The Assessment of Knowledge About Cervical Cancer, HPV Vaccinations, and Screening Programs Among Women as an Element of Cervical Cancer Prevention in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Issues
2.3. Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Maani, E.V.; Dunton, C.J.; Gasalberti, D.P.; Jack, B.W. Cervical Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 2, e161–e169, Erratum in Lancet Glob. Health 2021, 9, e119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203, Erratum in Lancet Glob. Health 2022, 10, e41. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Du, J.; Lu, H.; Xiang, F.; Mei, H.; Xiao, H. Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: An international comparative study based on the Global Burden of Disease. BMJ Open 2022, 12, e055470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Li, C. Recent global burden of cervical cancer incidence and mortality, predictors, and temporal trends. Gynecol. Oncol. 2021, 163, 583–592. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Cancer-Preventive Strategies, and International Agency for Research on Cancer. Cervix Cancer Screening; IARC: Lyon, France, 2005; Volume 10. [Google Scholar]
- Bouvard, V.; Wentzensen, N.; Mackie, A.; Berkhof, J.; Brotherton, J.; Giorgi-Rossi, P.; Kupets, R.; Smith, R.; Arrossi, S.; Bendahhou, K.; et al. The IARC Perspective on Cervical Cancer Screening. N. Engl. J. Med. 2021, 385, 1908–1918. [Google Scholar] [CrossRef]
- Bowden, S.J.; Doulgeraki, T.; Bouras, E.; Markozannes, G.; Athanasiou, A.; Grout-Smith, H.; Kechagias, K.S.; Ellis, L.B.; Zuber, V.; Chadeau-Hyam, M.; et al. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: An umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023, 21, 274. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.P.; Paliwal, S.; Kosta, S. Genotypic Diversity of Human Papillomavirus (HPV) Types and Its Prevalence With Cervical Cancer (CC) in Central India. Cureus 2023, 15, e35227. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann. Ig. 2018, 30 (Suppl. S1), 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X.; Zhang, Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front. Microbiol. 2018, 9, 2896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Majerciak, V.; Lobanov, A.; Mirza, S.; Band, V.; Liu, H.; Cam, M.; Hughes, S.H.; Lowy, D.R.; Zheng, Z.-M. HPV oncogenes expressed from only one of multiple integrated HPV DNA copies drive clonal cell expansion in cervical cancer. mBio 2024, 15, e0072924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, S.H.; Coffin, J.M. What Integration Sites Tell Us about HIV Persistence. Cell Host Microbe 2016, 19, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Leeman, J.E.; Li, Y.; Bell, A.; Hussain, S.S.; Majumdar, R.; Rong-Mullins, X.; Blecua, P.; Damerla, R.; Narang, H.; Ravindran, P.T.; et al. Human papillomavirus 16 promotes microhomology-mediated end-joining. Proc. Natl. Acad. Sci. USA 2019, 116, 21573–21579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Majerciak, V.; Xue, X.Y.; Uberoi, A.; Lobanov, A.; Chen, X.; Cam, M.; Hughes, S.H.; Lambert, P.F.; Zheng, Z.M. Mouse papillomavirus type 1 (MmuPV1) DNA is frequently integrated in benign tumors by microhomology-mediated end-joining. PLoS Pathog. 2021, 17, e1009812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bowden, S.J.; Bodinier, B.; Kalliala, I.; Zuber, V.; Vuckovic, D.; Doulgeraki, T.; Whitaker, M.D.; Wielscher, M.; Cartwright, R.; Tsilidis, K.K.; et al. Genetic variation in cervical preinvasive and invasive disease: A genome-wide association study. Lancet Oncol. 2021, 22, 548–557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, H.; Weiss, H.A.; Benavente, Y.; de Sanjose, S.; Mayaud, P.; ART and HPV Review Group. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV 2018, 5, e45–e58. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.S.; Chan, P.K.; Ho, S.C.; Yu, M.M.; Yim, S.F.; Cheung, T.H.; Wong, M.C.; Li, E.K. Natural history of cervical papilloma virus infection in systemic lupus erythematosus—A prospective cohort study. J. Rheumatol. 2010, 37, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Thompson, A.; Gandhi, K.K.; Hochberg, M.C.; Suissa, S. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Res. Ther. 2015, 17, 212, Erratum in Arthritis Res. Ther. 2016, 18, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.B.; Kyrgiou, M. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef] [PubMed]
