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bhandari, R.; Lorch, D.G.; Meyers, M.; Schippers, F.; Bernstein, D. Safety and efficacy of remimazolam in high risk colonoscopy: A randomized trial. Dig. Liver Dis. 2021, 53, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Liu, J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: Single-centre randomized controlled trial. BMC Anesthesiol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Dong, S.A.; Guo, Y.; Liu, S.S.; Wu, L.L.; Wu, L.N.; Song, K.; Wang, J.H.; Chen, H.R.; Li, W.Z.; Li, H.X.; et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J. Clin. Anesth. 2023, 86, 111077. [Google Scholar] [CrossRef]
- Ito, T.; Utsumi, N.; Baba, Y.; Matsumura, T.; Wakita, R.; Maeda, S. Considerations for Satisfactory Sedation during Dental Implant Surgery. J. Pers. Med. 2023, 13, 461. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, Y.T.; Chi, K.Y.; Huang, Y.T. Remimazolam versus propofol for procedural sedation: A meta-analysis of randomized controlled trials. PeerJ 2023, 11, e15495. [Google Scholar] [CrossRef]
- Eldawlatly, A.A.; Delvi, M.B.; Ahmad, A. Procedural sedation analgesia in the elderly patient. Saudi J. Anaesth. 2023, 17, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Lei, L.; Qiu, D.; Chen, S.; Xing, L.K.; Zhao, J.W.; Mao, Y.Y.; Yang, J.J. Effect of Remimazolam on Postoperative Delirium in Older Adult Patients Undergoing Orthopedic Surgery: A Prospective Randomized Controlled Clinical Trial. Drug Des. Dev. Ther. 2023, 17, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, B.; Hu, L.; Han, D.; Wu, J. Bibliometric Analysis of Global Trends in Remimazolam-Related Research Over the Past 15 Years: Compared with Propofol. Drug Des. Dev. Ther. 2023, 17, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, T.; Mitsuta, Y.; Uemura, Y.; Tajima, K.; Hirata, N. Anesthetic management with remimazolam in very elderly patients undergoing hybrid surgery of transcatheter aortic valve implantation plus off-pump coronary artery bypass grafting: Report of two cases. JA Clin. Rep. 2023, 9, 69. [Google Scholar] [CrossRef]
