Analysis of Thyroid Function in ANCA-Associated Vasculitis Patients with Renal Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Classification
2.3. Research Design
2.4. Follow-Up
2.5. Statistics
3. Results
3.1. The General Information of Included AAV Patients with Renal Injury
3.2. Comparison of Clinical Parameters between Normal Thyroid Function Group and Thyroid Dysfunction Group in AAV Patients with Renal Injury
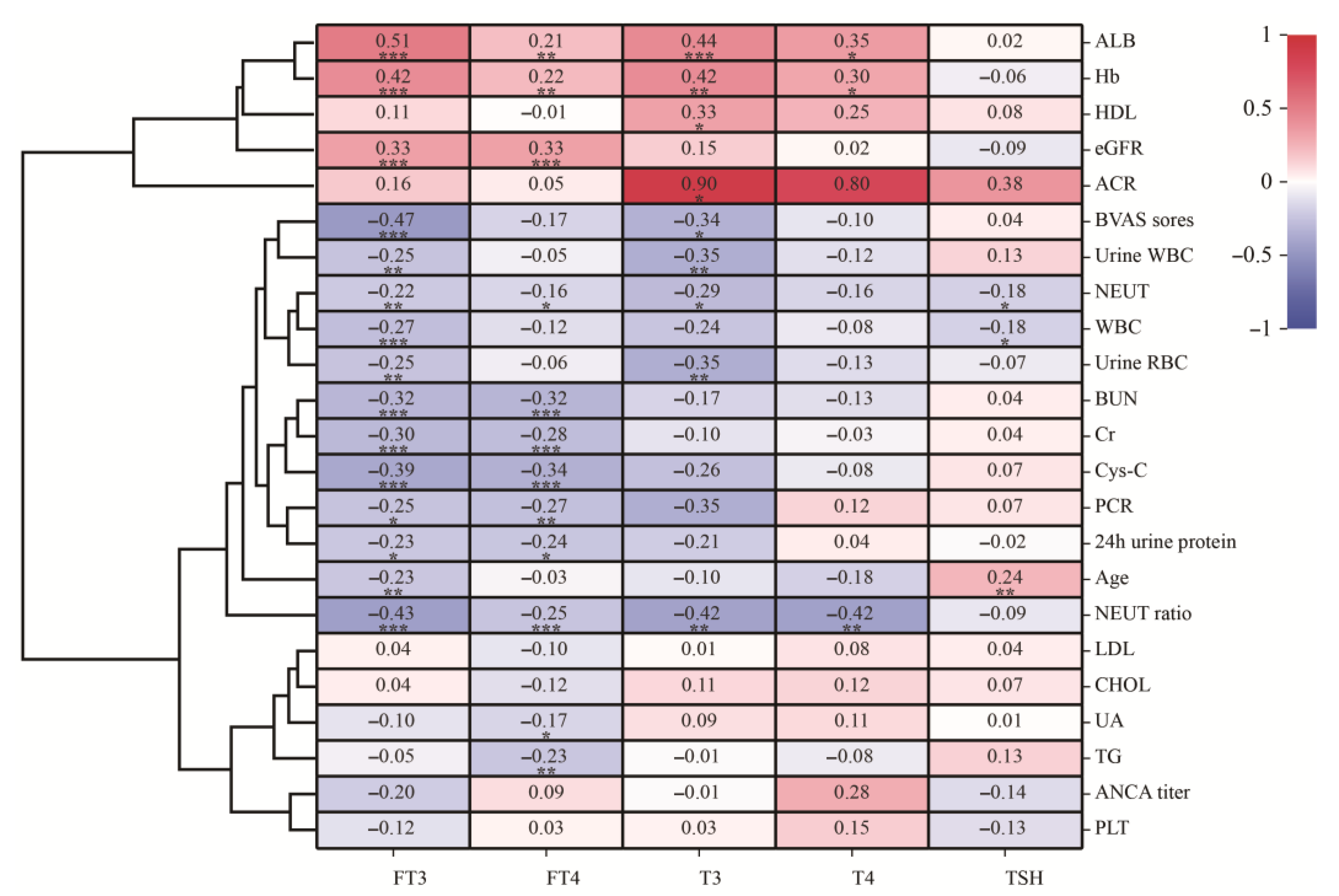
3.3. Relationship between Thyroid Hormone and Clinical Parameters in AAV Patients with Renal Injury
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvador, F. ANCA associated vasculitis. Eur. J. Intern. Med. 2020, 74, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Trivioli, G.; Marquez, A.; Martorana, D.; Tesi, M.; Kronbichler, A.; Lyons, P.A.; Vaglio, A. Genetics of ANCA-associated vasculitis: Role in pathogenesis, classification and management. Nat. Rev. Rheumatol. 2022, 18, 559–574. [Google Scholar] [CrossRef]
- Tan, J.A.; Dehghan, N.; Chen, W.; Xie, H.; Esdaile, J.M.; Avina-Zubieta, J.A. Mortality in ANCA-associated vasculitis: Ameta-analysis of observational studies. Ann. Rheum Dis. 2017, 76, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, F.; Zhang, Y.; Zhao, M.H. Clinical and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitides: A study of 426 patients from a single centre. Postgrad. Med. J. 2005, 81, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Geetha, D.; Jefferson, J.A. ANCA-Associated Vasculitis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 124–137. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, T.-T.; Liu, X.-M.; Liu, B.-L.; Hu, Y.; Liu, S.; Wang, J.; Li, G.-Q.; Mao, X.-M. High Levels of Thyroid Hormone Impair Regulatory T Cell Function Via Reduced PD-1 Expression. J. Clin. Endocrinol. Metab. 2021, 106, 2738–2753. [Google Scholar] [CrossRef]
- Van Der Spek, A.H.; Fliers, E.; Boelen, A. Thyroid Hormone and Deiodination in Innate Immune Cells. Endocrinology 2021, 162, bqaa200. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Camat, M.D.; James, A.; Wu, Y.; Ching, J.; Kovalik, J.-P.; et al. Thyroid Hormone Decreases Hepatic Steatosis, Inflammation, and Fibrosis in a Dietary Mouse Model of Nonalcoholic Steatohepatitis. Thyroid 2022, 32, 725–738. [Google Scholar] [CrossRef]