- Naldini, G.; Grisci, C.; Chiavarini, M.; Fabiani, R. Association between human papillomavirus and chlamydia trachomatis infection risk in women: A systematic review and meta-analysis. Int. J. Public Health 2019, 64, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.; Welp, A.; Stoler, M.H.; Cantrell, L.A. Cervical Cancer Screening Recommendations: Now and for the Future. Healthcare 2023, 11, 2273. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Krishnamurti, C. George Papanicolaou (1883–1962): Discoverer of the Pap Smear. J. Obstet. Gynaecol. India 2018, 68, 232–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karnon, J.; Peters, J.; Platt, J.; Chilcott, J.; McGoogan, E.; Brewer, N. Liquid-based cytology in cervical screening: An updated rapid and systematic review and economic analysis. Health Technol. Assess. 2004, 8, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Naz, S.; Ahmed, O.; Yaqeen, S.R.; Irfan, M.; Asif, M.G.; Kamal, A.; Faridi, N. Comparison of Liquid-Based Cytology and Conventional Papanicolaou Smear for Cervical Cancer Screening: An Experience from Pakistan. Cureus 2020, 12, e12293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115–e1127, Erratum in Lancet Glob. Health 2023, 11, e1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebrahimi, N.; Yousefi, Z.; Khosravi, G.; Malayeri, F.E.; Golabi, M.; Askarzadeh, M.; Shams, M.H.; Ghezelbash, B.; Eskandari, N. Human papillomavirus vaccination in low- and middle-income countries: Progression, barriers, and future prospective. Front. Immunol. 2023, 14, 1150238. [Google Scholar] [CrossRef] [PubMed]
- Spayne, J.; Hesketh, T. Estimate of global human papillomavirus vaccination coverage: Analysis of country-level indicators. BMJ Open 2021, 11, e052016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrull-Guardeño, J.; Sebastiá-Laguarda, C.; Donat-Colomer, F.; Sánchez-Martínez, V. Women’s knowledge and attitudes towards cervical cancer prevention: A qualitative study in the Spanish context. J. Clin. Nurs. 2021, 30, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Qayum, M.O.; Billah, M.M.; Akhter, R.; Flora, M.S. Women’s Knowledge, Attitude and Practice on Cervical Cancer and Its Screening in Dhaka, Bangladesh. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 3327–3335. [Google Scholar] [CrossRef]
- Weng, Q.; Jiang, J.; Haji, F.M.; Nondo, L.H.; Zhou, H. Women’s knowledge of and attitudes toward cervical cancer and cervical cancer screening in Zanzibar, Tanzania: A cross-sectional study. BMC Cancer 2020, 20, 63. [Google Scholar] [CrossRef]
- Garg, P.R.; Srivastava, S.; Shumayla, S.; Kurian, K.; Rehman, A.; Garg, R.; Rathi, S.K.; Mehra, S. Women’s Knowledge on Cervical Cancer Risk Factors and Symptoms: A Cross Sectional Study from Urban India. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 1083–1090. [Google Scholar] [CrossRef]
- Brewer, N.; Foliaki, S.; Gray, M.; Potter, J.D.; Douwes, J. Pasifika women’s knowledge and perceptions of cervical-cancer screening and the implementation of self-testing in Aotearoa New Zealand: A qualitative study. Lancet Reg. Health West. Pac. 2022, 28, 100551. [Google Scholar] [CrossRef]
- Lam, J.U.; Rebolj, M.; Dugué, P.A.; Bonde, J.; von Euler-Chelpin, M.; Lynge, E. Condom use in prevention of Human Papillomavirus infections and cervical neoplasia: Systematic review of longitudinal studies. J. Med. Screen. 2014, 21, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Pierce Campbell, C.M.; Lin, H.Y.; Fulp, W.; Papenfuss, M.R.; Salmerón, J.J.; Quiterio, M.M.; Lazcano-Ponce, E.; Villa, L.L.; Giuliano, A.R. Consistent condom use reduces the genital human papillomavirus burden among high-risk men: The HPV infection in men study. J. Infect. Dis. 2013, 208, 373–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Álvarez, M.I.; Gómez-Urquiza, J.L.; Husein-El Ahmed, H.; Albendín-García, L.; Gómez-Salgado, J.; Cañadas-De la Fuente, G.A. Prevalence and Risk Factors of Human Papillomavirus in Male Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valasoulis, G.; Michail, G.; Pouliakis, A.; Androutsopoulos, G.; Panayiotides, I.G.; Kyrgiou, M.; Daponte, A.; Paraskevaidis, E. Effect of Condom Use after CIN Treatment on Cervical HPV Biomarkers Positivity: Prolonged Follow Up Study. Cancers 2022, 14, 3530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Drolet, M.; Lewis, R.M.; Lemieux-Mellouki, P.; Pérez, N.; Jit, M.; Brotherton, J.M.; Ogilvie, G.; Kreimer, A.R.; Brisson, M. Human papillomavirus vaccine effectiveness by number of doses: Updated systematic review of data from national immunization programs. Vaccine 2022, 40, 5413–5432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S2), S367–S378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Staadegaard, L.; Rönn, M.M.; Soni, N.; Bellerose, M.E.; Bloem, P.; Brisson, M.; Maheu-Giroux, M.; Barnabas, R.V.; Drolet, M.; Mayaud, P.; et al. Immunogenicity, safety, and efficacy of the HPV vaccines among people living with HIV: A systematic review and meta-analysis. eClinicalMedicine 2022, 52, 101585. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Lagheden, C.; Luostarinen, T.; Eriksson, T.; Apter, D.; Bly, A.; Gray, P.; Harjula, K.; Heikkilä, K.; Hokkanen, M.; et al. Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: Population-based follow-up of a cluster-randomised trial. BMJ Open 2021, 11, e050669. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Otero, C.; Thompson, E.L.; Daley, E.M.; Griner, S.B.; Logan, R.; Vamos, C.A. Dispelling the myth: Exploring associations between the HPV vaccine and inconsistent condom use among college students. Prev. Med. 2016, 93, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, P.; Pierre-Victor, D.; Mukherjee, S.; Bhoite, P.; Powell, B.; Jean-Baptiste, N.; Clarke, R.; Avent, T.; Krupp, K. Human Papillomavirus Vaccination and Sexual Disinhibition in Females: A Systematic Review. Am. J. Prev. Med. 2016, 51, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jakowczyk, M. Low Popularity of HPV Vaccinations—Only 20% of the Target Population Has Taken Advantage of Them. Onkonet.pl. 2024. Available online: www.onkonet.pl/n_n_szczepienia_przeciw_hpv_problem.php (accessed on 30 October 2024).
- Borowska, M.; Koczkodaj, P.; Mańczuk, M. HPV vaccination coverage in the European Region. Nowotw. J. Oncol. 2024, 74, 191–196. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. 4 September 1998. [Updated 2023]. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993–2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 24 July 2024).
- Lee, Y.C.; Lee, Y.L.; Li, C.Y. BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina 2021, 57, 905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tekalegn, Y.; Sahiledengle, B.; Woldeyohannes, D.; Atlaw, D.; Degno, S.; Desta, F.; Bekele, K.; Aseffa, T.; Gezahegn, H.; Kene, C. High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case-control studies. Women’s Health 2022, 18, 17455065221075904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castellsagué, X.; Muñoz, N. Chapter 3: Cofactors in human papillomavirus carcinogenesis—Role of parity, oral contraceptives, and tobacco smoking. J. Natl. Cancer Inst. Monogr. 2003, 31, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Green, J.; Berrington de Gonzalez, A.; Appleby, P.; Peto, J.; Plummer, M.; Franceschi, S.; Beral, V. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet 2003, 361, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, A.; Herrero, R.; Castle, P.E.; Wacholder, S.; Bratti, M.C.; Sherman, M.E.; Lorincz, A.T.; Burk, R.D.; Morales, J.; Rodriguez, A.C.; et al. HPV co-factors related to the development of cervical cancer: Results from a population-based study in Costa Rica. Br. J. Cancer 2001, 84, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Kang, H.; Son, M. Association between breastfeeding and breast, thyroid, and cervical cancer among Korean adult women based on the Korean Genome and Epidemiology Study: A cohort study. Korean J. Women Health Nurs. 2021, 27, 368–378. [Google Scholar] [CrossRef]
- Maamri, A.; El Hfid, M.; Chafi, A.; Boutayeb, A. Cervix and breast cancers in Oujda city in Eastern Morocco: Determinants and risk factors. Open J. Prev. Med. 2012, 2, 9–15. [Google Scholar] [CrossRef]
- WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, Second Edition. Available online: https://www.who.int/publications-detail-redirect/9789240030824 (accessed on 30 October 2024).
- European Commission. Council Recommendation on Strengthening Prevention Through Early Detection: A New EU Approach on Cancer Screening Replacing Council Recommendation 2003/878/EC; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Cervical Cancer Prevention Program (Cytology)—Polish Ministry of Health—Gov.pl Polish Ministry of Health. 2023. Available online: www.gov.pl/web/zdrowie/program-profilaktyki-raka-szyjki-macicy-cytologia- (accessed on 30 October 2024).
- United States Preventive Services Taskforce Recommendation: Cervical Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/cervical-cancer-screening-adults-adolescents (accessed on 30 October 2024).
