Abstract
Background: Extracorporeal life support (ECLS) is pivotal for sustaining the function of failing hearts and lungs, and its utilization has risen. In cases where conventional cannulation strategies prove ineffective for providing adequate ECLS support, the implementation of an enhanced system with a third cannula may become necessary. Hybrid ECLS may be warranted in situations characterized by severe hypoxemia of the upper extremity, left ventricular congestion, and dilatation. Additionally, it may also be considered for patients requiring respiratory support or experiencing hemodynamic instability. Method: All hybrid ECLS cases of adults at the University Hospital Zurich, Switzerland, between January 2007 and December 2019 with initial triple cannulation were included. Data were collected via a retrospective review of patient records and direct export of the clinical information system. Results: 28 out of 903 ECLS cases were initially hybrid cannulated (3.1%). The median age was 57 (48.2 to 60.8) years, and the sex was equally distributed. The in-hospital mortality of hybrid ECLS was high (67.9%). In-hospital mortality rates differ depending on the indication (ARDS: 36.4%, refractory cardiogenic shock: 88.9%, cardiopulmonary resuscitation: 100%, post-cardiotomy: 100%, others: 75%). Survivors exhibited a lower SAPS II level compared with non-survivors (20.0 (12.0 to 65.0) vs. 55.0 (45.0 to 73.0)), and the allogenic transfusion of platelet concentrate was observed to be less frequent for survivors (0.0 (0.0) vs. 1.8 (2.5) units). Conclusion: The in-hospital mortality rate for hybrid ECLS was high. Different indications showed varying mortality rates, with survivors having lower SAPS II scores and requiring fewer platelet concentrate transfusions. These findings highlight the complexities of hybrid ECLS outcomes in different clinical scenarios and underline the importance of rigorous patient selection.
1. Introduction
Extracorporeal life support (ECLS) plays a critical role in cardiopulmonary assistance once conventional measures have been exhausted. It has become a key support instrument during organ recovery or the onset of destination therapy [1,2]. Veno-venous (V-V) and veno-arterial (VA) ECLS modifications are standard. If oxygenation or ventilation is predominantly impaired, support is provided by passing venous blood through an artificial lung membrane. The afferent limb returns the decarboxylated and oxygenated blood back to the venous system. If a patient has cardiovascular disease and consequent hemodynamic instability, the blood from the membrane lung can be returned to the arterial system. In addition to oxygenation, it provides a pumping function to maintain perfusion pressure. Specific limitations such as age, malignancy, or patient preference, as well as structural and staffing requirements, must be considered [3].
During ECLS therapy, the supply of oxygenated blood to the coronary arteries and brain is dependent on pulmonary function (V-V) or retrograde aortal flow (VA) [4]. However, a modified system with an additional third (sometimes even fourth) cannula is required in some cases in which ineffective ECLS support presents with a mixed picture of hypoperfusion and hypoxemia due to competing or insufficient circuits [5]. Unfortunately, only few data are available on the adaption of the ECLS toward a hybrid cannulation [6,7,8,9,10,11,12]. According to the Extracorporeal Life Support Organization (ELSO) report 2017, 2% of adults supported with ECLS for cardiac failure and less than 1% on ECMO for respiratory failure are managed with a hybrid cannulation [13]. The analysis of the Chinese Extracorporeal Life Support Registry (CSECLS) showed 19 (0.6%) initial hybrid configurations out of 3102 adult ECLS cases between 2017 and 2019 [6].
