Reconstruction of an Extensive Segmental Radial Shaft Bone Defect by Vascularized 3D-Printed Graft Cage
Abstract
1. Introduction
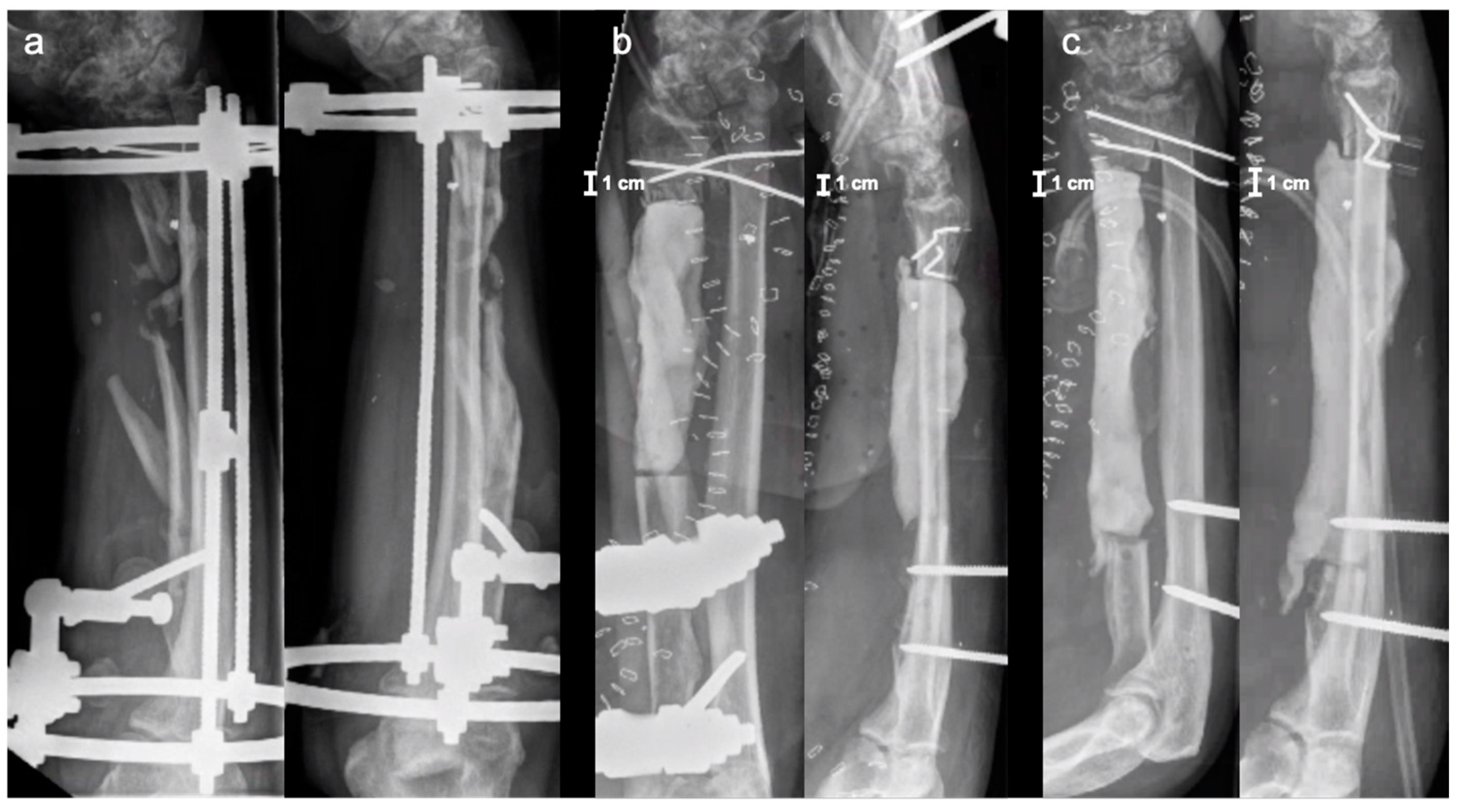
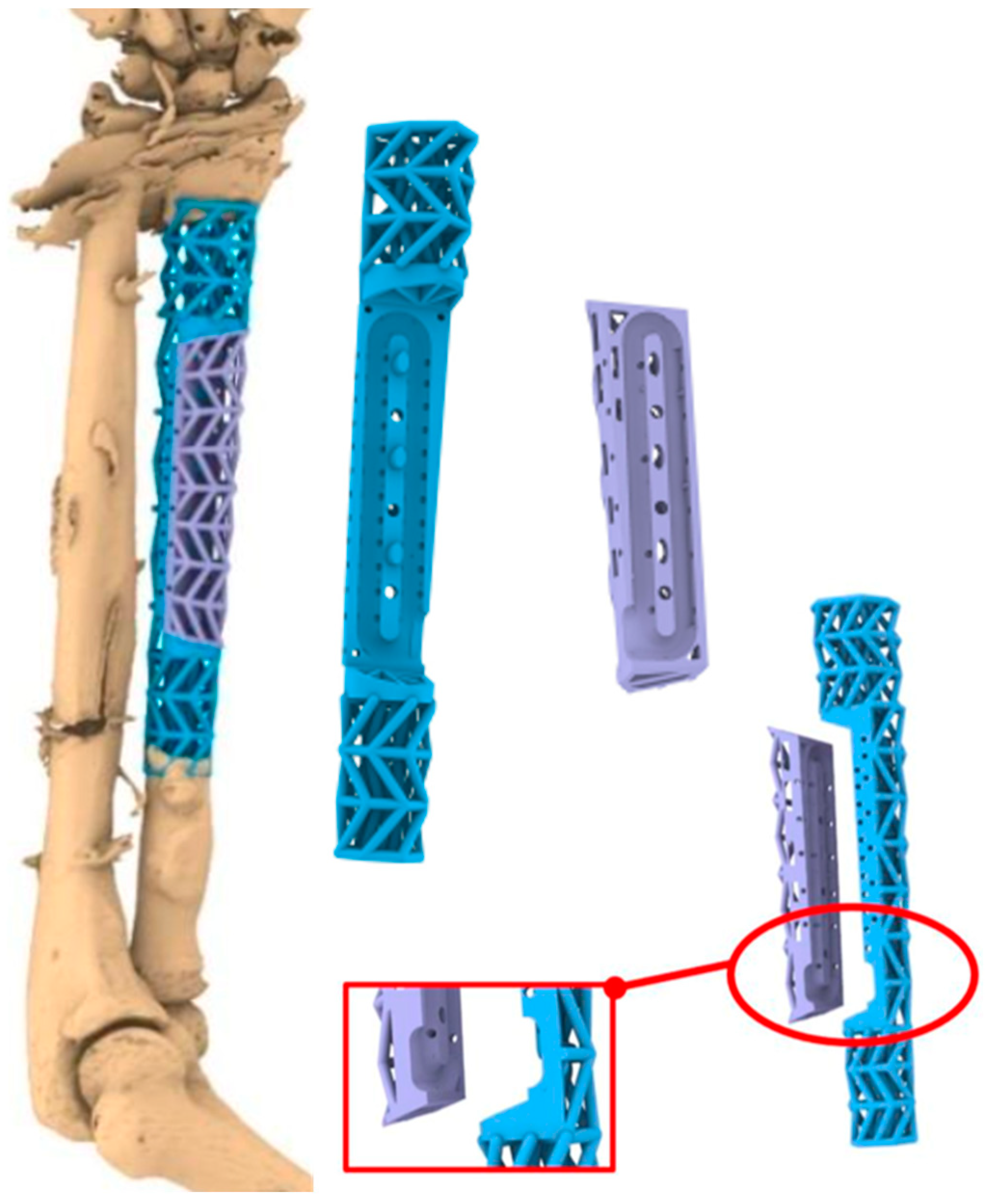
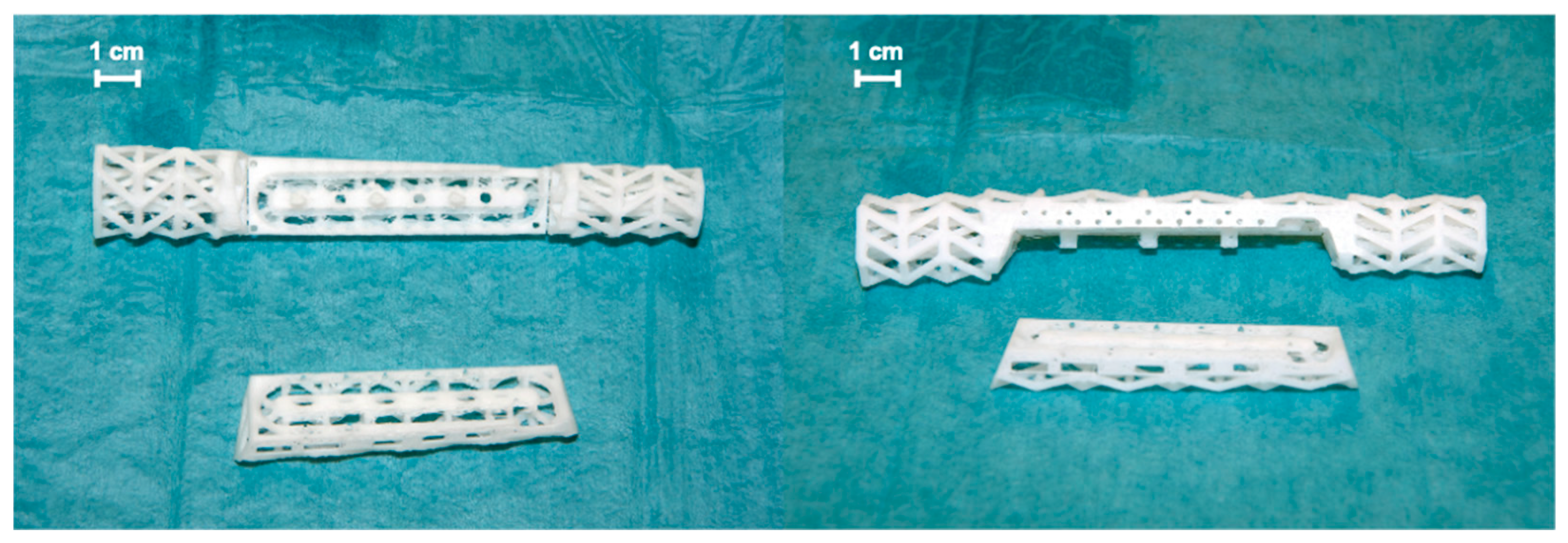
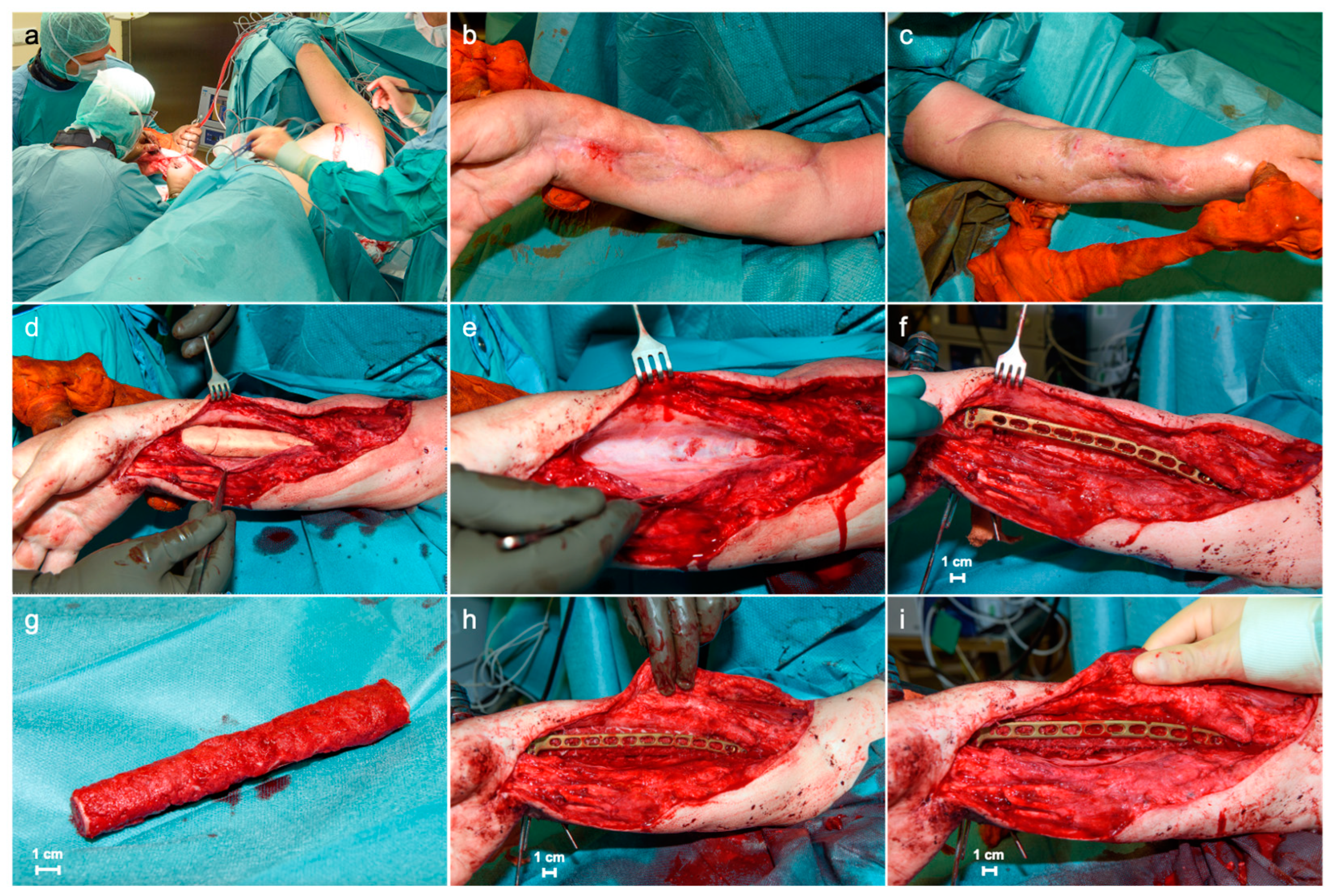
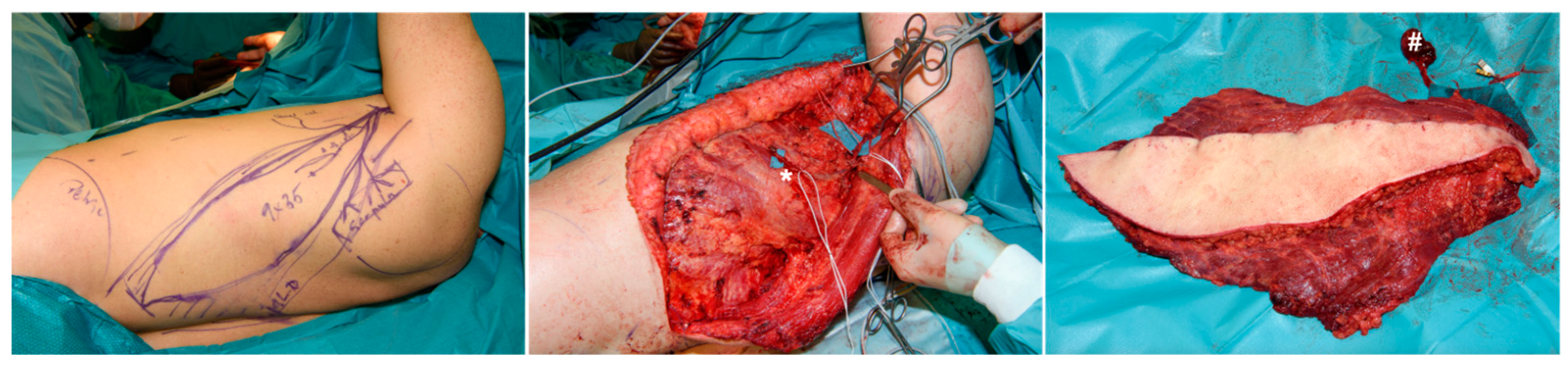
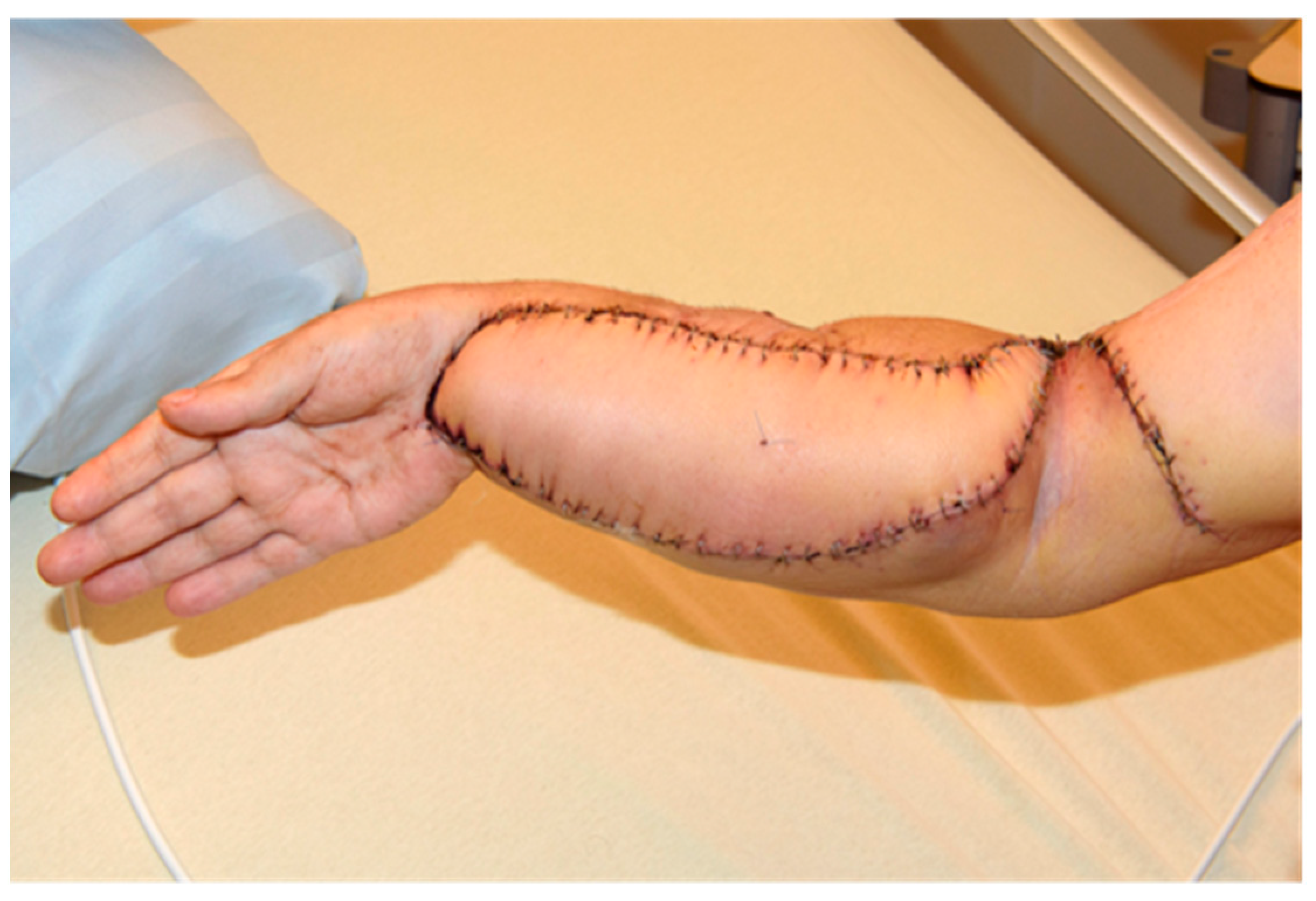
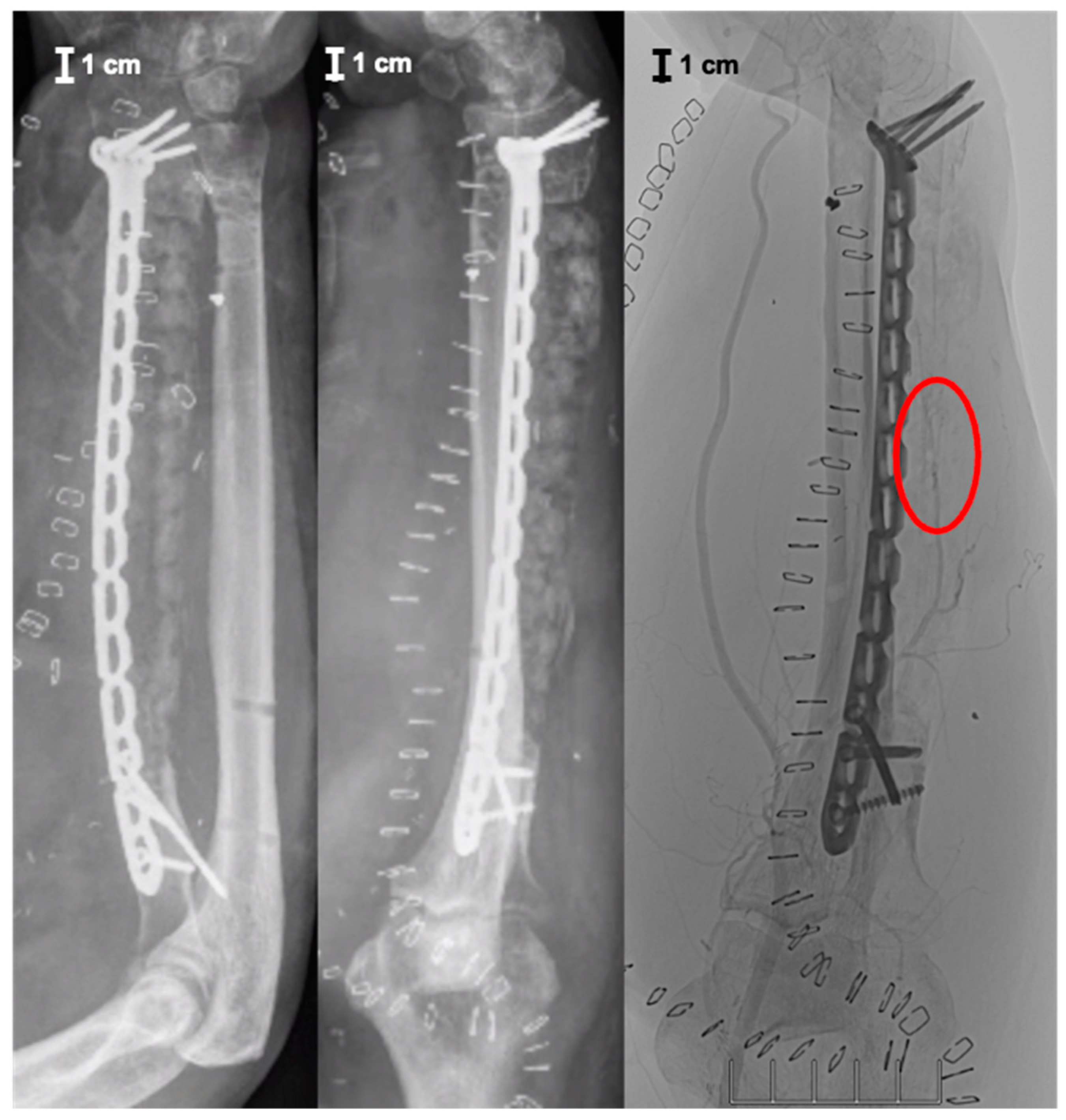
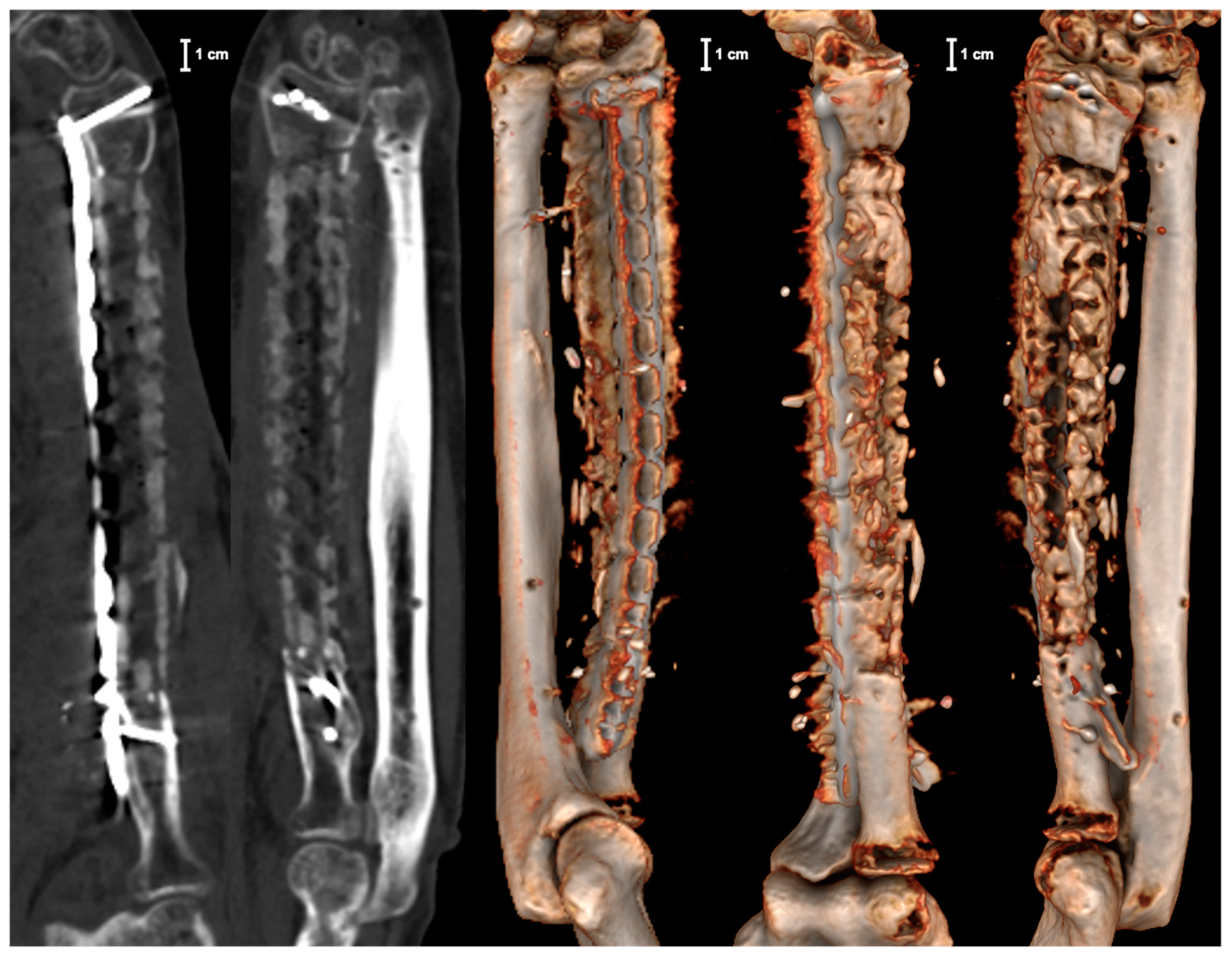
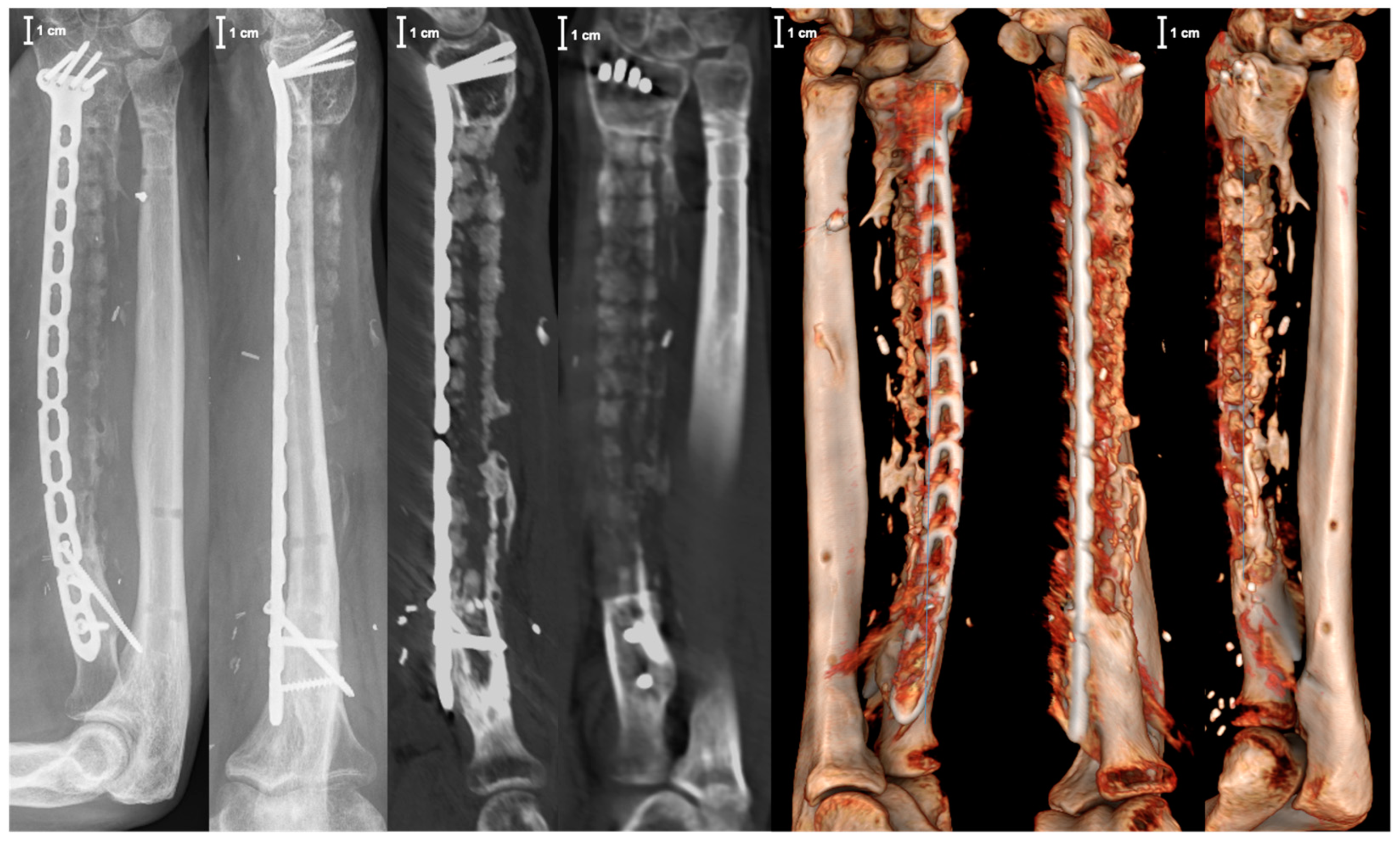
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Q.; Xu, Y.B.; Ren, C.; Li, M.; Zhang, C.C.; Liu, L.; Wang, Q.; Lu, Y.; Lin, H.; Li, Z. Bone transport combined with bone graft and internal fixation versus simple bone transport in the treatment of large bone defects of lower limbs after trauma. BMC Musculoskelet. Disord. 2022, 23, 157. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.; Rui, Y.; Wang, J.; Liu, J.; Ma, Y. Masquelet technique for reconstructing bone defects in open lower limb fracture: Analysis of the relationship between bone defect and bone graft. Injury 2021, 52, 988–995. [Google Scholar] [CrossRef]
