Abstract
Introduction: While the phenotypic diversity of childhood wheezing is well described, the subsequent life course of such phenotypes and their adult outcomes remain poorly understood. We hypothesized that different childhood wheezing phenotypes have varying longitudinal outcomes at age 26. We sought to identify factors associated with wheezing persistence, clinical remission, and new onset in adulthood. Methods: Participants were seen at birth and at 1, 2, 4, 10, 18, and 26 years in the Isle of Wight Birth Cohort (n = 1456). Information was collected prospectively on wheeze prevalence and phenotypic characteristics at each assessment. Wheeze phenotypes at 10 years were defined as participants wheezing (CW10) or not wheezing at 10 (CNW10). Multivariable regression analyses were undertaken to identify factors associated with wheezing persistence/remission in CW10 and wheeze development in CNW10 at age 26 years. Results: Childhood wheezing phenotypes showed different subsequent outcomes and associated risk factors. Adult wheeze developed in 17.8% of CNW10. Factors independently associated with adult wheeze development in CNW10 included eczema at age 4 years, family history of rhinitis, and parental smoking at birth. Conversely, 56.1% of CW10 had remission of wheeze by 26 years. Factors predicting adult wheezing remission in CW10 included absence of both atopy at age 4 years and family history of rhinitis. Conclusion: Early-life factors influence adult outcomes for childhood wheezing phenotypes, both with respect to later development of adult wheezing in asymptomatic participants and of wheeze remission in childhood wheezers. This suggests potential areas that could be targeted by early-life interventions to alleviate adult disease burden.
1. Introduction
Wheezing is commonly encountered in childhood, reflecting both virus-associated and multitrigger effects. While not synonymous with asthma in childhood, childhood wheezing, if persistent, may reflect the presence of childhood asthma [1]. Phenotypic classifications of childhood wheezing have provided key insights into the diverse nature of this symptom, its association with childhood health outcomes and its association with diagnosed asthma [2,3,4,5].
A landmark temporally defined phenotypic classification from the Tucson Children’s Respiratory Study (TCRS) [6], categorized participants into four distinct phenotype groups based on their wheezing status during the first six years of life. In the Isle of Wight Birth Cohort (IOWBC), we adapted this classification for timepoints over the first decade of life [7]. While an enhanced understanding of childhood wheeze and asthma has evolved from such phenotypic approaches [2,3,4,5], a key gap in knowledge is how childhood wheezing phenotypes track across the wider life course and to what extent they are related to adult wheezing and asthma status. A small number of studies have previously assessed the trajectory of childhood wheeze and asthma into adolescence and adulthood [8,9,10,11,12,13,14,15,16,17,18,19]. Early-life factors recognized to be associated with persistence of early-life wheezing into adolescence include family history of asthma or atopy, personal history of atopic conditions, early-life allergic sensitization, demonstration of Type-2 (T2) inflammatory signals, and the frequency and severity of wheezing [8]. Studies of disease trajectories into adulthood have identified a variety of patterns ranging from remission to persistence and incident disease. They have shown that most children with mild early-life wheezing outgrow their disease by adulthood [5,19,20]. Conversely, emerging evidence indicates that more severe childhood asthma is often associated with more severe adulthood asthma [20,21,22]. Studies of wheeze/asthma pathways from childhood to adulthood have identified persistent trajectories [17,18,19,20]. These have shown association with more severe asthma, impaired lung function, bronchial hyperresponsiveness and allergic sensitization in childhood, and adult T2 expression and lung function impairment [17,18,19,20]. However, none of these studies have examined childhood wheezing phenotypes within the framework of longitudinal birth cohorts. This knowledge gap is highly relevant given the increasingly recognized concepts of the early-life origins of adult wheeze/asthma and the tracking of impaired lung function from childhood into adulthood in some childhood asthmatics [5,8,16,19].
In this paper, we determine the longitudinal outcomes at the age of 26 years for childhood wheezing phenotypes defined at 10 years of age in the IOWBC [7,23,24,25,26]. Our hypotheses were that (a) childhood wheezing phenotypes show differing associations with young adult wheeze, (b) a proportion of childhood wheezers show remission by adulthood, (c) a proportion of participants who did not wheeze start to do so by young adulthood, and (d) factors in early life and adolescence are associated with wheeze remission, persistence, and new onset by adulthood.
2. Methods
2.1. The Study Cohort
In 1989, a whole-population-based birth cohort was initiated on the Isle of Wight to investigate the natural history of asthma and allergic conditions across the life course [7]. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Isle of Wight Post Graduate Medical Federation (No 05/89; dated 22 August 1988).which was updated at each subsequent follow-up. Consent was secured from 1456 out of 1536 participants born between January 1989 and February 1990. The cohort was followed from birth through follow-ups at ages 1, 2, 4, 10, 18, and 26 years (Figure 1) [7,23,24,25,26]. Detailed parent-completed questionnaires assessed asthma and allergies in early childhood. From age 10 years, International Study of Asthma and Allergies in Childhood (ISAAC) [27] questionnaires evaluated symptoms, alongside detailed supplementary questionnaires at each study visit. A cut-off of 18 years or greater was used to define adult status.