- Kido, T.; Suzuki, Y.; Nishida, K. General Anesthesia With Remimazolam and Peripheral Nerve Blocks Is Useful for Femoral Surgery in Patients With Severe Aortic Stenosis: A Case Report. A&A Pract. 2023, 17, e01702. [Google Scholar] [CrossRef]



| Author, Year, Country | Participants | Sample Size/Intervention | Primary Outcome | Major Finding | ||
|---|---|---|---|---|---|---|
| Age (Year) | Procedure | ASA | ||||
| Liu et al., 2021, China [10] | 65–75 | Colonoscopy | I–II | Remimazolam (n = 115) initial bolus 0.15 mg/kg (over 1 min), followed by 0.075 mg/kg Propofol plus etomidate (n = 117) bolus 0.1 mL/kg (over 1 min), followed by 0.05 mL/kg | Success of the procedure | Remimazolam may have noninferior efficacy and a higher safety profile than etomidate-propofol in older colonoscopy |
| Tan et al., 2022, China [11] | >60 | Upper GI endoscopy | I–II | Remimazolam (n = 66) initial bolus 0.1 or 0.2 mg, followed by 0.05 mg/kg Propofol (n = 33) bolus 1.0–1.5 mg/kg, followed by 0.5 mg/kg | Recovery of the cognitive domain | Remimazolam is a safe and effective sedative in older upper GI endoscopy |
| Lu et al., 2022, China [13] | 65–85 | Upper GI endoscopy | I–III | Remimazolam (n = 200) 300 mg/h, followed by 2.5 mg Propofol (n = 200) 3.0 g/h, followed by 0.5 mg/kg | Rate of hypotension, defined as SBP ≤ 90 mmHg or a >30% decline from the baseline | Remimazolam is associated with a low rate of hypotension in older upper GI endoscopy |
| Hu et al., 2022, China [14] | ≥65 | Gastroscopy | I–III | Remimazolam (n = 173) initial bolus 0.2 mg/kg (slow IV injection, over 1 min), followed by one-third of the initial dose Propofol (n = 173) initial bolus 1.5 mg/kg (slow IV injection, over 1 min), followed by one-third of the initial dose | Incidence of respiratory depression, defined as respiratory rate < 8/min and/or oxygen saturation <90% | The incidence of respiratory depression is significantly reduced in patients administered remimazolam compared with the patients administered propofol |