- Kazmirchuk, A.; Yarmoliuk, Y.; Lurin, I.; Gybalo, R.; Burianov, O.; Derkach, S.; Karpenko, K. Ukraine’s Experience with Management of Combat Casualties Using NATO’s Four-Tier “Changing as Needed” Healthcare System. World J. Surg. 2022, 46, 2858–2862. [Google Scholar] [CrossRef]
- Cillóniz, C.; Rodríguez-Hurtado, D.; Torres, A. Characteristics and Management of Community-Acquired Pneumonia in the Era of Global Aging. Med. Sci. 2018, 6, 35. [Google Scholar] [CrossRef]
- Friedrich, J.B.; Moran, S.L.; Bishop, A.T.; Shin, A.Y. Free vascularized fibula grafts for salvage of failed oncologic long bone reconstruction and pathologic fractures. Microsurgery 2009, 29, 385–392. [Google Scholar] [CrossRef]
- Pedersen, W.C.; Person, D.W. Long bone reconstruction with vascularized bone grafts. Orthop. Clin. N. Am. 2007, 38, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ghert, M.; Colterjohn, N.; Manfrini, M. The use of free vascularized fibular grafts in skeletal reconstruction for bone tumors in children. J. Acad. Orthop. Surg. 2007, 15, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-C.; Li, H.A.; Kang, Q.-L.; Li, G. An update to the advances in understanding distraction histogenesis: From biological mechanisms to novel clinical applications. J. Orthop. Translat. 2020, 25, 3–10. [Google Scholar] [CrossRef]