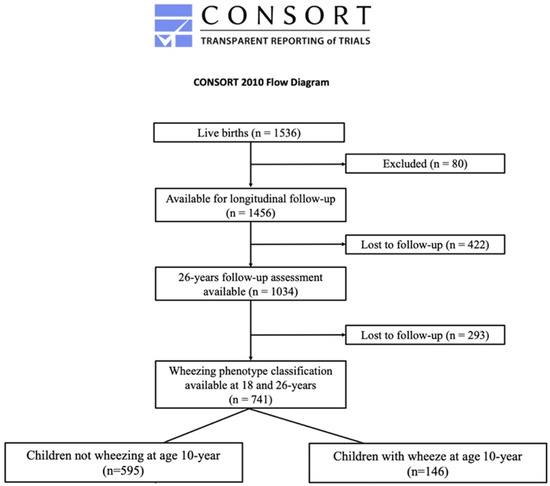
Figure 1.
CONSORT diagram showing participants recruited to the study and how their wheezing phenotypes were classified.
2.2. Variables
Current wheeze was defined as “wheeze or whistling in the last 12 months” as reported by either the parent (in childhood) or the participant (in adolescence/adulthood) in response to investigator-administered ISAAC questionnaires. Asthma was diagnosed as a composite diagnosis of “physician-diagnosed asthma ever plus either current wheeze in the past 12 months or taking asthma medications in the past 12 months”.
Spirometry was measured at ages 10, 18, and 26 years. Absolute values for forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and forced midexpiratory flow (FEF25–75) were used for analysis. Bronchial hyperresponsiveness (BHR) was assessed from age 10 onwards using methacholine challenge in a subgroup. The methodology for spirometry and bronchial challenge has been previously described [7,28]. FeNO (fractional exhaled nitric oxide) was measured at 18 and 26 years.
Skin prick testing (SPT) from age 4 assessed atopy (with mean wheal diameter >3 mm greater than the negative control to individual allergen defining sensitization) using a standardized battery of common allergens (ALK, Hørsholm, Denmark). Serum total IgE (Immunoglobulin E) was measured at 10 and 18 years.
BHR was categorized aligning with the American Thoracic Society BHR guideline categories, with “Definite BHR” classified as provoking methacholine concentration causing 20% fall in FEV1 (PC20) < 4 mg/mL [28]. We also used a continuous dose–response slope (DRS) estimated by log-transformed least-square regression of FEV1 changes to methacholine doses, since not all subjects demonstrated a 20% fall in FEV1 during the bronchial challenge. A transformation of Log10 (DRS+ 10) was required to satisfy the distributional assumption of normal data.
2.3. Statistical Analysis
Data were entered into SPSS (Statistical Package for the Social Sciences) (v24, IBM statistics, Armonk, USA) using a double-entry method and analysed by the R software (R Core Team, Vienna, Austria, 2022).
First, we undertook descriptive assessment of baseline characteristics and outcomes at 10 years for both CNW10 (participants not wheezing at age 10 years) and CW10 (participants wheezing at age 10 years). We then performed similar analyses for those participants (CNW10 and CW10) again at 26 years of age. For these analyses, categorical variables were assessed by chi-square tests (with Fisher’s exact test where low cell counts occurred), while continuous variables were assessed by appropriate parametric tests (e.g., t-tests) when the normality assumption reasonably held or nonparametric (e.g., Mann–Whitney U for two-sample comparisons) when the assumption was violated.
Next, we implemented time-lagged univariate analyses to assess the association of each of the early-life factors with wheeze status at 26 years for CNW10 and CW10 for the purpose of selecting potentially informative variables for subsequent analyses. These early-life factors included family history of asthma, eczema, rhinitis, low birthweight <2.5 kgs, exclusively breastfed newborn for at least 3 months, lower socioeconomic status at birth, recurrent chest infections at 1 and 2 years of age, parental smoking at birth and at 4 years of age, eczema at 4 years of age, atopy at 4 years of age, and rhinoconjunctivitis at 4 years of age. In this analysis, factors with p-value < 0.2 were then selected and included in multivariable time-lagged regression models [29] to examine their associations with (a) wheezing persistence in CW10 to age 26 years and (b) wheezing development at age 26 years in CNW10.
In the final phase, we used backward variable selection to identify early-life factors associated with the two outcomes, wheezing persistence and wheezing development. Given the use of longitudinal data and the multivariable regression modelling with prospective time-order, we minimized potential reverse causation.
3. Results
3.1. Study Population Description
A total of 1034 IOWBC participants (70.9% of the original birth cohort) were assessed at 26 years. Of these, 741 (71.7%) were reassessed at all 3 (10-, 18-, and 26-year) follow-ups from 10 years onwards. That subgroup formed the study population for this analysis (Figure 1). Subjects included in this analysis differed from the 293 (28.3%) excluded because of missing follow-up participation only with respect to male sex (p < 0.001; Table 1).

Table 1.
Characteristics at 10 and 26 years with statistically significant differences between childhood wheeze phenotypes.
Most of this study population was in the CNW10 group (n = 595, 80.3%), while 19.7% (n = 146) were in the CW10 group (Figure 2). Among CNW10, wheeze prevalence rose from 0% to about 20% by age 26 years. Conversely, among CW10, wheeze prevalence fell from 100% to about 50% at 26 years (Figure 2).
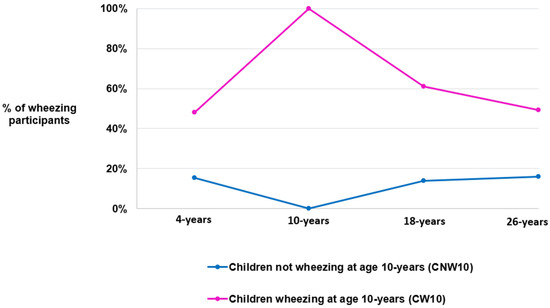
Figure 2.
Changes in the percentage of individuals with an active current wheezing status over time, categorized by their childhood wheeze phenotype group.
