Regulatory T Cells in Multiple Sclerosis Diagnostics—What Do We Know So Far?
Abstract
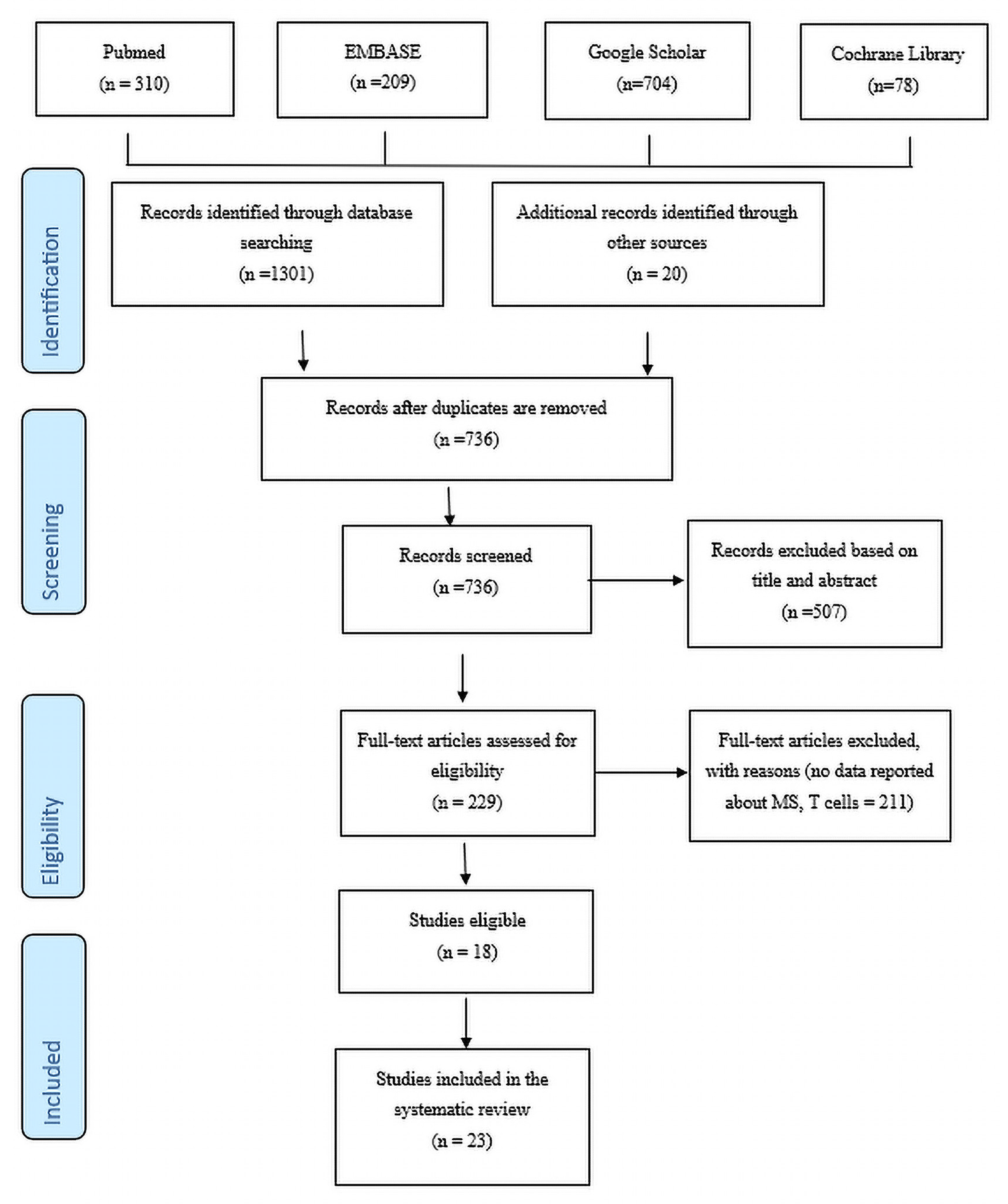
:1. Introduction
- (i)
- Regulatory T cells OR Treg cells OR T regulatory cells OR CD4+CD25+ regulatory T cells
- (ii)
- Regulatory T cells or Treg cells + experimental autoimmune encephalomyelitis (EAE)
- (iii)
- Multiple sclerosis OR MS OR central nervous system autoimmune disease
- (iv)
- Regulatory T cells or Treg cells for diagnosing multiple sclerosis
- (v)
- Regulatory T cells or Treg cells in therapy monitoring of multiple sclerosis.