- Dugan, T.R.; Hubert, M.G.; Siska, P.A.; Pape, H.-C.; Tarkin, I.S. Open supracondylar femur fractures with bone loss in the polytraumatized patient—Timing is everything! Injury 2013, 44, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Catagni, M.A.; Azzam, W.; Guerreschi, F.; Lovisetti, L.; Poli, P.; Khan, M.S.; Di Giacomo, L.M. Trifocal versus bifocal bone transport in treatment of long segmental tibial bone defects. Bone Jt. J. 2019, 101-B, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Stolberg-Stolberg, J.; Michel, P.A.; Garcia, P.; Amler, S.; Wähnert, D.; Raschke, M.J. Effect of Bone Morphogenetic Protein-2 in the Treatment of Long Bone Non-Unions. J. Clin. Med. 2021, 10, 4597. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.P.; Beason, A.M. Plate-Assisted Bone Segment Transport Versus Precice Bone Transport Nail. J. Orthop. Trauma 2021, 35, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. A 2008, 85, 777–786. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Henkel, J.; Medeiros Savi, F.; Berner, A.; Fountain, S.; Saifzadeh, S.; Steck, R.; Epari, D.R.; Woodruff, M.A.; Knackstedt, M.; Schuetz, M.A. Scaffold-guided bone regeneration in large volume tibial segmental defects. Bone 2021, 153, 116163. [Google Scholar] [CrossRef] [PubMed]
