Resolved Proteinuria May Attenuate the Risk of Heart Failure: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
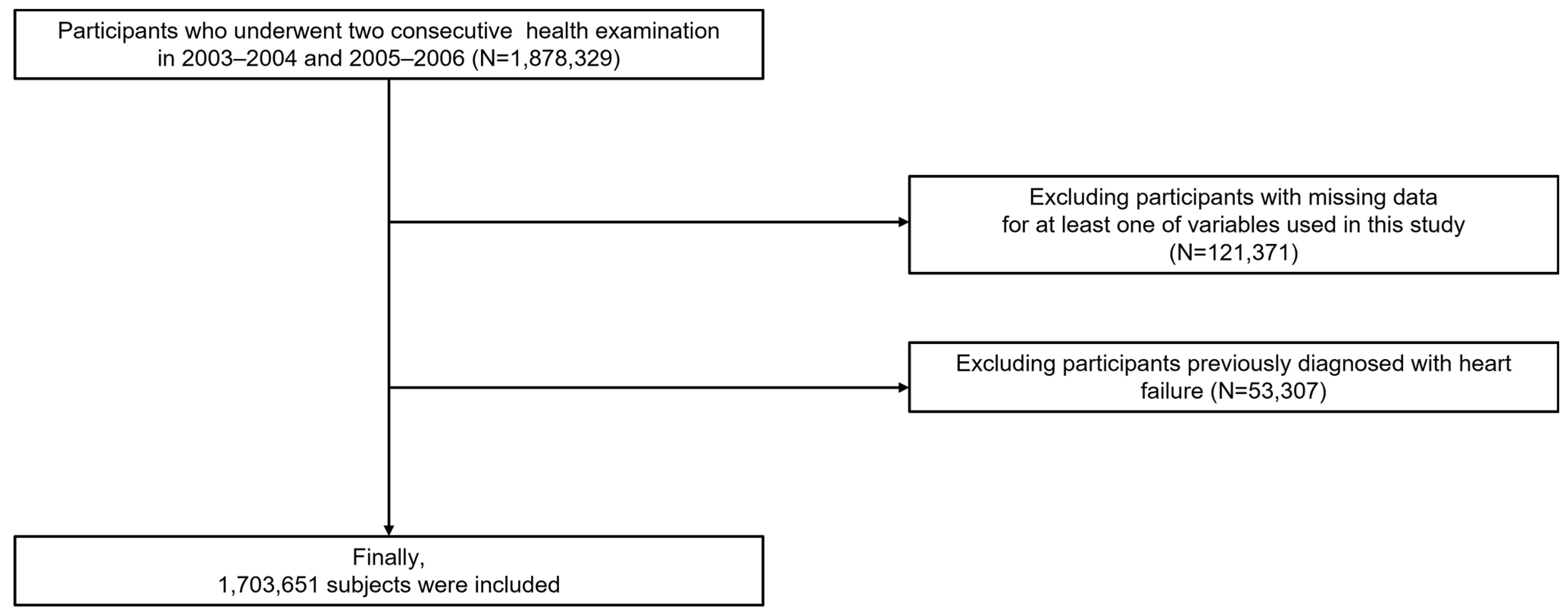
2. Materials and Methods
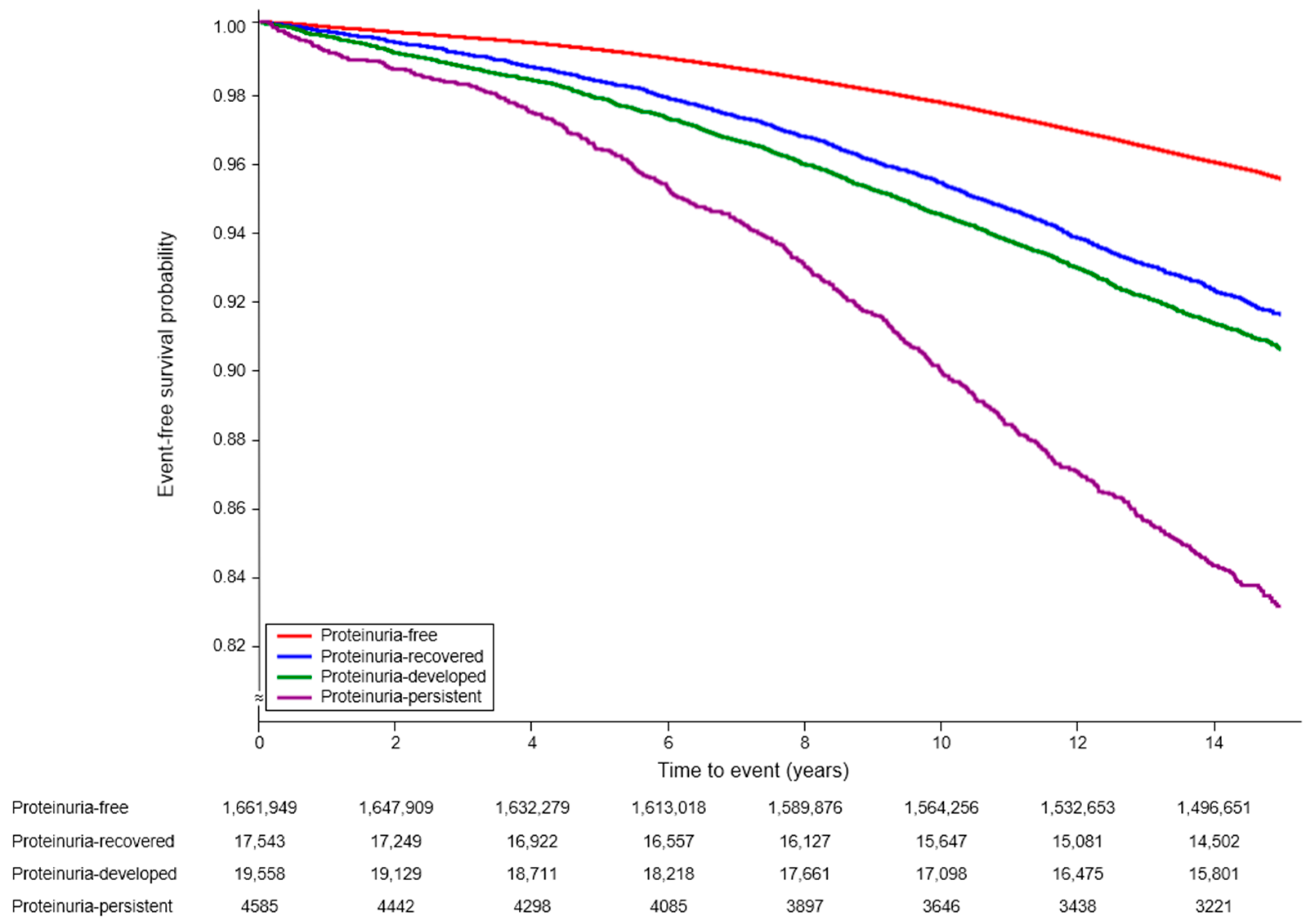
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, L.B.; Williams, S.G.; Tan, D.K.; Cohen-Solal, A. So many definitions of heart failure: Are they all universally valid? A critical appraisal. Expert Rev. Cardiovasc. Ther. 2010, 8, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Driver, J.A.; Gaziano, J.M. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009, 302, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Horwich, T.B.; Fonarow, G.C. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011, 8, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.A.; Inamdar, A.C. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef]
- Currie, G.; Delles, C. Proteinuria and its relation to cardiovascular disease. Int. J. Nephrol. Renovasc. Dis. 2013, 7, 13–24. [Google Scholar] [CrossRef]
- Liang, W.; Liu, Q.; Wang, Q.Y.; Yu, H.; Yu, J. Albuminuria and Dipstick Proteinuria for Predicting Mortality in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 665831. [Google Scholar] [CrossRef]
- Kumai, Y.; Kamouchi, M.; Hata, J.; Ago, T.; Kitayama, J.; Nakane, H.; Sugimori, H.; Kitazono, T. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012, 78, 1909–1915. [Google Scholar] [CrossRef]
- Kelly, D.M.; Rothwell, P.M. Proteinuria as an independent predictor of stroke: Systematic review and meta-analysis. Int. J. Stroke 2020, 15, 29–38. [Google Scholar] [CrossRef]
- Wen, C.P.; Yang, Y.C.; Tsai, M.K.; Wen, S.F. Urine dipstick to detect trace proteinuria: An underused tool for an underappreciated risk marker. Am. J. Kidney Dis. 2011, 58, 1–3. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, R.; Su, Z.; Zhang, J.; Zhao, X.; Wu, S.; Guo, X. Association of Persistent, Incident, and Remittent Proteinuria With Stroke Risk in Patients With Diabetes Mellitus or Prediabetes Mellitus. J. Am. Heart Assoc. 2017, 6, e006178. [Google Scholar] [CrossRef] [PubMed]