Criteria for Inclusion and Exclusion of Studies
2. Results
Study Characteristics
3. Discussion
3.1. Treg Cells and EAE
3.2. Clinical Guidelines Using Regulatory T Cells Measurement in MS Patients
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sürücü, L.; Maslakci, A. Validity and reliability in quantitative research. Bus. Manag. Stud. Int. J. 2020, 8, 2694–2726. [Google Scholar] [CrossRef]
- Verma, N.D.; Lam, A.D.; Chiu, C.; Tran, G.T.; Hall, B.M.; Hodgkinson, S.J. Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+T regulatory cells. Sci. Rep. 2021, 11, 10476. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, S.S.; Kundu, S.; Ahmed, S.; Sultana, S.; Patar, A.; Hossan, T.; Krogias, C. Mesenchymal Stem Cell Therapy in Multiple Sclerosis. J. Clin. Med. 2023, 12, 6311. [Google Scholar] [CrossRef] [PubMed]
- The George Washington University. Finding Resources: Using Journals for Your Research; The George Washington University: Washington, DC, USA, 2021. [Google Scholar]
- Calahorra, L.; Camacho-Toledano, C.; Serrano-Regal, M.P.; Ortega, M.C.; Clemente, D. Regulatory cells in multiple sclerosis: From blood to brain. Biomedicines 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, M.R.; Ammitzbøll, C.; Hansen, R.H.; Petersen, E.R.; McWilliam, O.; Marquart, H.V.; Damm, P.; Sellebjerg, F. Proinflammatory CD20+ T cells in the pathogenesis of multiple sclerosis. Brain 2019, 142, 120–132. [Google Scholar] [CrossRef] [PubMed]
- McKinney, E.F.; Cuthbertson, I.; Harris, K.M.; Smilek, D.E.; Connor, C.; Manferrari, G.; Carr, E.J.; Zamvil, S.S.; Smith, K.G. A CD8+ NK cell transcriptomic signature associated with clinical outcome in relapsing-remitting multiple sclerosis. Nat. Commun. 2021, 12, 635. [Google Scholar] [CrossRef]
- Sabatino, J.J., Jr.; Wilson, M.R.; Calabresi, P.A.; Hauser, S.L.; Schneck, J.P.; Zamvil, S.S. Anti-CD20 therapy depletes activated myelin-specific CD8+ T cells in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 25800–25807. [Google Scholar] [CrossRef]
- Kitz, A.; Singer, E.; Hafler, D. Regulatory T cells: From discovery to autoimmunity. Cold Spring Harb. Perspect. Med. 2018, 8, a029041. [Google Scholar] [CrossRef]
- Kimura, K. Regulatory T cells in multiple sclerosis. Clin. Exp. Neuroimmunol. 2020, 11, 148–155. [Google Scholar] [CrossRef]
- Canto-Gomes, J.; Silva, C.S.; Rb-Silva, R.; Boleixa, D.; da Silva, A.M.; Cheynier, R.; Costa, P.; González-Suárez, I.; Correia-Neves, M.; Cerqueira, J.J.; et al. Low memory T cells blood counts and high naïve regulatory T cells percentage at relapsing remitting multiple sclerosis diagnosis. Front. Immunol. 2022, 13, 901165. [Google Scholar] [CrossRef] [PubMed]
- Visweswaran, M.; Hendrawan, K.; Massey, J.C.; Khoo, M.L.; Ford, C.D.; Zaunders, J.J.; Withers, B.; Sutton, I.J.; Ma, D.D.F.; Moore, J.J. Sustained immunotolerance in multiple sclerosis after stem cell transplant. Ann. Clin. Transl. Neurol. 2022, 9, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Csurhes, P.A.; Smith, C.; Douglas, N.L.; Neller, M.A.; Matthews, K.K.; Beagley, L.; Rehan, S.; Crooks, P.; Hopkins, T.J.; et al. Ep-stein-Barr virus–specific T cell therapy for progressive multiple sclerosis. JCI Insight 2018, 3, e124714. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Bhargava, P.; Smith, M.D.; Vizthum, D.; Henry-Barron, B.; Kornberg, M.D.; Cassard, S.D.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. eBioMedicine 2022, 82, 104124. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; De Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Tavaf, M.J.; Soltanmohammadi, A.; Zargarani, S.; Yazdanpanah, E.; Sadighimoghaddam, B.; Yousefi, B.; Sameni, H.R.; Haghmorad, D. Berberine promotes immunological outcomes and decreases neuroinflammation in the experimental model of multiple sclerosis through the expansion of Treg and Th2 cells. Immunity Inflamm. Dis. 2023, 11, e766. [Google Scholar] [CrossRef] [PubMed]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Moalem-Taylor, G. Adoptive transfer of regulatory T cells as a promising immunotherapy for treating multiple sclerosis. Front. Neurosci. 2019, 13, 1107. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Traxinger, B.R.; Richert-Spuhler, L.E.; Lund, J.M. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2022, 15, 398–407. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, W.; Lund, B.T.; Li, P.; Levy, A.M.; Kelland, E.E.; Akbari, O.; Groshen, S.; Cen, S.Y.; Pelletier, D.; Weiner, L.P.; et al. Repopulation of T, B, and NK cells following alemtuzumab treatment in relapsing-remitting multiple sclerosis. J. Neuroinflammation 2020, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.; Hernandez, G.; Hartman, L.; Maksimovic, J.; Nace, S.; Lawler, B.; Risa, T.; Cook, T.; Agni, R.; Reichelderfer, M.; et al. Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: Results of the HINT 2 clinical trial. Mult. Scler. J. 2019, 25, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Tench, C.R.; Constantinescu, C.S.; Telford, G.; Singh, S.; Frakich, N.; Onion, D.; Auer, D.P.; Gran, B.; Evangelou, N.; et al. Hook-worm treatment for relapsing multiple sclerosis: A randomized double-blinded placebo-controlled trial. JAMA Neurol. 2020, 77, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, A.; Tenbrock, K. Regulatory T cell function in autoimmune disease. J. Transl. Autoimmun. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Bin Emran, T.; Rabaan, A.A.; Al Mutair, A.; Al Alawi, Z.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccines Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Schlöder, J.; Shahneh, F.; Schneider, F.-J.; Wieschendorf, B. Boosting regulatory T cell function for the treatment of autoimmune diseases—That’s only half the battle! Front. Immunol. 2022, 13, 973813. [Google Scholar] [CrossRef]
- Mikami, N.; Kawakami, R.; Sakaguchi, S. New Treg cell-based therapies of autoimmune diseases: Towards antigen-specific immune sup-pression. Curr. Opin. Immunol. 2020, 67, 36–41. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg enhancing therapies to treat autoimmune diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Glatigny, S.; Höllbacher, B.; Motley, S.J.; Tan, C.; Hundhausen, C.; Buckner, J.H.; Smilek, D.; Khoury, S.J.; Ding, L.; Qin, T.; et al. Abatacept targets T follicular helper and regulatory T cells, disrupting molecular pathways that regulate their proliferation and maintenance. J. Immunol. 2019, 202, 1373–1382. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B-and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: Immunological patterns and clinical implications. Front. Immunol. 2022, 12, 796482. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Van der Veeken, J.; Gonzalez, A.J.; Cho, H.; Arvey, A.; Hemmers, S.; Leslie, C.S.; Rudensky, A.Y. Memory of inflammation in regulatory T cells. Cell 2016, 166, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.Y.; Sadlon, T.; Hope, C.M.; Wong, Y.Y.; Wong, S.; Liu, N.; Withers, H.; Brown, K.; Bandara, V.; Gundsambuu, B.; et al. Molecular insights into regulatory T-cell adaptation to self, environment, and host tissues: Plasticity or loss of function in autoimmune disease. Front. Immunol. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Chaudhry, A.; Rudensky, A.Y. Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Investig. 2013, 123, 939–944. [Google Scholar] [CrossRef]