In recent years, there has been increasing awareness of the need for a standardized nomenclature for ECLS therapy. The use of standardized descriptions of the ECLS modification allows for comparison and analysis (also from center to center). For this purpose, the ELSO position papers provide concrete rules for the ECLS nomenclature [14,15]. The use of a hyphen to differentiate drainage cannulas to the left of the hyphen from return cannulas to the right of the hyphen is the core of any cannulation abbreviation. The hyphen itself symbolizes the membrane lung (ML). According to the Maastricht Treaty for ECLS nomenclature [14], the third added cannula is defined by an additional capital letter placed outside the existing cannulas. For example, if a second venous drain cannula is added, the letter “V” is placed to the left of the hyphen and outside the first drain cannula, resulting in “VV-A”. In a V-A setup, if an additional re-entry cannula is inserted alongside the initial arterial cannula to improve systemic oxygenation, the letter “V” is added to the outer right to indicate post-ML flow (return): “V-AV”. The insertion of an arterial cannula into a “V-V” configuration is indicated as “V-VA”. Consequently, the order of configurations during ECLS therapy reflects the patient’s temporal support needs and conveys a chronological sequence. But the nomenclature lacks differentiation between V-V and V-A proportional support. And from a physiological standpoint, V-VA is indistinguishable from V-AV. Besides the cannula hierarchy and localization, the position of the cannula tip and its dimensions are also relevant.
The hybrid ECLS configuration supports patients with combined cardiopulmonary failure who cannot be successfully assisted with the conventional veno-venous or veno-arterial alone. Hybrid ECLS may be required in conditions of severe hypoxemia of the upper extremity, also known as Harlequin syndrome or north–south syndrome [2], but also in cases of left ventricular congestion and dilatation [5]. A supplemental inflow cannula can be placed in the internal jugular vein to deliver oxygenated blood to the pulmonary circulation. This helps correct differential hypoxemia by directing oxygenated blood back to the right ventricle then through the pulmonary circulation, left ventricle, and out to the coronary arteries and aortic arch vessels [16]. Another relevant indication includes ECMO patients receiving respiratory support who also exhibit concurrent hemodynamic instability, particularly in cases of right heart failure [5] but also in left or biventricular failure. For hemodynamic support, an arterial perfusion cannula is inserted into the circuit, usually via the femoral or subclavian artery.
This study investigates the factors that determine the association between hybrid ECLS and patient outcomes. We analyzed all initially hybrid cannulated ECLS cases between the period from 2007 to 2019 at the University Hospital of Zurich.
2. Materials and Methods
2.1. Study Design
All hybrid ECLS cases of adults at the University Hospital Zurich (USZ) in Switzerland between January 2007 and December 2019 with initial triple cannulation were included. We differentiated V-AV (efferent limb venous; afferent arteriovenous) and VV-A (efferent limb veno-venous; afferent arterial) configuration. Modifications at a later stage of V-A ECLS and V-V ECMO were not included. Further exclusion criteria were age below 18 years and documented refusal of general consent. We grouped indications for hybrid ECLS therapy into five common categories according to the current literature [1,2,6,8]: “acute respiratory distress syndrome”, “refractory cardiogenic shock”, “cardiopulmonary resuscitation”, “post-cardiotomy”, and “other”.
The study was approved, and the requirement for written informed consent was waived by the Cantonal Ethics Commission of Zurich, Switzerland (BASEC-Nr. 2019-01926).
2.2. Assessment of Hybrid Modification
Based on the underlying disease, the indication was for either V-V or V-A ECLS therapy. In cases where the medical team, consisting of a cardiac anesthesiologist or intensivist, a cardiac surgeon, and a perfusionist, identified inadequate drainage or perfusion during initialization of the baseline configuration, a hybrid procedure was adapted. As recommended in the ELSO nomenclature, when a VVA mode configuration is implemented primarily for cardiac support, it is expressed as V-AV (initial indication V-A).
2.3. Study Endpoints
We recorded the in-hospital mortality to compare survivors and non-survivors. Furthermore, 1-year mortality was assessed.
2.4. Data Collection and Variables
Data were collected via a retrospective review of the medical records of all included patients with hybrid ECLS (medical history, last laboratory values, ECLS configuration and duration, complications, length of ICU stay, and outcomes) and direct export of the clinical information system via medical controlling (age, gender, number of transfused red blood cells, fresh frozen plasma, and platelet concentrate, and length of hospital stay).
2.5. Statistical Analysis
For descriptive statistics, we show the median and interquartile range (IQR 25% to 75%) for continuous variables. For categorical variables, we show counts (n) and proportions (%). Due to the small sample size, we chose not to present statistical tests, as this would quickly lead to a multiple-testing problem, which in turn would make any meaningful statistical interpretation impossible.
