The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases
Abstract
1. Introduction
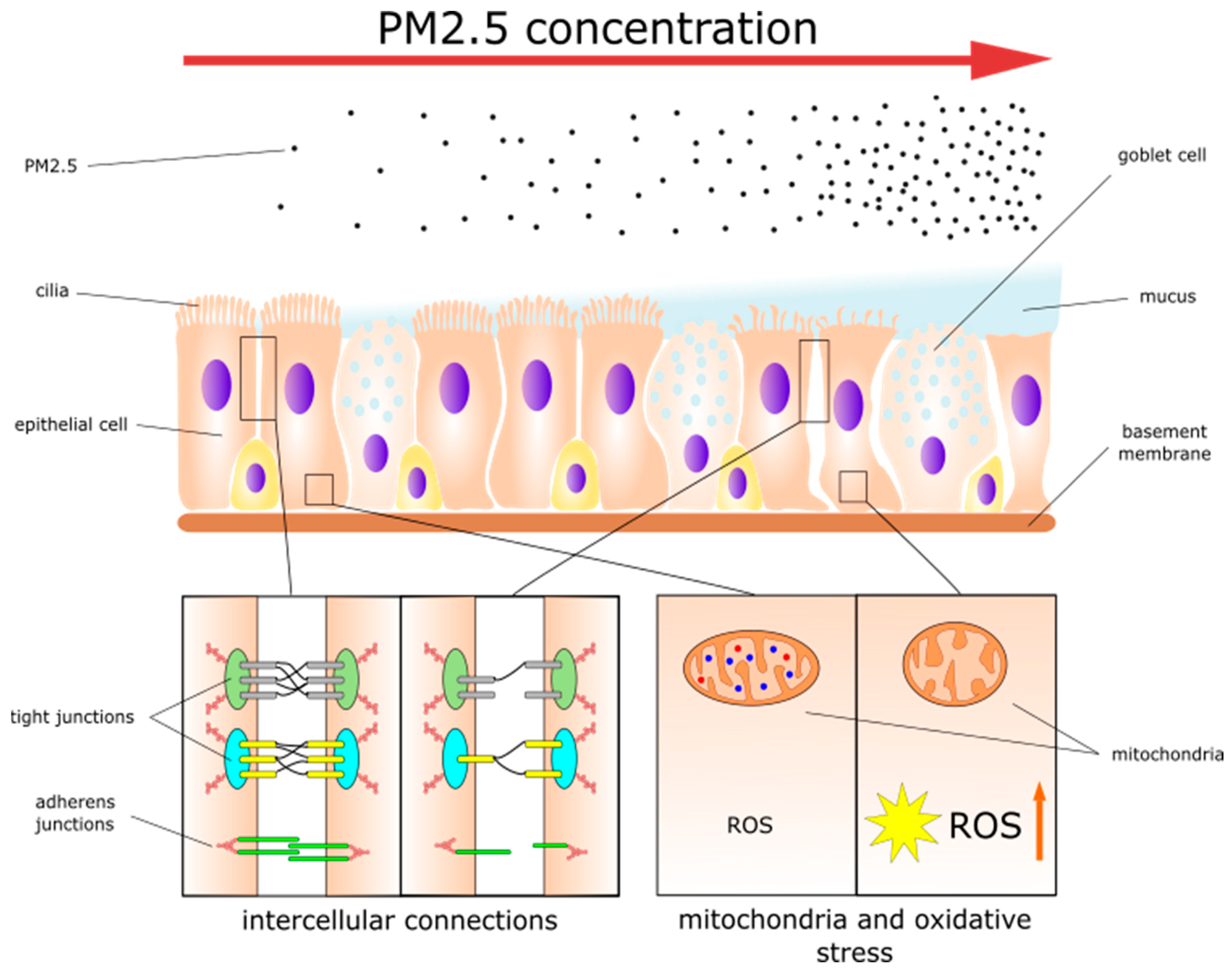
2. PM2.5 Effects on Healthy Nasal and Sinonasal Epithelia
2.1. Disruption of Epithelial Cell Metabolism
2.2. Dysfunction of Intercellular Connections
2.3. Mucociliary Clearance Dysfunction
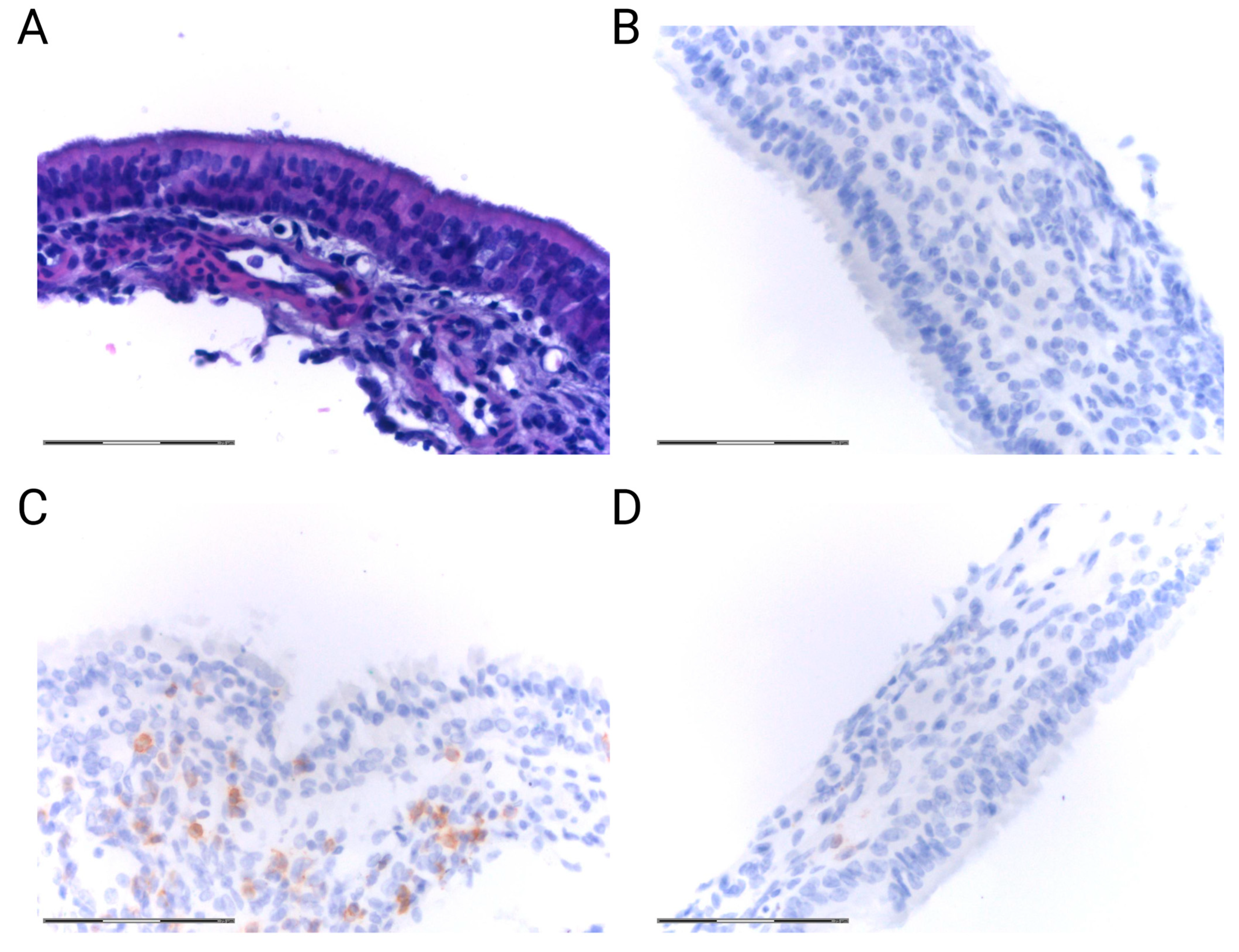
2.4. Inflammatory Response in the Epithelium
3. Contribution of PM2.5 to Upper Airway Disorders
3.1. Allergic Rhinitis
3.2. Rhinosinusitis
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| AJs | Adherence junctions |
| AKT | Protein kinase B |
| AMPKα | AMP-activated protein kinase alpha |
| AR | Allergic rhinitis |
| ARIA | Allergic Rhinitis and its impact on Asthma classification |
| BC | Black carbon |
| CAT | Catalase |
| CBF | Ciliary beat frequency |
| CCL20 | Chemokine (C-C motif) ligand 20 |
| CLDN | Claudin |
| CLDN-1 | Claudin-1 |
| CLDN-4 | Claudin-4 |
| CLDN-7 | Claudin-7 |
| CO | Carbon monoxide |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CRS | Chronic rhinosinusitis |
| CRSsNP | Chronic rhinosinusitis without nasal polyps |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| DCs | Dendritic cells |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| Drp1 | Dynamin-related protein 1 |
| ECP | Eosinophil cationic protein |
| ECs | Epithelial cells |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-activated kinase |
| Fis1 | Mitochondrial fission 1 protein |
| FTH1 | Ferritin heavy chain 1 |
| FTL | Ferritin light chain |
| GATA-3 | GATA binding protein 3 |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GPx4 | Glutathione peroxidase 4 |
| GRα | Glucocorticoid receptor-alpha |
| GSH-Px | Glutathione peroxidase |
| H&E | Hematoxylin and eosin |
| HNEpCs | Human nasal epithelial cells |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IgE | Immunoglobulin E |
| IL-1α | Interleukin-1 alpha |
| IL-1 β | Interleukin-1 beta |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-12p40 | The p40 subunit of interleukin 12 |
| IL-13 | Interleukin 13 |
| IL-17 | Interleukin 17 |
| IL-25 | Interleukin 25 |
| IL-33 | Interleukin 33 |
| ILC2s | Group 2 innate lymphoid cells |
| IFN-γ | Interferon gamma |
| MCC | Mucociliary clearance |
| MDA | Malondialdehyde |
| MFN1 | Mitofusin 1 |
| miR | microRNA |
| mRNAs | Messenger ribonucleic acid |
| mTOR | Mammalian target of rapamycin |
| MUC5A | Mucin 5A |
| MUC5B | Mucin 5B |
| MUC5AC | Mucin 5AC |
| NaCl | Sodium chloride |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLF | Nasal lavage fluid |
| NO2 | Nitrogen dioxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OPA1 | Mitochondrial dynamin-like GTPase |
| OVA | Ovalbumin |
| PI3K | Phosphoinositide 3-kinase |
| PM | Particulate matter |
| ROS | Reactive oxygen species |
| RosA | Rosmarinic acid |
| SO2 | Sulfur dioxide |
| SOD | Superoxide dismutase |
| Sos1 | Son of sevenless 1 |
| T-bet | T-box transcription factor TBX21 |
| TGF-β1 | Transforming growth factor beta 1 |
| Th1 | T helper 1 cells |
| Th2 | T helper 2 cells |
| Th17 | T helper 17 cells |
| Treg | T regulatory cells |