3.2. Comparison of Characteristics for CW10 and CNW10 in Childhood and Adulthood
Characteristics and outcomes with statistical significance between CW10 and CNW10 at 10 and 26 years are shown in Table 1. Statistically insignificant findings are provided in Table 2. CW10 was associated with higher current wheeze at 26 years and higher asthma prevalence at 10 and 26 years (p < 0.001, Table 1). At the age of 18 years, the prevalence of atopy was higher than at the age of 10 years in the two groups, CNW10 and CW10. (Table 1). Similar findings were found for current rhinitis. Current eczema was also higher at 10 years in CW10 than CNW10 (p = 0.046), but this difference had disappeared by 26 years of age. CW10 had higher prevalence of asthma treatment than CNW10 at 10 years (p< 0.001) and at 26 years (p = 0.051) (Table 1). Treatment with inhaled corticosteroids was greater in the CW10 group at 10 (p = 0.0013) but not at 26 years (p = 0.0802) (Table 1). Current active and passive smoking did not differ between CW10 and CNW10 at 10 years of age (Table 2).

Table 2.
Prebronchodilator lung function, FeNO, and BHR dose–response slope characteristics of 10 years childhood wheezing phenotypes at 10, 18, and 26 years.
3.3. Lung Function
FEV1-FVC-FEV1/FVC
For both groups, from 10 to 26 years, FEV1 showed a pattern of an adolescent increase followed by a plateau, while FVC had a pattern of consistent increase, FEF25–75 demonstrated an adolescent increase followed by decline in young adulthood, and FEV1/FVC seemed to decrease (Figure 3). Compared with CW10, CNW10 showed higher FEV1 at 10 years (p = 0.04), FEV1/FVC at 10 (p = 0.0002), 18 (p < 0.0001), and 26 years (p < 0.0005), and FEF25–75 at 10 (p = 0.0002) and 18 years (p = 0.01) (Table 2). However, at 26 years, FVC was higher in the CW10 group (p = 0.04) (Table 2). The FEV1, FVC, and FEV1/FVC ratio changed between ages 10–18 and 18–26 for the CW10 and CNW10 groups, as shown in Table 3.
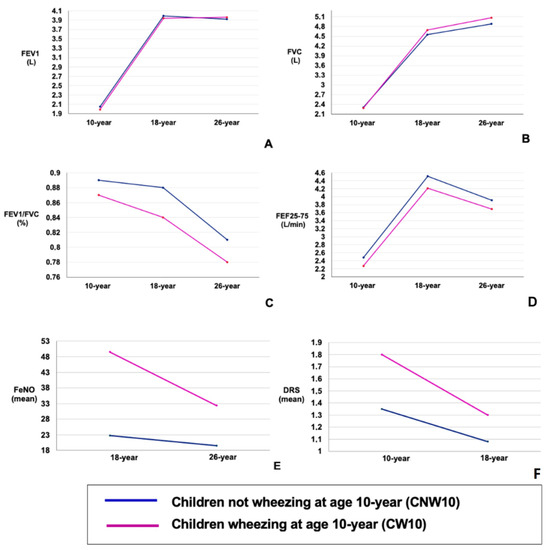
Figure 3.
(A–F): Longitudinal trajectories in prebronchodilator lung function at 10, 18, and 26 years of age for childhood wheezing phenotypes. Data are presented for childhood wheezing phenotypes. (A) Longitudinal prebronchodilator FEV1 (L) outcomes. (B) Longitudinal prebronchodilator FVC (L) outcomes. (C) Longitudinal prebronchodilator FEV1/FVC (%) outcomes. (D) Longitudinal prebronchodilator FEF25–75 (L/min) outcomes. (E) Longitudinal mean FeNO outcomes. (F) Longitudinal mean of dose–response scale at methacholine challenge outcomes.

Table 3.
Evolution of lung function between 10 and 18 years and between 18 and 26 years for childhood wheezing phenotypes at 10 years.
3.4. FeNO
At each age, FeNO was higher in those from the CW10 group than in those from the CNW10 group (Table 2). Both the CW10 and CNW10 groups showed declining trends between 18 and 26 years for FeNO. This decrease differed between the two groups, being more pronounced for the 26–18-year part in the CW10 group than in the CNW10 group (p < 0.002) (Table 3).
3.5. Bronchial Hyperresponsiveness
The proportion of participants with BHR (PC20 <4 mg/mL) was greater in the CW10 group at 10 years compared with the CNW10 group (p < 0.001) (Table 1). DRS was greater (indicating higher BHR) in CW10 at both ages (Table 2). A declining trend for DRS seemed to exist for both CW10 and CNW10 from 10 to 18 years. Figure 3. The decline in the DRS rate was more pronounced in the CW10 group between 18 and 10 years of age than in the CNW10 group (p < 0.002) (Table 3).
3.6. Longitudinal Wheeze Outcomes of CW10 and CNW10 at Age 26 Years and Associated Factors
For further longitudinal outcome analysis, 9.2% (55/595) of participants in the CNW10 group and 9.6% of participants (14/146) in the CW10 group were excluded from assessment because of transitory isolated wheezing in those participants at 18 years (see Table 4 notes).

Table 4.
Comparison of early-life, 10-year, and 26-year characteristics of wheezing and nonwheezing phenotypes at 10 years that show significant association to wheezing outcome at 26 years.
