Abstract
Maintenance of brain structure is essential for neurocognitive health. Precision medicine has interests in understanding how maintenance of an individual person’s brain, including cerebral cortical structure, interacts with lifestyle factors like physical activity. Cortical structure, including cortical thickness, has recognized relationships with physical activity, but concepts of these relationships come from group, not individual, focused findings. Whether or how group-focused concepts apply to an individual person is fundamental to precision medicine interests but remains unclear. This issue was studied in a healthy man using concurrent micro-longitudinal tracking of magnetic resonance imaging-defined cortical thickness and accelerometer-defined steps/day over six months. These data permitted detailed examination of temporal relationships between thickness maintenance and physical activity at an individual level. Regression analyses revealed graded significant and trend-level temporal interactions between preceding activity vs. subsequent thickness maintenance and between preceding thickness maintenance vs. subsequent activity. Interactions were bidirectional, delayed/prolonged over days/weeks, positive, bilateral, directionally asymmetric, and limited in strength. These novel individual-focused findings in some ways are predicted, but in other ways remain unaddressed or undetected, by group-focused work. We suggest that individual-focused concepts of temporal interactions between maintenance of cortical structure and activity can provide needed new insight for personalized tailoring of physical activity, cortical, and neurocognitive health.
1. Introduction
Adult cerebral cortical structure requires constant maintenance. Distinguished from normal aging of cortical structure over a lifespan of years/decades, normal maintenance, which has received little attention, ensures continuity in cortical structural viability and integrity over short intervals of, e.g., days/weeks.
Working from an idiographic perspective that recognizes the importance of human individuality, precision medicine approaches can potentially impact maintenance of an individual person’s cortical structure and related neurocognitive health through personalized tailoring of modifiable lifestyle factors, including physical activity [1,2,3]. Physical activity has recognized relationships with cortical structure, including thickness of the cortex [4,5,6,7]. Concepts that characterize these relationships come from group-based (nomothetic) approaches. Whether or how current group-based concepts apply to an individual person is basic to precision medicine interests but has not been studied.
We have reported magnetic resonance imaging (MRI) data that micro-longitudinally tracked normal maintenance of cortical thickness in a healthy man at weekly intervals over several months [8,9]. The results suggested that hemispheric mean thicknesses of his left and right cortices underwent reversing incremental and decremental fluctuations that appeared to reflect normal maintenance remodeling/turnover of cortical substrates. These findings raise the possibility that fluctuations in the maintenance of his cortical thickness may interact with ongoing fluctuations in his physical activity.
The present investigation explored this possibility with the use of this individual’s micro-longitudinal cortical thickness maintenance data and concurrent micro-longitudinally tracked physical activity in terms of steps/day. A hope was that an individual person micro-longitudinal approach might complement existing group-based work on relationships between cortical thickness and physical activity in the following ways.
First, existing group work is based on designs that used limited thickness sampling per individual and group analyses of inter-individual average measures of thickness. In contrast, the present study used micro-longitudinal thickness sampling at regular short intervals over several months from a single individual, and intra-individual analyses of thickness. Although unconventional and underused, recent reviews suggest individual-focused analyses are well suited for investigating concepts of human brain–behavior relationships [10,11,12].
Second, group work has focused on the effects of physical activity on cortical thickness, whereas potential reverse direction influences of cortical thickness on physical activity have received little attention. The present micro-longitudinal tracking independently assessed relationships in each direction.
Finally, group work does not systematically address time scales over which activity and thickness maintenance may be related. The present micro-longitudinal analyses were designed to empirically test windows of time when activity and maintenance of thickness were and were not related.
Our study addressed two specific questions that have not been studied at an individual person level. Question 1: Was preceding physical activity related to subsequent maintenance of cortical thickness and, if so, over what times? Question 2: Was preceding maintenance of cortical thickness related to subsequent physical activity and, if so, over what times?
Thickness was used as an index of cortical structural maintenance because it can be objectively and repeatedly measured in an individual person with automated programs. Hemispheric mean thickness was used as a measure because it is appropriate for assessing the spatially ubiquitous nature of the maintenance of cortical structure across the hemisphere. Micro-longitudinal sampling of thickness was used to continuously track maintenance fluctuations at short intervals over months.
Step count was used as an index of physical activity because it: (a) has been used in group-based work on relationships with cortical thickness, (b) is a fundamental unit of energy expenditure in an individual, and (c) has been successfully used as a motivator to increase activity and, thus, is of interest for precision medicine intervention. Accelerometer measures were used because they provide accurate measures of steps. Micro-longitudinal sampling was used to continuously track activity fluctuations at repeated regular short intervals over months.
The present study explores whether maintenance of the cerebral cortical structure of an individual person’s brain interacts with their physical activity. The individual-based focus and questions we address have received little attention; however, we felt an exploratory investigation was justified given (a) their basic significance for understanding temporal interactions between physical activity and maintenance of cortical structure at an individual level and (b) the rare availability of concurrent micro-longitudinal thickness maintenance and physical activity measures over a several-month period in an individual person.
2. Materials and Methods
Details of the studied individual’s medical history and health monitoring measures during the study have been published [8,9,13]. Salient points are briefly re-reviewed next.
2.1. Studied Individual
The subject is a 66-year-old man. He was selected based on simple criteria that he had a lifelong good medical history with no chronic illness and was highly motivated to undergo necessary weekly MRI scanning, daily health monitoring, and daily tracking of accelerometer-measured step counts.
2.2. Medical History
He has been active across life and had no history of medical problems, childhood abuse, psychiatric illness, concussion, or head trauma. He has been a vegetarian since 2000 and had not used tobacco since 1980 or alcohol since 2000. Prior to those times, he was a sporadic pipe smoker and minimal alcohol consumer. He had never used recreational drugs. Other than wisdom teeth removal and stitches for a minor childhood finger incision, he had no surgeries. The MRI scans indicated no brain abnormalities.
2.3. Health during the Study
During the study he experienced no illnesses or trauma, and his daily schedule involved the usual work and home routines. Health monitoring included daily measures of pulse, blood pressure, blood glucose, oral temperature, and weight. Further measures taken at the end of the study included waist circumference, body mass index, hemoglobin A1c (HbA1c), C-reactive protein, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides. Three physicians independently reviewed these measures and rated all to be within, or approximate, healthy ranges (marginally low pulse (57 ± 3 bpm) and marginally high systolic pressure (124 ± 7 mmHg) on arising in mornings).
Daily questionnaire scores indicated he was within normal ranges for anxiety, depression, and happiness. He did not suffer from sleep disorders, and sleep durations across the study fluctuated within normal adult guidelines. Questionnaire responses indicated he felt mentally alert, healthy, and regularly woke up feeling rested and with high energy. Further assessments indicated he did not suffer from metabolic syndrome and had low allostatic (stress) load. Doppler ultrasound measures at the end of the study indicated that blood flow velocities for cortical arteries were within normal ranges.
Finally, his left and right hemisphere thickness means and ranges were encompassed within thickness measures reported in 11 recent investigations that defined normal adult hemispheric thicknesses with the Freesurfer procedures used in the present study [13].
2.4. Prospective Micro-Longitudinal Design
Details of the micro-longitudinal time series measures of hemispheric thickness have been described [8,9]. In brief, MRI scans were made on 22 dates across a 25-week scan period. Except for missed scans at weeks 2, 6, and 7, scans were taken at 1-week intervals on Sundays around the same mid-day start time (mean ± SD: 13:55 ± 2.1 h). On each date, two scans were taken in one session, with removal from the scanner between the first (scan A) and second (scan B) scans (≈5 min interscan time; 11.2 min/scan). This provided 44 hemispheric thickness maintenance measures taken over a total scan time of 8.2 h.
Micro-longitudinal tracking of steps/day was done with a Fitbit One triaxial accelerometer. Activity during windows of time that both preceded and followed scan dates were of interest. Steps/day measures were not available for weeks that immediately preceded, and the first 6 days of, the 25-week scan period. Steps/day activity measures were available each day beginning on day 7 and extending over the remaining days of the scan period, as well as over a further 78 days after the scan period that became of interest. This provided a total of 247 sequential measures of steps/day activity.
2.5. MRI Scans, Scan Processing, and Thickness Maintenance Measures
Procedures for MRI scanning, scan processing, thickness maintenance measurement, and measurement error control and assessment have been described in detail [8,9]. Salient points are as follows. T1-weighted scans of the entire brain were made with a 3T GE Signa scanner (164 continuous axial slices, voxel size 1 mm × 1 mm × 1 mm). All scans were made with the same scanner, head coil, and scan parameters. Regular quality assurance tests during the study identified no problems, and scanner upgrades were not done. Scan checks ruled out motion and other artifacts. Scan processing was done with automated FreeSurfer procedures (http://surfer.nmr.mgh.harvard.edu; accessed on 16 January 2023). To treat data from all scans as equal and independent measures, each scan was processed individually without cross-scan registration or averaging. Thickness measures were taken in native space without transformation to a template. Cortical thickness was defined at ≈150,000 vertex locations/hemisphere, and mean hemispheric cortical thickness (mm) was determined for each hemisphere using all vertex measures. To ensure uniform processing, all scans were processed at one time, after collection of all data, using one workstation, operating system, and FreeSurfer (version 4.5.1) program. Cortical borders were visually checked and judged to not require manual correction.
2.6. Physical Activity Measures
Step counts were done with one Fitbit One accelerometer worn on the right front waistline between arising in the morning and retiring in the evening, with exceptions for showering. The Fitbit One has been shown to provide valid and reliable counts of step activity [14,15,16]. The individual had no gait irregularities or physical limitations. Steps were free-living and, accordingly, speed, bout durations, and other factors varied with daily needs. Step counts from the device readout were entered into a database each evening and subsequently not accessed or processed until after study completion.
Accuracies of the Fitbit One step counts in our specific subject were of interest. Tests from the studied individual during the study indicated an average 1% (±0.7) difference (836 comparison trials for 28 days) between Fitbit counts and corresponding direct counts of steps taken at variable rates over flat, hilly, irregular, and stair environments. This difference is within reported Fitbit One step count accuracy measures for nonimpaired subjects [14,16,17,18,19,20], including for free-living conditions [21,22,23] and older adult age groups [15,24,25].
Taken together, the above considerations suggest accuracies of daily step counts in the studied individual were high. A limitation is that these step counts come from one individual and, thus, do not represent population measures.
A runs test assessed potential shifting in activity over the study. In addition, Kolmogorov–Smirnov (K-S) uniform distribution analyses tested if step activity differed across days of the week.