- Madison, J.R.; Spies, C.; Schatz, I.J.; Masaki, K.; Chen, R.; Yano, K.; Curb, J.D. Proteinuria and risk for stroke and coronary heart disease during 27 years of follow-up: The Honolulu Heart Program. Arch. Intern. Med. 2006, 166, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yi, H.; Jang, M.; Kim, J.G.; Kwon, S.U.; Kim, N.; Lee, E.J. Air Pollution and Subarachnoid Hemorrhage Mortality: A Stronger Association in Women than in Men. J. Stroke 2022, 24, 429–432. [Google Scholar] [CrossRef]
- Jung, S.; Jung, G.; Kim, D.; Oh, J.; Choi, K. Epidemiology of Chronic Inflammatory Demyelinating Polyneuropathy in South Korea: A Population-Based Study. J. Clin. Neurol. 2023, 19, 558–564. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Fonarow, G.C.; Fudim, M.; Hall, M.E.; Hernandez, A.; Morris, A.A.; Shafi, T.; Weir, M.R.; et al. Albuminuria and Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 270–282. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Mann, J.F.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Hallé, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef]
- Blecker, S.; Matsushita, K.; Köttgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-normal albuminuria and risk of heart failure in the community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef]
- Ikeda, S.; An, Y.; Iguchi, M.; Ogawa, H.; Nakanishi, Y.; Minami, K.; Ishigami, K.; Aono, Y.; Doi, K.; Hamatani, Y.; et al. Proteinuria is independently associated with heart failure events in patients with atrial fibrillation: The Fushimi AF registry. Eur. Heart J. Qual. Care Clin. Outcomes, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Hamo, C.E.; Kwak, L.; Wang, D.; Florido, R.; Echouffo-Tcheugui, J.B.; Blumenthal, R.S.; Loehr, L.; Matsushita, K.; Nambi, V.; Ballantyne, C.M.; et al. Heart Failure Risk Associated With Severity of Modifiable Heart Failure Risk Factors: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e021583. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, W.; Ye, K.; Li, Y.; He, M.; Lu, B.; Hu, R. Albuminuria Is Associated with Left Ventricular Hypertrophy in Patients with Early Diabetic Kidney Disease. Int. J. Endocrinol. 2014, 2014, 351945. [Google Scholar] [CrossRef] [PubMed]
- Lorell, B.H.; Carabello, B.A. Left Ventricular Hypertrophy. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Farré, A.L.; Casado, S. Heart Failure, Redox Alterations, and Endothelial Dysfunction. Hypertension 2001, 38, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Yu, R.; Craig, J.C.; Polkinghorne, K.R.; Atkins, R.C.; Chadban, S.J. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am. J. Kidney Dis. 2011, 58, 19–28. [Google Scholar] [CrossRef]
| Variable | Total | Proteinuria-Free (−/−) | Proteinuria-Resolved (+/−) | Proteinuria-Developed (−/+) | Proteinuria-Persistent (+/+) | p-Value |
|---|---|---|---|---|---|---|
| Number of participants (%) | 1,703,651 | 1,661,965 (97.55) | 17,543 (1.03) | 19,558 (1.15) | 4585 (0.27) | |
| Age, years | 43.94 ± 12.05 | 43.86 ± 12.01 | 46.74 ± 13.11 | 46.73 ± 12.99 | 49.26 ± 12.6 | <0.001 |
| Sex | <0.001 | |||||
| Men | 1,177,934 (69.14) | 1,150,367 (69.22) | 11,031 (62.88) | 12,928 (66.10) | 3608 (78.69) | |
| Women | 525,717 (30.86) | 511,598 (30.78) | 6512 (37.12) | 6630 (33.90) | 977 (21.31) | |
| Body mass index (kg/m2) | 23.62 ± 3.03 | 23.61 ± 3.02 | 24.13 ± 3.32 | 24.17 ± 3.44 | 24.94 ± 3.37 | <0.001 |
| Household income | <0.001 | |||||
| Q1, lowest | 254,366 (14.93) | 247,563 (14.90) | 3038 (17.32) | 3135 (16.03) | 630 (13.74) | |
| Q2 | 632,196 (37.11) | 617,708 (37.17) | 6240 (35.57) | 6872 (35.14) | 1376 (30.01) | |
| Q3 | 562,916 (33.04) | 549,527 (33.06) | 5498 (31.34) | 6282 (32.12) | 1609 (35.09) | |
| Q4, highest | 254,173 (14.92) | 247,167 (14.87) | 2767 (15.77) | 3269 (16.71) | 970 (21.16) | |
| Smoking | <0.001 | |||||