Adult wheeze developed in 17.8% (96/540) of CNW10. Among CNW10, compared with those who wheezed at 26 years, those who did not wheeze at 26 years had lower prevalence of asthma and rhinitis at 26 years (Table 4). In the CW10 group, 43.9% (58/132) continued wheezing into adulthood, while 56.1% (74/132) experienced symptom remission. Compared with participants who lost wheezing by adulthood, CW10 subjects who continued wheezing up to adulthood had higher prevalences of wheezing, asthma diagnosis, rhinitis, atopy, asthma treatment at 10 and 26 years, and BHR at 10 years, as well as higher BMI (body mass index) at 26 years (Table 4). Prevalence of eczema was higher at 26 years among those still wheezing at 26 years compared with those with wheeze remission (Table 4). Other assessed variables that did not show statistically significant differences between the studied groups are presented in Table 3 and Table 4.
3.7. Early-Childhood Risk Factors for Wheezing Appearance/Reappearance or Remission in Adulthood
When considering associations with early-life risk factors, CNW10 with wheezing in adulthood had higher prevalences of respiratory infections at 2 years, eczema at 4 years, and family history of rhinitis (Table 4). Conversely, among CW10, those who lost wheezing by adulthood had lower prevalences of atopy at 4 years and positive family history of rhinitis compared with the other group of participants who continued to wheeze (Table 4).
The factors that met the inclusion criterion (p-value < 0.2) in the multivariate model for adult wheezing development were recurrent chest infections by age 2, eczema by age 4, family history of rhinitis, parental tobacco smoking at birth, and rhinitis at age 4. For wheezing remission in adulthood, the variables that qualified for inclusion were atopy at age 4, family history of rhinitis, low birth weight (<2.5 kg), and exposure to tobacco smoke at birth. Following multivariable logistic regression analysis, factors adjusted for other covariates (Table 5A, notes) with the development of adult wheezing in CNW10 included a history of eczema at age 4 years (p-value < 0.0005), family history of rhinitis (p-value = 0.02), and parental tobacco usage at birth (p-value = 0.04) (Table 5A). Factors adjusted for other covariates (Table 5B, notes) with wheezing remission by adulthood in CW10 included a lack of atopy at age 4 years (p = 0.04) and the absence of familial predisposition to rhinitis (p-value = 0.0006) (Table 5B).

Table 5.
Factors associated at multivariable regression with wheeze development or remission in adulthood among childhood wheeze phenotypes at 10 years.
4. Discussion
To address our original hypotheses, we characterized wheezing phenotypes among 10-year-olds in the IOWBC, defined their longitudinal adult wheezing outcomes at 26 years, and identified associated risk factors for those outcomes. Our childhood wheezing phenotypes were defined by the presence or absence of current wheezing at 10 years, CW10 or CNW10. CW10 encompassed previously characterized late-onset and persistent childhood wheeze, while CNW10 represented transient and never-wheezed “early childhood phenotypes” [24]. With respect to our first hypothesis, we found that CW10 had higher prevalences of young adulthood wheeze, diagnosed asthma, worse airflow limitation, FeNO, and BHR than CNW10. Addressing our second hypothesis, half of CW10 no longer wheezed at 26 years. In the context of our fourth hypothesis, we found that such wheeze remission by age 26 years in CW10 was independently associated with absence of atopy at 4 years and lack of rhinitis family history. Conversely, we found that a majority (57%) of current wheezers at 26 years were asymptomatic at 10 years. Further, addressing our third hypothesis, we found that nearly one-fifth of CNW10 developed wheezing at 26 years. Returning to our fourth hypothesis, this outcome was independently associated with parental smoking at birth, eczema at 4 years, and rhinitis family history. While our findings showed clear associations with potentially relevant risk factors, they cannot be used to infer definitive causality. Nevertheless, associations of early-life factors with adult outcomes of childhood wheeze phenotypes provide opportunities to potentially intervene in early life to influence their subsequent life course impacts.
While wheeze and asthma are not synonymous entities, a diagnostic label of adult asthma infers the presence of a more significant wheezing state. Despite numerous studies suggesting that adult wheeze and asthma often originate in early childhood [5,8,19,30,31,32,33,34,35,36], few have used prospectively collected data to specifically investigate early-life risk factors related to adult-onset wheeze or asthma in the context of childhood wheezing phenotypic classification. Factors such as shorter duration of breastfeeding or having ≥ 2 siblings have been associated with higher rates of adult-onset asthma [37,38], but information on time of onset of wheeze/asthma and early-life factors was collected retrospectively and thus prone to recall bias. We identified that family history of rhinitis, parental smoking at birth, and eczema at age 4 years were associated with young-adult wheeze in those who were asymptomatic at 10 years. Previous studies have linked family history of rhinitis to a quadruple rise in the likelihood of wheeze or asthma development in childhood [39,40]. We found that rhinitis family history conferred a twofold increased risk of adult wheeze in asymptomatic 10-year-olds. Family history of rhinitis may be considered as a proxy for inherited predisposition to atopy and could be used as a marker to identify infants at high risk of asthma for early intervention [41]. Such family history of rhinitis may indicate a genetic or environmental predisposition to airway hyperresponsiveness, potentially driving chronic inflammation and shared inflammatory pathways between rhinitis and the lower respiratory tract. Passive smoke exposures in early life represent a clear modifiable risk factor [42,43]. They constitute a pivotal risk factor for respiratory ailments, stunted lung growth, manifestation of wheezing symptoms in childhood, and chronic obstructive pulmonary disease (COPD) in adulthood [42,44,45,46,47].
Furthermore, recently reported findings demonstrated associations of exposure to maternal smoking in pregnancy with accelerated lung function decline in adulthood [48]. Such findings align with our observed associations of early-life passive smoke exposure with new-onset wheezing in adulthood [49,50]. Our results further emphasize the need to eliminate exposure to tobacco smoke in early childhood, as the consequences can be far reaching and may appear several decades after exposure.