| TIMP-1 | Tissue inhibitor of metalloproteinase |
| TJs | Tight junctions |
| TNF-α | Tumor necrosis factor alpha |
| TSLP | Thymic stromal lymphopoietin |
| UA | Ursolic Acid |
| UBE2Q1 | A member of ubiquitin-conjugating enzyme family |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| WHO | World Health Organization |
| xCT | SLC7A11—cystine/glutamate antiporter |
| ZO | Zonula occludens |
| ZO-1 | Zonula occludens 1 |
| ZO-2 | Zonula occludens 2 |
References
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 10 June 2023).
- Katsouyanni, K. Ambient air pollution and health. Br. Med. Bull. 2003, 68, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Franchini, M. Health Effects of Ambient Air Pollution in Developing Countries. Int. J. Environ. Res. Public Health 2017, 14, 1048. [Google Scholar] [CrossRef] [PubMed]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef] [PubMed]
- Saraga, D.; Maggos, T.; Degrendele, C.; Klanova, J.; Horvat, M.; Kocman, D.; Kanduc, T.; Garcia Dos Santos, S.; Franco, R.; Gomez, P.M.; et al. Multi-city comparative PM2.5 source apportionment for fifteen sites in Europe: The ICARUS project. Sci. Total Environ. 2021, 751, 141855. [Google Scholar] [CrossRef]
- Salameh, D.; Detournay, A.; Pey, J.; Pérez, N.; Liguori, F.; Saraga, D.; Bove, M.C.; Brotto, P.; Cassola, F.; Massabò, D.; et al. PM2.5 chemical composition in five European Mediterranean cities: A 1-year study. Atmos. Res. 2015, 155, 102–117. [Google Scholar] [CrossRef]
- Majumdar, D. How are the Two Most Polluted Metro-cities of India Combating Air Pollution? Way Forward after Lifting of COVID-19 Lockdown. Aerosol Air Qual. Res. 2021, 21, 200463. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Chen, J.; Zhang, F.; He, C.; Zhao, J.; Yin, L. Seasonal variations and chemical compositions of PM2.5 aerosol in the urban area of Fuzhou, China. Atmos. Res. 2012, 104–105, 264–272. [Google Scholar] [CrossRef]
- Juda-Rezler, K.; Zajusz-Zubek, E.; Reizer, M.; Maciejewska, K.; Kurek, E.; Bulska, E.; Klejnowski, K. Bioavailability of elements in atmospheric PM2.5 during winter episodes at Central Eastern European urban background site. Atmos. Environ. 2021, 245, 117993. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R.P. Decline in PM2.5 concentrations over major cities around the world associated with COVID-19. Environ. Res. 2020, 187, 109634. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Y.; Yu, Z.; Lu, R. PM2.5 and Cardiovascular Diseases in the Elderly: An Overview. Int. J. Environ. Res. Public. Health 2015, 12, 8187–8197. [Google Scholar] [CrossRef] [PubMed]
- Carre, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Leandri, R. Does air pollution play a role in infertility?: A systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, C.; Tang, X. The Impact of PM2.5 on the Host Defense of Respiratory System. Front. Cell Dev. Biol. 2020, 8, 91. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef]
- Zhang, S.; Routledge, M.N. The contribution of PM2.5 to cardiovascular disease in China. Environ. Sci. Pollut. Res. Int. 2020, 27, 37502–37513. [Google Scholar] [CrossRef]