- Kobbe, P.; Laubach, M.; Hutmacher, D.W.; Alabdulrahman, H.; Sellei, R.M.; Hildebrand, F. Convergence of scaffold-guided bone regeneration and RIA bone grafting for the treatment of a critical-sized bone defect of the femoral shaft. Eur. J. Med. Res. 2020, 25, 70. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Translat. 2022, 34, 73–84. [Google Scholar] [CrossRef]
- Dawson, J.; Kiner, D.; Gardner, W., 2nd; Swafford, R.; Nowotarski, P.J. The reamer-irrigator-aspirator as a device for harvesting bone graft compared with iliac crest bone graft: Union rates and complications. J. Orthop. Trauma 2014, 28, 584–590. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Lai, S.; Cao, Q.; Liu, Y.; Li, J.; Zhu, X.; Fu, W.; Zhang, X. Construction of Vascularized Tissue Engineered Bone with nHA-Coated BCP Bioceramics Loaded with Peripheral Blood-Derived MSC and EPC to Repair Large Segmental Femoral Bone Defect. ACS Appl. Mater. Interfaces 2023, 15, 249–264. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Sun, Y.; Wu, T.; Sun, Y.; Chai, Y.; Kang, Q.; Rui, B.; Li, G. In vivo prevascularization strategy enhances neovascularization of β-tricalcium phosphate scaffolds in bone regeneration. J. Orthop. Translat. 