Eczema in infancy is a recognized risk factor for wheeze or asthma development in childhood [51]. Recent findings have indicated that the proposed linear progression of the atopic march [52] fails to encompass the diversity of allergic phenotypes and their trajectories. Nevertheless, atopic dermatitis frequently serves as the initial manifestation of a progressive atopic syndrome, and our results show that it also indicates risk for development of pulmonary symptoms in adulthood. It may simply reflect atopic predisposition of these participants; however, could prevention of eczema reduce the risk of adult wheeze or asthma? The administration of probiotics during pregnancy seemed to reduce the likelihood of developing eczema in childhood by approximately 20% [53], but it was not associated with a reduction in the onset of asthma symptoms [53,54]. Several studies have also assessed restoration of the epithelial skin barrier and its effect on the onset of pulmonary asthma symptoms, but none has yet shown a significant effect [55]. Further studies are needed to identify preventive strategies to target the expression of atopic diseases in the skin and the respiratory tract at the same time. One such strategy might be to correct an evolving Type 2 inflammatory imbalance by using prophylactic allergen immunotherapy [56].
Of wheezing subjects at age 10, 56.1% no longer had pulmonary symptoms at age 26, defined as clinical remission. Past findings have indicated that remission of childhood wheezing is more common with milder childhood wheezing [20,21,22]. They have also shown that clinical remission is less frequent for those with a label of childhood asthma and much less so for severe childhood asthma. This probably reflects that a diagnosis of childhood asthma aligns with a more severe wheezing phenotype that is more likely to persist. Previous studies have revealed that clinical remission of wheezing symptoms in childhood or adolescence is favoured by lower initial BHR, significant improvement in small-airway function, male sex, milder disease (with less frequent and severe symptoms), and less atopic sensitization [56,57,58]. One possible focus for preventive measures to promote clinical wheeze remission among childhood wheezers is on mitigation against allergen sensitization. Early-life allergic sensitization, especially multiple sensitizations, poses heightened risk of impaired lung function and enhanced BHR by adolescence [59]. Future primary prevention strategies might target infancy, leveraging early allergen exposure to induce immune tolerance [56]. Indeed, our recent primary prevention study of allergen immunotherapy suggested that early administration of house dust mite sublingual immunotherapy in high-risk, nonsensitized participants could potentially reduce childhood wheeze and asthma incidence [56]. Recently, we also developed the ASPIRE (asthma predictive risk score) system [60], aiming to predict adult asthma status from early childhood. Our present study is in line with the ASPIRE score, implying the same risk factors for persistent wheezing in adulthood (atopy at 4 years and a family history of rhinitis). This underscores the potential of early intervention based on a few routinely collected factors. Better prediction of disease outcome can lead to better therapeutic adherence and, consequently, a more favourable evolution of symptoms.
A core strength of our study is the rich longitudinal dataset that enabled extensive characterization of childhood wheeze phenotypes and their longitudinal outcomes. High cohort retention and nondifferential loss to follow-up in core parameters permitted meaningful investigation of long-term outcomes. One limitation of our study is a lack of objective data such as lung function in the first 4 years of life, though this was available from 10 years onwards. As recently shown in the Vitamin D Antenatal Asthma Reduction Trial, earlier lung function measures in the pre-school phase may have offered unique perspectives on subsequent respiratory morbidity and lung function [61]. As shown in other studies, impaired lung function trajectories may establish early in childhood, and factors associated with that merit attention in future studies [22]. Another limitation is that our population was very homogeneous, and replication in other populations is indicated to assess consistency of findings in other geographic areas and ethnicities. However, at present, comparable study cohorts for replication to age 26 are limited. Finally, as with any epidemiological study, our findings cannot infer causality but should be viewed as indicative of associations.
5. Conclusions
Our IOWBC study provides valuable insights into childhood wheezing outcomes up to age 26. A substantial proportion of CW10 individuals no longer experienced wheezing by age 26, while a significant number of CNW10 individuals developed wheezing during this period. Absence of pulmonary symptoms in late childhood does not guarantee their absence in adulthood. We have identified clinical factors in early childhood associated with the onset or clinical remission of wheezing in adults in a birth cohort. This underscores the importance of pursuing early-life interventions that could have profound and lasting impacts on life course wheezing outcomes.
Author Contributions
S.C. is the paper’s principal author and was involved in data analysis, statistical analyses, figure creation, and writing of the paper. R.J.K. is the paper’s main correspondent; with S.H.A., they were involved in data collection, paper writing, statistical analyses, and overall proofreading/consistency of the paper. H.Z. took part in the statistical analyses and proofreading/consistency of the paper. L.K.T. was involved in proofreading the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, U.S.A. (grant no. R01 HL082925, R01 AI121226); Asthma U.K. (grant no. 364); and the David Hide Asthma and Allergy Centre Charity. Sophie Carra received a grant from the University of Montpellier for the realization of this project. The APC was self-funded.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Isle of Wight Post Graduate Medical Federation (No 05/89; dated 22 August 1988).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
After publication, the data will be made available to others on reasonable requests to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. Deidentified participant data will be provided after approval from the corresponding authors.
Acknowledgments
We would like to acknowledge the help of all the staff at the David Hide Asthma and Allergy Centre in undertaking the Isle of Wight Birth Cohort assessments. We are specifically indebted to the research team including Stephen Potter, Susan Grevatt, Gill Glasby, Kaisha Bennett, Nicky Tongue, and Sharon Matthews. We also thank the participants and their families who helped us with this project over the last three decades.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Statements
Ethics approval was obtained at initiation (No 05/89; dated 08/22/1988) and updated at each subsequent follow-up (ages 1, 2, 4, 10, 18, and 26 years).