- Orellano, P.; Quaranta, N.; Reynoso, J.; Balbi, B.; Vasquez, J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS ONE 2017, 12, e0174050. [Google Scholar] [CrossRef]
- Ni, L.; Chuang, C.C.; Zuo, L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015, 6, 294. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Zhang, R.; Han, Z.; Huang, Y.; Deng, C.; Dong, W.; Zhuang, G. Effects of N-acetylcysteine on oxidative stress and inflammation reactions in a rat model of allergic rhinitis after PM2.5 exposure. Biochem. Biophys. Res. Commun. 2020, 533, 275–281. [Google Scholar] [CrossRef]
- Mady, L.J.; Schwarzbach, H.L.; Moore, J.A.; Boudreau, R.M.; Tripathy, S.; Kinnee, E.; Dodson, Z.M.; Willson, T.J.; Clougherty, J.E.; Lee, S.E. Air pollutants may be environmental risk factors in chronic rhinosinusitis disease progression. Int. Forum Allergy Rhinol. 2018, 8, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Ma, S.; Wang, K.; Lou, H.; Wang, Y.; Zhang, L.; Wang, C.; Akdis, C.A. Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells. Allergy Asthma Immunol. Res. 2020, 12, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M., Jr.; London, N.R., Jr.; Tharakan, A.; Surya, N.; Sussan, T.E.; Rao, X.; Lin, S.Y.; Toskala, E.; Rajagopalan, S.; Biswal, S. Airborne Particulate Matter Induces Nonallergic Eosinophilic Sinonasal Inflammation in Mice. Am. J. Respir. Cell Mol. Biol. 2017, 57, 59–65. [Google Scholar] [CrossRef]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-Induced Oxidative Stress and Mitochondrial Damage in the Nasal Mucosa of Rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Gao, W.; Li, Y.; Wang, Y.-F. Concentration-dependent effects of PM2.5 mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Favero, C.; Spinazze, A.; Cavallo, D.M.; Carugno, M.; Motta, V.; Bonzini, M.; Cattaneo, A.; Pesatori, A.C.; Bollati, V. Short-term particulate matter exposure influences nasal microbiota in a population of healthy subjects. Environ. Res. 2018, 162, 119–126. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, F.; Zhou, H.; Ren, H.; Du, Y.; Liang, S.; Wang, F.; Cheng, L.; Xie, X.; Jin, A.; et al. High-Level PM2.5/PM10 Exposure Is Associated With Alterations in the Human Pharyngeal Microbiota Composition. Front. Microbiol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Elam, T.; Raiculescu, S.; Biswal, S.; Zhang, Z.; Orestes, M.; Ramanathan, M. Air Pollution Exposure and the Development of Chronic Rhinosinusitis in the Active Duty Population. Mil. Med. 2022, 188, e1965–e1969. [Google Scholar] [CrossRef]
- Chu, H.; Xin, J.; Yuan, Q.; Wang, M.; Cheng, L.; Zhang, Z.; Lu, M. The effects of particulate matters on allergic rhinitis in Nanjing, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 11452–11457. [Google Scholar] [CrossRef]
- Duchen, M.R. Roles of mitochondria in health and disease. Diabetes 2004, 53 (Suppl. 1), S96–S102. [Google Scholar] [CrossRef]
- Pole, A.; Dimri, M.; Dimri, G.P. Oxidative stress, cellular senescence and ageing. AIMS Mol. Sci. 2016, 3, 300–324. [Google Scholar] [CrossRef]