- Burns, R.B.; Potter, J.E.; Ricciotti, H.A.; Reynolds, E.E. Screening Pelvic Examinations in Adult Women: Grand Rounds Discussion From the Beth Israel Deaconess Medical Center. Ann. Intern. Med. 2015, 163, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.; Guthmann, R.; Plonka, C. Clinical inquiry: Do annual pelvic exams benefit asymptomatic women who receive regular Pap smears? J. Fam. Pract. 2015, 64, 51–52,65. [Google Scholar] [PubMed]
- Henderson, J.T.; Yu, J.M.; Harper, C.C.; Sawaya, G.F. U. S. clinicians’ perspectives on less frequent routine gynecologic examinations. Prev. Med. 2014, 62, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, J.; Levi, A. The Annual Gynecologic Examination Updated for the 21st Century. Nurs. Women’s Health 2016, 20, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kling, J.M.; Vegunta, S.; Al-Badri, M.; Faubion, S.S.; Fields, H.E.; Shah, A.A.; Wallace, M.R.; Ruddy, B.E.; Bryan, M.J.; Temkit, M.; et al. Routine pelvic examinations: A descriptive cross-sectional survey of women’s attitudes and beliefs after new guidelines. Prev. Med. 2017, 94, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Mwaka, A.D.; Orach, C.G.; Were, E.M.; Lyratzopoulos, G.; Wabinga, H.; Roland, M. Awareness of cervical cancer risk factors and symptoms: Cross-sectional community survey in post-conflict northern Uganda. Health Expect. Int. J. Public Particip. Health Care Health Policy 2016, 19, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Chepkorir, J.; Guillaume, D.; Lee, J.; Duroseau, B.; Xia, Z.; Wyche, S.; Anderson, J.; Han, H.R. The Role of Health Information Sources on Cervical Cancer Literacy, Knowledge, Attitudes and Screening Practices in Sub-Saharan African Women: A Systematic Review. Int. J. Environ. Res. Public Health 2024, 21, 872. [Google Scholar] [CrossRef] [PubMed]
- Ayanto, S.Y.; Belachew, T.; Wordofa, M.A. Determinants of cervical cancer screening utilization among women in Southern Ethiopia. Sci. Rep. 2022, 12, 14830. [Google Scholar] [CrossRef] [PubMed]
- Tiiti, T.A.; Bogers, J.; Lebelo, R.L. Knowledge of Human Papillomavirus and Cervical Cancer among Women Attending Gynecology Clinics in Pretoria, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 4210. [Google Scholar] [CrossRef] [PubMed]
- Tekle, T.; Wolka, E.; Nega, B.; Kumma, W.P.; Koyira, M.M. Knowledge, Attitude and Practice Towards Cervical Cancer Screening Among Women and Associated Factors in Hospitals of Wolaita Zone, Southern Ethiopia. Cancer Manag. Res. 2020, 12, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Ndejjo, R.; Mukama, T.; Musabyimana, A.; Musoke, D. Uptake of Cervical Cancer Screening and Associated Factors among Women in Rural Uganda: A Cross Sectional Study. PLoS ONE 2016, 11, e0149696. [Google Scholar] [CrossRef] [PubMed]
- Kangmennaang, J.; Thogarapalli, N.; Mkandawire, P.; Luginaah, I. Investigating the disparities in cervical cancer screening among Namibian women. Gynecol. Oncol. 2015, 138, 411–416. [Google Scholar] [CrossRef]