3. Results
We screened 903 ECLS cases between 2007 and 2019, 28 of whom (3.1%) were instances of initial hybrid ECLS cannulation and matched the inclusion criteria (Figure 1).
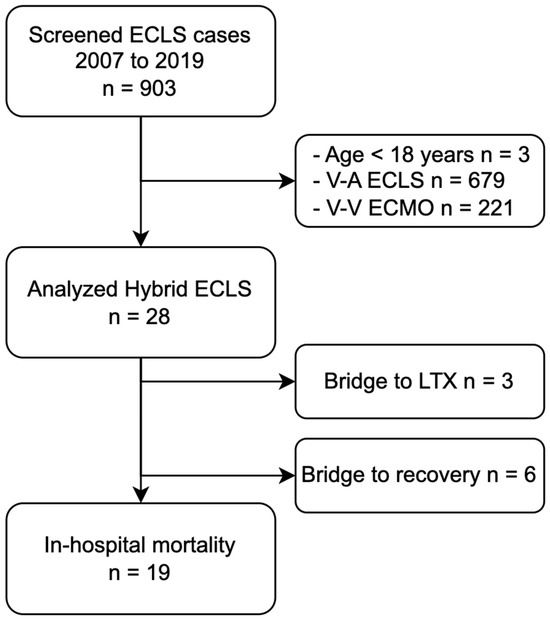
Figure 1.
Flowchart of patient selection and hybrid ECLS outcome. Abbreviations: ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; LTX, lung transplantation; V-A, veno-arterial; V-V, veno-venous.
3.1. Patient Characteristics and Mortality
The median age was 57 (48.2 to 60.8) years, and the sex was equally distributed. The most frequent comorbidity at the time of ECLS cannulation was coronary artery disease (17.9%). Patients had a high SAPS II within the first 24 h of ICU admission, and survivors exhibited a lower SAPS II level compared with non-survivors (20.0 (12.0 to 65.0) vs. 55.0 (45.0 to 73.0)) (Table 1). Fifteen patients died during hybrid ECLS therapy, and four patients died after weaning. Overall, the in-hospital mortality was high (19 out of 28, 67.9%) (Table 2).

Table 1.
Characteristics of hybrid ECLS patients stratified for survivors and non-survivors.

Table 2.
Description of hybrid ECLS cases stratified for survivors and non-survivors.
3.2. Outcomes of Survivors
Six patients were successfully weaned, and three patients had bridged-to-lung transplantation. Except for two patients who were lost to follow-up, all patients were alive at one year (Table 3).

Table 3.
Outcome of survivors.
3.3. Hybrid ECLS Indication
The largest group of hybrid ECLS indications was represented by patients suffering from ARDS who showed a higher survival at hospital discharge (7 out of 11 cases), resulting in an in-hospital mortality of 36.4% (Figure 2). Patients with refractory cardiogenic shock showed a high in-hospital mortality of 88.9% (eight out of nine). Patients with hybrid ECLS indications for cardiopulmonary resuscitation and post-cardiotomy showed 100% in-hospital mortality (two out of two each). The indication “other” consisted of two patients suffering from acute respiratory insufficiency (not defined as ARDS), one patient with pulmonary scleroderma, and one patient diagnosed with obstructive shock due to an atrial tumor. Only one of the four patients in the “other” group survived, resulting in an in-hospital mortality of 75%.
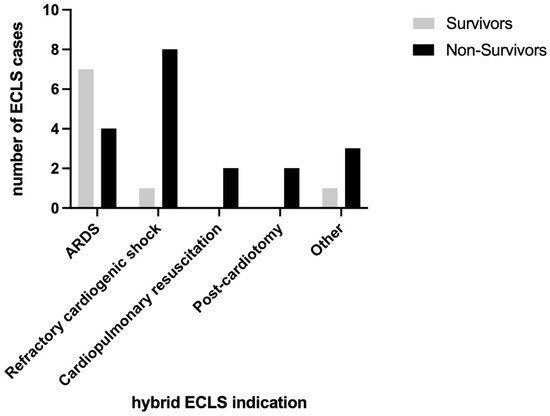
Figure 2.