- Billroth-MacLurg, A.C.; Ford, J.; Rosenberg, A.; Miller, J.; Fowell, D.J. Regulatory T cell numbers in inflamed skin are controlled by local in-flammatory cues that upregulate CD25 and facilitate antigen-driven local proliferation. J. Immunol. 2016, 197, 2208–2218. [Google Scholar] [CrossRef]
- Alvarez, F.; Al-Aubodah, T.-A.; Yang, Y.H.; Piccirillo, C.A. Mechanisms of TREG cell adaptation to inflammation. J. Leukoc. Biol. 2020, 108, 559–571. [Google Scholar] [CrossRef]
- Niec, R.E.; Rudensky, A.Y.; Fuchs, E. Inflammatory adaptation in barrier tissues. Cell 2021, 184, 3361–3375. [Google Scholar] [CrossRef]
- Vucic, S.; Ryder, J.; Mekhael, L.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.W.; Kiernan, M.C. Phase 2 randomized placebo con-trolled double blind study to assess the efficacy and safety of tecfidera in patients with amyotrophic lateral sclerosis (TEALS Study): Study protocol clinical trial (SPIRIT Compliant). Medicine 2020, 99, e18904. [Google Scholar] [CrossRef]
- Kojima, H.; Kashiwakura, Y.; Kanno, Y.; Hashiguchi, M.; Kobata, T. Fine-tuning of antigen-specific immune responses by regulatory T cells activated via antigen recognition–independent and humoral factor–dependent mechanisms. Scand. J. Immunol. 2021, 93, e13020. [Google Scholar] [CrossRef] [PubMed]
- Sambucci, M.; Gargano, F.; Guerrera, G.; Battistini, L.; Borsellino, G. One, no one, and one hundred thousand: T regulatory cells’ multiple identities in neuroimmunity. Front. Immunol. 2019, 10, 2947. [Google Scholar] [CrossRef] [PubMed]
- Verreycken, J.; Baeten, P.; Broux, B. Regulatory T cell therapy for multiple sclerosis: Breaching (blood-brain) barriers. Hum. Vaccines Immunother. 2022, 18, 2153534. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, C.B.; Huntemann, N.; Bock, S.; Nelke, C.; Kremer, D.; Pfeffer, K.; Meuth, S.G.; Ruck, T. Crosstalk of microorganisms and immune responses in autoimmune neuroinflammation: A focus on regulatory t cells. Front. Immunol. 2021, 12, 747143. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Hawiger, D. Peripherally induced regulatory T cells: Recruited protectors of the central nervous system against autoimmune neuroinflammation. Front. Immunol. 2017, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Howlett-Prieto, Q.; Feng, X.; Kramer, J.F.; Kramer, K.J.; Houston, T.W.; Reder, A.T. Anti-CD20 therapy corrects a CD8 regulatory T cell deficit in multiple sclerosis. Mult. Scler. J. 2021, 27, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, A.-H.; Bol, Y.; Damoiseaux, J.; Smolders, J.; Hupperts, R. Vitamin D 3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J. Neurol. Sci. 2017, 378, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Balogh, A.; Csizmadia, I.; Klug, L.; Kleinewietfeld, M.; Steiniger, J.; Šušnjar, U.; Müller, D.N.; Boschmann, M.; Paul, F. Metabolic, mental and immunological effects of normoxic and hypoxic training in multiple sclerosis patients: A pilot study. Front. Immunol. 2018, 9, 2819. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T cells driving multiple sclerosis: Identity, mechanisms and potential triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Rossi, B.; Constantin, G. Live imaging of immune responses in experimental models of multiple sclerosis. Front. Immunol. 2016, 7, 506. [Google Scholar] [CrossRef]