- Tapera, O.; Kadzatsa, W.; Nyakabau, A.M.; Mavhu, W.; Dreyer, G.; Stray-Pedersen, B.; Sjh, H. Sociodemographic inequities in cervical cancer screening, treatment and care amongst women aged at least 25 years: Evidence from surveys in Harare, Zimbabwe. BMC Public Health 2019, 19, 428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Endalew, D.A.; Moti, D.; Mohammed, N.; Redi, S.; Wassihun Alemu, B. Knowledge and practice of cervical cancer screening and associated factors among reproductive age group women in districts of Gurage zone, Southern Ethiopia. A cross-sectional study. PLoS ONE 2020, 15, e0238869. [Google Scholar] [CrossRef] [PubMed]
- Jatho, A.; Bikaitwoha, M.E.; Mugisha, N.M. Socio-culturally mediated factors and lower level of education are the main influencers of functional cervical cancer literacy among women in Mayuge, Eastern Uganda. Ecancermedicalscience 2020, 14, 1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seweryn, M.; Leszczyńska, A.; Jakubowicz, J.; Banaś, T. Cervical Cancer in Poland—Epidemiology, Prevention, and Treatment Pathways. In Oncology in Clinical Practice; VM Media Group sp. z o.o: Gdańsk, Poland, 2024. [Google Scholar] [CrossRef]
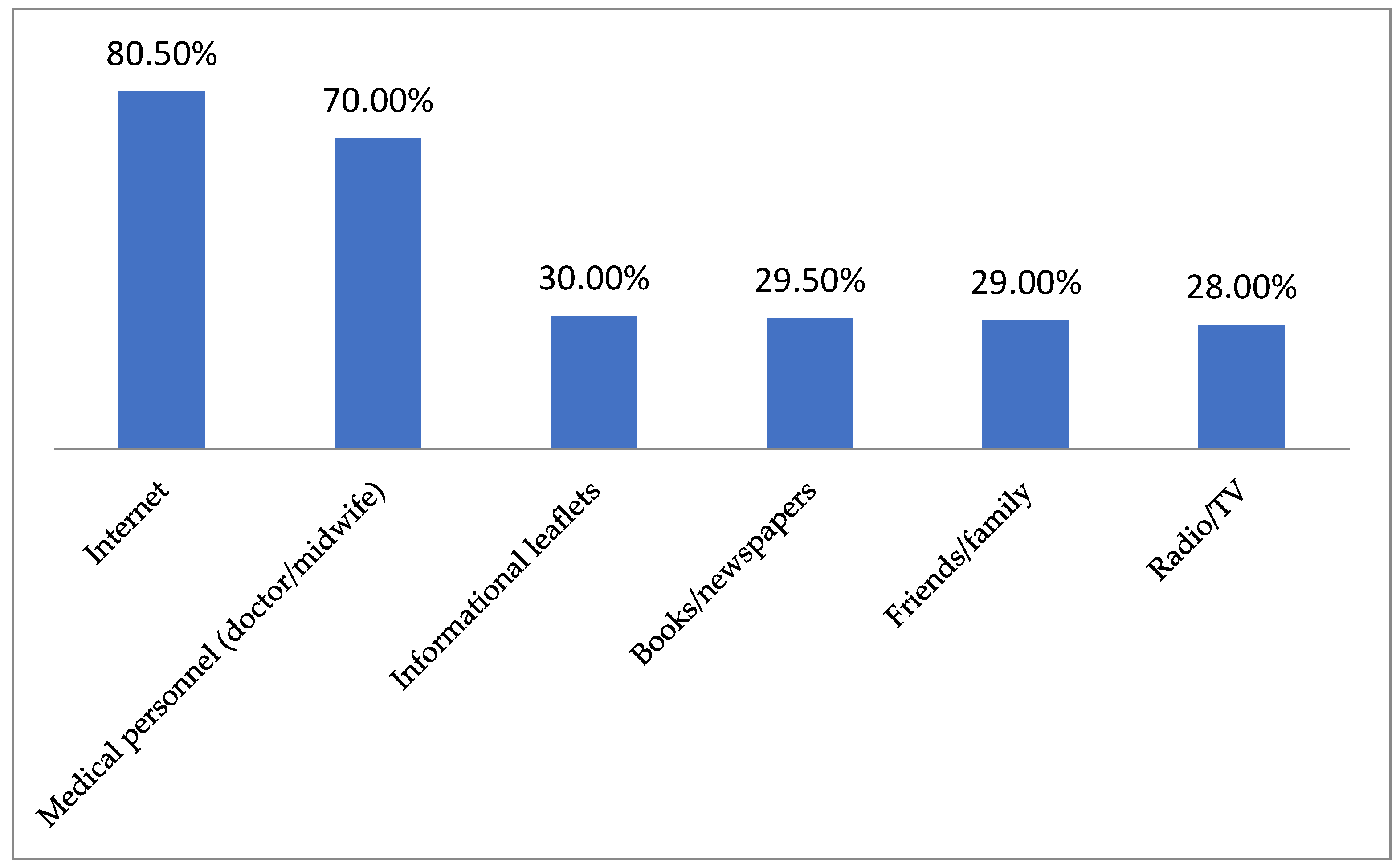
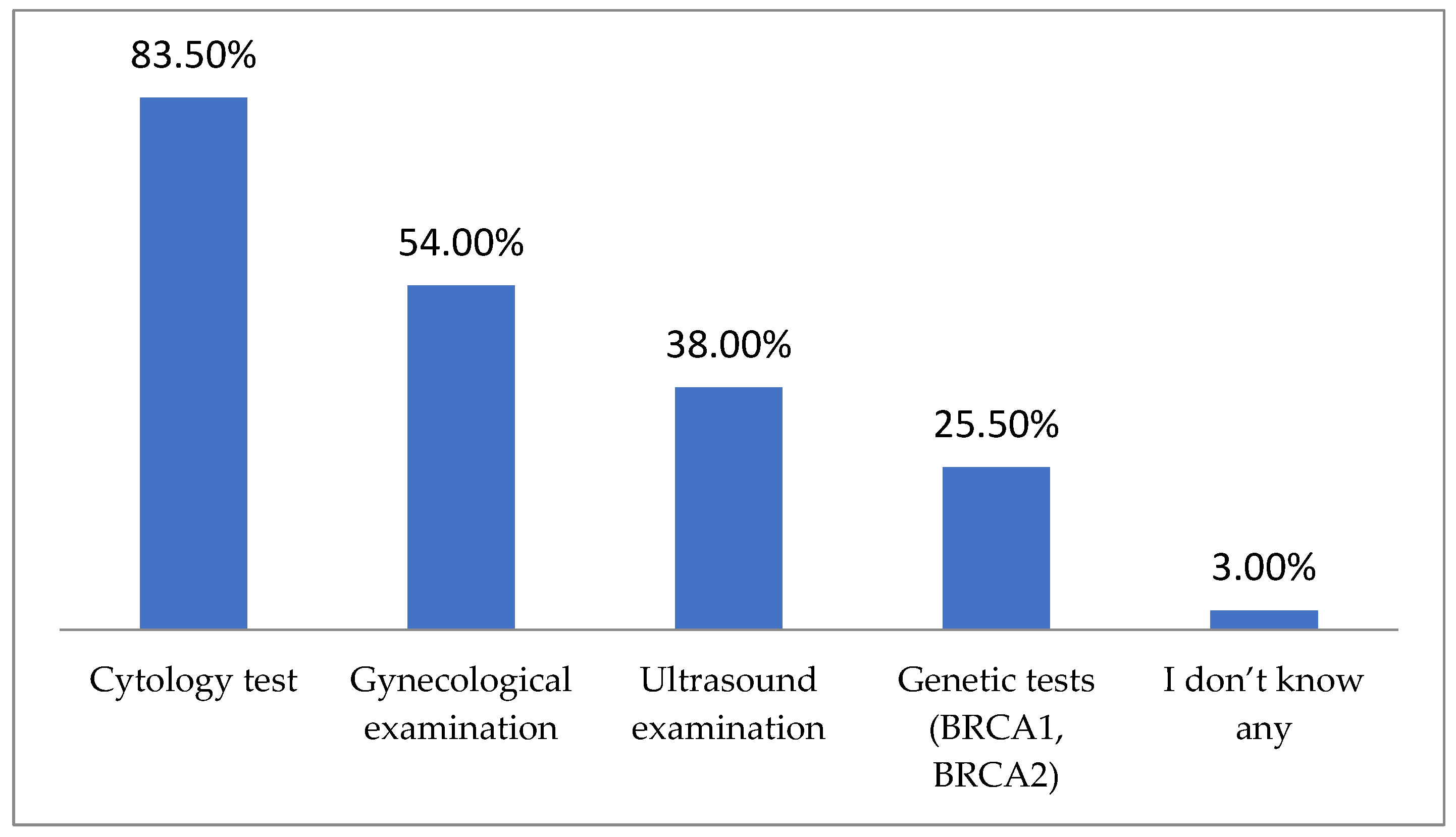
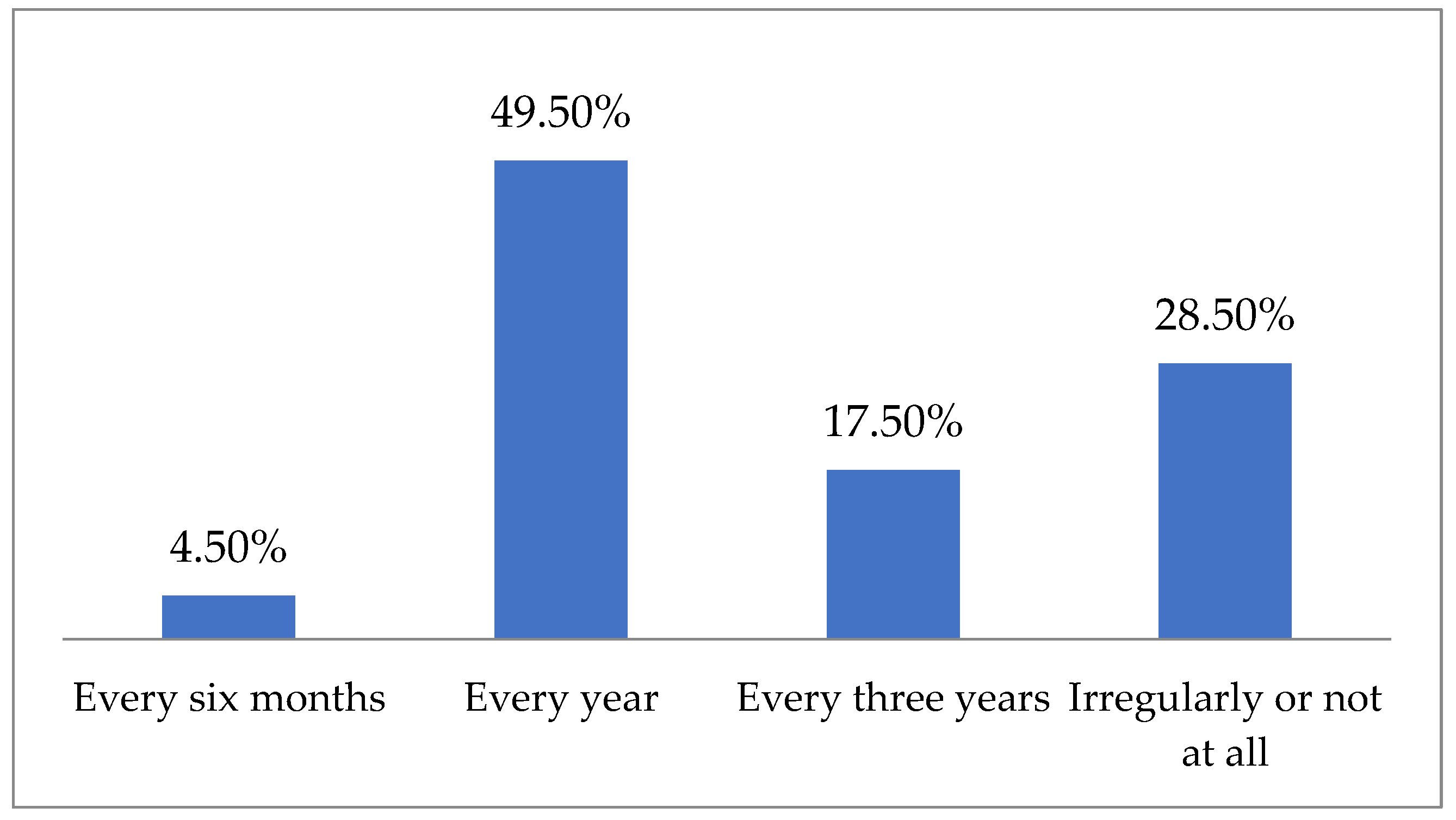
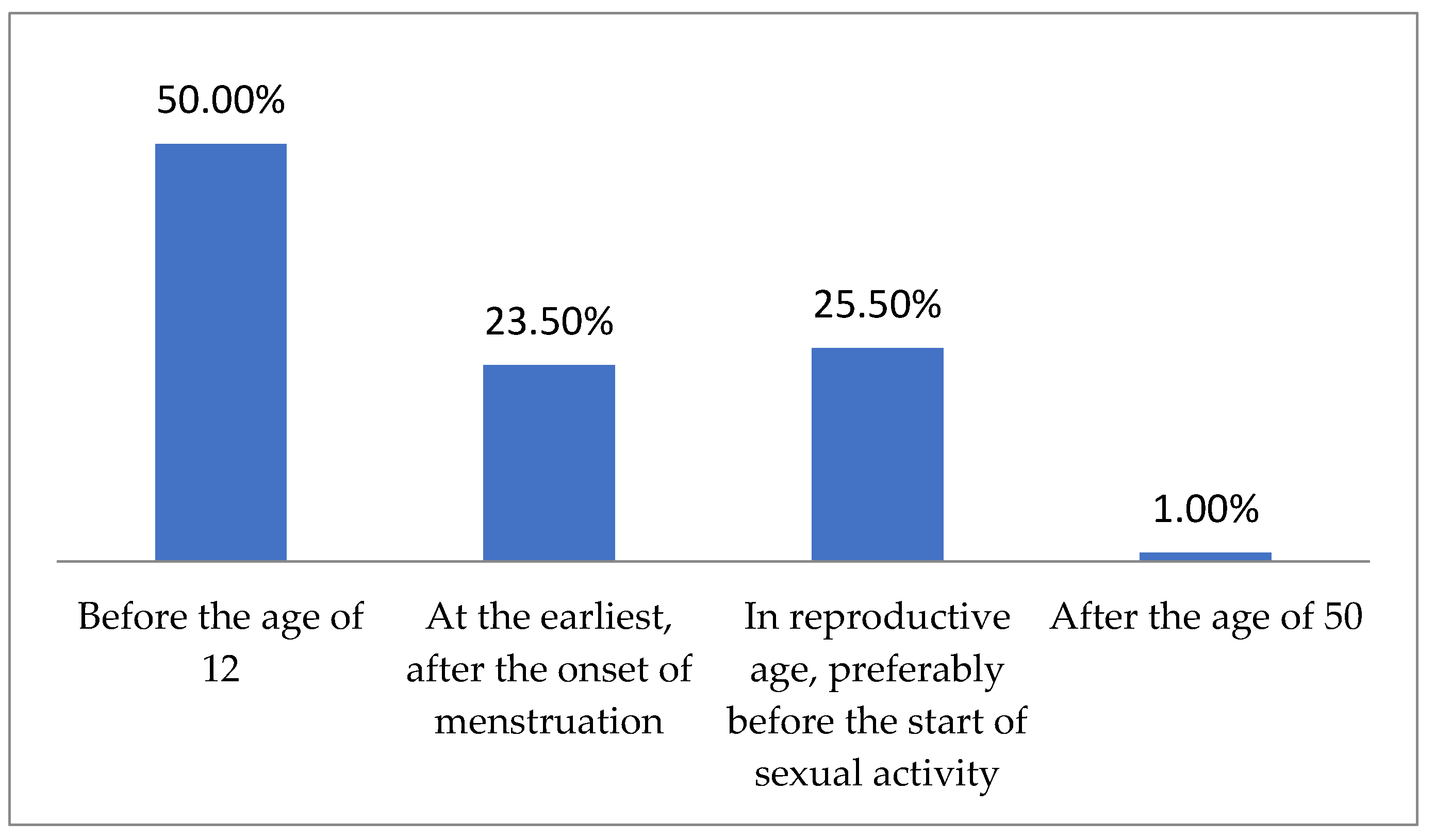
| Study Group (n = 200) | |
|---|---|
| Age (years) | |
| 18–25 | 124 (62.00%) |
| 26–35 | 42 (21.00%) |
| 36–45 | 34 (17.00%) |