2.7. Regression Analyses
Regression analyses were used to assess relationships, first, between preceding physical activity and subsequent cortical thickness maintenance (Question 1) and, second, in the reverse direction, between preceding cortical thickness maintenance and subsequent physical activity (Question 2). For both questions, initial analyses pooled left and right thickness measures. Follow-up analyses considered left and right cortices separately. Data were plotted as scatterplots with associated linear regression lines, R2, and p values. The analyses provided tests of “if-then”, i.e., “if” this occurred earlier, “then” this occurred later, relationships in each direction.
2.8. Temporal Analyses
To our knowledge, no prior attempts have been made to examine relationships between maintenance of cortical thickness and steps/day activity over continuous short intervals for an extended period in an individual person. A main interest was assessment of windows of time that did and did not contribute to relationships in each direction. This necessitated trial-and-error empirical testing of variable time windows.
As an a priori plan for systematic testing, a strategy was applied that first tested longer time windows of multiple weeks, which was followed by tests for shorter windows of individual weeks, week segments, and days. This provided tests of relationships over a range of time scales and bracketing of periods that had graded degrees of relationships. This plan was used to successfully assess bidirectional temporal relationships between cortical thickness maintenance and sleep duration in this individual [13].
2.9. Significance Levels
Plots of data and regression analyses were done using SPSS. Analyses of different time windows involved different numbers of tests. For analyses involving multiple tests, conservative Bonferroni multiple test-adjusted p values were applied, whereby p = 0.05/n, where n = number of tests done in that analysis. Use of Bonferroni adjustments impacted outcomes by reducing chances of false positive results and by increasing the difficulty of obtaining relationships that were interpreted to be significant. Following Bonferroni correction, a test result was considered significant if p ≤ 0.05/n, and a trend if p > 0.05/n but ≤0.05. The number of tests associated with Bonferroni correction and the applicable p value are indicated in pertinent Results sections.
Given the conservative nature of Bonferroni-adjusted significance levels, trend level outcomes were considered. This was done in view of the possibility that time windows with conservative significant relationships may extend in graded fashion to times with further meaningful but weaker trend-level relationships, and to yet further times with no relationships. This allowed considerations which, on the one hand, did not increase or exaggerate significant outcomes and, on the other hand, did not disregard outcomes at trend levels which may be meaningful for assessing potential graded temporal relationships.
2.10. Blind Controls
Controls were applied to support blinded assessments. (1) Thickness measures were not defined until after the study. (2) Similarly, steps/day activity measures were not accessed until after the study. (3) Thickness and activity measures were each processed and defined independently by different investigators who did not have knowledge of the other measure, and were not altered in subsequent analyses.
3. Results
3.1. Cortical Thickness Maintenance
Thickness maintenance profiles for each scan series have been previously described and are plotted across weeks for each hemisphere (Figure 1A,B) [8,9]. Mean thickness measures for each hemisphere, taken on Sundays, underwent reversing incremental and decremental fluctuations from week to week that were not attributable to measurement error. Right thickness was consistently maintained at a higher level on each scan date, leading to a larger mean (±SD) right (2.574 ± 0.025 mm) than left thickness (2.549 ± 0.025 mm). Previous runs tests indicated cortical thickness maintenance of each hemisphere did not progressively shift over time.
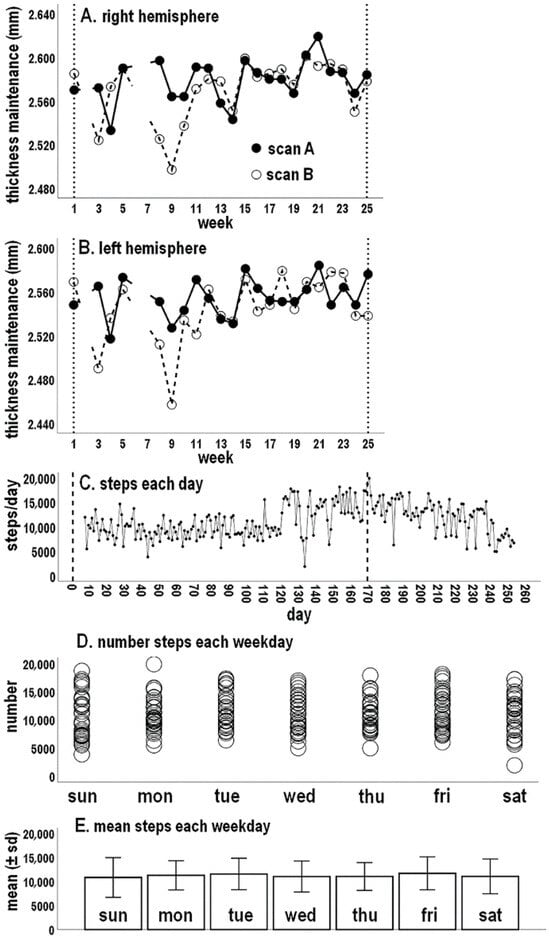
Figure 1.
Concurrent micro-longitudinal tracking of maintenance of right (A) and left (B) hemisphere mean cortical thicknesses and step-per-day activity (C). In (A,B) filled circles indicate scan A, and open circles indicate scan B; breaks in the lines indicate missing data at weeks 2, 6, and 7. Vertical dashed lines in (A–C) correspond to first and last scan days. Scatterplots of number of steps (D) and graphs of mean (±SD) steps (E) for each day of the week.
3.2. Activity across the Study Period
Except for the initial 6 days during which activity measures were not recorded, the number of steps taken each day between the first and last scan dates are shown in Figure 1C. Mean (±SD) activity over this time was 10,714 (±3260) steps/day. In addition, in addressing Question 2, activity for 78 days after the last scan became of interest when assessing temporal relationships between preceding thickness maintenance and subsequent activity. These days became part of the study period (Figure 1C). With these days, mean activity was 11,159 (±3354) steps/day.
A runs test of steps/day over the study period was significant (p < 0.001), indicating activity underwent progressive shifting. This appeared largely due to a spontaneous upward shift beginning around day 120, which subsequently drifted down to earlier levels (Figure 1C).
3.3. Activity for Different Days of the Week
Scatterplots of the number of steps and graphs indicating mean (±SD) steps are shown for each day of the week (Figure 1D,E). Mean step levels across the 7 days did not differ from a null horizontal distribution indicating a no cross-day difference (K-S, p = 0.502). Similarly, standard deviation levels across days did not differ from a null horizontal distribution (K-S, p = 0.682). This suggests steps activity was similar for different days of the week.
3.4. Question 1: Was Preceding Physical Activity Related to Subsequent Maintenance of Cortical Thickness and, if so, over What Times?
Using the empirical broad-to-narrow time window approach discussed in the Methods, initial analyses focused on activity over preceding broad multi-week periods.
3.4.1. Analysis 1: Activity during Preceding Three-Week Periods vs. Subsequent Thickness Maintenance (n = 2 Tests; Bonferroni Adjusted p ≤ 0.025)
There was a significant positive relationship between average steps/day across the immediately preceding 3-week period, i.e., across the three Sunday–Saturday periods for the preceding weeks 1–3, and thickness maintenances on the subsequent Sunday (Figure 2A,C). In contrast, average steps/day across the next earlier 3-week period, i.e., preceding weeks 4–6, was not related to subsequent thickness maintenances (Figure 2B,C).

Figure 2.
Scatterplots, linear regression lines, and associated R2 for relationships between average steps/day during preceding weeks 1–3 (A) and 4–6 (B) vs. subsequent thickness maintenance. (C) R2 profile summary for these preceding multi-week periods. The R2 profile suggests associations were delayed/prolonged over weeks, with a temporal gradient involving a significant positive relationship for weeks 1–3 and no relationship for weeks 4–6.
3.4.2. Analysis 2: Activity during Preceding Individual Week Periods vs. Subsequent Thickness Maintenance (n = 4 Tests; Bonferroni Adjusted p ≤ 0.0125)
Analyses next focused on average activity for preceding individual week (Sunday–Saturday) periods. There was a nonsignificant relationship between average steps/day for the preceding 1st week vs. subsequent thickness maintenance (Figure 3A,D). Steps/day averages for the preceding 2nd and 3rd weeks each had positive relationships at trend levels (Figure 3B–D). In contrast, the relationship for the preceding week 4 dropped off sharply and was not significant (R2 = 0.002, p = 0.677; Figure 3D). These results provided a further suggestion of positive relationships between activity at some time(s) during preceding weeks and subsequent thickness maintenance (Figure 3D).
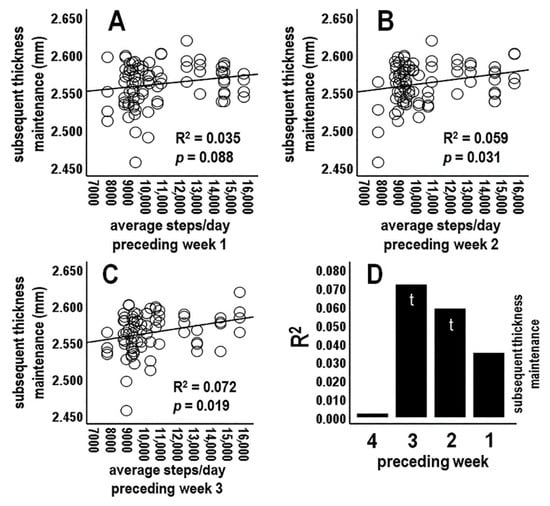
Figure 3.
Scatterplots, linear regression lines, and associated R2 for relationships between average steps/day during preceding individual week 1 (A), 2 (B), and 3 (C) vs. subsequent thickness maintenance. (D) R2 profile summary for preceding individual weeks 1–4.
3.4.3. Analysis 3: Activity during Preceding Week Segment Periods vs. Subsequent Thickness Maintenance (n = 6 Tests; Bonferroni Adjusted p ≤ 0.008)
The focus next shifted to average daily activity across early week, i.e., Sunday–Wednesday, and latter week, i.e., Thursday–Saturday, segments of preceding weeks 1, 2, and 3.
Steps/day averages for early week segments were positively related to subsequent thickness maintenances at a significant level for the preceding week 2 and at trend levels for both week 1 and week 3 (Figure 4A–C,F). Noticeably different, activities for latter-week segments (Figure 4D,E) were not related to subsequent thickness maintenance: i.e., compare distributions and regression lines in Figure 4A–C vs. Figure 4D,E, and R2 in Figure 4F.

Figure 4.