- Ghosh, D.; Curtis, A.D.; Wilkinson, D.S.; Mannie, M.D. Depletion of CD4+ CD25+ regulatory T cells confers susceptibility to experimental autoimmune encephalomyelitis (EAE) in GM-CSF-deficient Csf2−/− mice. J. Leukoc. Biol. 2016, 100, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Leavenworth, J.W.; Luo, L.; Hu, X.; Dixon, M.L.; Pope, B.J.; Leavenworth, J.D.; Raman, C.; Meador, W.R. Dysregulated follicular regulatory T cells and antibody responses exacerbate CNS autoimmunity. J. Immunol. 2021, 206, 51.11. [Google Scholar] [CrossRef]
- Dong, C. Cytokine regulation and function in T cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Uzonna, J.E. The pivotal role of regulatory T cells in the regulation of innate immune cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Koutrolos, M.; Berer, K.; Kawakami, N.; Wekerle, H.; Krishnamoorthy, G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol. Commun. 2014, 2, 163. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.L.; Greilach, S.A.; Othy, S.; Sears-Kraxberger, I.; Wi, B.; Ayala-Angulo, J.; Vu, E.; Pham, Q.; Silva, J.; Dang, K.; et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 2020, 140, 104868. [Google Scholar] [CrossRef] [PubMed]
- Hosseinalizadeh, H.; Rabiee, F.; Eghbalifard, N.; Rajabi, H.; Klionsky, D.J.; Rezaee, A. Regulating the regulatory T cells as cell therapies in autoimmunity and cancer. Front. Med. 2023, 10, 1244298. [Google Scholar] [CrossRef] [PubMed]
- Baeten, P.; Van Zeebroeck, L.; Kleinewietfeld, M.; Hellings, N.; Broux, B. Improving the efficacy of regulatory T cell therapy. Clin. Rev. Allergy Immunol. 2021, 62, 363–381. [Google Scholar] [CrossRef]
- Kashi, V.P.; Ortega, S.B.; Karandikar, N.J. Neuroantigen-specific autoregulatory CD8+ T cells inhibit autoimmune demyelination through modulation of dendritic cell function. PLoS ONE 2014, 9, e105763. [Google Scholar] [CrossRef]
- Wang, Y.; Sadike, D.; Huang, B.; Li, P.; Wu, Q.; Jiang, N.; Fang, Y.; Song, G.; Xu, L.; Wang, W.; et al. Regulatory T cells alleviate myelin loss and cognitive dysfunction by regulating neuroinflammation and microglial pyroptosis via TLR4/MyD88/NF-κB pathway in LPC-induced demyelination. J. Neuroinflammation 2023, 20, 41. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, F.; Lanuti, P.; Pierdomenico, L.; Simeone, P.; Bologna, G.; Ercolino, E.; Buttari, F.; Fantozzi, R.; Thomas, A.; Onofrj, M.; et al. The characterization of regulatory T-cell profiles in alzheimer’s disease and multiple sclerosis. Sci. Rep. 2019, 9, 8788. [Google Scholar] [CrossRef]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef] [PubMed]
- Docampo, M.J.; Lutterotti, A.; Sospedra, M.; Martin, R. Mechanistic and biomarker studies to demonstrate immune tolerance in multiple sclerosis. Front. Immunol. 2022, 12, 787498. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, R.; Zhang, M.; Qian, B.; Wang, B.; Yang, W. Recent progress in multiple sclerosis treatment using immune cells as targets. Pharmaceutics 2023, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Buc, M. Role of regulatory T cells in pathogenesis and biological therapy of multiple sclerosis. Mediat. Inflamm. 2013, 2013, 963748. [Google Scholar] [CrossRef]
- Khosravi, M.; Majdinasab, N.; Amari, A.; Ghadiri, A.A. Increased frequency of CD4+CD25high CD127low regulatory T cells in patients with multiple sclerosis. Gene Rep. 2019, 17, 100456. [Google Scholar] [CrossRef]
- Pohl, A.D.P.; Schmidt, A.; Zhang, A.-H.; Maldonado, T.; Königs, C.; Scott, D.W. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell. Immunol. 2020, 358, 104222. [Google Scholar] [CrossRef]
