Age Is a Predictor of In-Hospital Outcomes for Left Ventricular Assist Device Implantation: A Nationwide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patients and Variables
2.3. Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Device and Surgical Techniques Improvement
4.2. Organ Availability and Patients’ Status
4.3. Shift from BTT to DT
4.4. LOS and Impact of Age
4.5. Increased Utilization of Nonhome Destination
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colvin, M.; Smith, J.M.; Hadley, N.; Skeans, M.A.; Uccellini, K.; Goff, R.; Foutz, J.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 Annual Data Report: Heart. Am. J. Transpl. 2020, 20 (Suppl. S1), 340–426. [Google Scholar] [CrossRef]
- Estep, J.D.; Starling, R.C.; Horstmanshof, D.A.; Milano, C.A.; Selzman, C.H.; Shah, K.B.; Loebe, M.; Moazami, N.; Long, J.W.; Stehlik, J.; et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results from the ROADMAP Study. J. Am. Coll. Cardiol. 2015, 66, 1747–1761. [Google Scholar] [CrossRef]
- Nordan, T.; Critsinelis, A.C.; Ortoleva, J.; Kiernan, M.S.; Vest, A.; DeNofrio, D.; Chen, F.Y.; Couper, G.S.; Kawabori, M. Durable Left Ventricular Assist Device as a Bridge to Heart Transplantation under the New Donor Heart Allocation System. ASAIO J. 2021, 68, 890–898. [Google Scholar] [CrossRef]
- Goff, R.R.; Uccellini, K.; Lindblad, K.; Hall, S.; Davies, R.; Farr, M.; Silvestry, S.; Rogers, J.G. A change of heart: Preliminary results of the US 2018 adult heart allocation revision. Am. J. Transpl. 2020, 20, 2781–2790. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network. Adult Heart Allocation. Available online: https://optn.transplant.hrsa.gov/professionals/by-organ/heart-lung/adult-heart-allocation/ (accessed on 22 May 2023).
- Uriel, M.H.; Clerkin, K.J.; Takeda, K.; Naka, Y.; Sayer, G.T.; Uriel, N.; Topkara, V.K. Bridging to transplant with HeartMate 3 left ventricular assist devices in the new heart organ allocation system: An individualized approach. J. Heart Lung Transpl. 2023, 42, 124–133. [Google Scholar] [CrossRef]
- Gonuguntla, K.; Patil, S.; Cowden, R.G.; Kumar, M.; Rojulpote, C.; Bhattaru, A.; Tiu, J.G.; Robinson, P. Predictors of in-hospital mortality in patients with left ventricular assist device. Ir. J. Med. Sci. 2020, 189, 1275–1281. [Google Scholar] [CrossRef]
- Khalil, F.; Asleh, R.; Perue, R.K.; Weinstein, J.-M.; Solomon, A.; Betesh-Abay, B.; Briasoulis, A.; Alnsasra, H. Vascular Function in Continuous Flow LVADs: Implications for Clinical Practice. Biomedicines 2023, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Doulamis, I.P.; Inampudi, C.; Kourek, C.; Mandarada, T.; Kuno, T.; Asleh, R.; Briasoulis, A. Characteristics and outcomes of left ventricular assist device recipients transplanted before and after the new donor heart allocation system. Artif. Organs 2022, 46, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Bostic, R.; Tong, K.; Russo, M.; Rogers, J.G. Temporal changes in hospital costs for left ventricular assist device implantation. J. Card. Surg. 2011, 26, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Cost and Utilization Project (HCUP): HCUP Nationwide Inpatient Sample. Available online: http://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 22 April 2023).