Scatterplots, linear regression lines, and associated R2 for relationships between average steps/day during preceding early week segments of weeks 1 (A), 2 (B), and 3 (C), and for preceding latter week segments of weeks 1 (D) and 2 (E) vs. subsequent thickness maintenance. (F) R2 profile summary for week segments of preceding weeks 1, 2, and 3. The R2 profile suggests a delayed/prolonged gradient in associations involving: first, early week segments that had a significant (s) positive relationship during preceding week 2 and trend (t)-level positive relationships during preceding weeks 1 and 3, and second, no relationships for latter week segments.
Early week segment positive relationships appeared to be graded, with a relatively higher relationship during the 2nd as compared to the 1st and 3rd preceding weeks (Figure 4F). These positive relationships involved delayed and/or prolonged associations because the early-week activity for preceding week 1 was separated from subsequent thickness maintenance measures by intervening week 1 latter week days, and early week activities for preceding weeks 2 and 3 were separated from subsequent thickness maintenance measures by the intervening weeks (Figure 4F).
3.4.4. Analysis 4: Activity during Preceding Individual Days vs. Subsequent Thickness Maintenance (n = 21 Tests; Bonferroni Adjusted p ≤ 0.002)
Steps during individual days of preceding weeks 1, 2, and 3 were next assessed. For preceding week 1, steps on early week segment Wednesdays and Sundays, i.e., the respective 4th and 7th days prior to thickness maintenance measures, were each positively related at trend levels to thickness maintenance on the subsequent Sunday (Table 1; Figure 5A,B,I). Relationships for early week 1 Mondays and Tuesdays were not significant (Table 1; Figure 5I).

Table 1.
Relationships between steps each day of preceding weeks 1, 2, and 3 vs. subsequent thickness maintenance.
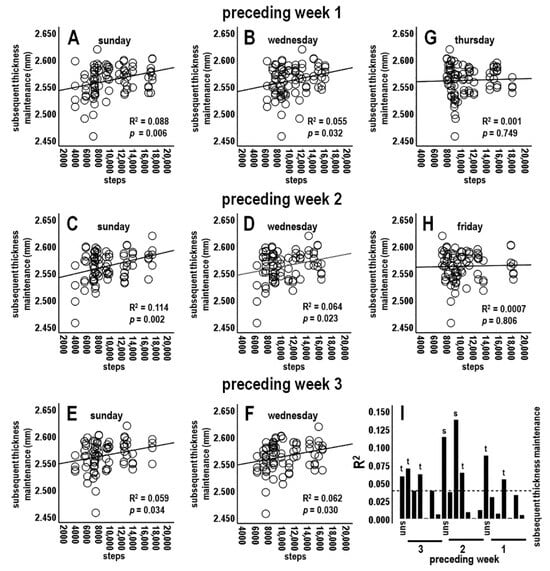
Figure 5.
Scatterplots, linear regression lines, and associated R2 for relationships between steps during indicated individual early week segment days (A–F) and latter week segment days (G,H) of preceding week 1 (top), 2 (middle), and 3 (lower) vs. subsequent thickness maintenance. (I) R2 profile summary indicating significant (s) and trend (t)-level positive relationships for preceding individual days. R2 at and below the dashed line are not significant. In (I), for each week Sunday is labeled and followed in sequence by Monday–Saturday.
For preceding week 2, steps on early week segment Sundays and Tuesdays were each significantly positively related to thickness maintenance on the subsequent Sunday (Table 1; Figure 5C,I). Steps on Wednesdays were positively related at a trend level, whereas relationships for Mondays were not significant (Table 1; Figure 5D,I).
For preceding week 3, steps on early week segment Sundays, Mondays, and Wednesdays were each positively related at trend levels to subsequent thickness maintenance (Table 1; Figure 5E,F,I), whereas relationships for early week Tuesdays were not significant (Table 1; Figure 5I).
In contrast to the above findings for early week segment days, steps on latter week segment Thursdays, Fridays, and Saturdays of preceding weeks 1, 2, and 3 were not related to subsequent thickness maintenance (Table 1; Figure 5G–I). These nonsignificant relationships noticeably differed from positive relationships of early week segment days (e.g., compare positive scatterplot distributions and regression lines for early week segment days in Figure 5A–F to flat distributions and regression lines for latter week segment days in Figure 5G,H).
Analysis 4 results suggest that activity for some preceding early week segment individual days had positive relationships with subsequent thickness maintenance and that these associations were temporally delayed and/or prolonged across shortest to longer times of respectively 4–7 days to 2–3 weeks (Figure 5I). Relationships across days were graded, e.g., with significant to trend-level positive relationships for early week segment days but no significant relationships for latter week segment days (Table 1; Figure 5I).
3.4.5. Analysis 5: Left and Right Cortex Laterality Analyses (n = 42 Tests; Bonferroni Adjusted p ≤ 0.001)
The above analyses used pooled thickness maintenances from both hemispheres. To test if preceding individual day activities over preceding weeks 1–3 were related to subsequent thickness maintenances of each cortex, thickness maintenances of right and left cortices were considered separately.
Preceding steps during individual early week segment days could be positively related to subsequent thickness maintenance of each cortex. For example, preceding early week segment days at which significant or trend-level positive relations were seen in Analysis 4 had further trend-level positive relations for each cortex (e.g., Figure 6A–C). Steps during preceding other early week segment days where trends for positive relations were seen in Analysis 4 had trend-level positive relations with one but not both cortices (e.g., Figure 6D).

Figure 6.
Scatterplots, linear regression lines, and associated R2 for relationships between steps during early week segment individual days of preceding weeks 1 (A), 2 (B,C), and 3 (D) and latter week segment individual days of preceding weeks 1 (E) and 2 (F) vs. subsequent thickness maintenances of separate right and left cortices. Identification of right and left cortices as indicated in (A) also applies to (B–F).
Contrasting with the above positive trend relationships for preceding early week segment days, no relationships were seen between daily activity for preceding latter week segment days and thickness maintenances of each cortex (e.g., Figure 6E,F). Relationships for early week segment days noticeably differed from relationships for latter week segment days (e.g., compare the positive scatterplot distributions and regression lines for each cortex in Figure 6A–C to the flat distributions and regression lines for each cortex in Figure 6E,F).
3.4.6. Question 1 Summary
The above analyses suggest preceding activity during (a) the prior 3 but not earlier weeks had a significant positive relationship with subsequent thickness maintenance (Figure 2C). In addition, preceding activity during (b) individual week periods of prior weeks 2–3 (Figure 3D), (c) early but not latter segments of prior weeks 1–3 (Figure 4F), and (d) early but not latter week individual days of weeks 1–3 (Figure 5I) had graded significant and/or trend-level positive relationships with subsequent thickness maintenance. Preceding activity relationships with subsequent thickness maintenance involved associations that were delayed/prolonged over shortest to longer times of respectively 4–7 days to 2–3 weeks (Figure 4F and Figure 5I). Trend-level positive relationships were seen for each cortex (Figure 6).
3.5. Question 2: Was Preceding Maintenance of Cortical Thickness Related to Subsequent Physical Activity and, if So, over What Times?
Question 2 examined physical activity and cortical thickness maintenance relationships in the reverse direction to that addressed in Question 1, using the approach of assessing broader to narrower periods of activity.
3.5.1. Analysis 6: Preceding Thickness Maintenance vs. Activity for Subsequent Three-Week Periods (n = 4 Tests; Bonferroni Adjusted p ≤ 0.0125)
Preceding thickness maintenance was significantly positively related to average steps/day over subsequent sequential 1st–3rd, 4th–6th, and 7th–9th three-week periods (Figure 7A). In contrast, preceding thickness maintenance was not related to activity over the subsequent 10th–12th three-week period (Figure 7A).

Figure 7.
R2 profile summaries for preceding thickness maintenance vs. average steps/day during subsequent 3-week periods (A), individual week periods (B), and early- and latter-week segments (C). Significant (s) and trend (t)-level associations are indicated; measures at and below the dashed lines were not significant. In (C), sequence of early (left bar) and latter (right bar) week segments indicated for week 1 also applies to each other week.
3.5.2. Analysis 7: Preceding Thickness Maintenance vs. Activity for Subsequent Individual Weeks (n = 9; Bonferroni Adjusted p ≤ 0.005)
Preceding thickness maintenance was significantly positively related to average steps/day during the subsequent individual 1st, 3rd, 4th, and 6th–8th weeks and positively related at trend levels to activity during the subsequent 2nd and 5th weeks (Figure 7B; Table 2). In contrast, thickness maintenance was not related to activity during the 9th week (Figure 7B; Table 2). This suggested that preceding thickness maintenance had graded, delayed/prolonged relationships with activity during the subsequent first 8 individual-week periods.

Table 2.
Relationships between preceding thickness maintenance and average daily steps for each of subsequent weeks 1–9.
3.5.3. Analysis 8: Preceding Thickness Maintenance vs. Activity for Subsequent Week Segments (n = 16; Bonferroni Adjusted p ≤ 0.003)
The above results led to tests of relationships between preceding thickness maintenance and activities during early week, i.e., Monday–Thursday, and latter week, i.e., Friday–Sunday, segments of the subsequent 8 weeks.
Preceding thickness maintenance was positively related to subsequent average steps/day for 81% (13/16) of the 16 total early and latter week segments, with 50% (8/16) significantly related, and 31% (5/16) related at trend levels (Table 3; Figure 7C). Relationships for the remaining 19% (3/16) were not significant (Table 3; Figure 7C).

Table 3.
Relationships between preceding thickness maintenance and average daily steps for early and latter week segments of subsequent weeks 1–8.
The interval for initial positive relationships was short, and gradients in positive relationships were seen for early vs. latter week segment activities. Specifically, beginning with a significant positive relationship with activity during the early week segment of subsequent week 1, i.e., within the 1st–4th days after thickness maintenance measures, activity during early week segments of all other weeks also had significant or trend-level positive relationships with preceding thickness maintenance (100%, 8/8) (Table 3; Figure 7C). In addition, activity during a majority (62.5%, 5/8) of latter week segments had significant or trend-level positive relationships with preceding thickness maintenance (Table 3; Figure 7C).
These results suggest preceding thickness maintenance relationships with subsequent activity involved graded positive associations that were temporally delayed/prolonged, with shortest to longer times between associations of respectively 1–4 days to 8 weeks (Figure 7C).
Analyses of activity during separate days were not pursued based on thinking that individual day associations across 8 weeks would not provide more meaningful bracketing of relationships than these week segment results.