| Study Identification | Study Design | Research Subjects | Number of MS Cases | Number of Healthy Controls | Regulatory T Cell Definition | MS Sclerosis Duration | EDSS Criteria Reported by Authors | EDSS Score | Mean Age of MS | Mean Age in Healthy Controls | Conclusion | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Verma, N. D., Lam, A. D., Chiu, C., Tran, G. T., Hall, B. M., & Hodgkinson, S. J. (2021). Multiple sclerosis patients have reduced resting and increased activated CD4+ CD25+ FOXP3+ T regulatory cells. Scientific Reports, 11(1), 10476. | Cohort Studies | Human Subjects | N = 36 | N = 20 | CD4+ T | 8.9 (0.1–25.2) Years | McDonald Criteria 2017 | Baseline (MS) = 3.71 After Treatment = 3.04 No Treatment = 4.21 | MS = 42.5 (21–64) | HD = 41.7 (21–69) | The study found an increased proportion of CD25 Treg in most MS patients. | [3] |
| Choi, I. Y., Piccio, L., Childress, P., Bollman, B., Ghosh, A., Brandhorst, S., … & Longo, V. D. (2016). A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Reports, 15(10), 2136–2146. | Experimental Trial | EAE (Mice) | Experimental autoimmune encephalomyelitis (EAE) N = 23 Therapeutic FMD cycles (FMD(T); N = 23) | EAE CTRL N = 23 Ketogenic diet (EAE KD); N = 13 Semi-therapeutic FMD cycles (EAE FMD(S); N = 7 | CD4+ or CD8+ T cells in the spinal cord | 6 Months | Not Stated | CD (Baseline) = 0 (0 to 0) After Treatment = 0 (0 to 0.5) FMD (Baseline) = 0 (−1 to 0) After Treatment = 0 (−0.5 to 0.1) KD (Baseline) = 0 (−0.5 to 0) After Treatment = 0 (−0.5 to 0) (p < 0.05) 3 | Not Indicated | Not Indicated | The fasting-mimicking diet (FMD) increased Treg cell level, causing autoimmune cells to regenerate. | [22] |
| Gilmore, W., Lund, B. T., Li, P., Levy, A. M., Kelland, E. E., Akbari, O., … & Traboulsee, A. L. (2020). Repopulation of T, B, and NK cells following alemtuzumab treatment in relapsing-remitting multiple sclerosis. Journal of Neuroinflammation, 17(1), 1–21. | Clinical Trial | Human Subjects | N = 28 | None | CD4+ CD8+ | 48 Months | McDonald Criteria | Baseline = 5.5 After Treatment = 2.2 | Not Indicated | Not Indicated | The study found that 11 patients developed new T2 lesions while 8 had relapses. The findings point to a positive impact in decreasing levels of T cells to treat MS. | [23] |
| Fleming, J., Hernandez, G., Hartman, L., Maksimovic, J., Nace, S., Lawler, B., … & Fabry, Z. (2019). Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: Results of the HINT 2 clinical trial. Multiple Sclerosis Journal, 25(1), 81–91. | Clinical Trial | Human Subjects | N = 16 | None | CD25+, CD127−, CD4+ | 14 Months | McDonald Criteria | Baseline = 1.3 ± 0.9 Last Treatment = 1.1 ± 1.3 | 32 (±8) | 32 | The Trichuris suis (TSO) proved effective in relapsing MS symptoms, and the T cell volume significantly improved. | [24] |
| Tanasescu, R., Tench, C. R., Constantinescu, C. S., Telford, G., Singh, S., Frakich, N., … & Pritchard, D. I. (2020). Hookworm treatment for relapsing multiple sclerosis: A randomised double-blinded placebo-controlled trial. JAMA Neurology, 77(9), 1089–1098. | Randomized Double-Blinded Placebo-Controlled Trial | Human Subjects | N = 35 | N = 36 | CD4+ | 36 Weeks | McDonald Criteria | Placebo 3 (1.5–5) Hookworm 3 (1.5–5) | 45 | 36 | The group undergoing the Hookworm treatment experienced relief in symptoms and a subsequent increase in T cells. | [25] |