References
- Edwards, L.R.; Borger, J. Pediatric Bronchospasm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Fitzpatrick, A.M.; Bacharier, L.B.; Guilbert, T.W.; Jackson, D.J.; Szefler, S.J.; Beigelman, A.; Cabana, M.D.; Covar, R.; Holguin, F.; Lemanske, R.F., Jr.; et al. Phenotypes of Recurrent Wheezing in Preschool Participants: Identification by Latent Class Analysis and Utility in Prediction of Future Exacerbation. J. Allergy Clin. Immunol. Pract. 2019, 7, 915–924.e7. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Mauger, D.T.; Lemanske, R.F. Childhood asthma-predictive phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 66470. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Beigelman, A.; Calatroni, A.; Jackson, D.J.; Gergen, P.J.; O’connor, G.T.; Kattan, M.; Wood, R.A.; Sandel, M.T.; Lynch, S.V.; et al. Longitudinal Phenotypes of Respiratory Health in a High-Risk Urban Birth Cohort. Am. J. Respir. Crit. Care Med. 2019, 199, 7182. [Google Scholar] [CrossRef] [PubMed]
- McCready, C.; Haider, S.; Little, F.; Nicol, M.P.; Workman, L.; Gray, D.M.; Granell, R.; Stein, D.J.; Custovic, A.; Zar, H.J. Early childhood wheezing phenotypes and determinants in a South African birth cohort: Longitudinal analysis of the Drakenstein Child Health Study. Lancet Child Adolesc. Health 2023, 7, 12735. [Google Scholar] [CrossRef]
- Martinez, F.D.; Wright, A.L.; Taussig, L.M.; Holberg, C.J.; Halonen, M.; Morgan, W.J. The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 1995, 332, 133–138. [Google Scholar] [CrossRef]
- Arshad, S.H.; Holloway, J.W.; Karmaus, W.; Zhang, H.; Ewart, S.; Mansfield, L.; Matthews, S.; Hodgekiss, C.; Roberts, G.; Kurukulaaratchy, R. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int. J. Epidemiol. 2018, 47, 10431044i. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, C.E.; Sossa-Briceño, M.P.; Castro-Rodriguez, J.A. Factors predicting persistence of early wheezing through childhood and adolescence: A systematic review of the literature. J. Asthma Allergy 2017, 10, 8398. [Google Scholar] [CrossRef]
- Granell, R.; Henderson, A.J.; Sterne, J.A. Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Participants: A population-based birth cohort. J. Allergy Clin. Immunol. 2016, 138, 1060–1070.e11. [Google Scholar] [CrossRef]
- Oksel, C.; Granell, R.; Haider, S.; Fontanella, S.; Simpson, A.; Turner, S.; Devereux, G.; Arshad, S.H.; Murray, C.S.; Roberts, G.; et al. Distinguishing Wheezing Phenotypes from Infancy to Adolescence. A Pooled Analysis of Five Birth Cohorts. Ann. Am. Thorac. Soc. 2019, 16, 868–876. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Coull, B.A.; Rifas-Shiman, S.L.; Wu, A.C.; Lutz, S.M.; Hivert, M.-F.; Oken, E.; Gold, D.R. Characterization of longitudinal wheeze phenotypes from infancy to adolescence in Project Viva, a prebirth cohort study. J. Allergy Clin. Immunol. 2020, 145, 716–719.e8. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Jarvis, D.; Baptista Menezes, A.M.; Gonçalves, H.; Duarte de Oliveira, P.; Wehrmeister, F.C. Wheezing trajectories from childhood to adulthood in a population-based cohort. Allergol. Int. 2022, 71, 2006. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef]
- Tai, A.; Tran, H.; Roberts, M.; Clarke, N.; Gibson, A.-M.; Vidmar, S.; Wilson, J.; Robertson, C.F. Outcomes of childhood asthma to the age of 50 years. J. Allergy Clin. Immunol. 2014, 133, 1572–1578.e3. [Google Scholar] [CrossRef]
- Butland, B.K.; Strachan, D.P. Asthma onset and relapse in adult life: The British 1958 birth cohort study. Ann. Allergy Asthma Immunol. 2007, 98, 33743. [Google Scholar] [CrossRef]
- Tan, D.J.; Walters, E.H.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Matheson, M.C.; Dharmage, S.C. Age-of-asthma onset as a determinant of different asthma phenotypes in adults: A systematic review and meta-analysis of the literature. Expert Rev. Respir. Med. 2015, 9, 10923. [Google Scholar] [CrossRef] [PubMed]
- Ödling, M.; Wang, G.; Andersson, N.; Hallberg, J.; Janson, C.; Bergström, A.; Melén, E.; Kull, I. Characterization of Asthma Trajectories from Infancy to Young Adulthood. J. Allergy Clin. Immunol. Pract. 2021, 9, 2368–2376 e2363. [Google Scholar] [CrossRef]
- Tan, D.J.; Lodge, C.J.; Walters, E.H.; Lowe, A.J.; Bui, D.S.; Bowatte, G.; Pham, J.; Erbas, B.; Hui, J.; Hamilton, G.S.; et al. Longitudinal Asthma Phenotypes from Childhood to Middle-Age: A Population-based Cohort Study. Am. J. Respir. Crit. Care Med. 2022, 208, 132–141. [Google Scholar] [CrossRef]