3.5.4. Analysis 9: Left and Right Cortex Laterality Analyses (n = 6; Bonferroni Adjusted p ≤ 0.008)
The above analyses used pooled thickness maintenances of both hemispheres. To test if preceding thickness maintenances of each cortex could be related to subsequent activity, thickness maintenances of left and right cortices were considered separately. Because relationships for 3-week periods (Analysis 6) encompassed relationships for shorter periods (Analyses 7–8), activity for 3-week periods were assessed.
Preceding thickness maintenances of the left and right cortices were each significantly positively related to subsequent mean activity over weeks 1–3 (Figure 8A) and 4–6 (Figure 8B). Over weeks 7–9, preceding thickness maintenance of the right cortex was significantly positively related to subsequent mean activity, whereas maintenance of the left cortex was positively related to activity at a trend level (Figure 8C). These results suggest preceding thickness maintenances of each cortex were positively related to subsequent activity.

Figure 8.
Scatterplots, linear regression lines, and associated R2 of relationships between preceding thickness maintenances of separate left and right cortices vs. average steps/day during subsequent weeks 1–3 (A), 4–6 (B), and 7–9 (C). The convention distinguishing right and left cortices in (A) also applies to (B,C).
3.5.5. Question 2 Summary
The above analyses suggest preceding thickness maintenance had significant positive relationships with activity during the subsequent (a) 1st–3rd, 4th–6th, and 7th–9th but not later week periods (Figure 7A) and graded significant to trend-level positive relationships with activity during (b) the related first 8 individual-week periods (Figure 7B) and (c) all early week segments and most latter week segments of these 8 weeks (Figure 7C). Preceding thickness maintenance to subsequent activity associations were graded and delayed/prolonged over shortest to longer times of respectively 1–4 days to 8 weeks (Figure 7C). Significant or trend-level positive relationships were seen for each cortex (Figure 8).
4. Discussion
4.1. Present Results
Maintenance of the adult cerebral cortex involves continuous structural rebuilding over day/week periods that is needed to revitalize structure. Normal maintenance of cortical thickness in an individual person potentially interacts with their physical activity but, to our knowledge, temporal interactions have not been studied at an individual person level. This study used micro-longitudinal tracking to address two questions regarding potential temporal interactions at an individual level. Given the absence of available data and frank uncertainty about whether or when interactions might occur in an individual, testing of activity from empirically varied times was necessary to address our questions.
Question 1: Was preceding physical activity related to subsequent maintenance of cortical thickness and, if so, over what times? In the studied individual, preceding physical activity over the prior 3 but not earlier weeks was significantly positively related to subsequent thickness maintenance. Graded significant and/or trend-level positive relationships were seen between preceding activity and subsequent thickness maintenance for activity that occurred during early but not latter segments of preceding weeks 1–3 and early but not latter week individual days of these weeks. Relationships in this direction involved associations that were delayed/prolonged over shortest to longer times of respectively 4–7 days to 2–3 weeks and that were bilateral, i.e., involved thickness maintenances of both cortices.
Question 2: Was preceding maintenance of cortical thickness related to subsequent physical activity and, if so, over what times? In the studied individual, preceding thickness maintenance was significantly positively related to physical activity that occurred during the subsequent 1st–3rd, 4th–6th, and 7th–9th but not latter week periods. Graded significant and/or trend-level positive relationships were seen between preceding thickness maintenance and activity for all individual-week periods of subsequent weeks 1–8 and for all early week segments and most latter week segments of these weeks. Relationships in this direction involved associations that were delayed/prolonged over shortest to longer times of respectively 1–4 days to 8 weeks and that were bilateral, i.e., involved thickness maintenances of both cortices.
In addressing Questions 1 and 2, we note that conservative Bonferroni adjustments were applied to reduce false positive results and that results that reached significant and trend levels of significance are considered and clearly distinguished. This is useful for defining associations where periods with conservatively defined significant relationships may have extended to yet earlier/later periods with trend-level relationships which, together, may comprise graded, delayed/prolonged associations that are meaningful from a micro-longitudinal, individual person perspective. Bonferroni-adjusted significance levels and distinction of periods with related significant and trend-level associations arguably permitted transparent consideration of this possibility without either exaggerating or overlooking associations.
4.2. Concepts from the Present Results
Taken together, the findings of Questions 1 and 2 suggest that the following concepts characterized temporal interactions between physical activity and cortical thickness maintenance in the studied individual.
Interactions were bidirectional. Preceding activity interacted with subsequent thickness maintenance and, conversely, preceding thickness maintenance interacted with subsequent activity. Interactions were positive. Interactions in both directions were consistently positive. Interactions involved both cortices. Each cortex was involved in interactions in both directions. Interactions had limits. Significant and trend-level relationships in the two directions ranged within modest R2 magnitudes. This suggests that in each direction, the preceding factor did not, by itself, exclusively dictate the subsequent measure. Interactions involved multi-week periods. Interactions extended over periods of 3 (Question 1) and 8 (Question 2) weeks. Interactions were graded. There were gradients in interactions for different multi-week, week, week segment, and day periods as reflected by variability in scatterplot distributions, related regression lines, and significant or trend-level relationships. Interactions were temporally asymmetric. Interactions in the preceding thickness maintenance to subsequent activity direction had a shorter initial onset (1–4 days) and longer duration (8 weeks) than the initial onset (4–7 days) and duration (3 weeks) of interactions in the reverse preceding activity to subsequent thickness maintenance direction. Interactions were prolonged and/or delayed. The findings of significant relationships across weeks and that periods with significant or trend-level relationships were separated by intervening periods that had no relationships suggest interactions were delayed and/or prolonged. Interactions were continuous. Interactions extended micro-longitudinally across the study. Overall, the observed bidirectional positive interactions suggest that physical activity may have continuous protective effects on cortical substrate maintenance which, in turn, promotes physical activity.
The above concepts come from one person, which raises the generalizability issue. Due to individual person specificity (see Implication 2 below), selection of a generally representative individual would appear to be very difficult. However, it is unlikely the present study serendipitously assessed the only individual to whom one or more of these concepts pertain; thus, they arguably generalize to some extent to other individuals. On further consideration, physical activity and cortical thickness vary across individuals, thus indicating that different expressions of the above concepts or different concepts apply in other individuals. This suggests the merit of exploring generalizability with further micro-longitudinal individual person analyses.
4.3. Comparison of the Present Individual-Focused Concepts to Existing Group-Based Concepts of Interactions between Cortical Thickness and Physical Activity
Cortical thickness has rarely been studied with a micro-longitudinal individual person analysis design, i.e., using intraindividual analysis of sequential thickness measures taken from an individual person at regular short intervals over a period of several months [8,9]. Recent reviews point to a need for brain studies using such designs [10,11,12]. Moreover, this type of design has not been used to investigate interactions between cortical thickness and physical activity.
Existing studies have, however, extensively assessed relationships between cortical thickness and physical activity with group-focused analyses. Concepts of relationships from the present individual-focused findings in some ways are similar to and predicted, but in other ways have not been detected, by group-focused work.
Similarities in concepts from group- and the present individual-focused findings include the following.
Positive relationships. Most group studies report positive relationships between physical activity and cortical thickness [4,5,6,26,27,28,29,30,31], with fewer studies finding either mixed positive and negative [32] or no [33,34] relationships. Aligning with many group-based results, interactions in the studied individual were positive.
Relationship bilaterality. Group-based work has reported relationships between physical activity and cortical thicknesses of both cortices [27,28,30,32]. Similarly, interactions were bilateral in the presently studied individual.
Long periods for relationships. Group analyses indicate activity over long periods can be related to cortical thickness [4,5,26,28,29,30,31,32]. Consistent with these findings, in the studied individual, interactions extended over multi-week periods.
Relationship limitations. Group work suggests physical activity operates collectively with other factors to affect cortical thickness [5,27]. In the studied individual, the strengths of interactions had limitations that did not reflect exclusive control relationships and, thus, likely involved influences of other factors (see below Implication 1).
Beyond the above similarities in group- vs. the present individual-based concepts, group-based work has paid little or no attention to other concepts of interactions that were resolved with the present micro-longitudinal individual-focused analyses, including the following.
Interaction directionality. Establishing direction(s) of interaction(s) between cortical structure and physical activity is an important focus of health neuroscience [35,36,37,38]. Group-based studies indicate that physical activity affects cortical thickness [4,5,6,27,28,29,30,31,32]. To our knowledge, only one group study has examined the reverse direction (“reverse causality”) possibility that cortical thickness affects physical activity [39]. The novel findings of that study suggest cortical thickness affects subsequent adherence to physical activity. Given limited attention to reverse direction effects, group-based conceptualization of directionality of physical activity and cortical thickness relationships is still being worked out. Distinction of direction(s) of interaction(s) is arguably improved with micro-longitudinal tracking and corresponding within-individual tests for independent relationships in each direction over sequentially continuous time periods. The present study provides such tests and suggests interactions in the studied individual were bidirectional.
Temporal interaction dynamics. Of further importance, group-based work has not attempted to address concepts that pertain to micro-longitudinal temporal characteristics regarding the presently observed (a) gradients, (b) time asymmetries, (c) delays and/or prolongations, and (d) ongoing continuous nature of interactions. The present findings of interactions that are: continuously ongoing, graded in strength over prolonged/delayed day to multi-week periods, and asymmetric in the two directions provide original insight into temporal interaction dynamics that can operate at an individual person level.
In summary, concepts from the present study agree with concepts from group analyses in some respects. In addition, the present findings complement current understanding by identifying concepts of temporal interactions that have received little or no attention in group work but that are arguably relevant for understanding relationships between maintenance of cortical thickness and physical activity at an individual level. This raises the possibility that an individual-focused approach can contribute to individualized precision medicine tailoring of physical activity recommendations by applying individual-focused concepts that have not been recognized or readily resolved by group-based work.
4.4. Normality of Interactions
This issue requires considering whether the individual’s steps/day activity and thickness maintenance measures were or were not normal.
The studied individual’s steps/day activity. What constitutes normal daily steps for a particular individual person remains enigmatic and must be indirectly judged from diverse findings from population/group-focused work, including the following.
First, adults normally take ≈3000–18,000+ steps/day, with group average rates in different countries ranging, e.g., from ≈5000–9600 steps/day [40,41,42]. The world-wide population’s average rate is estimated to be ≈4961 steps/day [43].
Second, classification schemes for group data categorize steps/day activity. For example, in one scheme, “sedentary” and “low activity” categories ranged from <5000–7499 steps/day, a “moderate activity” category ranged from 7500–9999, and “active” and “very active” categories ranged from ≥10,000 and ≥12,500 steps/day [44].
Third, longstanding mass media and national promotions suggest a population goal for adults to walk 10,000 steps/day [45,46,47,48,49].