2022, 37, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Toh, S.; Tsubo, K.; Nishikawa, S.; Narita, S.; Miura, H. Complications of vascularized fibula graft for reconstruction of long bones. Plast. Reconstr. Surg. 2002, 109, 2301–2306. [Google Scholar] [CrossRef]
- Lenze, U.; Pohlig, F.; Knebel, C.; Lenze, F.; Harrasser, N.; Mühlhofer, H.; Toepfer, A.; Rechl, H.; von Eisenhart-Rothe, R. Autologous fibula transplantation for reconstruction of bone defects. Orthopäde 2017, 46, 648–655. [Google Scholar] [CrossRef]
- Miska, M.; Schmidmaier, G. Diamond concept for treatment of nonunions and bone defects. Unfallchirurg 2020, 123, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Migliorini, F.; Cuozzo, F.; Torsiello, E.; Hildebrand, F.; Maffulli, N. Outcomes and complications of the reamer irrigator aspirator versus traditional iliac crest bone graft harvesting: A systematic review and meta-analysis. J. Orthop. Traumatol. 2021, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Mendel, T.; Hückstädt, M.; Hofmann, G.O.; Klauke, F. Reconstruction of a metadiaphyseal bone defect after open comminuted fracture of the proximal femur using a modified Masquelet technique. Unfallchirurgie 2023, 126, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Weiland, A.J.; Phillips, T.W.; Randolph, M.A. Bone