| Permanent residence | |
| Village | 60 (30.00%) |
| Town/city | 140 (70.00%) |
| Marital status | |
| Single | 103 (51.50%) |
| Informal/marital relationship | 96 (48.00%) |
| Widow | 1 (0.50%) |
| Education | |
| Completed primary school | 0 (0.00%) |
| Completed vocational school | 5 (2.50%) |
| Completed secondary education | 62 (31.00%) |
| Completed higher education | 133 (66.50%) |
| Main occupation | |
| Student | 99 (49.50%) |
| Unemployed | 6 (3.00%) |
| Working professional | 90 (45.00%) |
| Household | 5 (2.50%) |
| Frequency of Gynecologist Visits vs. Age of Respondents | ||||||
|---|---|---|---|---|---|---|
| Age Range | Once a Year n (%) | Every Six Months n (%) | Every Few Years n (%) | I Don’t Go at All n (%) | Only When Concerning Symptoms Appear n (%) | Row Total n |
| 18–25 years | 46 (37.10%) | 27 (21.77%) | 9 (7.26%) | 16 (12.90%) | 26 (20.97%) | 124 |
| 26–35 years | 23 (53.49%) | 9 (20.93%) | 6 (13.95%) | 2 (4.65%) | 3 (6.98%) | 43 |
| 36–45 years | 21 (63.64%) | 6 (18.18%) | 6 18.18% | 0 (0.00%) | 0 (0.00%) | 33 |
| Total | 90 | 42 | 21 | 18 | 29 | 200 |
| Pearson’s chi-square: 24.71460, df = 8, p < 0.0017 | ||||||
| The Most Common Symptom of Cervical Cancer According to Respondents | |||||
|---|---|---|---|---|---|
| Age Range | Vaginal Bleeding n (%) | Pain During Intercourse n (%) | Discharge n (%) | Irregular Menstrual Bleeding n (%) | Row Total n |
| 18–25 years | 54 (43.55%) | 25 (20.16%) | 18 (14.52%) | 27 (21.77%) | 124 |