Fourth, numerous reports suggest that average rates in the range of ≈4400–10,000 steps/day are associated with increased longevity and health benefits [50,51,52,53,54,55,56,57,58,59,60,61]. Less studied, activity above this range also has beneficial health effects [54,59,61,62,63,64].
Finally, it is proposed that a population-level threshold of ≈7000–8000 steps/day relates to public health guidelines for getting 30 min/day or 150 min/week of moderate-to-vigorous physical activity [40,57,65,66]. There also appears to be variation in this relationship across individuals [67].
The above work, although comprehensive with respect to normal group activity and public health guidelines, was not designed to identify what constitutes a normal number of steps for any specific person. However, the findings from this work currently provide the most relevant indices for gauging if an individual’s daily step activity is within normal expectations. The studied individual averaged 11,159 (±3354) steps/day; his pattern of activity over days, weeks, and months reflected a continuously active profile (Figure 1C); and his measures equaled or exceeded the above normal population/group indices. This suggests his steps/day activity fit normal expectations.
The studied individual’s cortical thickness maintenance measures. As previously reported [8,13] and briefly reviewed in Methods, the studied individual’s medical history and daily health measures attested to his good health and fitness over life and during the study. Moreover, we have previously presented detailed comparisons which show that his hemispheric thickness mean and variation measures were encompassed within the range of hemispheric thickness mean and variation measures reported in 11 studies that applied FreeSurfer thickness measurement procedures in normal adult groups of his age and younger [13]. From this, his cortical thickness maintenance measures arguably fit normal expectations.
Summary. The above views suggest that the studied individual’s steps/day activity and cortical thickness measures were within normal expectations and that the observed results likely reflect normal interactions.
4.5. Implications
The present findings have useful implications.
Implication 1: At an individual person level, normal maintenance of cortical thickness, physical activity, and their micro-longitudinal temporal interactions are likely affected by multiple factors.
The observed R2 magnitudes suggest that maintenance of cortical thickness in the studied individual was not exclusively dictated by preceding physical activity and that physical activity was not exclusively dictated by preceding maintenance of thickness.
Consistent with these possibilities, group-level work has shown cortical thickness to be associated with, e.g., body mass index [68], genetic [69], hormonal [70], stress [71], diet [72], allostatic load [73], time of day [74], gut microbiome [75], cardiovascular [76], and metabolic [77] factors.
Similarly, group-level work indicates that physical activity has associations with multiple factors, including, e.g., diet [78], gut microbiome [79], emotion regulation [80], fitness [81], weather [82], and metabolic [83] factors. Adding to group-level findings, studies using micro-longitudinal tracking and within-individual analyses have shown physical activity’s associations with, e.g., sleep [84,85,86,87] and stress [88].
Moreover, directly pertinent to the present findings, cortical thickness maintenance in the studied individual has been shown to be associated with both sleep duration and thickness maintenance during prior days/weeks [9,13]. Thus, other factors were likely in play in the studied individual and arguably co-influenced and perhaps limited the presently observed interactions. Taken with other recent proposals [89], this suggests a need for precision medicine research on how maintenance of cortical thickness, physical activity, and other factors micro-longitudinally co-interact at an individual level and whether, e.g., cortical thickness maintenance may mediate/moderate or be mediated/moderated by co-interactions.
Implication 2: Identifying concepts of micro-longitudinal temporal interactions between cortical thickness maintenance and lifestyle factors like physical activity at the individual level can improve individualized precision medicine tailoring of cortical structural maintenance and neurocognitive health.
To our knowledge, no previous work has investigated micro-longitudinal temporal interactions between fluctuations in cortical thickness maintenance and physical activity at an individual person level. This is an overlooked area of investigation.
Precision medicine has interests in maximizing cortical structural and related neurocognitive health, in part through individualized tailoring of potentially influential lifestyle factors like physical activity that may continuously affect brain maintenance. This focus on the individual person derives from a recognition of the importance of human variability and individual specificity.
Pertinent to the present investigation and precision medicine interests, cortical structure, including thickness, varies across individuals [90,91,92,93] and is individual specific [94,95,96,97,98,99]. Analogous to cortical thickness, physical activity also varies across individuals [100,101,102,103,104] and is individual specific [84,105,106,107].
At a group level, physical activity has been shown to be associated with cortical thickness [6,7,27], and activity interventions can change cortical thickness [4,5,31,108,109].
Differences in cortical structure, including thickness, are associated with differences in neurocognitive functions [92,94,110,111,112,113]. Similarly, although contrary results have been reported [114,115,116], much work indicates physical activity also has associations with neurocognitive functions [117,118,119,120,121,122,123,124,125,126].
The present findings complement the above group-level work by providing a beginning perspective on intraindividual micro-longitudinal temporal interactions between physical activity and cortical thickness maintenance that potentially contribute to individual specificity of physical activity, cortical thickness, and neurocognition. Recognition of these interactions may be useful for personalized tailoring of physical activity. It is possible, for example, that knowledge of an individual’s temporal delays/prolongations in interaction between preceding physical activity and subsequent thickness maintenance and between preceding thickness maintenance and subsequent activity might be used to inform tailoring of personalized recommendations for activity scheduling that will optimally affect that individual’s (a) thickness maintenance, (b) neurocognitive health, and (c) self-motivation to maintain healthful physical activity. Considered with recent views on personalized health maintenance [1,3,38], there is hope that further understanding of these interactions can improve individualized precision medicine tailoring of activity, brain, and neurocognitive health.
5. Limitations
This study has clear limitations. (1) The data and related interaction concepts are from one person. (2) Cortical structure is assessed only in terms of hemispheric mean thickness. (3) Physical activity is assessed only in terms of steps/day. (4) The data pertain to free-living and not intervention-related conditions. (5) The findings suggest “if-then”, i.e., if this occurs, then this follows, and not “cause-effect” interactions. (6) This work is an exploratory starting, not finishing, point for understanding interactions between maintenance of cortical structure and physical activity at an individual person level.
6. Conclusions
Maintenance of cerebral cortical structure, reflected in part by cortical thickness, is necessary to maintain neurocognitive health. Precision medicine has interests in using personalized adjustment of lifestyle factors, including physical activity, to optimize maintenance of a person’s cortical thickness and related neurocognitive health. However, whether or how maintenance of cortical thickness in an individual person micro-longitudinally interacts with their physical activity remains unclear. The present study used an unconventional micro-longitudinal tracking approach to assess temporal interactions between cortical thickness maintenance and physical activity over empirically varied time windows in a healthy adult man. These novel person-focused findings in some ways are predicted, but in other ways remain undetected, by existing group-focused work. We suggest that an understanding of person-focused interactions can complement group-focused findings and improve individualized precision medicine tailoring of cortical structural maintenance, physical activity, and related neurocognitive health.
Author Contributions
Conceptualization, J.W., H.X. and X.W.; methodology, J.W., H.X. and X.W.; software, H.X. and X.W.; validation, J.W. and H.X.; formal analysis, J.W., H.X. and X.W.; investigation, J.W. and X.W.; resources, J.W. and X.W.; data curation, J.W. and H.X.; writing—original draft preparation, J.W; writing—review and editing, J.W., H.X. and X.W.; visualization, J.W.; supervision, J.W.; project administration, J.W. and H.X.; funding acquisition, J.W. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by University Research Incentive Funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Toledo (200270, approved 25 July 2014).
Informed Consent Statement
Informed consent was obtained from the subject of this study. Written informed consent has been obtained from the subject to publish this paper.
Data Availability Statement
Raw data are not publicly available due to subject privacy and ethics concerns. Address questions to J.W. at john.wall@utoledo.edu.