Survivors and non-survivors stratified by indication of hybrid ECLS.
3.4. Hybrid ECLS Cannulation Details
Most cannulations were performed peripherally using the Seldinger technique (71.4%). Six ECLS cases were performed as V-AV configurations and the others as VV-A (Figure 3). Regarding the efferent ECLS limb, a venous femoral drain was installed in each case. The further combinations of V-AV and VV-A modifications are shown in Figure 4. The in-hospital mortality was higher in the VV-A group (17 of 22, 77.3%) compared to the V-AV group (2 of 6, 33.3%).
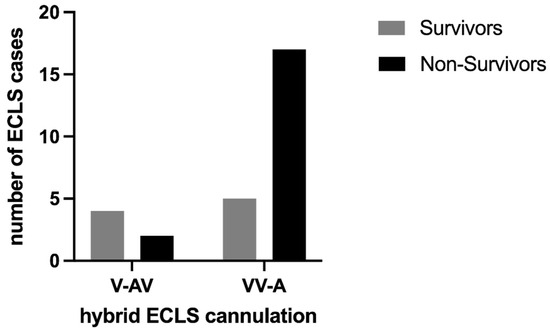
Figure 3.
Survivors and non-survivors stratified by cannulation of hybrid ECLS.

Figure 4.
Cannulation sites for hybrid configuration according to frequency. (All) All hybrid ECLS cannulation percentages together, (V-AV) venous-arteriovenous hybrid modification (6 cases), and (VV-A) venovenous-arterial hybrid modification (22 cases). The cannulation site of one case (efferent right atrium and afferent pulmonary artery, 3.6% each) is not shown due to visibility. The right or left site is arbitrary. Note that the sum of percentages is equal to 300% due to three cannulation sites each. Abbreviations: jv internal jugular vein; sa subclavian artery; fv femoral vein; fa femoral artery. Created with BioRender.com, accessed on 9 December 2023, with confirmation of publication and licensing rights.
3.5. Hybrid ECLS Complications
The allogenic transfusion of platelet concentrate was higher for non-survivors compared to survivors (1.8 vs. 0 units). Besides renal replacement therapy, major bleeding events were the most frequent complications and were also observed more frequently in the cohort of non-survivors (Table 2).
4. Discussion
This retrospective single-center study reports 28 initially hybrid-cannulated ECLS cases at the University Hospital of Zurich in the period from 2007 to 2019. We observed a high in-hospital mortality of 67.9% (n = 19); 4 of the 19 non-survivors died after ECLS weaning. The most frequent diagnosis at the time of hybrid ECLS installation was ARDS (11 patients), with more survivors than non-survivors in this group. Overall, non-survivors had a higher SAPS II and more frequently received transfusion of allogenic platelet concentrates.
Although only a few studies are available for hybrid ECLS with a low number of included patients (3 to 26 patients) [6,7,8,9,10,11,12,17], the in-hospital mortality observed at our institution is in line with that in recently published studies. Biscotti et al. [9] and Ius et al. [11] reported lower mortality rates of 57.1% and 50.0%, but they also included patients who were initially conventionally cannulated and only later modified to a hybrid strategy. ECLS indications in those studies were predominantly pulmonary diseases. Looking separately at the indication ARDS of our study, the in-hospital mortality is as low as 36.4%. This is in accordance with a study published in 2010 with a small number of cases [12]. A recently published retrospective study of patients with predominant ARDS on V-V ECMO support requiring a change to V-VA support showed an in-hospital mortality rate of 37% [18].
We recently published our outcome analysis of 679 V-A [19] and 221 V-V [20] ECLS cases. The frequency of hybrid ECLS in our institution (3%) is in line with published data of ELSO [13] (2% of adults supported for cardiac failure and less than 1% for respiratory failure). The Chinese Extracorporeal Life Support Registry (CSECLS) states that 0.6% of initial hybrid configurations between 2017 and 2019 [6].