| Never | 980,235 (57.54) | 954,998 (57.46) | 10,904 (62.16) | 11,807 (60.37) | 2526 (55.09) | |
| Former | 212,652 (12.48) | 207,536 (12.49) | 2071 (11.81) | 2356 (12.05) | 689 (15.03) | |
| Current | 510,764 (29.98) | 499,431 (30.05) | 4568 (26.04) | 5395 (27.58) | 1370 (29.88) | |
| Alcohol consumption (days/week) | <0.001 | |||||
| <3 | 1,139,835 (66.91) | 1,111,388 (66.87) | 12,133 (69.16) | 13,292 (67.96) | 3022 (65.91) | |
| ≥3 | 563,816 (33.09) | 550,577 (33.13) | 5410 (30.84) | 6266 (32.04) | 1563 (34.09) | |
| Regular exercise (days/week) | <0.001 | |||||
| <3 | 1,374,142 (80.66) | 1341,444 (80.71) | 13,597 (77.51) | 15,552 (79.52) | 3549 (77.40) | |
| ≥3 | 329,509 (19.34) | 320,521 (19.29) | 3946 (22.49) | 4006 (20.48) | 1036 (22.60) | |
| Comorbidities (%) | ||||||
| Hypertension | 769,339 (45.16) | 744,298 (44.78) | 10,067 (57.38) | 11,373 (58.15) | 3601 (78.54) | <0.001 |
| Diabetes mellitus | 239,866 (14.08) | 228,364 (13.74) | 4482 (25.55) | 5188 (26.53) | 1832 (39.96) | <0.001 |
| Dyslipidemia | 421,156 (24.72) | 405,688 (24.41) | 6250 (35.63) | 6871 (35.13) | 2347 (51.19) | <0.001 |
| Atrial fibrillation | 4448 (0.26) | 4234 (0.25) | 76 (0.43) | 104 (0.53) | 34 (0.74) | <0.001 |
| Cancer | 31,454 (1.85) | 30,290 (1.82) | 493 (2.81) | 504 (2.58) | 167 (3.64) | <0.001 |
| Renal disease | 16,806 (0.99) | 14,682 (0.88) | 785 (4.47) | 699 (3.57) | 640 (13.96) | <0.001 |
| Charlson Comorbidity Index | <0.001 | |||||
| 0 | 677,492 (39.77) | 664,172 (39.96) | 5674 (32.34) | 6459 (33.02) | 1187 (25.89) | |
| 1 | 691,773 (40.61) | 676,615 (40.71) | 6467 (36.86) | 7314 (37.4) | 1377 (30.03) | |
| ≥2 | 334,386 (19.63) | 321,178 (19.33) | 5402 (30.79) | 5785 (29.58) | 2021 (44.08) | |
| Follow-up duration (years) | 14.04 ± 2.36 | 14.06 ± 2.33 | 13.56 ± 3.08 | 13.38 ± 3.33 | 12.6 ± 3.90 | <0.001 |
| Proteinuria Status | Total (N) | Heart Failure (N) | IR (per 1000) | HR (95% Confidence Interval) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Free | 1,661,965 | 71,276 | 3.05 | 1 (ref) | 1 (ref) | 1 (ref) |