Acknowledgments
We thank Cindy Grey, Sue Yeager, Michelle Hanus, and Lindsey Katschke for their technical expertise, and W. Bauer, H. Li, and M. Gerken for their assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shan, Z.Y.; Lagopoulos, J. Precision Medicine for Brain Disorders: New and Emerging Approaches. J. Pers. Med. 2023, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.K.; Hsueh, P.S.; Qian, M.; Yoon, S.; Meli, L.; Diaz, K.M.; Schwartz, J.E.; Kronish, I.M.; Davidson, K.W. Are Nomothetic or Ideographic Approaches Superior in Predicting Daily Exercise Behaviors? Methods Inf. Med. 2017, 56, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zuidersma, M.; Riese, H.; Snippe, E.; Booij, S.H.; Wichers, M.; Bos, E.H. Single-Subject Research in Psychiatry: Facts and Fictions. Front. Psychiatry 2020, 11, 539777. [Google Scholar] [CrossRef] [PubMed]
- Rektorova, I.; Klobusiakova, P.; Balazova, Z.; Kropacova, S.; Sejnoha Minsterova, A.; Grmela, R.; Skotakova, A.; Rektor, I. Brain structure changes in nondemented seniors after six-month dance-exercise intervention. Acta Neurol. Scand. 2020, 141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Harada, K.; Lee, S.; Harada, K.; Makino, K.; Chiba, I.; Park, H.; Shimada, H. The Effect of a Multicomponent Dual-Task Exercise on Cortical Thickness in Older Adults with Cognitive Decline: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Hsu, C.L.; Best, J.R.; Li, L.C.; Egbert, A.R.; Liu-Ambrose, T. Not Just for Joints: The Associations of Moderate-to-Vigorous Physical Activity and Sedentary Behavior with Brain Cortical Thickness. Med. Sci. Sports Exerc. 2020, 52, 2217–2223. [Google Scholar]
- Angelo, B.C.; DeFendis, A.; Yau, A.; Alves, J.M.; Thompson, P.M.; Xiang, A.H.; Page, K.A.; Luo, S. Relationships between physical activity, healthy eating and cortical thickness in children and young adults. Brain Imaging Behav. 2022, 16, 2690–2704. [Google Scholar]
- Wall, J.; Xie, H.; Wang, X. An exploration into short-interval maintenance of adult hemispheric cortical thickness at an individual brain level. J. Exp. Neurosci. 2017, 11, 1179069517733453. [Google Scholar] [CrossRef]
- Xie, H.; Wall, J.; Wang, X. Relationships in ongoing structural maintenances of the two cerebral cortices of an individual brain. J. Exp. Neurosci. 2018, 12, 1179069518795875. [Google Scholar] [CrossRef]
- Gratton, C.; Braga, R. Editorial overview: Deep imaging of the individual brain: Past, practice, and promise. Curr. Opin. Behav. Sci. 2021, 40, iii–vi. [Google Scholar]
- Fischer, H.; Nilsson, M.E.; Ebner, N.C. Why the Single-N Design Should Be the Default in Affective Neuroscience. Affect. Sci. 2023. [Google Scholar] [CrossRef]
- Naselaris, T.; Allen, E.; Kay, K. Extensive sampling for complete models of individual brains. Curr. Opin. Behav. Sci. 2021, 40, 45–51. [Google Scholar] [CrossRef]
- Wall, J.; Xie, H.; Wang, X. Interaction of Sleep and Cortical Structural Maintenance from an Individual Person Microlongitudinal Perspective and Implications for Precision Medicine Research. Front. Neurosci. 2020, 14, 769. [Google Scholar] [CrossRef]
- Mora-Gonzalez, J.; Gould, Z.R.; Moore, C.C.; Aguiar, E.J.; Ducharme, S.W.; Schuna, J.M., Jr.; Barreira, T.V.; Staudenmayer, J.; McAvoy, C.R.; Boikova, M.; et al. A catalog of validity indices for step counting wearable technologies during treadmill walking: The CADENCE-adults study. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 117. [Google Scholar] [CrossRef]
- Maganja, S.A.; Clarke, D.C.; Lear, S.A.; Mackey, D.C. Formative Evaluation of Consumer-Grade Activity Monitors Worn by Older Adults: Test-Retest Reliability and Criterion Validity of Step Counts. JMIR Form. Res. 2020, 4, e16537. [Google Scholar] [CrossRef] [PubMed]
- Takacs, J.; Pollock, C.L.; Guenther, J.R.; Bahar, M.; Napier, C.; Hunt, M.A. Validation of the Fitbit One activity monitor device during treadmill walking. J. Sci. Med. Sport. 2014, 17, 496–500. [Google Scholar] [PubMed]
- Floegel, T.A.; Florez-Pregonero, A.; Hekler, E.B.; Buman, M.P. Validation of Consumer-Based Hip and Wrist Activity Monitors in Older Adults with Varied Ambulatory Abilities. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 229–236. [Google Scholar] [CrossRef]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar]
- Diaz, K.M.; Krupka, D.J.; Chang, M.J.; Peacock, J.; Ma, Y.; Goldsmith, J.; Schwartz, J.E.; Davidson, K.W. Fitbit®: An accurate and reliable device for wireless physical activity tracking. Int. J. Cardiol. 2015, 185, 138–140. [Google Scholar] [CrossRef]
- Case, M.A.; Burwick, H.A.; Volpp, K.G.; Patel, M.S. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015, 313, 625–626. [Google Scholar]
- Middelweerd, A.; Vanderploeg, H.P.; Vanhalteren, A.; Twisk, J.W.R.; Brug, J.; Te Velde, S.J. A Validation Study of the Fitbit One in Daily Life Using Different Time Intervals. Med. Sci. Sports Exerc. 2017, 49, 1270–1279. [Google Scholar] [CrossRef]
- Ferguson, T.; Rowlands, A.V.; Olds, T.; Maher, C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 42. [Google Scholar] [PubMed]
- Reid, R.E.R.; Insogna, J.A.; Carver, T.E.; Comptour, A.M.; Bewski, N.A.; Sciortino, C.; Andersen, R.E. Validity and reliability of Fitbit activity monitors compared to ActiGraph GT3X+ with female adults in a free-living environment. J. Sci. Med. Sport. 2017, 20, 578–582. [Google Scholar] [CrossRef]
- Straiton, N.; Alharbi, M.; Bauman, A.; Neubeck, L.; Gullick, J.; Bhindi, R.; Gallagher, R. The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas 2018, 112, 85–93. [Google Scholar]
- Paul, S.S.; Tiedemann, A.; Hassett, L.M.; Ramsay, E.; Kirkham, C.; Chagpar, S.; Sherrington, C. Validity of the Fitbit activity tracker for measuring steps in community-dwelling older adults. BMJ Open Sport. Exerc. Med. 2015, 1, e000013. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Storsve, A.B.; Westlye, L.T.; Drevon, C.A.; Fjell, A.M. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol. Aging 2014, 35, 1055–1064. [Google Scholar] [PubMed]
- Lee, J.S.; Shin, H.Y.; Kim, H.J.; Jang, Y.K.; Jung, N.Y.; Lee, J.; Kim, Y.J.; Chun, P.; Yang, J.J.; Lee, J.M.; et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci. Rep. 2016, 6, 24284. [Google Scholar] [CrossRef]
- Rogge, A.K.; Röder, B.; Zech, A.; Hötting, K. Exercise-induced neuroplasticity: Balance training increases cortical thickness in visual and vestibular cortical regions. Neuroimage 2018, 179, 471–479. [Google Scholar]
- Gu, Y.; Beato, J.M.; Amarante, E.; Chesebro, A.G.; Manly, J.J.; Schupf, N.; Mayeux, R.P.; Brickman, A.M. Assessment of Leisure Time Physical Activity and Brain Health in a Multiethnic Cohort of Older Adults. JAMA Netw. Open 2020, 3, e2026506. [Google Scholar] [CrossRef]
- Um, Y.H.; Wang, S.M.; Kim, N.Y.; Kang, D.W.; Na, H.R.; Lee, C.U.; Lim, H.K. Effects of Moderate Intensity Exercise on the Cortical Thickness and Subcortical Volumes of Preclinical Alzheimer’s Disease Patients: A Pilot Study. Psychiatry Investig. 2020, 17, 613–619. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Q.; Herold, F.; Cheval, B.; Dong, X.; Cui, L.; Xiong, X.; Chen, A.; Yin, H.; Kong, Z.; et al. Brain Structure, Cardiorespiratory Fitness, and Executive Control Changes after a 9-Week Exercise Intervention in Young Adults: A Randomized Controlled Trial. Life 2021, 11, 292. [Google Scholar]
- Tarumi, T.; Tomoto, T.; Repshas, J.; Wang, C.; Hynan, L.S.; Cullum, C.M.; Zhu, D.C.; Zhang, R. Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness. Neuroimage 2021, 225, 117512. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2016, 8, 336. [Google Scholar] [CrossRef]
- Siddarth, P.; Burggren, A.C.; Eyre, H.A.; Small, G.W.; Merrill, D.A. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS ONE 2018, 13, e0195549. [Google Scholar]
- Best, J.R.; Chiu, B.K.; Hall, P.A.; Liu-Ambrose, T. Larger Lateral Prefrontal Cortex Volume Predicts Better Exercise Adherence Among Older Women: Evidence from Two Exercise Training Studies. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 804–810. [Google Scholar]
- Gujral, S.; McAuley, E.; Oberlin, L.E.; Kramer, A.F.; Erickson, K.I. Role of Brain Structure in Predicting Adherence to a Physical Activity Regimen. Psychosom. Med. 2018, 80, 69–77. [Google Scholar]
- Hofman, A.; Rodriguez-Ayllon, M.; Vernooij, M.W.; Croll, P.H.; Luik, A.I.; Neumann, A.; Niessen, W.J.; Ikram, M.A.; Voortman, T.; Muetzel, R.L. Physical activity levels and brain structure in middle-aged and older adults: A bidirectional longitudinal population-based study. Neurobiol. Aging 2023, 121, 28–37. [Google Scholar] [CrossRef]
- Stillman, C.M.; Erickson, K.I. Physical activity as a model for health neuroscience. Ann. N. Y. Acad. Sci. 2018, 1428, 103–111. [Google Scholar]
- Morris, T.P.; Burzynska, A.; Voss, M.; Fanning, J.; Salerno, E.A.; Prakash, R.; Gothe, N.P.; Whitfield-Gabrieli, S.; Hillman, C.H.; McAuley, E.; et al. Brain Structure and Function Predict Adherence to an Exercise Intervention in Older Adults. Med. Sci. Sports Exerc. 2022, 54, 1483–1492. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; De Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough? For adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. [Google Scholar] [CrossRef]
- Beagle, A.J.; Tison, G.H.; Aschbacher, K.; Olgin, J.E.; Marcus, G.M.; Pletcher, M.J. Comparison of the Physical Activity Measured by a Consumer Wearable Activity Tracker and That Measured by Self-Report: Cross-Sectional Analysis of the Health eHeart Study. JMIR Mhealth Uhealth 2020, 8, e22090. [Google Scholar] [PubMed]
- Amagasa, S.; Fukushima, N.; Kikuchi, H.; Oka, K.; Chastin, S.; Tudor-Locke, C.; Owen, N.; Inoue, S. Older Adults’ Daily Step Counts and Time in Sedentary Behavior and Different Intensities of Physical Activity. J. Epidemiol. 2021, 31, 350–355. [Google Scholar] [PubMed]
- Althoff, T.; Sosič, R.; Hicks, J.L.; King, A.C.; Delp, S.L.; Leskovec, J. Large-scale physical activity data reveal worldwide activity inequality. Nature 2017, 547, 336–339. [Google Scholar] [PubMed]