| Guo et al., 2022, China [12] | ≥65 | GI endoscopy | I–II | Remimazolam (n = 39) initial bolus 0.15 mg/kg (slow IV injection, completed in 30 s), followed by one-third of the initial dose Propofol (n = 38) initial bolus 1.5 mg/kg (slow IV injection, completed in 30 s), followed by one-third of the initial dose | Success rate of sedation, the time to loss of consciousness, the recovery time between the remimazolam and propofol | Compared with propofol, remimazolam can be safely and effectively used in older GI endoscopy |
| Ye et al., 2023, China [16] | ≥65 | Gastroscopy | I–II | Remimazolam (n = 64) initial bolus 0.2 mg/kg, followed by 3 mg Propofol (n = 65) initial bolus 2.0 mg/kg, followed by 30 mg | Incidence of adverse events to assess safety (hypotension, hypertension, bradycardia, tachycardia, respiratory depression, hypoxia, injection pain) | Remimazolam is safer alternative than propofol in older gastroscopy |
| Liu et al., 2023, China [15] | 60–80 | GI endoscopy | I–II | Remimazolam (n = 107) initial bolus 0.10 mg/kg, followed by 2.5 mg Propofol (n = 109) initial bolus 1.5 mg/kg, followed by 0.5 mg/kg | Incidence of moderate hypoxemia, defined as SpO2 ≥ 85% and <90%, duration > 15 s | Remimazolam improves risk of moderate hypoxemia and hypotension in older GI endoscopy |
| Author, Year | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Intervention | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Results | Overall Bias |
|---|---|---|---|---|---|---|
| Liu et al., 2021, China [10] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Tan et al., 2022, China [11] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Lu et al., 2022, China [13] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Hu et al., 2022, China [14] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Guo et al., 2022, China [12] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ye et al., 2023, China [16] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Liu et al., 2023, China [15] | Some concern | Low risk | Low risk | Low risk | Low risk | Some concern |
| Outcome | No. of Studies | No. of Patients | Conventional Meta-Analysis | Trial Sequential Analysis | NNT | |||
|---|---|---|---|---|---|---|---|---|