| Mähler, A., Balogh, A., Csizmadia, I., Klug, L., Kleinewietfeld, M., Steiniger, J., … & Paul, F. (2018). Metabolic, mental and immunological effects of normoxic and hypoxic training in multiple sclerosis patients: A pilot study. Frontiers in Immunology, 9, 2819. | Randomized Single-Blinded Parallel-Group Study | Human Subjects | N = 34 | N = 16 | CD4+ CD31+ | 4 Weeks | McDonald Criteria | Baseline = <4.5 | 40 | 40 | The training did not increase or decrease the amount of CD39+ and CD31+ Tregs. Therefore, the T cells could not account for the increase in erythropoietin. | [49] |
| Glatigny, S., Höllbacher, B., Motley, S. J., Tan, C., Hundhausen, C., Buckner, J. H., … & Bettelli, E. (2019). Abatacept targets T follicular helper and regulatory T cells, disrupting molecular pathways that regulate their proliferation and maintenance. The Journal of Immunology, 202(5), 1373–1382. | Double-Blinded Placebo-Controlled Trial | Human Subjects | N = 65 | N = 19 | CD4+ | 52 Weeks | Not Stated | Not Indicated | Not Indicated | Not Indicated | The Abatacept treatment showed a selective decrease in CD4+ T follicular helper (Tfh) and regulatory T cells, revealing its inability to support sustained tolerance. However, the generation of the T cells improved the patient’s symptoms. | [31] |
| McKinney, E. F., Cuthbertson, I., Harris, K. M., Smilek, D. E., Connor, C., Manferrari, G., … & Smith, K. G. (2021). A CD8+ NK cell transcriptomic signature associated with clinical outcome in relapsing-remitting multiple sclerosis. Nature Communications, 12(1), 635. | Clinical Trial | Human Subjects | N = 79 | N = 225 | CD4+ CD8+ | 18 Months | Not Stated | Not Indicated | Not Indicated | Not Indicated | The findings revealed that NK8+ killer cells, a subset of CD8+, have surrogate markers that indicate a relapse in remitting multiple sclerosis. | [8] |
| Iannetta, M., Landi, D., Cola, G., Campogiani, L., Malagnino, V., Teti, E., Coppola, L., Di Lorenzo, A., Fraboni, D., Buccisano, F., Grelli, S., Mozzani, M., Zingaropoli, M. A., Ciardi, M. R., Nisini, R., Bernardini, S., Andreoni, M., Marfia, G. A., & Sarmati, L. (2022). B- and T-Cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease-modifying therapies: Immunological patterns and clinical implications. Frontiers in Immunology, 12, 796482. | Clinical Trial | Human Subjects | N = 40 | N = 30 | CD4+ or CD8+ T cells in the spinal cord | 6 Months | McDonald Criteria | Baseline = 2 (0–3.0) Ocrelizumab (OCR) = 2 (2.0–4.5) Fingolimod (FTY) = 1.5 (1.0–3.0) Natalizumab (NAT) = 0.5 (0.0–2.5) | Not Indicated | Not Indicated | The SARS-CoV-2 vaccination triggered T cell responses, leading to improved symptoms. | [32] |
| Rolf, L., Muris, A. H., Bol, Y., Damoiseaux, J., Smolders, J., & Hupperts, R. (2017). Vitamin D3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. Journal of the Neurological Sciences, 378, 30–35. | Randomized Pilot Study | Human Subjects | N = 20 | N = 20 | CD8+ T cells | 48 Weeks | Placebo = 2.0 (1.5–2.3) Vitamin D3 = 2.0 (1.5–2.5) | Vitamin D3 = 38.5 | Placebo 37.6 | The study found that the stimulated CD8+ T cells caused no significant differences in pro- and anti-inflammatory cytokine balances. | [48] | |
| Von Essen, M. R., Ammitzbøll, C., Hansen, R. H., Petersen, E. R. S., McWilliam, O., Marquart, H. V., Damm, P., & Sellebjerg, F. (2019). Proinflammatory CD20+ T cells In the pathogenesis of multiple sclerosis. Brain: A Journal of Neurology, 142(1), 120–132. | Clinical Trial | Human Subjects | N = 25 | N = 25 | CD3 CD4 CD20+ | 2 Years | McDonald Criteria 2010 | CDSS Improved from the Baseline | 37 | 36 | The findings showed an increased amount of CD20+ T cells in the blood of MS patients. | [7] |