- Li, L.Z.; Hu, Y.; Ai, S.-L.; Cheng, L.; Liu, J.; Morris, E.; Li, Y.; Gou, S.-J.; Fu, P. The relationship between thyroid dysfunction and nephrotic syndrome: A clinicopathological study. Sci. Rep. 2019, 9, 6421. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.; Isenberg, D.A. Autoimmune thyroid disease in systemic lupus erythematosus. Ann. Rheum Dis. 2002, 61, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Merkel, P.A.; Tomasson, G.; Segelmark, M.; Mohammad, A.J. Comorbidities in Patients with Antineutrophil Cytoplasmic Antibody-associated Vasculitis versus the General Population. J. Rheumatol. 2016, 43, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Sarica, S.H.; Gallacher, P.J.; Dhaun, N.; Sznajd, J.; Harvie, J.; McLaren, J.; Basu, N. Multimorbidity in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Results From a Longitudinal, Multicenter Data Linkage Study. Arthritis Rheumatol. 2021, 73, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Kermani, T.A.; Cuthbertson, D.; Carette, S.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; Monach, P.A.; Moreland, L.; Pagnoux, C.; et al. Hypothyroidism in vasculitis. Rheumatology 2022, 61, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, S.; Hogan, S.L.; Falk, R.J.; Joy, M.S.; Chin, H.; Jennette, C.E.; Jennette, J.C.; Nachman, P.H. Association between thyroid disease and its treatment with ANCA small-vessel vasculitis: A case-control study. Nephrol. Dial. Transplant. 2007, 22, 3508–3515. [Google Scholar] [CrossRef]
- Prendecki, M.; Martin, L.; Tanna, A.; Antonelou, M.; Pusey, C.D. Increased Prevalence of Thyroid Disease in Patients with Antineutrophil Cytoplasmic Antibodies-associated Vasculitis. J. Rheumatol. 2018, 45, 686–689. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.B.; Lee, S.W. Thyroid Dysfunction in Patients with Antineutrophil Cytoplasmic Antibody-associated Vasculitis: A Monocentric Retrospective Study. J. Rheumatol. 2019, 46, 1248–1250. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.-H.; Yu, J.; Xin, G.; Liu, Y.-C.; Zhang, Y.-K.; Wang, H.-Y. The clinical and pathological characteristics of Chinese elderly patients with anti-neutrophil cytoplasmic autoantibodies associated small vessel vasculitis. Exp. Gerontol. 2004, 39, 1401–1405. [Google Scholar] [CrossRef]
- Baier, E.; Tampe, D.; Hakroush, S.; Tampe, B. Low levels of hemoglobin associate with critical illness and predict disease course in patients with ANCA-associated renal vasculitis. Sci. Rep. 2022, 12, 18736. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Usui, J.; Kaneko, S.; Tsunoda, R.; Imai, E.; Kai, H.; Morito, N.; Saito, C.; Nagata, M.; Yamagata, K. Anaemia is an essential complication of ANCA-associated renal vasculitis: A single center cohort study. BMC Nephrol. 