- Bassett, D.R., Jr.; Toth, L.P.; LaMunion, S.R.; Crouter, S.E. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sports Med. 2017, 47, 1303–1315. [Google Scholar] [CrossRef]
- Mair, J.L.; Aguiar, E.J.; Stamatakis, E.; Edney, S.M. Moving Toward the Inclusion of Step-Based Metrics in Physical Activity Guidelines and Surveillance. J. Phys. Act. Health 2023, 20, 575–577. [Google Scholar]
- Choi, B.C.; Pak, A.W.; Choi, J.C.; Choi, E.C. Daily step goal of 10,000 steps: A literature review. Clin. Investig. Med. 2007, 30, E146–E151. [Google Scholar] [CrossRef]
- Orlov, A.; Rotar, O.; Vigl, M.; Konradi, A.; Boeing, H. Objective measurement of physical activity in a random sample of Saint-Petersburg inhabitants. Arter. Hypertens. 2020, 24, 135–141. [Google Scholar]
- Vandelanotte, C.; Van Itallie, A.; Brown, W.; Mummery, W.K.; Duncan, M.J. Every Step Counts: Understanding the Success of Implementing The 10,000 Steps Project. Stud. Health Technol. Inform. 2020, 268, 15–30. [Google Scholar]
- Denworth, L. The “10,000 Steps” Gimmick. Sci. Am. 2023, 328, 25. [Google Scholar]
- Del Pozo Cruz, B.; Ahmadi, M.N.; Lee, I.M.; Stamatakis, E. Prospective Associations of Daily Step Counts and Intensity with Cancer and Cardiovascular Disease Incidence and Mortality and All-Cause Mortality. JAMA Intern. Med. 2022, 182, 1139–1148. [Google Scholar]
- Lee, I.M.; Shiroma, E.J.; Kamada, M.; Bassett, D.R.; Matthews, C.E.; Buring, J.E. Association of Step Volume and Intensity with All-Cause Mortality in Older Women. JAMA Intern. Med. 2019, 179, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.S.; Hyde, E.T.; Bassett, D.R.; Carlson, S.A.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Kraus, W.E.; Lee, I.M.; et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 78. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kadota, A.; Segawa, H.; Kondo, K.; Torii, S.; Miyagawa, N.; Fujiyoshi, A.; Hisamatsu, T.; Watanabe, Y.; Shiino, A.; et al. Relationship Between Step Counts and Cerebral Small Vessel Disease in Japanese Men. Stroke 2020, 51, 3584–3591. [Google Scholar] [PubMed]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R., Jr.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality Among US Adults. J. Am. Med. Assoc. 2020, 323, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Aho, S.; Vuoristo, M.S.; Raitanen, J.; Mansikkamäki, K.; Alanko, J.; Vähä-Ypyä, H.; Luoto, R.; Kellokumpu-Lehtinen, P.L.; Vasankari, T. Higher number of steps and breaks during sedentary behaviour are associated with better lipid profiles. BMC Public Health 2021, 21, 629. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Fukuda, H.; Takebayashi, M.; Mori, M.; Matsushima, R.; Nakano, K.; Miyake, K.; Tani, Y.; Yokokawa, H. Effects of an mHealth App (Kencom) With Integrated Functions for Healthy Lifestyles on Physical Activity Levels and Cardiovascular Risk Biomarkers: Observational Study of 12,602 Users. J. Med. Internet Res. 2021, 23, e21622. [Google Scholar] [CrossRef]
- Hsueh, M.C.; Stubbs, B.; Lai, Y.J.; Sun, C.K.; Chen, L.J.; Ku, P.W. A dose response relationship between accelerometer assessed daily steps and depressive symptoms in older adults: A two-year cohort study. Age Ageing 2021, 50, 519–526. [Google Scholar] [CrossRef]
- Paluch, A.E.; Gabriel, K.P.; Fulton, J.E.; Lewis, C.E.; Schreiner, P.J.; Sternfeld, B.; Sidney, S.; Siddique, J.; Whitaker, K.M.; Carnethon, M.R. Steps per Day and All-Cause Mortality in Middle-aged Adults in the Coronary Artery Risk Development in Young Adults Study. J. Am. Med. Assoc. Netw. Open 2021, 4, e2124516. [Google Scholar]
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.M.; et al. Daily steps and all-cause mortality: A meta-analysis of 15 international cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Yang, J.; Bao, M.; Chen, T.; Cai, R.; Zhang, N.; Chen, H.; Liu, M.; Wu, X.; Zhang, B.; et al. The relationships between step count and all-cause mortality and cardiovascular events: A dose-response meta-analysis. J. Sport. Health Sci. 2021, 10, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Gohari, A.; Shab-Bidar, S. Daily Step Count and All-Cause Mortality: A Dose-Response Meta-analysis of Prospective Cohort Studies. Sports Med. 2022, 52, 89–99. [Google Scholar] [CrossRef]
- Cocate, P.G.; de Oliveira, A.; Hermsdorff, H.H.; Alfenas Rde, C.; Amorim, P.R.; Longo, G.Z.; Peluzio Mdo, C.; Faria, F.R.; Natali, A.J. Benefits and relationship of steps walked per day to cardiometabolic risk factor in Brazilian middle-aged men. J. Sci. Med. Sport. 2014, 17, 283–287. [Google Scholar] [CrossRef]
- Ewald, B.; Attia, J.; McElduff, P. How many steps are enough? Dose-response curves for pedometer steps and multiple health markers in a community-based sample of older Australians. J. Phys. Act. Health 2014, 11, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Zając-Gawlak, I.; Pelclová, J.; Groffik, D.; Přidalová, M.; Nawrat-Szołtysik, A.; Kroemeke, A.; Gába, A.; Sadowska-Krępa, E. Does physical activity lower the risk for metabolic syndrome: A longitudinal study of physically active older women. BMC Geriatr. 2021, 21, 11. [Google Scholar]
- Tudor-Locke, C.; Leonardi, C.; Johnson, W.D.; Katzmarzyk, P.T.; Church, T.S. Accelerometer steps/day translation of moderate-to-vigorous activity. Prev. Med. 2011, 53, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.B.; Oh, T.; Miyatake, N.; Tsushita, K.; Higuchi, M.; Tabata, I. Steps per day required for meeting physical activity guidelines in Japanese adults. J. Phys. Act. Health 2014, 11, 1367–1372. [Google Scholar] [CrossRef]
- Kumahara, H.; Ayabe, M. Individual variations in steps per day for meeting physical activity guidelines in young adult women. Appl. Physiol. Nutr. Metab. 2019, 44, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.E.; Xian, H.; Lew, D.; Hatton, S.N.; Puckett, O.; Whitsel, N.; Beck, A.; Dale, A.M.; Fang, B.; Fennema-Notestine, C.; et al. Body mass trajectories and cortical thickness in middle-aged men: A 42-year longitudinal study starting in young adulthood. Neurobiol. Aging 2019, 79, 11–21. [Google Scholar] [PubMed]
- Doucet, G.E.; Moser, D.A.; Rodrigue, A.; Bassett, D.S.; Glahn, D.C.; Frangou, S. Person-based brain morphometric similarity is heritable and correlates with biological features. Cereb. Cortex 2019, 29, 852–862. [Google Scholar] [PubMed]
- Klinger-König, J.; Frenzel, S.; Hannemann, A.; Wittfeld, K.; Bülow, R.; Friedrich, N.; Nauck, M.; Völzke, H.; Grabe, H.J. Sex differences in the association between basal serum cortisol concentrations and cortical thickness. Neurobiol. Stress. 2021, 15, 100416. [Google Scholar] [CrossRef] [PubMed]
- Michalski, L.J.; Demers, C.H.; Baranger, D.A.A.; Barch, D.M.; Harms, M.P.; Burgess, G.C.; Bogdan, R. Perceived stress is associated with increased rostral middle frontal gyrus cortical thickness: A family-based and discordant-sibling investigation. Genes. Brain Behav. 2017, 16, 781–789. [Google Scholar] [PubMed]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ottino-Gonzalez, J.; Jurado, M.A.; Garcia-Garcia, I.; Segura, B.; Marques-Iturria, I.; Sender-Palacios, M.J.; Tor, E.; Prats-Soteras, X.; Caldu, X.; Junque, C.; et al. Allostatic load is linked to cortical thickness changes depending on body-weight status. Front. Hum. Neurosci. 2017, 11, 639. [Google Scholar] [CrossRef]
- Trefler, A.; Sadeghi, N.; Thomas, A.G.; Pierpaoli, C.; Baker, C.I.; Thomas, C. Impact of time-of-day on brain morphometric measures derived from T1-weighted magnetic resonance imaging. Neuroimage 2016, 133, 41–52. [Google Scholar]
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J.; et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017, 5, 49. [Google Scholar]
- España-Irla, G.; Gomes-Osman, J.; Cattaneo, G.; Albu, S.; Cabello-Toscano, M.; Solana-Sanchéz, J.; Redondo-Camós, M.; Delgado-Gallén, S.; Alviarez-Schulze, V.; Pachón-García, C.; et al. Associations Between Cardiorespiratory Fitness, Cardiovascular Risk, and Cognition Are Mediated by Structural Brain Health in Midlife. J. Am. Heart Assoc. 2021, 10, e020688. [Google Scholar] [CrossRef]
- Coutinho, A.M.; Coutu, J.P.; Lindemer, E.R.; Rosas, H.D.; Rosen, B.R.; Salat, D.H. Differential associations between systemic markers of disease and cortical thickness in healthy middle-aged and older adults. Neuroimage 2017, 146, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Furber, M.; Pyle, S.; Roberts, M.; Roberts, J. Comparing Acute, High Dietary Protein and Carbohydrate Intake on Transcriptional Biomarkers, Fuel Utilisation and Exercise Performance in Trained Male Runners. Nutrients 2021, 13, 4391. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar]
- Semplonius, T.; Willoughby, T. Long-Term Links between Physical Activity and Sleep Quality. Med. Sci. Sports Exerc. 2018, 50, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Kinkorova, I.; Vodicka, P.; Mika, T. Physiological Profiles of Recreational Runners and Cyclists Aged 20 to 60 Years. Appl. Sci. 2022, 12, 3252. [Google Scholar]
- Ferguson, T.; Curtis, R.; Fraysse, F.; Olds, T.; Dumuid, D.; Brown, W.; Esterman, A.; Maher, C. Weather associations with physical activity, sedentary behaviour and sleep patterns of Australian adults: A longitudinal study with implications for climate change. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, J.; Yang, W.; Dong, T.; Zhang, X.; Cheng, S.; Zhou, X.; Zhou, M.; Niu, L.; Yi, G.; et al. Prevalence of metabolic syndrome among the adult population in western China and the association with socioeconomic and individual factors: Four cross-sectional studies. BMJ Open 2022, 12, e052457. [Google Scholar] [CrossRef]
- Chevance, G.; Baretta, D.; Romain, A.J.; Godino, J.G.; Bernard, P. Day-to-day associations between sleep and physical activity: A set of person-specific analyses in adults with overweight and obesity. J. Behav. Med. 2022, 45, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Robertson, M.C.; Winne, A.; Wu, I.H.C.; Le, T.A.; Balachandran, D.D.; Basen-Engquist, K.M. Investigating the within-person relationships between activity levels and sleep duration using Fitbit data. Transl. Behav. Med. 2021, 11, 619–624. [Google Scholar]