| 26–35 years | 19 (44.19%) | 8 (18.60%) | 7 (16.28%) | 9 (20.93%) | 43 |
| 36–45 years | 20 (60.61%) | 2 (6.06%) | 5 (15.15%) | 6 (18.18%) | 33 |
| Total | 93 | 35 | 30 | 42 | 200 |
| Pearson’s chi-square: 4.989188, df = 6, p < 0.005 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Drab, A.; Filipek, P.; Religioni, U. The Assessment of Knowledge About Cervical Cancer, HPV Vaccinations, and Screening Programs Among Women as an Element of Cervical Cancer Prevention in Poland. J. Pers. Med. 2024, 14, 1139. https://doi.org/10.3390/jpm14121139
Wdowiak K, Drab A, Filipek P, Religioni U. The Assessment of Knowledge About Cervical Cancer, HPV Vaccinations, and Screening Programs Among Women as an Element of Cervical Cancer Prevention in Poland. Journal of Personalized Medicine. 2024; 14(12):1139. https://doi.org/10.3390/jpm14121139
Chicago/Turabian StyleWdowiak, Krystian, Agnieszka Drab, Paulina Filipek, and Urszula Religioni. 2024. "The Assessment of Knowledge About Cervical Cancer, HPV Vaccinations, and Screening Programs Among Women as an Element of Cervical Cancer Prevention in Poland" Journal of Personalized Medicine 14, no. 12: 1139. https://doi.org/10.3390/jpm14121139
APA StyleWdowiak, K., Drab, A., Filipek, P., & Religioni, U. (2024). The Assessment of Knowledge About Cervical Cancer, HPV Vaccinations, and Screening Programs Among Women as an Element of Cervical Cancer Prevention in Poland. Journal of Personalized Medicine, 14(12), 1139. https://doi.org/10.3390/jpm14121139