The institutional comparison shows a higher in-hospital mortality rate between conventionally V-A-assisted and hybrid-assisted ECLS cases (V-A vs. hybrid: indication post-cardiotomy 70.7% vs. 100%, cardiopulmonary resuscitation 67.9% vs. 100%, refractory cardiogenic shock 47.0% vs. 88.9%). Mortality is nearly identical between V-V and hybrid-assisted patients (V-V vs. hybrid: indication ARDS 36.2% vs. 36.4%). It is likely that the sicker patients needed more support initially. In terms of selection bias, the initial hybrid cannulated patients also have a higher mortality rate. This finding should certainly be taken into account when the treatment team is considering hybrid cannulation. As compared to V-A and hybrid cannulation, V-V cannulated cases have fewer major bleeding complications (16.7%, 25.2%, and 28.6%) or leg ischemias (6.3%, 13.4%, and 10.7%) at our institution. Complication rates are similar between V-A and hybrid. But the small number of hybrid cannulated cases must be considered.
In our study, we describe three patients who were treated with a hybrid ECLS bridging procedure and who were finally treated with a lung transplantation. Bridging to heart transplantation was not observed. It is crucial to note that ECLS therapy is not a definitive treatment but rather a bridge to recovery in the context of significant lung or heart failure, as well as a bridge to decision-making. ECLS often necessitates high doses of anticoagulation, posing a risk of bleeding, particularly intracranial, and requiring massive transfusion [21]. This is associated with adverse effects on patient morbidity and mortality [22], not to mention the additional immunologic effects and increased risk of a positive cross-match in planned transplants. On the positive side, advancements in treatment protocols and expertise in specialized centers over the past decade have made bridging to lung transplantation feasible for patients with end-stage lung disease, yielding reasonable outcomes [23]. Apart from optimized gas exchange, these patients experience reduced sedation requirements and less confinement to the hospital bed, allowing for better-organized physiotherapy and nutrition. While pharmacological treatments for end-stage heart failure have improved, the majority of patients succumb to the disease, with heart transplantation being the sole option [24]. However, the scarcity of donor organs has led to the establishment of ECLS as the primary alternative, serving as a bridge to recovery or as a bridge-to-bridge, allowing for the subsequent implantation of a long-term device, or as a bridge-to-transplant [25]. It is important to recognize ECLS as a high-risk procedure with significant morbidity and mortality. Additionally, the limited availability of healthcare resources and ethical considerations must be taken into account.
The cannulation strategy depends on the underlying pathology and is implemented in various ways. Nevertheless, principles are now emerging to improve systemic oxygenation or cardiac unloading [26,27]. A V-VA configuration has been proposed in patients with differential hypoxia (Harlequin syndrome) or secondary heart failure (e. g., right ventricular impairment) after V-V ECMO initiation [5]. We describe six cases with an in-hospital mortality of 33.3%. The other cannulation mode, VV-A, combines the need for higher venous drainage with the possibility of providing both circulatory and respiratory support and is more complex [5]. Interestingly, this cannulation strategy is predominant in our study. Their markedly higher in-hospital mortality of 77.3% reflects this statement.
Besides renal replacement therapy, major bleeding events were the most prevalent complication during hybrid ECLS therapy. With a frequency of 28.6%, major bleeding occurred less frequently than described in the literature, with almost every second patient suffering from major bleeding [8]. In contrast to our expectation, complications like leg ischemia and intracranial bleeding were rare in our cohort. Werner et al. [7] and Mihu et al. [8] reported higher incidences of these complications, despite a conservative anticoagulation regimen. As a possible reason, they mention that more than half of their patients were externally cannulated and that there was no radiological imaging of the brain prior to initiation of ECLS. A patient-oriented balance needs to be struck between the postulated benefits of hybrid cannulation and the potential harm it may cause. There is an increased risk of bleeding, infection, and thrombosis. Additional technical problems arise, such as the difficulty of flow measurement with parallel systems, as well as their flow regulation.