2017, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Baier, E.; Kluge, I.A.; Hakroush, S.; Tampe, B. Low hemoglobin levels are associated with Bowman’s capsule rupture and peritubular capillaritis in ANCA-associated renal vasculitis: A link of vascular injury to anemia? J. Nephrol. 2023, 36, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Taeib, S.H.; Kanoun, F.; Hammami, M.B.; Kamoun, S.; Ben Romdhane, N.; Feki, M.; Slimane, H.; Kaabachi, N. Erythrocyte abnormalities in thyroid dysfunction. Tunis Med. 2010, 88, 783–788. [Google Scholar] [PubMed]
- Dilek, M.; Akpolat, T.; Cengiz, K. Hypothyroidism as a cause of resistance to erythropoietin. Nephron 2002, 92, 248. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Teumer, A.; Marouli, E.; Deloukas, P.; Kuś, A.; Sterenborg, R.; Åsvold, B.O.; Medici, M.; Ellervik, C. Thyroid function, pernicious anemia and erythropoiesis: A two-sample Mendelian randomization study. Hum. Mol. Genet. 2022, 31, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Anemia in thyroid diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef]
- Ni, J.; Li, J.; Wang, Y.; Guan, L.; Lin, H.; Zhang, L.; Zhang, H. Systemic Lupus Erythematosus Patients With Related Organic Damage Are at High Risk of Hypothyroidism. Front. Endocrinol. 2022, 13, 920283. [Google Scholar] [CrossRef]
- Reinhardt, W.; Mülling, N.; Behrendt, S.; Benson, S.; Dolff, S.; Führer, D.; Tan, S. Association between albuminuria and thyroid function in patients with chronic kidney disease. Endocrine 2021, 73, 367–373. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kalantar-Zadeh, K.; Streja, E.; Carrero, J.-J.; Ma, J.Z.; Lu, J.L.; Kovesdy, C.P. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 282–287. [Google Scholar] [CrossRef]
- Vargas, F.; Rodríguez-Gómez, I.; Vargas-Tendero, P.; Jimenez, E.; Montiel, M. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J. Endocrinol. 2012, 213, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Schairer, B.; Jungreithmayr, V.; Schuster, M.; Reiter, T.; Herkner, H.; Gessl, A.; Sengölge, G.; Winnicki, W. Effect of Thyroid Hormones on Kidney Function in Patients after Kidney Transplantation. Sci. Rep. 2020, 10, 2156. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, J.G.; Wulkan, R.W.; Mantel, M.J.; Berghout, A. Is cystatin C a marker of glomerular filtration rate in thyroid dysfunction? Clin. Chem. 2003, 49, 1558–1559. [Google Scholar] [CrossRef]