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Angraal, S.; Couch, T.; Welsh, J.W.; Nallamothu, B.K.; Girotra, S.; Chan, P.S.; Krumholz, H.M. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA 2017, 318, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.; Narroway, H.; Williams, M.; Brookes, J.; Farag, J.; Cistulli, D.; Bannon, P.; Marasco, S.; Potapov, E.; Loforte, A. Contemporary outcomes of continuous-flow left ventricular assist devices-a systematic review. Ann. Cardiothorac. Surg. 2021, 10, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.S.; DeFilippis, E.M.; Cowger, J.A.; Netuka, I.; Pinney, S.P.; Givertz, M.M. Trends and Outcomes of Left Ventricular Assist Device Therapy: JACC Focus Seminar. J. Am. Coll Cardiol. 2022, 79, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Holley, C.T.; Eckman, P.; Roy, S.S.; Cogswell, R.; Harvey, L.; Shumway, S.; Liao, K. A Decade of Experience with Continuous-Flow Left Ventricular Assist Devices. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Starling, R.C. Patient Population and Selection Criteria for Mechanical Circulatory Support. In Mechanical Support for Heart Failure: Current Solutions and New Technologies; Karimov, J.H., Fukamachi, K., Starling, R.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 131–139. [Google Scholar]
- Özer, T.; Gunay, D.; Hancer, H.; Yerlikhan, O.A.; Ozgur, M.M.; Aksut, M.; Sarikaya, S.; Kirali, K. Transition from Conventional Technique to Less Invasive Approach in Left Ventricular Assist Device Implantations. ASAIO J. 2020, 66, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Funamoto, M.; Osho, A.; Wolfe, S.; Kubi, B.; Singh, R.; D’Alessandro, D.A. Effect of the 2018 heart allocation system on patients with durable left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2024, 167, 217–230.e9. [Google Scholar] [CrossRef]
- Mullan, C.W.; Chouairi, F.; Sen, S.; Mori, M.; Clark, K.A.; Reinhardt, S.W.; Miller, P.E.; Fuery, M.A.; Jacoby, D.; Maulion, C.; et al. Changes in Use of Left Ventricular Assist Devices as Bridge to Transplantation with New Heart Allocation Policy. JACC Heart Fail 2021, 9, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, R.; John, R.; Estep, J.D.; Duval, S.; Tedford, R.J.; Pagani, F.D.; Martin, C.M.; Mehra, M.R. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J. Heart Lung Transpl. 2020, 39, 1–4. [Google Scholar] [CrossRef]