Retrograde aortic flow may lead to impaired myocardial function and pulmonary congestion as an inherent disadvantage of VA treatment. In addition to hybrid cannulation, another strategy to improve hemodynamics in cardiogenic shock is venting [28]. The first systematic review of left ventricular unloading with Impella in addition to VA-ECMO (“ECMELLA”) described an improved survival and neurological outcome despite higher complication rates compared to VA-ECMO alone [29].
5. Conclusions
The in-hospital mortality rate for hybrid ECLS was high. Different indications showed varying mortality rates, with survivors having lower SAPS II scores and requiring fewer platelet concentrate transfusions. These findings highlight the complexities of hybrid ECLS outcomes in different clinical scenarios and underline the need for rigorous patient selection.
6. Limitations
The small sample size of the study presents challenges in generalizing the results. Results from a single high-volume ECMO center may have limited applicability to broader populations. In addition, the inclusion of patients over an extended period of time includes the potential for changes in both the patient population and standards of care that may have evolved over time. Finally, the analysis focuses on a heterogeneous cohort, reflecting the inherent complexity of this specialized therapy. Despite these limitations, retrospective studies provide valuable insights into associations between variables and serve as a basis for generating hypotheses that merit further investigation.
Author Contributions
Conceptualization, S.D.S., A.K., D.R.S. and M.J.W.; formal analysis, S.D.S. and J.B.; data curation, S.D.S.; writing—original draft preparation, S.D.S.; writing—review and editing, S.D.S., A.K., J.B., R.A., D.R.S. and M.J.W.; visualization, S.D.S.; supervision, A.K.; project administration, S.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study was reviewed, and the requirement for written informed consent was waived by the Cantonal Ethics Commission of Zurich, Switzerland (BASEC No. 2019-01926).
Informed Consent Statement
The study was reviewed by the Cantonal Ethics Commission of Zurich, Switzerland. Patients with documented refused informed consent were excluded.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
S.D.S., J.B., R.A. and M.J.W. have no conflicts of interest to declare. A.K. has received support from Bayer AG (Switzerland) and CSL Behring GmbH (Switzerland) for lecturing. D.R.S.’s former academic department receives grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Perioperative Medicine (SSAPM), Berne, Switzerland; the Swiss Foundation for Anesthesia Research, Zurich, Switzerland; and CSL Vifor (International) AG, St. Gallen, Switzerland. D.R.S. is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Alexion Pharma Germany GmbH, Munich, Germany; CSL Behring GmbH, Marburg, Germany; and LFB Biomédicaments, Courtaboeuf Cedex, France. D.R.S. received honoraria/travel support for consulting or lecturing from Alliance Rouge, Bern, Switzerland; Danube University of Krems, Austria; European Society of Anesthesiology and Intensive Care, Brussels, BE; International Foundation for Patient Blood Management, Basel, Switzerland; Korean Society of Anesthesiologists, Seoul, Korea; Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France; Society for the Advancement of Blood Management, Mount Royal NJ, Alexion Pharmaceuticals Inc., Boston, MA; AstraZeneca AG, Baar, Switzerland; Bayer AG, Zürich, Switzerland; B. Braun Melsungen AG, Melsungen, Germany; Baxter AG, Glattpark, Switzerland; CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland; CSL Vifor (Switzerland) Villars-sur-Glâne, Switzerland; CSL Vifor (International), St. Gallen, Switzerland; Celgene International II Sàrl, Couvet, Switzerland; Daiichi Sankyo AG, Thalwil, Switzerland; Haemonetics, Braintree, MA, USA; iSEP, Nantes, France, LFB Biomédicaments, Courtaboeuf Cedex, France; Merck Sharp & Dohme, Kenilworth, New Jersey, USA; Novo Nordisk Health Care AG, Zurich, Switzerland; Octapharma AG, Lachen, Switzerland; Pharmacosmos A/S, Holbaek, Denmark; Pierre Fabre Pharma, Alschwil, Switzerland; Portola Schweiz GmbH, Aarau, Switzerland; Roche Diagnostics International Ltd., Reinach, Switzerland; Sarstedt AG & Co., Sevelen, Switzerland, and Nümbrecht, Germany; Shire Switzerland GmbH, Zug, Switzerland; Takeda, Glattpark, Switzerland; Werfen, Bedford, MA; and Zuellig Pharma Holdings, Singapore, Singapore.