- Kim, K.A.; Jung, J.H.; Kang, I.G.; Choi, Y.S.; Kim, S.T. ROS Is Involved in Disruption of Tight Junctions of Human Nasal Epithelial Cells Induced by HRV16. Laryngoscope 2018, 128, E393–E401. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Yeon, S.H.; Choi, M.R.; Jang, Y.S.; Kim, J.A.; Oh, H.W.; Jun, X.; Park, S.K.; Heo, J.Y.; Rha, K.S.; et al. Altered Mitochondrial Functions and Morphologies in Epithelial Cells Are Associated With Pathogenesis of Chronic Rhinosinusitis With Nasal Polyps. Allergy Asthma Immunol. Res. 2020, 12, 653–668. [Google Scholar] [CrossRef]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne Fine Particulate Matter Induces Oxidative Stress and Inflammation in Human Nasal Epithelial Cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zeng, P.; Zhuang, G.; Guo, Q.; Cai, C. Toxicological Effects of Artificial Fine Particulate Matter in Rats through Induction of Oxidative Stress and Inflammation. Tohoku J. Exp. Med. 2021, 255, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Gu, W.; Hou, T.; Zhou, H.; Zhu, L.; Zhu, W.; Wang, Y. Ferroptosis is involved in PM2.5-induced acute nasal epithelial injury via AMPK-mediated autophagy. Int. Immunopharmacol. 2023, 115, 109658. [Google Scholar] [CrossRef]
- Fariss, M.W.; Chan, C.B.; Patel, M.; Van Houten, B.; Orrenius, S. Role of mitochondria in toxic oxidative stress. Mol. Interv. 2005, 5, 94–111. [Google Scholar] [CrossRef]
- Jia, J.; Xia, J.; Zhang, R.; Bai, Y.; Liu, S.; Dan, M.; Li, T.; Yan, T.; Chen, L.; Gong, S.; et al. Investigation of the impact of PM2.5 on the ciliary motion of human nasal epithelial cells. Chemosphere 2019, 233, 309–318. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, Z.; Zhang, R.; Deng, C.; Xu, J.; Dong, W.; Hong, Z.; Yu, H.; Situ, H.; Liu, C.; et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J. Appl. Toxicol. 2018, 38, 678–687. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics. Pharmaceuticals 2011, 4, 429–456. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, C.; Zhang, L. Epithelial physical barrier defects in chronic rhinosinusitis. Expert. Rev. Clin. Immunol. 2019, 15, 679–688. [Google Scholar] [CrossRef]
- Kojima, T.; Go, M.; Takano, K.; Kurose, M.; Ohkuni, T.; Koizumi, J.; Kamekura, R.; Ogasawara, N.; Masaki, T.; Fuchimoto, J.; et al. Regulation of tight junctions in upper airway epithelium. Biomed. Res. Int. 2013, 2013, 947072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Van Crombruggen, K.; Gevaert, E.; Bachert, C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy 2016, 71, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.S. The apical junctional complex in respiratory diseases. Chonnam Med. J. 2014, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Inui, T.A.; Hirano, S.; Asano, S.; Okazaki, T.; Inui, T.; Marunaka, Y.; Nakahari, T. Intracellular Cl− Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy. Int. J. Mol. Sci. 2020, 21, 4052. [Google Scholar] [CrossRef]
- Bergougnoux, A.; Claustres, M.; De Sario, A. Nasal epithelial cells: A tool to study DNA methylation in airway diseases. Epigenomics 2015, 7, 119–126. [Google Scholar] [CrossRef]
- Rubin, B.K. Physiology of airway mucus clearance. Respir. Care 2002, 47, 761–768. [Google Scholar]