- Pesonen, A.K.; Kahn, M.; Kuula, L.; Korhonen, T.; Leinonen, L.; Martinmäki, K.; Gradisar, M.; Lipsanen, J. Sleep and physical activity—The dynamics of bi-directional influences over a fortnight. BMC Public Health 2022, 22, 1160. [Google Scholar]
- Heiland, E.G.; Ekblom, Ö.; Bojsen-Møller, E.; Larisch, L.M.; Blom, V.; Ekblom, M.M. Bi-Directional, Day-to-Day Associations between Objectively-Measured Physical Activity, Sedentary Behavior, and Sleep among Office Workers. Int. J. Environ. Res. Public Health 2021, 18, 7999. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.M.; Schwartz, J.E.; Kronish, I.M.; Diaz, K.M.; Alcantara, C.; Duer-Hefele, J.; Davidson, K.W. Does Stress Result in You Exercising Less? Or Does Exercising Result in You Being Less Stressed? Or Is It Both? Testing the Bi-directional Stress-Exercise Association at the Group and Person (N of 1) Level. Ann. Behav. Med. 2017, 51, 799–809. [Google Scholar]
- Oberlin, L.E.; Jaywant, A.; Wolff, A.; Gunning, F.M. Strategies to Promote Cognitive Health in Aging: Recent Evidence and Innovations. Curr. Psychiatry Rep. 2022, 24, 441–450. [Google Scholar] [CrossRef]
- Di Biase, M.A.; Tian, Y.E.; Bethlehem, R.A.I.; Seidlitz, J.; Alexander-Bloch, A.F.; Yeo, B.T.T.; Zalesky, A. Mapping human brain charts cross-sectionally and longitudinally. Proc. Natl. Acad. Sci. USA. 2023, 120, e2216798120. [Google Scholar] [CrossRef] [PubMed]
- Frangou, S.; Modabbernia, A.; Williams, S.C.R.; Papachristou, E.; Doucet, G.E.; Agartz, I.; Aghajani, M.; Akudjedu, T.N.; Albajes-Eizagirre, A.; Alnaes, D.; et al. Cortical thickness across the lifespan: Data from 17,075 healthy individuals aged 3–90 years. Hum. Brain Mapp. 2022, 43, 431–451. [Google Scholar] [CrossRef]
- Matuz-Budai, T.; Lábadi, B.; Kohn, E.; Matuz, A.; Zsidó, A.N.; Inhóf, O.; Kállai, J.; Szolcsányi, T.; Perlaki, G.; Orsi, G.; et al. Individual differences in the experience of body ownership are related to cortical thickness. Sci. Rep. 2022, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Koten, J.W.; Schüppen, A.; Morozova, M.; Lehofer, A.; Koschutnig, K.; Wood, G. On the homogeneity and heterogeneity of cortical thickness profiles in Homo sapiens sapiens. Sci. Rep. 2017, 7, 17937. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Arnatkevičiūtė, A.; McTavish, E.; Pang, J.C.; Chopra, S.; Suo, C.; Fornito, A.; Aquino, K.M. The individuality of shape asymmetries of the human cerebral cortex. eLife 2022, 11, e75056. [Google Scholar] [CrossRef]
- Jäncke, L.; Valizadeh, S.A. Identification of individual subjects based on neuroanatomical measures obtained 7 years earlier. Eur. J. Neurosci. 2022, 56, 4642–4652. [Google Scholar] [CrossRef]
- Wachinger, C.; Golland, P.; Reuter, M. BrainPrint: Identifying subjects by their brain. Med. Image Comput. Comput. Assist. Interv. 2014, 17 Pt 3, 41–48. [Google Scholar] [PubMed]
- Valizadeh, S.A.; Liem, F.; Mérillat, S.; Hänggi, J.; Jäncke, L. Identification of individual subjects on the basis of their brain anatomical features. Sci. Rep. 2018, 8, 5611. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, L.; Kumar, K.; Wachinger, C.; Vangel, M.; de Guise, J.; Desrosiers, C.; Wells, W.; Toews, M. Neuroimage signature from salient keypoints is highly specific to individuals and shared by close relatives. Neuroimage 2020, 204, 116208. [Google Scholar] [CrossRef]
- Takao, H.; Hayashi, N.; Ohtomo, K. Brain morphology is individual-specific information. Magn. Reson. Imaging 2015, 33, 816–821. [Google Scholar] [CrossRef]
- Ross, R.; Goodpaster, B.H.; Koch, L.G.; Sarzynski, M.A.; Kohrt, W.M.; Johannsen, N.M.; Skinner, J.S.; Castro, A.; Irving, B.A.; Noland, R.C.; et al. Precision exercise medicine: Understanding exercise response variability. Br. J. Sports Med. 2019, 53, 1141–1153. [Google Scholar]
- Loyen, A.; Wendel-Vos, G.C.W.; Shekoh, M.I.; Verschuren, W.M.M.; Picavet, H.S.J. 20-year individual physical activity patterns and related characteristics. BMC Public Health 2022, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wijndaele, K.; Sharp, S.J.; Strain, T.; Pearce, M.; White, T.; Wareham, N.; Brage, S. Specific physical activities, sedentary behaviours and sleep as long-term predictors of accelerometer-measured physical activity in 91,648 adults: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 41. [Google Scholar]
- Jansen, F.M.; van Kollenburg, G.H.; Kamphuis, C.B.M.; Pierik, F.H.; Ettema, D.F. Hour-by-hour physical activity patterns of adults aged 45–65 years: A cross-sectional study. J. Public Health 2018, 40, 787–796. [Google Scholar] [CrossRef]
- Jacques, M.; Landen, S.; Alvarez Romero, J.; Yan, X.; Garnham, A.; Hiam, D.; Siegwald, M.; Mercier, E.; Hecksteden, A.; Eynon, N.; et al. Individual physiological and mitochondrial responses during 12 weeks of intensified exercise. Physiol. Rep. 2021, 9, e14962. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Peacock, O.; Western, M.; Batterham, A.M. Multidimensional physical activity: An opportunity, not a problem. Exerc. Sport. Sci. Rev. 2015, 43, 67–74. [Google Scholar] [PubMed]
- Marsh, C.E.; Thomas, H.J.; Naylor, L.H.; Scurrah, K.J.; Green, D.J. Fitness and strength responses to distinct exercise modes in twins: Studies of Twin Responses to Understand Exercise as a THerapy (STRUETH) study. J. Physiol. 2020, 598, 3845–3858. [Google Scholar]
- Chiang, J.-H.; Yang, P.-C.; Tu, H. Pattern analysis in daily physical activity data for personal health management. Pervasive Mob. Comput. 2014, 13, 13–25. [Google Scholar]
- Stern, Y.; MacKay-Brandt, A.; Lee, S.; McKinley, P.; McIntyre, K.; Razlighi, Q.; Agarunov, E.; Bartels, M.; Sloan, R.P. Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology 2019, 92, e905–e916. [Google Scholar]
- Bashir, S.; Al-Sultan, F.; Jamea, A.A.; Almousa, A.; Alzahrani, M.S.; Alhargan, F.A.; Abualait, T.; Yoo, W.K. Physical exercise and cortical thickness in healthy controls: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7375–7379. [Google Scholar]
- de Manzano, O.; Ullen, F. Same genes, different brains: Neuroanatomical differences between monozygotic twins discordant for musical training. Cereb. Cortex 2018, 28, 387–394. [Google Scholar]
- Mitko, A.; Rothlein, D.; Poole, V.; Robinson, M.; McGlinchey, R.; DeGutis, J.; Salat, D.; Esterman, M. Individual differences in sustained attention are associated with cortical thickness. Hum. Brain Mapp. 2019, 40, 3243–3253. [Google Scholar]
- Oschwald, J.; Guye, S.; Liem, F.; Rast, P.; Willis, S.; Röcke, C.; Jäncke, L.; Martin, M.; Mérillat, S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 2020, 31, 1–57. [Google Scholar]
- Tadayon, E.; Pascual-Leone, A.; Santarnecchi, E. Differential Contribution of Cortical Thickness, Surface Area, and Gyrification to Fluid and Crystallized Intelligence. Cereb. Cortex 2020, 30, 215–225. [Google Scholar]
- Ciria, L.F.; Román-Caballero, R.; Vadillo, M.A.; Holgado, D.; Luque-Casado, A.; Perakakis, P.; Sanabria, D. An umbrella review of randomized control trials on the effects of physical exercise on cognition. Nat. Hum. Behav. 2023, 7, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Ling, D.S. Aerobic-Exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019, 37, 100572. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Osorio, F.; Ramirez-Campillo, R.; Cerda-Vega, E.; Campos-Jara, R.; Martínez-Salazar, C.; Araneda, R.; Ebner-Karestinos, D.; Arellano-Roco, C.; Campos-Jara, C. Effects of Sport-Based Exercise Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12573. [Google Scholar] [CrossRef]
- Paolillo, E.W.; Lee, S.Y.; VandeBunte, A.; Saloner, R.; Gaynor, L.S.; Djukic, N.; Tsuei, T.; Cobigo, Y.; Kramer, J.H.; Casaletto, K.B. Data-driven physical actigraphy patterns relate to cognitive and vascular health in older adults. Exp. Gerontol. 2023, 178, 112231. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.A.; Deijen, J.B.; Milders, M.V.; De Greef, M.H.G.; Scherder, E.J.A.; van Duijn, C.M.; Drent, M.L. The effects of a moderate physical activity intervention on physical fitness and cognition in healthy elderly with low levels of physical activity: A randomized controlled trial. Alzheimers Res. Ther. 2023, 15, 12. [Google Scholar] [CrossRef]
- Pucci, V.; Guerra, C.; Barsi, A.; Nucci, M.; Mondini, S. How long have you exercised in your life? The effect of motor reserve and current physical activity on cognitive performance. J. Int. Neuropsychol. Soc. 2023, 1–7. [Google Scholar] [CrossRef]
- Yuan, X.; Li, D.; Hu, Y.; Qi, M.; Kong, Y.; Zhao, C.; Huang, J.; Song, Y. Neural and behavioral evidence supporting the relationship between habitual exercise and working memory precision in healthy young adults. Front. Neurosci. 2023, 17, 1146465. [Google Scholar] [CrossRef]
- Cheval, B.; Darrous, L.; Choi, K.W.; Klimentidis, Y.C.; Raichlen, D.A.; Alexander, G.E.; Cullati, S.; Kutalik, Z.; Boisgontier, M.P. Genetic insights into the causal relationship between physical activity and cognitive functioning. Sci. Rep. 2023, 13, 5310. [Google Scholar] [PubMed]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [PubMed]
- Festa, F.; Medori, S.; Macrì, M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines 2023, 11, 1765. [Google Scholar]
- Augusto-Oliveira, M.; Arrifano, G.P.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Lopes-Araújo, A.; Royes, L.F.F.; Crespo-Lopez, M.E. Exercise Reshapes the Brain: Molecular, Cellular, and Structural Changes Associated with Cognitive Improvements. Mol. Neurobiol. 2023, 60, 6950–6974. [Google Scholar]
- Chen, C.; Nakagawa, S. Physical activity for cognitive health promotion: An overview of the underlying neurobiological mechanisms. Ageing Res. Rev. 2023, 86, 101868. [Google Scholar] [CrossRef]
- Erickson, K.I.; Donofry, S.D.; Sewell, K.R.; Brown, B.M.; Stillman, C.M. Cognitive Aging and the Promise of Physical Activity. Annu. Rev. Clin. Psychol. 2022, 18, 417–442. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