References
- Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Life Support for Adults with Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Assmann, A.; Beckmann, A.; Schmid, C.; Werdan, K.; Michels, G.; Miera, O.; Schmidt, F.; Klotz, S.; Starck, C.; Pilarczyk, K.; et al. Use of extracorporeal circulation (ECLS/ECMO) for cardiac and circulatory failure -A clinical practice Guideline Level 3. ESC Heart Fail 2022, 9, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Frenckner, B.; Broman, M.; Broomé, M. Position of draining venous cannula in extracorporeal membrane oxygenation for respiratory and respiratory/circulatory support in adult patients. Crit. Care 2018, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, A.; Scolletta, S.; Lorusso, R.; Taccone, F.S. Hybrid extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S707–S715. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, H.; Li, J.; Qin, B.; Lu, J.; Zhang, J.; Lv, L.; Li, B.; Zhou, C.; Yin, Y.; et al. Dynamic and Hybrid Configurations for Extracorporeal Membrane Oxygenation: An Analysis of the Chinese Extracorporeal Life Support Registry. ASAIO J. 2022, 68, 547–552. [Google Scholar] [CrossRef]
- Werner, N.L.; Coughlin, M.; Cooley, E.; Haft, J.W.; Hirschl, R.B.; Bartlett, R.H.; Mychaliska, G.B. The University of Michigan Experience with Veno-Venoarterial Hybrid Mode of Extracorporeal Membrane Oxygenation. ASAIO J. 2016, 62, 578–583. [Google Scholar] [CrossRef]
- Mihu, M.R.; Mageka, D.; Swant, L.V.; El Banayosy, A.; Maybauer, M.O.; Harper, M.D.; Koerner, M.M.; El Banayosy, A. Veno-arteriovenous extracorporeal membrane oxygenation-A single center experience. Artif. Organs. 2021, 45, 1554–1561. [Google Scholar] [CrossRef]
- Biscotti, M.; Lee, A.; Basner, R.C.; Agerstrand, C.; Abrams, D.; Brodie, D.; Bacchetta, M. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: A single-center experience. ASAIO J. 2014, 60, 635–642. [Google Scholar] [CrossRef]
- Cakici, M.; Gumus, F.; Ozcinar, E.; Baran, C.; Bermede, O.; Inan, M.B.; Durdu, M.S.; Sirlak, M.; Akar, A.R. Controlled flow diversion in hybrid venoarterial-venous extracorporeal membrane oxygenation. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 112–118. [Google Scholar] [CrossRef]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Puntigam, J.; Optenhoefel, J.; Greer, M.; Welte, T.; et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: Technique and early outcomes. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 761–767. [Google Scholar] [CrossRef]
- Stöhr, F.; Emmert, M.Y.; Lachat, M.L.; Stocker, R.; Maggiorini, M.; Falk, V.; Wilhelm, M.J. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: Is the configuration mode an important predictor for the outcome? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 676–680. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Broman, L.M.; Taccone, F.S.; Lorusso, R.; Malfertheiner, M.V.; Pappalardo, F.; Di Nardo, M.; Belliato, M.; Bembea, M.M.; Barbaro, R.P.; Diaz, R.; et al. The ELSO Maastricht Treaty for ECLS Nomenclature: Abbreviations for cannulation configuration in extracorporeal life support—A position paper of the Extracorporeal Life Support Organization. Crit. Care 2019, 23, 36. [Google Scholar] [CrossRef]