- Jiao, J.; Zhang, L. Influence of Intranasal Drugs on Human Nasal Mucociliary Clearance and Ciliary Beat Frequency. Allergy Asthma Immunol. Res. 2019, 11, 306–319. [Google Scholar] [CrossRef]
- Sobiesk, J.L.; Munakomi, S. Anatomy, Head and Neck, Nasal Cavity; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kaliner, M.; Marom, Z.; Patow, C.; Shelhamer, J. Human respiratory mucus. J. Allergy Clin. Immunol. 1984, 73, 318–323. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Peiser, C.; Dinh, Q.T.; Matthias, J.; Eynott, P.R.; Heppt, W.; Carlstedt, I.; Witt, C.; Fischer, A.; Chung, K.F. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope 2003, 113, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Camargo Pires-Neto, R.; Júlia Lichtenfels, A.; Regina Soares, S.; Macchione, M.; Hilário Nascimento Saldiva, P.; Dolhnikoff, M. Effects of São Paulo air pollution on the upper airways of mice. Environ. Res. 2006, 101, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.S.; Meyerholz, D.K.; Tang, X.X.; Reznikov, L.; Abou Alaiwa, M.; Ernst, S.E.; Karp, P.H.; Wohlford-Lenane, C.L.; Heilmann, K.P.; Leidinger, M.R.; et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 2016, 351, 503–507. [Google Scholar] [CrossRef]
- Paplinska-Goryca, M.; Misiukiewicz-Stepien, P.; Proboszcz, M.; Nejman-Gryz, P.; Gorska, K.; Zajusz-Zubek, E.; Krenke, R. Interactions of nasal epithelium with macrophages and dendritic cells variously alter urban PM-induced inflammation in healthy, asthma and COPD. Sci. Rep. 2021, 11, 13259. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, N.; Matsuda, N. Alert cell strategy: Mechanisms of inflammatory response and organ protection. Curr. Pharm. Des. 2014, 20, 5766–5778. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, F.C.L.; Nelson, H.S. Recent advances in allergic rhinitis. F1000Research 2018, 7, 1333. [Google Scholar] [CrossRef]
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. N. Am. 2016, 36, 235–248. [Google Scholar] [CrossRef]
- Kakli, H.A.; Riley, T.D. Allergic Rhinitis. Prim. Care 2016, 43, 465–475. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melen, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Drazdauskaite, G.; Layhadi, J.A.; Shamji, M.H. Mechanisms of Allergen Immunotherapy in Allergic Rhinitis. Curr. Allergy Asthma Rep. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Schwartz, G.; Bernstein, J.A. Allergic Rhinitis: Mechanisms and Treatment. Immunol. Allergy Clin. N. Am. 2016, 36, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.M.; Cripps, A.W.; West, N.P.; Cox, A.J. Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents. Front. Pharmacol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.W.; Eifan, A.; Penagos, M.; Dumitru, A.; Switzer, A.; McMahon, O.; Phippard, D.; Togias, A.; Durham, S.R.; Shamji, M.H. Local and systemic effects of cat allergen nasal provocation. Clin. Exp. Allergy 2015, 45, 613–623. [Google Scholar] [CrossRef]
- Baraniuk, J.N. Pathogenesis of allergic rhinitis. J. Allergy Clin. Immunol. 1997, 99, S763–S772. [Google Scholar] [CrossRef]