- Radhoe, S.P.; Veenis, J.F.; Jakus, N.; Timmermans, P.; Pouleur, A.C.; Rubís, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Barge-Caballero, E.; Paolillo, S.; et al. How does age affect outcomes after left ventricular assist device implantation: Results from the PCHF-VAD registry. ESC Heart Fail 2023, 10, 884–894. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Schroeder, S.E.; Houston, B.A.; Robinson, M.R.; Gosev, I.; Reyentovich, A.; Koehl, D.; Cantor, R.; Jorde, U.P.; Kirklin, J.K.; et al. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: Focus on the 2018 Heart Transplant Allocation System. Ann. Thorac. Surg. 2023, 115, 311–327. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Cantor, R.; Mohacsi, P.; Gummert, J.; De By, T.; Hannan, M.M.; Kormos, R.L.; Schueler, S.; Lund, L.H.; Nakatani, T.; et al. First Annual IMACS Report: A global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J. Heart Lung Transpl. 2016, 35, 407–412. [Google Scholar] [CrossRef]
- Melendo-Viu, M.; Dobarro, D.; Roubin, S.R.; Pernas, C.L.; Cordón, C.M.; Lamas, M.V.; Esteban, M.P.; Martínez, M.V.; Abu Assi, E.; Romero, R.P.; et al. Left Ventricular Assist Device as a Destination Therapy: Current Situation and the Importance of Patient Selection. Life 2023, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Nayak, A.; Morris, A.A.; Lanfear, D.E.; Nemeh, H.; Desai, S.; Bansal, A.; Guerrero-Miranda, C.; Hall, S.; Cleveland, J.C.; et al. Prediction of survival after implantation of a fully magnetically levitated left ventricular assist device. JACC Heart Fail 2022, 10, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Hall, S.A.; Uriel, N.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Crandall, D.; et al. Predictors of 5-Year Mortality in Patients Managed with a Magnetically Levitated Left Ventricular Assist Device. J. Am. Coll. Cardiol. 2023, 82, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ayers, B.C.; Wood, K.; McNitt, S.; Goldenberg, I.; Chen, L.; Alexis, J.; Vidula, H.; Thomas, S.; Storozynsky, E.; Barrus, B.; et al. Association of previous cardiac surgery with outcomes in left ventricular assist device patients. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Chou, B.B.P.; Critsinelis, A.; Lamba, H.K.; Long, G.; Civitello, A.B.; Delgado, R.M.; Chatterjee, S. Continuous-Flow Left Ventricular Assist Device Support in Patients with Ischemic Versus Nonischemic Cardiomyopathy. Tex. Heart Inst. J. 2021, 48, e207241. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Taha, M.; Pisipati, S.; Patel, K.; Al-Khafaji, J.; Desai, R.; Shah, J.; Gullapalli, N. Impact of chronic kidney disease on in-hospital outcomes following left ventricular assist device placement: A national perspective. Heart Lung 2020, 49, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, C.; Tran, D.; George, P.; Sorensen, E.; Kaczorowski, D.J.; Ton, V.-K.; Kon, Z.N.; Vorhees, H.; Sawan, M.; Griffith, B.P. Left ventricular assist device implantation may be feasible in appropriately selected patients with severe renal insufficiency. J. Thorac. Cardiovasc. Surg. 2020, 159, 1307–1319.e2. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Frogel, J.; Ram, E.; Jamal, T.; Peled-Potashnik, Y.; Maor, E.; Grupper, A.; Morgan, A.; Segev, A.; Raanani, E.; et al. The impact of diabetes on short-, intermediate- and long-term mortality following left ventricular assist device implantation. Eur. J. Cardio-Thoracic Surg. 2022, 61, 1432–1437. [Google Scholar] [CrossRef]
- Zhou, P.; Xiao, Z.; Zhu, P.; Nie, Z.; Pavan, D.; Zheng, S. Diabetes Mellitus Is Not a Risk Factor for Patients Supported with Left Ventricular Assist Device. Ann. Thorac. Surg. 2020, 109, 1614–1622. [Google Scholar] [CrossRef]
- Grant, J.K.; Ebner, B.; Vincent, L.; Maning, J.; Olorunfemi, O.; Olarte, N.I.; Colombo, R.; Munagala, M.; Chaparro, S. Assessing in-hospital cardiovascular, thrombotic and bleeding outcomes in patients with chronic liver disease undergoing left ventricular assist device implantation. Thromb. Res. 2021, 202, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Cotts, W.G.; McGee, E.C., Jr.; Myers, S.L.; Naftel, D.C.; Young, J.B.; Kirklin, J.K.; Grady, K.L. Predictors of hospital length of stay after implantation of a left ventricular assist device: An analysis of the INTERMACS registry. J. Heart Lung Transpl. 2014, 33, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Sanaiha, Y.; Downey, P.; Lyons, R.; Nsair, A.; Shemin, R.J.; Benharash, P. Trends in utilization, mortality, and resource use after implantation of left ventricular assist devices in the United States. J. Thorac. Cardiovasc. Surg. 2021, 161, 2083–2091.e4. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Givertz, M.M.; Stewart, G.C.; Mudge, G.H., Jr. Ventricular assist devices the challenges of outpatient management. J. Am. Coll. Cardiol. 2009, 54, 1647–1659. [Google Scholar] [CrossRef]
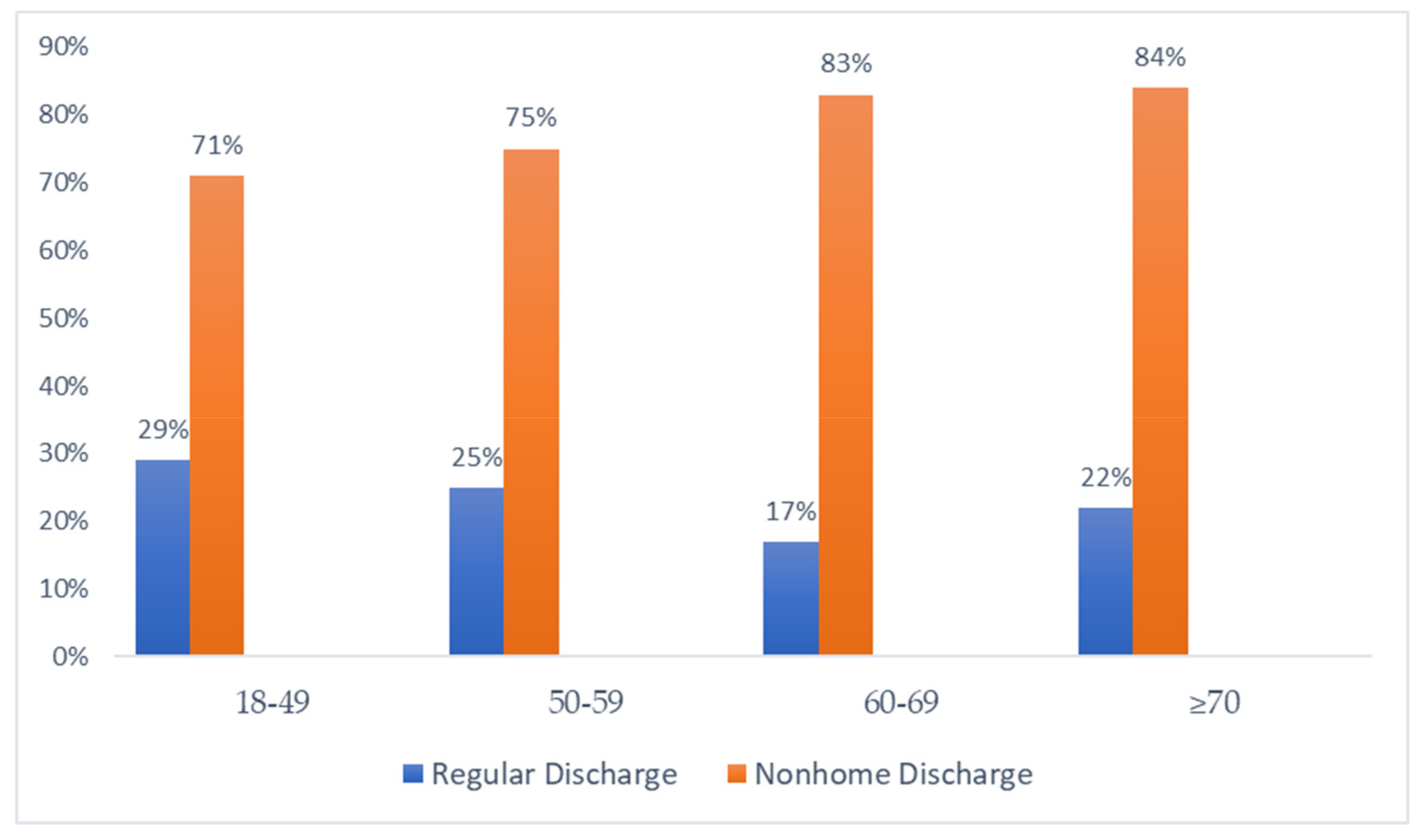
| 18–49 | 50–59 | 60–69 | ≥70 | p-Value | |
|---|---|---|---|---|---|
| Total (n = 7375) | 1935 | 1900 | 2300 | 1240 | |
| Mean Age | 38 | 55 | 64 | 73 | 0.000 |
| Male | 73% | 72% | 75% | 85% | 0.003 |
| Race | |||||
| White | 40% | 57% | 61% | 80% | 0.000 |