| Resolved | 17,543 | 1386 | 5.83 | 1.93 (1.83, 2.03) | 1.32 (1.25, 1.39) | 1.31 (1.24, 1.38) |
| Developed | 19,558 | 1708 | 6.53 | 2.16 (2.06, 2.27) | 1.53 (1.46, 1.60) | 1.52 (1.44, 1.59) |
| Persistent | 4585 | 694 | 12.02 | 4.06 (3.77, 4.38) | 2.23 (2.06, 2.40) | 2.19 (2.03, 2.36) |
| p-value | <0.001 | <0.001 | <0.001 | |||
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Resolved vs. Free (ref) | 1.93 | (1.83, 2.03) | <0.001 | 1.32 | (1.26, 1.40) | <0.001 | 1.31 | (1.24, 1.38) | <0.001 |
| Developed vs. Free (ref) | 2.17 | (2.07, 2.27) | <0.001 | 1.53 | (1.46, 1.60) | <0.001 | 1.52 | (1.45, 1.59) | <0.001 |
| Resolved vs. Persistent (ref) | 0.48 | (0.44, 0.52) | <0.001 | 0.64 | (0.58, 0.70) | <0.001 | 0.64 | (0.58, 0.70) | <0.001 |
| Developed vs. Persistent (ref) | 0.54 | (0.49, 0.59) | <0.001 | 0.73 | (0.67, 0.80) | <0.001 | 0.74 | (0.68, 0.81) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Kang, M.K.; Park, M.-S.; Leem, G.-H.; Song, T.-J. Resolved Proteinuria May Attenuate the Risk of Heart Failure: A Nationwide Population-Based Cohort Study. J. Pers. Med. 2023, 13, 1662. https://doi.org/10.3390/jpm13121662
Chang Y, Kang MK, Park M-S, Leem G-H, Song T-J. Resolved Proteinuria May Attenuate the Risk of Heart Failure: A Nationwide Population-Based Cohort Study. Journal of Personalized Medicine. 2023; 13(12):1662. https://doi.org/10.3390/jpm13121662
Chicago/Turabian StyleChang, Yoonkyung, Min Kyoung Kang, Moo-Seok Park, Gwang-Hyun Leem, and Tae-Jin Song. 2023. "Resolved Proteinuria May Attenuate the Risk of Heart Failure: A Nationwide Population-Based Cohort Study" Journal of Personalized Medicine 13, no. 12: 1662. https://doi.org/10.3390/jpm13121662
APA StyleChang, Y., Kang, M. K., Park, M.-S., Leem, G.-H., & Song, T.-J. (2023). Resolved Proteinuria May Attenuate the Risk of Heart Failure: A Nationwide Population-Based Cohort Study. Journal of Personalized Medicine, 13(12), 1662. https://doi.org/10.3390/jpm13121662