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Costache, A.; Berghi, O.N.; Cergan, R.; Dumitru, M.; Neagos, A.; Popa, L.G.; Giurcaneanu, C.; Vrinceanu, D. Respiratory allergies: Salicaceae sensitization (Review). Exp. Ther. Med. 2021, 21, 609. [Google Scholar] [CrossRef]
- Wise, S.K.; Lin, S.Y.; Toskala, E.; Orlandi, R.R.; Akdis, C.A.; Alt, J.A.; Azar, A.; Baroody, F.M.; Bachert, C.; Canonica, G.W.; et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int. Forum Allergy Rhinol. 2018, 8, 108–352. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gu, Z.W.; Cao, Z.W. Difference between CD25+Tregs and Helios+Tregs in a murine model of allergic rhinitis. Braz. J. Otorhinolaryngol. 2021, 87, 550–556. [Google Scholar] [CrossRef]
- Guo, Z.-Q.; Dong, W.-Y.; Xu, J.; Hong, Z.-C.; Zhao, R.-W.; Deng, C.-R.; Zhuang, G.-S.; Zhang, R.-X. T-helper type 1-T-helper type 2 shift and nasal remodeling after fine particulate matter exposure in a rat model of allergic rhinitis. Am. J. Rhinol. Allergy 2017, 31, 148–155. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Rui, X.; Zhou, L.; Mo, X. PM2.5 exposure exacerbates allergic rhinitis in mice by increasing DNA methylation in the IFN-γ gene promoter in CD4+T cells via the ERK-DNMT pathway. Toxicol. Lett. 2019, 301, 98–107. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM2.5 Exacerbates Oxidative Stress and Inflammatory Response through the Nrf2/NF-κB Signaling Pathway in OVA-Induced Allergic Rhinitis Mouse Model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef]
- Sun, N.; Han, Z.; Wang, H.; Guo, Z.; Deng, C.; Dong, W.; Zhuang, G.; Zhang, R. Effects of Ursolic Acid on the Expression of Th1-Th2-related Cytokines in a Rat Model of Allergic Rhinitis After PM2.5 Exposure. Am. J. Rhinol. Allergy 2020, 34, 587–596. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, L.; Chen, Y.; Chen, M. Sublingual immunotherapy increases Treg/Th17 ratio in allergic rhinitis. Open Med. (Wars) 2021, 16, 826–832. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, M.; Liu, Z. Th17 response and its regulation in inflammatory upper airway diseases. Clin. Exp. Allergy 2015, 45, 602–612. [Google Scholar] [CrossRef]
- Li, J.; Li, Y. Autophagy is involved in allergic rhinitis by inducing airway remodeling. Int. Forum Allergy Rhinol. 2019, 9, 1346–1351. [Google Scholar] [CrossRef]
- Wang, J.C.; Huang, Y.; Zhang, R.X.; Han, Z.J.; Zhou, L.L.; Sun, N.; Dong, W.Y.; Zhuang, G.S. miR-338-3p inhibits autophagy in a rat model of allergic rhinitis after PM2.5 exposure through AKT/mTOR signaling by targeting UBE2Q1. Biochem. Biophys. Res. Commun. 2021, 554, 1–6. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, Z.; Fan, E.; Li, Y.; Miyake, K.; Xu, X.; Zhang, L. Changes in gene expression in chronic allergy mouse model exposed to natural environmental PM2.5-rich ambient air pollution. Sci. Rep. 2018, 8, 6326. [Google Scholar] [CrossRef]
- Yang, G.; Suo, L.M.; Geng, X.R.; Zeng, X.H.; Liu, J.Q.; Liu, Z.Q.; Li, M.; Chen, Y.R.; Hong, J.Y.; Xue, J.M.; et al. An eosinophil-Sos1-RAS axis licenses corticosteroid resistance in patients with allergic rhinitis. Immunobiology 2022, 227, 152215. [Google Scholar] [CrossRef]