| RR or WMD, with 95% CI | Heterogeneity (I2/τ) | Conventional Test Boundary | Monitoring Boundary | RIS | ||||
| Sedation success | 7 | 1502 | Not significant (RR 0.999, 95% CI 0.995–1.003) | 0.0/0.000 | Not cross | Not cross | Exceed RIS (1502 of 599 patients) Enter futility border | Not significant (NNTH: 96; 95% CI NNTH 56 to ∞ NNTB 351) |
| Hypoxemia | 6 | 1422 | Significant (RR 0.302, 95% CI 0.187–0.489) | 20.595/0.573 | Cross | Cross | 36.0% (1422 of 3951 patients) | Significant (NNTB: 13; 95% CI NNTB 10 to NNTB 20) |
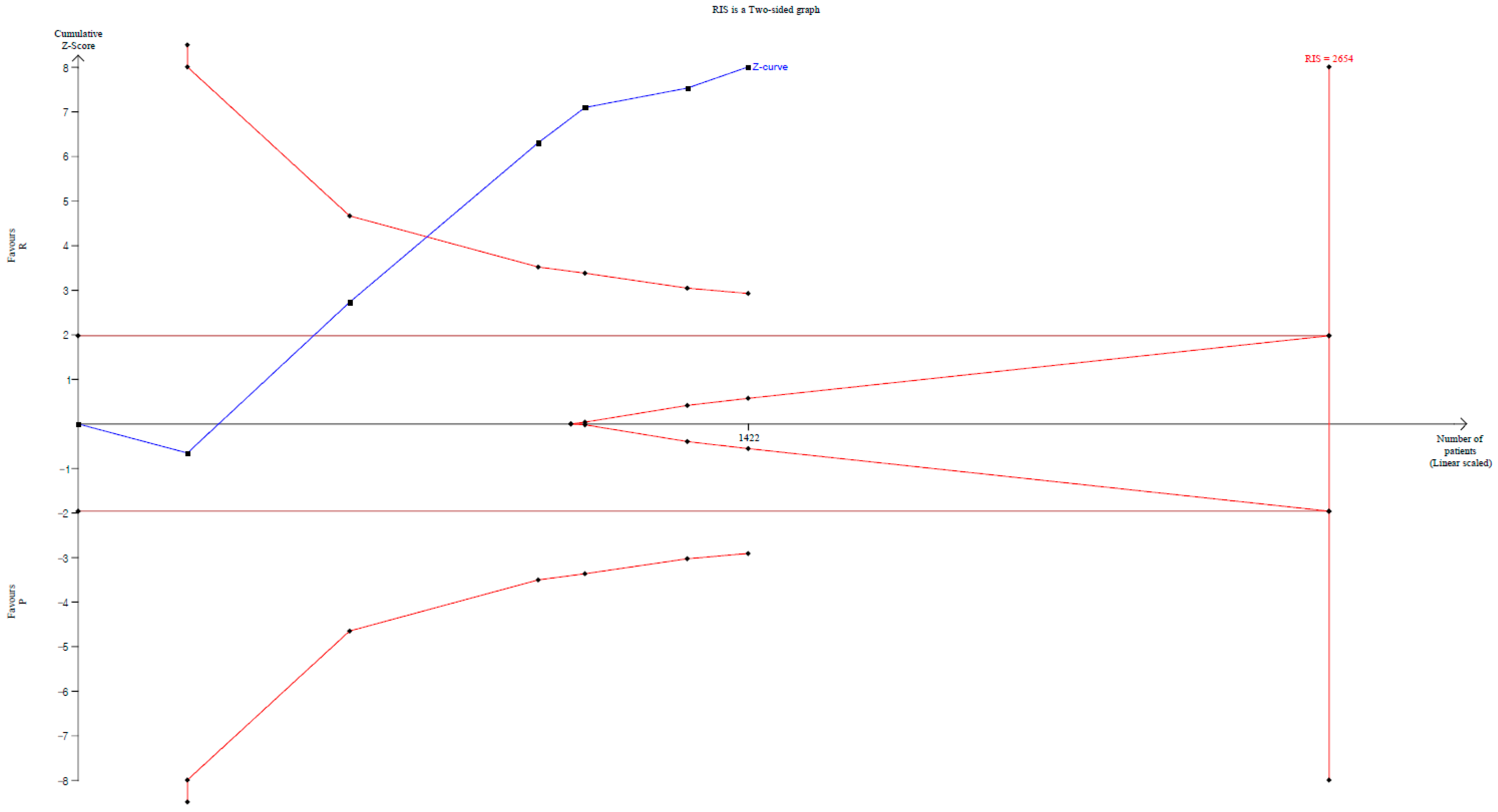
| Hypotension | 6 | 1422 | Significant (RR 0.477, 95% CI 0.330–0.690) | 72.634/0.593 | Cross | Cross | 53.6% (1422 of 2654 patients) | Significant (NNTB: 5; 95% CI NNTB 4 to NNTB 6) |
| Bradycardia | 5 | 1323 | Significant (RR 0.396, 95% CI 0.249–0.631) | 47.484/0.722 | Coss | Not cross | 22.6% (1323 of 5851 patients) | Significant (NNTB: 16; 95% CI NNTB 11 to NNTB 29) |
| Respiratory depression | 4 | 838 | Significant (RR 0.347, 95% CI 0.183–0.659) | 0.0/0.000 | Cross | Not cross | 26.9% (838 of 3118 patients) | Significant (NNTB: 17; 95% CI NNTB 11 to NNTB 36) |
| Time to LOC | 4 | 952 | Significant (WMD 5.385, 95% CI 2.082–8.689) | 65.420/1.641 | Cross | Cross | 99.5% (952 of 957 patients) | NR |
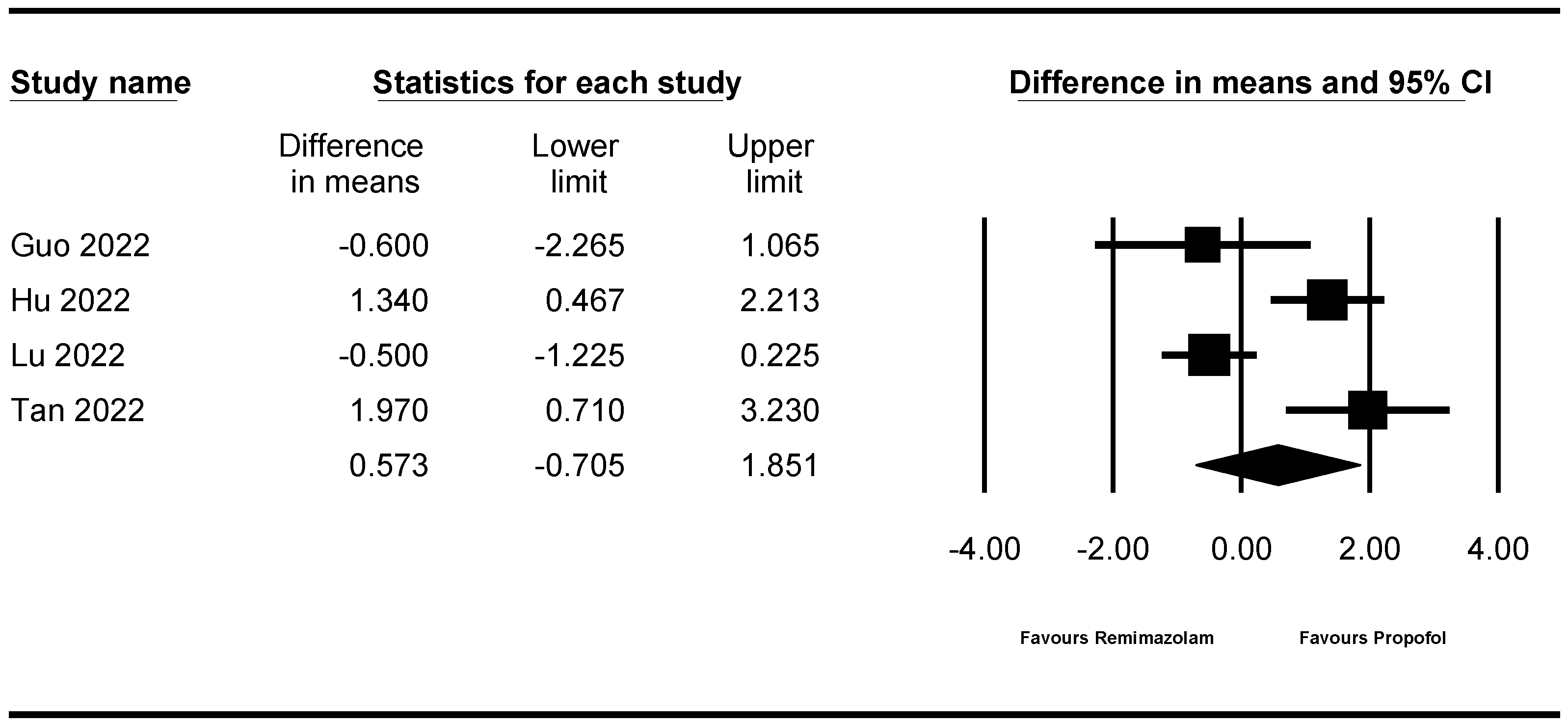
| Recovery time | 4 | 922 | Not significant (WMD 0.573, 95% CI −0.705 to 1.851) | 83.050/1.080 | Not cross | Not cross | 46.8% (922 of 1968 patients) | NR |
| Injection pain | 4 | 838 | Significant (RR 0.207, 95% CI 0.074–0.579) | 2.823/0.628 | Cross | Not cross | 3.8% (838 of 22,319 patients) | Not significant (NNTB: 428; 95% CI NNTH 86 to ∞ NNTB 61) |
| Dizziness/headache | 4 | 822 | Not significant (RR 0.726, 95% CI 0.419–1.257) | 0.0/0.000 | Not cross | Not cross | 17.2% (822 of 4774 patients) | Not significant (NNTB: 60; 95% CI NNTH 77 to ∞ NNTB 21) |
| PONV | 4 | 1039 | Not significant (RR 1.411, 95% CI 0.778–2.559) | 0.0/0.000 | Not cross | Not cross | 10.4% (1039 of 9973 patients) | Not significant (NNTH: 74; 95% CI NNTH 28 to ∞ NNTB 111) |
| Outcomes | Number of Studies | Quality Assessment | Quality | ||||