- Chadha, V.; Alon, U.S. Bilateral nephrectomy reverses hypothyroidism in congenital nephrotic syndrome. Pediatr. Nephrol. 1999, 13, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Rhee, C.M.; Leung, A.M.; Braverman, L.E.; Brent, G.A.; Pearce, E.N. A review: Radiographic iodinated contrast media-induced thyroid dysfunction. J. Clin. Endocrinol. Metab. 2015, 100, 376–383. [Google Scholar] [CrossRef]
- Den Hollander, J.G.; Wulkan, R.W.; Mantel, M.J.; Berghout, A. Correlation between severity of thyroid dysfunction and renal function. Clin. Endocrinol. 2005, 62, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kreisman, S.H.; Hennessey, J.V. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch. Intern. Med. 1999, 159, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, J.; González, O.; Saracho, R.; Aguirre, R.; González, Ó.; Martínez, I. Changes in renal function in primary hypothyroidism. Am. J. Kidney Dis. 1996, 27, 195–198. [Google Scholar] [CrossRef]
- Villabona, C.; Sahun, M.; Gómez, N.; Gómez, J.M.; Soler, J.; Roca, M.; Mora, J.; Puchal, R. Blood volumes and renal function in overt and subclinical primary hypothyroidism. Am. J. Med. Sci. 1999, 318, 277–280. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, M.J.; Lee, H.S.; Oh, H.J.; Ko, K.I.; Kim, C.H.; Doh, F.M.; Koo, H.M.; Kim, H.R.; Han, J.H.; et al. Thyroid hormone replacement therapy attenuates the decline of renal function in chronic kidney disease patients with subclinical hypothyroidism. Thyroid 2013, 23, 654–661. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, M.J.; Kim, S.J.; Oh, H.J.; Kim, H.R.; Han, J.H.; Koo, H.M.; Doh, F.M.; Park, J.T.; Han, S.H.; et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, R.; Chen, X.; Chen, Y.; Wang, D.; Zhang, F.; Wang, Y. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Curr. Med. Res. Opin. 2015, 31, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J. Update on the treatment of hypothyroidism. Curr. Opin. Oncol. 2016, 28, 18–25. [Google Scholar] [CrossRef] [PubMed]

| All (n = 174) | Normal Thyroid Group (n = 24) | Thyroid Dysfunction Group (n = 150) | p | |
|---|---|---|---|---|
| Age (year) | 56.9 ± 16.5 | 45.1 ± 16.2 | 58.8 ± 15.8 | 0.002 * |
| Elder (>60 years old, %) | 84 (48.3%) | 3 (12.5%) | 81 (54.0%) | <0.001 * |
| Female (%) | 102 (58.6%) | 12 (50.0%) | 90 (60.0%) | 0.356 |
| Hb (g/L) | 88.41 ± 22.26 | 104.00 ± 24.93 | 85.92 ± 20.8 | <0.001 * |
| NEUT ratio (%) | 75.34 ± 11.77 | 68.37 ± 9.66 | 76.28 ± 11.43 | 0.002 * |