grafts: A radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast. Reconstr. Surg. 1984, 74, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Hierner, R.; Täger, G.; Nast-Kolb, D. Vascularized bone transfer. Unfallchirurg 2009, 112, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Tetsworth, K.; Woloszyk, A.; Glatt, V. 3D printed titanium cages combined with the Masquelet technique for the reconstruction of segmental femoral defects: Preliminary clinical results and molecular analysis of the biological activity of human-induced membranes. OTA Int. 2019, 2, e016. [Google Scholar] [CrossRef]
- Gamieldien, H.; Ferreira, N.; Birkholtz, F.F.; Hilton, T.; Campbell, N.; Laubscher, M. Filling the gap: A series of 3D-printed titanium truss cages for the management of large, lower limb bone defects in a developing country setting. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 497–505. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Medeiros Savi, F.; Saifzadeh, S.; Wille, M.-L.; Wagels, M.; Hutmacher, D.W. Bone regeneration exploiting corticoperiosteal tissue transfer for scaffold-guided bone regeneration. Tissue Eng. Part C Methods 2022, 28, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Saifzadeh, S.; Medeiros Savi, F.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Savi, F.M.; Dlaska, C.E.; Saifzadeh, S.; Brierly, G.; Ren, E.; Cipitria, A.; Reichert, J.C.; Wille, M.-L.; Schuetz, M.A. Convergence of scaffold-guided bone reconstruction and surgical vascularization strategies-a quest for regenerative matching axial vascularization. Front. Bioeng. Biotechnol. 2020, 7, 448. [Google Scholar] [CrossRef]
- Sparks, D.S.; Savi, F.M.; Dlaska, C.E.; Saifzadeh, S.; Brierly, G.; Ren, E.; Cipitria, A.; Reichert, J.C.; Wille, M.-L.; Schuetz, M.A. Convergence of scaffold-guided bone regeneration principles and microvascular tissue transfer surgery. Sci. Adv. 2023, 9, eadd6071. [Google Scholar] [CrossRef]
- Capanna, R.; Bufalini, C.; Campanacci, M. A new technique for reconstructions of large metadiaphyseal bone defects. Orthop. Traumatol. 1993, 2, 159–177. [Google Scholar] [CrossRef]
- Castrisos, G.; Gonzalez Matheus, I.; Sparks, D.; Lowe, M.; Ward, N.; Sehu, M.; Wille, M.-L.; Phua, Y.; Medeiros Savi, F.; Hutmacher, D. Regenerative matching axial vascularisation of absorbable 3D-printed scaffold for large bone defects: A first in human series. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2108–2118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mommsen, P.; März, V.; Krezdorn, N.; Aktas, G.; Sehmisch, S.; Vogt, P.M.; Großner, T.; Omar Pacha, T. Reconstruction of an Extensive Segmental Radial Shaft Bone Defect by Vascularized 3D-Printed Graft Cage. J. Pers. Med. 2024, 14, 178. https://doi.org/10.3390/jpm14020178
Mommsen P, März V, Krezdorn N, Aktas G, Sehmisch S, Vogt PM, Großner T, Omar Pacha T. Reconstruction of an Extensive Segmental Radial Shaft Bone Defect by Vascularized 3D-Printed Graft Cage. Journal of Personalized Medicine. 2024; 14(2):178. https://doi.org/10.3390/jpm14020178
Chicago/Turabian StyleMommsen, Philipp, Vincent März, Nicco Krezdorn, Gökmen Aktas, Stephan Sehmisch, Peter Maria Vogt, Tobias Großner, and Tarek Omar Pacha. 2024. "Reconstruction of an Extensive Segmental Radial Shaft Bone Defect by Vascularized 3D-Printed Graft Cage" Journal of Personalized Medicine 14, no. 2: 178. https://doi.org/10.3390/jpm14020178
APA StyleMommsen, P., März, V., Krezdorn, N., Aktas, G., Sehmisch, S., Vogt, P. M., Großner, T., & Omar Pacha, T. (2024). Reconstruction of an Extensive Segmental Radial Shaft Bone Defect by Vascularized 3D-Printed Graft Cage. Journal of Personalized Medicine, 14(2), 178. https://doi.org/10.3390/jpm14020178