|---|---|---|---|---|---|---|---|
| ROB | Inconsistency | Indirectness | Imprecision | Publication Bias | |||
| Sedation success rate | 7 | not serious | not serious | not serious | not serious | NA | ⨁⨁⨁⨁ High |
| Hypoxemia | 6 | not serious | not serious | not serious | not serious | NA | ⨁⨁⨁⨁ High |
| Hypotension | 6 | not serious | serious a | not serious | not serious | NA | ⨁⨁⨁𐤏 Moderate |
| Bradycardia | 5 | not serious | not serious | not serious | not serious | NA | ⨁⨁⨁⨁ High |
| Respiratory depression | 4 | not serious | not serious | not serious | serious b | NA | ⨁⨁⨁𐤏 Moderate |
| Time to LOC | 4 | not serious | serious a | not serious | serious b | NA | ⨁⨁𐤏𐤏 Low |
| Recovery time | 4 | not serious | serious a | not serious | serious b | NA | ⨁⨁𐤏𐤏 Low |
| Injection pain | 4 | not serious | not serious | not serious | serious b | NA | ⨁⨁⨁𐤏 Moderate |
| Dizziness/headache | 4 | not serious | not serious | not serious | serious b | NA | ⨁⨁⨁𐤏 Moderate |
| PONV | 4 | not serious | not serious | not serious | serious b | NA | ⨁⨁⨁𐤏 Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Lee, C.; Choi, G.J.; Kang, H. Remimazolam for Procedural Sedation in Older Patients: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. J. Pers. Med. 2024, 14, 276. https://doi.org/10.3390/jpm14030276
Lee M, Lee C, Choi GJ, Kang H. Remimazolam for Procedural Sedation in Older Patients: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Journal of Personalized Medicine. 2024; 14(3):276. https://doi.org/10.3390/jpm14030276
Chicago/Turabian StyleLee, Myeongjong, Cheol Lee, Guen Joo Choi, and Hyun Kang. 2024. "Remimazolam for Procedural Sedation in Older Patients: A Systematic Review and Meta-Analysis with Trial Sequential Analysis" Journal of Personalized Medicine 14, no. 3: 276. https://doi.org/10.3390/jpm14030276
APA StyleLee, M., Lee, C., Choi, G. J., & Kang, H. (2024). Remimazolam for Procedural Sedation in Older Patients: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Journal of Personalized Medicine, 14(3), 276. https://doi.org/10.3390/jpm14030276