- Li, R.L.; Ho, Y.C.; Luo, C.W.; Lee, S.S.; Kuan, Y.H. Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, T.; Mao, J.H. Haze facilitates sensitization to house dust mites in children. Environ. Geochem. Health 2020, 42, 2195–2203. [Google Scholar] [CrossRef]
- Dabrowiecki, P.; Chcialowski, A.; Dabrowiecka, A.; Piorkowska, A.; Badyda, A. Exposure to ambient air pollutants and short-term risk for exacerbations of allergic rhinitis: A time-stratified, case-crossover study in the three largest urban agglomerations in Poland. Respir. Physiol. Neurobiol. 2023, 315, 104095. [Google Scholar] [CrossRef] [PubMed]
- Leland, E.M.; Zhang, Z.; Kelly, K.M.; Ramanathan, M., Jr. Role of Environmental Air Pollution in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2021, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef]
- Yang, X.; Shen, S.; Deng, Y.; Wang, C.; Zhang, L. Air Pollution Exposure Affects Severity and Cellular Endotype of Chronic Rhinosinusitis with Nasal Polyps. Laryngoscope 2021, 132, 2103–2110. [Google Scholar] [CrossRef]
- Ma, S.; Xian, M.; Wang, Y.; Wang, C.; Zhang, L. Budesonide repairs decreased barrier integrity of eosinophilic nasal polyp epithelial cells caused by PM2.5. Clin. Transl. Allergy 2021, 11, e12019. [Google Scholar] [CrossRef]
- Patel, T.R.; Tajudeen, B.A.; Brown, H.; Gattuso, P.; LoSavio, P.; Papagiannopoulos, P.; Batra, P.S.; Mahdavinia, M. Association of Air Pollutant Exposure and Sinonasal Histopathology Findings in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 761–767. [Google Scholar] [CrossRef]
- Qing, H.; Wang, X.; Zhang, N.; Zheng, K.; Du, K.; Zheng, M.; Li, Y.; Chang, Y.; Zhang, L.; Bachert, C. The Effect of Fine Particulate Matter on the Inflammatory Responses in Human Upper Airway Mucosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1315–1318. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, Z.; Dong, W.; Deng, C.; Han, Z.; Liu, J.; Wang, H.; Zhuang, G.; Zhang, R. Effects of PM2.5 on mucus secretion and tissue remodeling in a rabbit model of chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Hu, P.; Li, Y.; Cai, C.; Wang, X.; Zhang, L. PM2.5 Upregulates the Expression of MUC5AC via the EGFR-PI3K Pathway in Human Sinonasal Epithelial Cells. Int. Arch. Allergy Immunol. 2021, 183, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rowan, N.R.; Pinto, J.M.; London, N.R.; Lane, A.P.; Biswal, S.; Ramanathan, M., Jr. Exposure to Particulate Matter Air Pollution and Anosmia. JAMA Netw. Open 2021, 4, e2111606. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Deng, C.; Zhao, Q.; Han, Z.; Guo, Z.; Wang, H.; Dong, W.; Duan, Y.; Zhuang, G.; Zhang, R. Ursolic Acid Alleviates Mucus Secretion and Tissue Remodeling in Rat Model of Allergic Rhinitis After PM2.5 Exposure. Am. J. Rhinol. Allergy 2021, 35, 272–279. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Han, Z.; Wang, J.; Sun, N.; Zhang, R.; Dong, W.; Deng, C.; Zhuang, G. Effects of rosmarinic acid on the inflammatory response in allergic rhinitis rat models after PM2.5 exposure. J. Clin. Lab. Anal. 2022, 36, e24316. [Google Scholar] [CrossRef]