| Black | 44% | 32% | 27% | 14% | |
| Hispanic | 8% | 7% | 5% | 4% | |
| Asian or Pacific Islander | 3% | 1% | 2% | 1% | |
| Insurance | |||||
| Medicare | 28% | 32% | 62% | 88% | 0.000 |
| Medicaid | 32% | 18% | 8% | 0.0% | |
| Private | 37% | 47% | 30% | 11% | |
| Self-pay | 3% | 2% | 0.00% | 0.00% | |
| Median household income | |||||
| $1–$49,999 | 37% | 29% | 26% | 21% | 0.000 |
| $50,000–$64,999 | 24% | 31% | 30% | 24% | |
| $65,000–85,999 | 20% | 24% | 27% | 26% | |
| $86,000 or more | 19% | 16% | 17% | 28% | |
| Hospital Region | |||||
| Northeast | 18% | 17% | 20% | 26% | 0.143 |
| Midwest | 27% | 28% | 27% | 24% | |
| South | 44% | 43% | 42% | 35% | |
| West | 11% | 11% | 11% | 15% | |
| Relative bed size category of hospital | |||||
| Small | 2% | 3% | 2% | 7% | 0.008 |
| Medium | 6% | 6% | 9% | 10% | |
| Large | 92% | 91% | 89% | 83% | |
| Location/teaching status of hospital | |||||
| Rural | N/A | N/A | N/A | N/A | 0.054 |
| Urban non-teaching | 0% | 1% | 0% | 2% | |
| Urban teaching | 100% | 99% | 100% | 98% | |
| Charlson Comorbidity Index | |||||
| 0 | 0% | 0% | 0% | 1% | 0.000 |
| 1 | 20% | 14% | 9% | 10% | |
| 2 | 21% | 14% | 13% | 12% | |
| ≥3 | 59% | 71% | 78% | 78% | |
| Hypertension | 2% | 1% | 1% | 2% | 0.924 |
| Chronic heart failure | 97% | 97% | 99% | 98% | 0.143 |
| Coronary artery disease | 28% | 52% | 63% | 62% | 0.000 |
| Dyslipidemia | 19% | 34% | 36% | 37% | 0.000 |
| Previous myocardial infarction | 9% | 16% | 17% | 14% | 0.011 |
| Chronic obstructive pulmonary disease | 18% | 22% | 20% | 18% | 0.469 |
| Liver Disease | 14% | 12% | 9% | 8% | 0.019 |
| Chronic kidney disease | 9% | 7% | 7% | 10% | 0.281 |
| Peripheral vascular disease | 5% | 8% | 12% | 15% | 0.000 |
| Previous coronary artery bypass grafting | 1% | 5% | 10% | 10% | 0.000 |
| Smoking | 27% | 24% | 27% | 22% | 0.473 |
| 18–49 | 50–59 | 60–69 | ≥70 | p-Value | |
|---|---|---|---|---|---|
| In-hospital mortality | 140 (7%) | 115 (6%) | 265 (12%) | 215 (17%) | 0.000 |
| Non-elective admission | 405 (21%) | 500 (26%) | 665 (29%) | 435 (35%) | 0.000 |
| Driveline infection | 125 (6%) | 70 (4%) | 100 (4%) | 35 (3%) | 0.1 |
| Acute kidney injury | 1425 (74%) | 1335 (70%) | 1765 (77%) | 940 (76%) | 0.169 |
| Acute kidney injury requiring dialysis | 205 (11%) | 190 (10%) | 180 (8%) | 105 (8%) | 0.58 |
| Ischemic stroke | 70 (4%) | 110 (6%) | 105 (5%) | 80 (6%) | 0.325 |
| Postoperative bleeding | 390 (20%) | 395 (21%) | 570 (25%) | 170 (14%) | 0.005 |
| Intracranial hemorrhage | 60 (3%) | 40 (2%) | 80 (3%) | 25 (2%) | 0.55 |