- Koefoed, H.J.L.; Vonk, J.M.; Koppelman, G.H. Predicting the course of asthma from childhood until early adulthood. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 11522. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.; Carlin, J.B.; Oswald, H.; Olinsky, A.; Phelan, P.D.; Robertson, C.F. Association between allergy and asthma from childhood to middle adulthood in an Australian cohort study. Am. J. Respir. Crit. Care Med. 2000, 162, 2177–2181. [Google Scholar] [CrossRef]
- Oswald, H.; Phelan, P.D.; Lanigan, A.; Hibbert, M.; Bowes, G.; Olinsky, A. Outcome of childhood asthma in mid-adult life. BMJ 1994, 309, 95–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Phelan, P.D.; Robertson, C.F.; Olinsky, A. The Melbourne Asthma Study: 1964–1999. J. Allergy Clin. Immunol. 2002, 109, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kurukulaaratchy, R.J.; Matthews, S.; Holgate, S.T.; Arshad, S.H. Predicting persistent disease among participants who wheeze during early life. Eur. Respir. J. 2003, 22, 76771. [Google Scholar] [CrossRef] [PubMed]
- Kurukulaaratchy, R.J.; Fenn, M.H.; Waterhouse, L.M.; Matthews, S.M.; Holgate, S.T.; Arshad, S.H. Characterization of wheezing phenotypes in the first 10 years of life. Clin. Exp. Allergy 2003, 33, 5738. [Google Scholar] [CrossRef] [PubMed]
- Kurukulaaratchy, R.J.; Raza, A.; Scott, M.; Williams, P.; Ewart, S.; Matthews, S.; Roberts, G.; Arshad, S.H. Characterisation of asthma that develops during adolescence; findings from the Isle of Wight Birth Cohort. Respir. Med. 2012, 106, 32937. [Google Scholar] [CrossRef]
- Kurukulaaratchy, R.J.; Matthews, S.; Arshad, S.H. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics 2004, 113, 34550. [Google Scholar] [CrossRef]
- Shah, A.A. International Study of Asthma and Allergies in Childhood (ISAAC). J. Assoc. Physicians India 1994, 42, 265. [Google Scholar]
- Coates, A.L.; Wanger, J.; Cockcroft, D.W.; Culver, B.H.; Force, T.B.T.T.; Carlsen, K.-H.; Diamant, Z.; Gauvreau, G.; Hall, G.L.; Hallstrand, T.S.; et al. ERS technical standard on bronchial challenge testing: General considerations and performance of methacholine challenge tests. Eur. Respir. J. 2017, 49, 1601526. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Locatelli, F.; Sly, P.D.; White, E.; Blake, D.; Heyworth, J.; Hall, L.G.; Foong, R.E. Characterisation of lung function trajectories and associated early-life predictors in an Australian birth cohort study. ERJ Open Res. 2022, 8, 000722022. [Google Scholar] [CrossRef]
- Pijnenburg, M.W.; Frey, U.; De Jongste, J.C.; Saglani, S. Childhood asthma: Pathogenesis and phenotypes. Eur. Respir. J. 2022, 59, 2100731. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.-Y.; Yoon, J.; Cho, H.-J.; Park, M.J.; Song, K.B.; Choi, E.J.; Paek, E.Y.; Yang, S.-I.; Lee, E.; et al. Atopic Dermatitis With Coexisting Food Allergy in Early Life Is Associated With Childhood Asthma. Allergy Asthma Immunol. Res. 2022, 14, 56580. [Google Scholar] [CrossRef] [PubMed]
- León, B. Understanding the development of Th2 cell-driven allergic airway disease in early life. Front. Allergy 2022, 3, 1080153. [Google Scholar] [CrossRef] [PubMed]
- Steininger, H.; Moltzau-Anderson, J.; Lynch, S.V. Contributions of the early-life microbiome to childhood atopy and asthma development. Semin. Immunol. 2023, 69, 101795. [Google Scholar] [CrossRef]
- Ilmarinen, P.; Tuomisto, L.E.; Kankaanranta, H. Phenotypes, Risk Factors, and Mechanisms of Adult-Onset Asthma. Mediat. Inflamm. 2015, 2015, 514868. [Google Scholar] [CrossRef] [PubMed]
- Holtjer, J.C.; Bloemsma, L.D.; Beijers, R.J.; Cornelissen, M.E.; Hilvering, B.; Houweling, L.; Vermeulen, R.C.; Downward, G.S.; der Zee, A.-H.M.-V. Identifying risk factors for COPD and adult-onset asthma: An umbrella review. Eur. Respir. Rev. 2023, 32, 230009. [Google Scholar] [CrossRef] [PubMed]
- Hedman, L.; Almqvist, L.; Bjerg, A.; Andersson, M.; Backman, H.; Perzanowski, M.S.; Rönmark, E. Early-life risk factors for development of asthma from 8 to 28 years of age: A prospective cohort study. ERJ Open Res. 2022, 8, 000742022. [Google Scholar] [CrossRef]
- Toppila-Salmi, S.; Lemmetyinen, R.; Chanoine, S.; Karjalainen, J.; Pekkanen, J.; Bousquet, J.; Siroux, V. Risk factors for severe adult-onset asthma: A multi-factor approach. BMC Pulm. Med. 2021, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Dold, S.; Wjst, M.; von Mutius, E.; Reitmeir, P.; Stiepel, E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch. Dis. Child. 1992, 67, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Vignola, A.M.; Demoly, P. Links between rhinitis and asthma. Allergy 2003, 58, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Kilanowski, A.; Thiering, E.; Wang, G.; Kumar, A.; Kress, S.; Flexeder, C.; Bauer, C.; Berdel, D.; von Berg, A.; Bergström, A.; et al. Allergic disease trajectories up to adolescence: Characteristics, early-life, and genetic determinants. Allergy 2023, 78, 83650. [Google Scholar] [CrossRef] [PubMed]