- Cergan, R.; Berghi, O.N.; Dumitru, M.; Vrinceanu, D.; Manole, F.; Serboiu, C.S. Biologics for Chronic Rhinosinusitis-A Modern Option for Therapy. Life 2023, 13, 2165. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.-H.; Hu, D.; Ke, X.; Gu, Z.; Zou, Q.-Y.; Hu, G.-H.; Song, S.-H.; Kang, H.-Y.; Hong, S.-L. The airway inflammation induced by nasal inoculation of PM2.5 and the treatment of bacterial lysates in rats. Sci. Rep. 2018, 8, 9816. [Google Scholar] [CrossRef]
| Allergic Rhinitis | Rhinosinusitis | ||
|---|---|---|---|
| PM2.5 effects on nasal and sinonasal mucosa | |||
| Increased release of TSLP and IL-33 by nasal epithelial cells | [26] | Increased sinonasal inflammation and epithelial thickening | [88] |
| Increased NF-κB expression in nasal epithelial cells | [75] | Damage to sinonasal epithelium and cilia morphological dysfunction | [24,93] |
| Increased vasodilatation, mucosal edema, and gland hyperplasia | [21,26,76] | Downregulation of proteins involved in epithelial barrier integrity:
| [24,90] |
| Increased number of goblet cells and mucus production | [73,81] | Goblet cell hyperplasia and mucus overproduction Altered MUC5AC expression | [93,94] |
| Increased autophagy | [80] | Collagen deposition and increased levels of fibroblasts, tissue remodeling | [93] |
| Infiltration of inflammatory cells into mucosa | |||
| ↑ Adhesion molecules ICAM-1 and VCAM-1 | [26] | Increased migration factors: IL-8 | [90] |
| Increased infiltration of eosinophils | [21,26,73,74,75,76] | Increased infiltration of neutrophils and macrophages | [24] |
| PM2.5 effect on immunological processes | |||
Decreased Th1 cell activity:
| [21,73] | Decreased IFN-γ and IL-12p40 | [24] |
Increased Th2 cell activity:
| [21,73,76] | Increased IL-5, IL-6 | [92] |
| Increased Th17 cell activity | [75] | ||
Decreased Treg cell activity:
| [75] | Increased IL-10 | [90] |
Increased B cell activity:
| [21,26,73] | ||
Increased activity of eosinophils:
| [21,73,76] | Increased activity of eosinophils: ↑ IL-13, eotaxin-1 | [88] |
Increased pro-inflammatory factors:
| [24,90,92] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaręba, Ł.; Piszczatowska, K.; Dżaman, K.; Soroczynska, K.; Motamedi, P.; Szczepański, M.J.; Ludwig, N. The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases. J. Pers. Med. 2024, 14, 98. https://doi.org/10.3390/jpm14010098
Zaręba Ł, Piszczatowska K, Dżaman K, Soroczynska K, Motamedi P, Szczepański MJ, Ludwig N. The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases. Journal of Personalized Medicine. 2024; 14(1):98. https://doi.org/10.3390/jpm14010098
Chicago/Turabian StyleZaręba, Łukasz, Katarzyna Piszczatowska, Karolina Dżaman, Karolina Soroczynska, Parham Motamedi, Mirosław J. Szczepański, and Nils Ludwig. 2024. "The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases" Journal of Personalized Medicine 14, no. 1: 98. https://doi.org/10.3390/jpm14010098
APA StyleZaręba, Ł., Piszczatowska, K., Dżaman, K., Soroczynska, K., Motamedi, P., Szczepański, M. J., & Ludwig, N. (2024). The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases. Journal of Personalized Medicine, 14(1), 98. https://doi.org/10.3390/jpm14010098