| Gastrointestinal bleeding | 170 (9%) | 200 (11%) | 310 (13%) | 100 (8%) | 0.06 |
| Hemoptysis | 85 (4%) | 55 (3%) | 105 (5%) | 25 (2%) | 0.23 |
| Hematuria | 45 (2%) | 45 (2%) | 60 (3%) | 10 (1%) | 0.47 |
| Unspecified bleeding | 75 (4%) | 85 (4%) | 100 (4%) | 20 (2%) | 0.39 |
| Epistaxis | 0 (0.00%) | 10 (0.01%) | 5 (0.00%) | 0 (0.00%) | 0.24 |
| Bleeding requiring transfusion | 105 (5%) | 110 (6%) | 145 (6%) | 30 (2%) | 0.17 |
| Septic shock | 170 (9%) | 160 (8%) | 170 (7%) | 70 (6%) | 0.415 |
| Variables | Year | 18–49 | p-Value | 50–59 | p-Value | 60–69 | p-Value | ≥70 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| In-hospital mortality | 2019 | 5% | 0.1 | 6.3% | 0.84 | 11.1% | 0.77 | 16.1% | 0.6 |
| 2020 | 9.6% | 6% | 12% | 18.5% | |||||
| Acute kidney injury requiring dialysis | 2019 | 8% | 0.14 | 8.7% | 0.4 | 6% | 0.14 | 4% | 0.02 |
| 2020 | 13.3% | 11.5% | 10% | 13% | |||||
| Intracranial hemorrhage | 2019 | 3% | 0.92 | 2.4% | 0.62 | 3.1% | 0.7 | 2.4% | 0.65 |
| 2020 | 3.2% | 1.7% | 3.8% | 1.6% | |||||
| Gastrointestinal bleeding | 2019 | 7.5% | 0.36 | 10.2% | 0.83 | 14.3% | 0.58 | 9.6% | 0.33 |
| 2020 | 10.1% | 10.9% | 12.5% | 6.5% | |||||
| Postoperative bleeding | 2019 | 19.1% | 0.59 | 19.9% | 0.66 | 25.8% | 0.59 | 15.3% | 0.45 |
| 2020 | 21.3% | 21.8% | 23.6% | 12.1% | |||||
| Ischemic stroke | 2019 | 3.5% | 0.91 | 4.4% | 0.21 | 5% | 0.83 | 8.1% | 0.28 |
| 2020 | 3.7% | 7.5% | 4.3% | 5% | |||||
| Driveline infection | 2019 | 4.5% | 0.08 | 3.4% | 0.73 | 5% | 0.63 | 4% | 0.25 |
| 2020 | 8.6% | 4% | 4% | 2% | |||||
| Septic shock | 2019 | 8% | 0.33 | 6.3% | 0.11 | 6.7% | 0.57 | 4% | 0.25 |
| 2020 | 10% | 11% | 8.2% | 7.3% |
| Variable | Adjusted Odds Ratio | p-Value | Confidence Interval |
|---|---|---|---|
| Age category | |||
| 50–59 | 0.89 | 0.76 | 0.44–1.82 |
| 60–69 | 1.99 | 0.02 | 1.12–3.57 |
| ≥70 | 2.88 | 0.002 | 1.45–5.71 |
| Female | 0.97 | 0.88 | 0.64–1.47 |
| Race | |||
| Black | 1.01 | 0.97 | 0.63–1.63 |
| Hispanic | 0.78 | 0.57 | 0.32–1.87 |
| Asian or Pacific Islander | 1.75 | 0.32 | 0.58–5.29 |
| Native American | 1.77 | 0.59 | 0.22–14.37 |
| Other | 1.01 | 0.98 | 0.35–2.92 |
| Elective admission | 0.65 | 0.08 | 0.4–1.05 |
| Hospital region | |||
| Midwest | 1.16 | 0.63 | 0.63–2.13 |
| South | 1.09 | 0.76 | 0.64–1.86 |
| West | 1.07 | 0.83 | 0.57–2.0 |
| Teaching hospital | 0.32 | 0.14 | 0.07–1.44 |
| Hospital bed size | |||
| Medium | 0.56 | 0.42 | 0.14–2.26 |
| Large | 0.97 | 0.96 | 0.31–3.01 |
| Insurance | |||