- Jayes, L.; Haslam, P.L.; Gratziou, C.G.; Powell, P.; Britton, J.; Vardavas, C.; Jimenez-Ruiz, C.; Leonardi-Bee, J. Tobacco Control Committee of the European Respiratory Society. SmokeHaz: Systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest 2016, 150, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Plata, R.; Rojas-Martínez, R.; Martínez-Briseño, D.; García-Sancho, C.; Pérez-Padilla, R. Effect of Passive Smoking on the Growth of Pulmonary Function and Respiratory Symptoms in Schoolparticipants. Rev. Invest. Clin. 2016, 68, 11927. [Google Scholar]
- Diver, W.R.; Jacobs, E.J.; Gapstur, S.M. Secondhand Smoke Exposure in Childhood and Adulthood in Relation to Adult Mortality Among Never Smokers. Am. J. Prev. Med. 2018, 55, 34552. [Google Scholar] [CrossRef]
- Strachan, D.P.; Butland, B.K.; Anderson, H.R. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996, 312, 11959. [Google Scholar] [CrossRef]
- Bui, D.S.; Perret, J.L.; Abramson, M.J.; Walters, H.E.; Lowe, A.J.; Lodge, C.J.; Dharmage, S.C. Reply: Childhood Exposures, Asthma, Smoking, Interactions and the Catch-Up Hypothesis. Ann. ATS 2018, 15, 12424. [Google Scholar] [CrossRef] [PubMed]
- Kirkeleit, J.; Riise, T.; Wielscher, M.; Accordini, S.; Carsin, A.-E.; Dratva, J.; Franklin, K.A.; Garcia-Aymerich, J.; Jarvis, D.; Leynaert, B.; et al. Early life exposures contributing to accelerated lung function decline in adulthood—a follow-up study of 11, 000 adults from the general population. EClinicalMedicine 2023, 66, 102339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arshad, S.H.; Hodgekiss, C.; Holloway, J.W.; Kurukulaaratchy, R.; Karmaus, W.; Zhang Roberts, G. Association of asthma and smoking with lung function impairment in adolescence and early adulthood: The Isle of Wight Birth Cohort Study. Eur. Respir. J. 2020, 55, 1900477. [Google Scholar] [CrossRef] [PubMed]
- Kurukulaaratchy, R.J.; Evans, S.; Arshad, S.H. Early-life wheeze: “The Child is father of the Man”. Eur. Respir. J. 2014, 43, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, G.; Sharpe, R.A.; Fleming, L.E.; Depledge, M.; Osborne, N.J. Association of Infant Eczema with Childhood and Adult Asthma: Analysis of Data from the 1958 Birth Cohort Study. Int. J. Environ. Res. Public Health 2018, 15, 1415. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 1317. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chang, G.; Zhang, L. The prevention effect of probiotics against eczema in participants: An update systematic review and meta-analysis. J. Dermatolog. Treat. 2022, 33, 184454. [Google Scholar] [CrossRef]
- Zhao, M.; Shen, C.; Ma, L. Treatment efficacy of probiotics on atopic dermatitis, zooming in on infants: A systematic review and meta-analysis. Int. J. Dermatol. 2018, 57, 635–641. [Google Scholar] [CrossRef]
- Bradshaw, L.E.; Wyatt, L.A.; Brown, S.J.; Haines, R.H.; Montgomery, A.A.; Perkin, M.R.; Lawton, S.; Sach, T.H.; Chalmers, J.R.; Ridd, M.J.; et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy 2023, 78, 9951006. [Google Scholar] [CrossRef] [PubMed]
- Alviani, C.; Roberts, G.; Mitchell, F.; Martin, J.; Zolkipli, Z.; Michaelis, L.J.; Vijayanand, P.; Kurukulaaratchy, R.; Arshad, S.H. Primary prevention of asthma in high-risk participants using HDM SLIT; assessment at age 6 years. J. Allergy Clin. Immunol. 2020, 145, 17113. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Raza, A.; Lau, L.; Bawakid, K.; Karmaus, W.; Zhang, H.; Ewart, S.; Patil, V.; Roberts, G.; Kurukulaaratchy, R. Pathophysiological characterization of asthma transitions across adolescence. Respir. Res. 2014, 15, 153. [Google Scholar] [CrossRef]
- Sears, M.R. Predicting asthma outcomes. J. Allergy Clin. Immunol. 2015, 136, 829–837. [Google Scholar] [CrossRef]
- Illi, S.; von Mutius, E.; Lau, S.; Niggemann, B.; Gruber, C.; Wahn, U. Perennial allergen sensitisation early in life and chronic asthma in participants: A birth cohort study. Lancet 2006, 368, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.J.; Kothalawala, D.M.; Kurukulaaratchy, R.J.; Granell, R.; Simpson, A.; Murray, C.; Custovic, A.; Roberts, G.; Zhang, H.; Arshad, S.H. Prediction of Adult Asthma-risk in early childhood using novel adult asthma predictive risk scores. Allergy 2023, 78, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Knihtilä, H.M.; Stubbs, B.J.; Carey, V.J.; Laranjo, N.; Zeiger, R.S.; Bacharier, L.B.; O’Connor, G.T.; Weiss, S.T.; Litonjua, A.A. Preschool impulse oscillometry predicts active asthma and impaired lung function at school age. J. Allergy Clin. Immunol. 2024, 154, 94–100.e13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).