- Conrad, S.A.; Broman, L.M.; Taccone, F.S.; Lorusso, R.; Malfertheiner, M.V.; Pappalardo, F.; Di Nardo, M.; Belliato, M.; Grazioli, L.; Barbaro, R.P.; et al. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A Position Paper of the Extracorporeal Life Support Organization. Am. J. Respir. Crit. Care Med. 2018, 198, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.; MacLaren, G.; Vidanapathirana, P.C.; Delnoij, T.; Lorusso, R. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: The potential dynamic process of organ support and importance of hybrid modes. Eur. J. Heart Fail. 2017, 19 (Suppl. S2), 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kukielski, C.; Jarrett Davis, C.; Saberi, A.; Chaudhary, S. Veno-arteriovenous (V-AV) ECMO configuration: A single-center experience. J. Card. Surg. 2022, 37, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Erlebach, R.; Wild, L.C.; Seeliger, B.; Rath, A.K.; Andermatt, R.; Hofmaenner, D.A.; Schewe, J.C.; Ganter, C.C.; Müller, M.; Putensen, C.; et al. Outcomes of patients with acute respiratory failure on veno-venous extracorporeal membrane oxygenation requiring additional circulatory support by veno-venoarterial extracorporeal membrane oxygenation. Front. Med. 2022, 9, 1000084. [Google Scholar] [CrossRef]
- Sahli, S.D.; Kaserer, A.; Braun, J.; Halbe, M.; Dahlem, Y.; Spahn, M.A.; Rössler, J.; Krüger, B.; Maisano, F.; Spahn, D.R.; et al. Predictors associated with mortality of extracorporeal life support therapy for acute heart failure: Single-center experience with 679 patients. J. Thorac. Dis. 2022, 14, 1960–1971. [Google Scholar] [CrossRef]
- Neumann, E.; Sahli, S.D.; Kaserer, A.; Braun, J.; Spahn, M.A.; Aser, R.; Spahn, D.R.; Wilhelm, M.J. Predictors associated with mortality of veno-venous extracorporeal membrane oxygenation therapy. J. Thorac. Dis. 2023, 15, 2389–2401. [Google Scholar] [CrossRef]
- Javidfar, J.; Bacchetta, M. Bridge to lung transplantation with extracorporeal membrane oxygenation support. Curr. Opin. Organ. Transpl. 2012, 17, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Muñoz, M.; Klein, A.A.; Levy, J.H.; Zacharowski, K. Patient Blood Management: Effectiveness and Future Potential. Anesthesiology 2020, 133, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Tipograf, Y.; Salna, M.; Minko, E.; Grogan, E.L.; Agerstrand, C.; Sonett, J.; Brodie, D.; Bacchetta, M. Outcomes of Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation. Ann. Thorac. Surg. 2019, 107, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Combes, A. Mechanical circulatory support for end-stage heart failure. Metabolism 2017, 69, S30–S35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansen, B.; Singer Englar, T.; Cole, R.; Catarino, P.; Chang, D.; Czer, L.; Emerson, D.; Geft, D.; Kobashigawa, J.; Megna, D.; et al. Extracorporeal membrane oxygenation as a bridge to durable mechanical circulatory support or heart transplantation. Int. J. Artif. Organs. 2022, 45, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Dave, S.; Goerlich, C.E.; Kaczorowski, D.J. Hybrid and parallel extracorporeal membrane oxygenation circuits. JTCVS Tech. 2021, 8, 77–85. [Google Scholar] [CrossRef]
- Napp, L.C.; Kühn, C.; Hoeper, M.M.; Vogel-Claussen, J.; Haverich, A.; Schäfer, A.; Bauersachs, J. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2016, 105, 283–296. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Orban, M.; Kupka, D.; Scherer, C.; Hagl, C.; Zimmer, S.; Luedike, P.; Thiele, H.; Westermann, D.; Massberg, S.; et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur. Heart J. 2020, 41, 3753–3761. [Google Scholar] [CrossRef]
- Thevathasan, T.; Füreder, L.; Fechtner, M.; Mørk, S.R.; Schrage, B.; Westermann, D.; Linde, L.; Gregers, E.; Andreasen, J.B.; Gaisendrees, C.; et al. Left-Ventricular Unloading with Impella During Refractory Cardiac Arrest Treated with Extracorporeal Cardiopulmonary Resuscitation: A Systematic Review and Meta-Analysis. Crit. Care Med. 2024. online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).