| Medicaid | 0.99 | 0.99 | 0.56–1.76 |
| Private | 1.1 | 0.67 | 0.71–1.70 |
| Self-pay | 0.52 | 0.53 | 0.06–4.1 |
| Income | |||
| $50,000–64,999 | 0.87 | 0.6 | 0.52–1.46 |
| $65,000–85,999 | 0.98 | 0.94 | 0.58–1.64 |
| $86,000 or more | 1.46 | 0.19 | 0.83–2.55 |
| Hypertension | 1.52 | 0.53 | 0.41–5.62 |
| Chronic obstructive lung disease | 0.58 | 0.07 | 0.32–1.05 |
| Chronic kidney disease | 0.3 | 0.03 | 0.1–0.9 |
| Coronary artery disease | 0.6 | 0.03 | 0.39–0.94 |
| Previous myocardial infarction | 0.15 | 0.001 | 0.05–0.47 |
| Diabetes mellitus type 2 | 0.59 | 0.01 | 0.4–0.88 |
| Obesity | 1.21 | 0.47 | 0.72–2.06 |
| Peripheral vascular disease | 0.81 | 0.58 | 0.38–1.71 |
| Liver disease | 0.53 | 0.12 | 0.24–1.18 |
| Previous coronary artery bypass grafting | 1.1 | 0.86 | 0.44–2.7 |
| Charlson Comorbidity Index | 1.39 | 0.02 | 1.05–1.84 |
| Age Category | Mean Length of Stay | SD | p-Value |
|---|---|---|---|
| 18–49 | 39.1 | 36.3–41.9 | |
| 50–59 | 37.7 | 34.9–40.6 | <0.01 |
| 60–69 | 36.5 | 33.9–39.1 | |
| ≥70 | 31.9 | 29.2–34.5 |
| Age Category | Mean Total Charge (105) | SD | p-Value |
|---|---|---|---|
| 18–49 | 1.25 | 1.13–1.35 | |
| 50–59 | 1.14 | 1.04–1.23 | 0.23 |
| 60–69 | 1.17 | 1.09–1.26 | |
| ≥70 | 1.11 | 1.01–1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkawi, A.R.; Yamaguchi, A.; Shimamura, J.; Chehab, O.; Alvarez, P.; Kuno, T.; Briasoulis, A. Age Is a Predictor of In-Hospital Outcomes for Left Ventricular Assist Device Implantation: A Nationwide Analysis. J. Pers. Med. 2024, 14, 236. https://doi.org/10.3390/jpm14030236
Akkawi AR, Yamaguchi A, Shimamura J, Chehab O, Alvarez P, Kuno T, Briasoulis A. Age Is a Predictor of In-Hospital Outcomes for Left Ventricular Assist Device Implantation: A Nationwide Analysis. Journal of Personalized Medicine. 2024; 14(3):236. https://doi.org/10.3390/jpm14030236
Chicago/Turabian StyleAkkawi, Abdul Rahman, Akira Yamaguchi, Junichi Shimamura, Omar Chehab, Paulino Alvarez, Toshiki Kuno, and Alexandros Briasoulis. 2024. "Age Is a Predictor of In-Hospital Outcomes for Left Ventricular Assist Device Implantation: A Nationwide Analysis" Journal of Personalized Medicine 14, no. 3: 236. https://doi.org/10.3390/jpm14030236
APA StyleAkkawi, A. R., Yamaguchi, A., Shimamura, J., Chehab, O., Alvarez, P., Kuno, T., & Briasoulis, A. (2024). Age Is a Predictor of In-Hospital Outcomes for Left Ventricular Assist Device Implantation: A Nationwide Analysis. Journal of Personalized Medicine, 14(3), 236. https://doi.org/10.3390/jpm14030236