| Alb (g/L) | 33.75 ± 6.42 | 38.86 ± 4.68 | 33.05 ± 6.28 | 0.003 * |
| BUN (mmol/L) | 21.75 ± 15.20 | 12.50 ± 6.71 | 22.07 ± 13.15 | <0.001 * |
| Cr (μmol/L) | 431.02 ± 302.30 | 260.58 ± 215.55 | 457.14 ± 306.71 | 0.009 * |
| eGFR (mL/min/1.73 m2) | 12.69 (6.96, 27.72) | 44.32 (13.89, 68.75) | 11.00 (6.62, 23.44) | <0.001 * |
| BVAS score | 16.97 ± 6.22 | 13.67 ± 6.77 | 17.49 ± 5.98 | 0.005 * |
| FT3 (pmol/L) | 2.66 ± 0.89 | 4.13 ± 0.40 | 2.45 ± 0.73 | <0.001 * |
| FT4 (pmol/L) | 14.02 ± 3.78 | 16.86 ± 2.25 | 13.58 ± 3.78 | <0.001 * |
| TSH (mU/L) | 2.66 (1.33, 4.89) | 2.07 ± 1.27 | 2.80 (1.40, 5.45) | 0.008 * |
| TT3 (nmol/L) | 0.97 ± 0.39 | 1.55 ± 0.32 | 0.88 ± 0.32 | <0.001 * |
| TT4 (nmol/L) | 73.89 ± 24.40 | 101.93 ± 11.48 | 69.79 ± 23.08 | 0.003 * |
| PCR (g/mmol) | 0.30 (0.17, 0.54) | 0.251 ± 0.195 | 0.30 (0.17, 0.62) | 0.091 |
| 24 h urinary protein (g) | 1.61 (1.04, 3.52) | 1.54 (0.79, 2.37) | 1.94 (1.075, 4.065) | 0.197 |
| ACR (mg/mmol) | 1944.70 ± 1675.55 | 1458.78 ± 1327.56 | 2033.05 ± 1742.79 | 0.539 |
| Cys-C (mg/L) | 3.89 ± 1.80 | 2.56 ± 1.46 | 4.11 ± 1.76 | 0.150 |
| UA (μmol/L) | 451.73 ± 143.00 | 441.92 ± 128.94 | 453.31 ± 145.18 | 0.678 |
| TG (mmol/L) | 1.66 ± 1.04 | 1.49 ± 0.91 | 1.66 ± 1.02 | 0.276 |
| CHOL (mmol/L) | 4.30 ± 1.58 | 4.10 ± 1.46 | 4.32 ± 1.53 | 0.335 |
| HDL (mmol/L) | 1.21 ± 0.53 | 1.16 ± 0.42 | 1.22 ± 0.55 | 0.565 |
| LDL (mmol/L) | 2.31 ± 1.09 | 2.24 ± 1.01 | 2.32 ± 1.06 | 0.535 |
| PLT (×109) | 225.29 ± 121.95 | 197.54 ± 85.24 | 231.14 ± 124.00 | 0.813 |
| NEUT (×109) | 7.20 ± 5.86 | 5.12 ± 2.08 | 7.46 ± 6.24 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Wang, Y.; Ma, L.; Gou, S.; Fu, P. Analysis of Thyroid Function in ANCA-Associated Vasculitis Patients with Renal Injury. J. Pers. Med. 2024, 14, 99. https://doi.org/10.3390/jpm14010099
Yu W, Wang Y, Ma L, Gou S, Fu P. Analysis of Thyroid Function in ANCA-Associated Vasculitis Patients with Renal Injury. Journal of Personalized Medicine. 2024; 14(1):99. https://doi.org/10.3390/jpm14010099
Chicago/Turabian StyleYu, Wenhui, Yuelan Wang, Liang Ma, Shenju Gou, and Ping Fu. 2024. "Analysis of Thyroid Function in ANCA-Associated Vasculitis Patients with Renal Injury" Journal of Personalized Medicine 14, no. 1: 99. https://doi.org/10.3390/jpm14010099
APA StyleYu, W., Wang, Y., Ma, L., Gou, S., & Fu, P. (2024). Analysis of Thyroid Function in ANCA-Associated Vasculitis Patients with Renal Injury. Journal of Personalized Medicine, 14(1), 99. https://doi.org/10.3390/jpm14010099