| Vucic, S., Ryder, J., Mekhael, L., Henderson, R. D., Mathers, S., Needham, M., … & Kiernan, M. C. (2020). Phase 2 randomized placebo-controlled double-blind study to assess the efficacy and safety of tecfidera in patients with amyotrophic lateral sclerosis (TEALS Study): Study protocol clinical trial (SPIRIT Compliant). Medicine, 99(6). | Randomized Placebo-Controlled Double-Blinded Trial | Human Subjects | N = 60 | N = 30 | CD4+ T cells CD45RO+ | 40 Weeks | Not Stated | Not Indicated | Not Indicated | Not Indicated | The findings reveal that increasing CD4+ T cell count improves the body’s neurological functions and prolongs the patient’s survival. | [41] |
| Cignarella, F., Cantoni, C., Ghezzi, L., Salter, A., Dorsett, Y., Chen, L., … & Piccio, L. (2018). Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metabolism, 27(6), 1222–1235. | Randomized Controlled Trial | Human Subjects | N = 17 | N = 10 | CD4+ T cells | 4 Weeks | McDonald Criteria | AD Libitum (Baseline) = 3.7 (2.7–5.2) Intermittent Fasting (Baseline) = 3.7 (3.2–4) | 40 ± 12 | 42 ± 8.2 | The findings revealed that intermittent fasting (IF) increased the number of regulatory T cells, improving the MS symptoms. | [33] |
| Tavaf, M. J., Soltanmohammadi, A., Zargarani, S., Yazdanpanah, E., Sadighimoghaddam, B., Yousefi, B., … & Haghmorad, D. (2023). Berberine promotes immunological outcomes and decreases neuroinflammation in the experimental model of multiple sclerosis through the expansion of Treg and Th2 cells. Immunity, Inflammation and Disease, 11(1), e766. | Experimental Trial | Mice | 6 Mice 2 Mice—Low Dose Berberine 2 Mice—High Dose Berberine | 2 Mice Control | CD4+ T cells | 25 Days | Not Stated | Not Indicated | 8–10 Weeks | 8–10 Weeks | The treatment groups had decreased pro-inflammatory cytokines, which relieved the inhibition of Treg cells and hence improved symptoms. | [17] |
| Montalban, X., Hauser, S. L., Kappos, L., Arnold, D. L., Bar-Or, A., Comi, G., de Seze, J., Giovannoni, G., Hartung, H. P., Hemmer, B., Lublin, F., Rammohan, K. W., Selmaj, K., Traboulsee, A., Sauter, A., Masterman, D., Fontoura, P., Belachew, S., Garren, H., Mairon, N., … ORATORIO Clinical Investigators (2017). Ocrelizumab versus placebo in primary progressive multiple sclerosis. The New England Journal of Medicine, 376(3), 209–220. | Randomized Placebo-Controlled Trial | Human Subjects | N = 732 | N = 244 | CD3+ CD4+ CD8+ cells | 12 Weeks | McDonald Criteria | Baseline = 3.0–6.5 After Treatment = 1.5 (1.0–3.0) | 43.2 | 40.1 | The infusion of ocrelizumab was associated with reduced clinical disease progression compared with the placebo group. | [16] |
| Pender, M. P., Csurhes, P. A., Smith, C., Douglas, N. L., Neller, M. A., Matthews, K. K., Beagley, L., Rehan, S., Crooks, P., Hopkins, T. J., Blum, S., Green, K. A., Ioannides, Z. A., Swayne, A., Aftab, B. T., Hooper, K. D., Burrows, S. R., Thompson, K. M., Coulthard, A., & Khanna, R. (2018). Epstein–Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight, 3(22), e124714. | Clinical Trial | Human Subjects | N = 13 | N = 13 | CD4+ CD8+ T cells | 27 Weeks | Revised McDonald Criteria | Baseline = 8.0 After Treatment ≤ 6.5 | Not Indicated | Not Indicated | The EBV-specific T cell therapy reduced the EDSS score, preventing further autoimmune attacks. | [14] |
| Fitzgerald, K. C., Bhargava, P., Smith, M. D., Vizthum, D., Henry-Barron, B., Kornberg, M. D., Cassard, S. D., Kapogiannis, D., Sullivan, P., Baer, D. J., Calabresi, P. A., & Mowry, E. M. (2022). Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. eBioMedicine, 82, 104124. | Randomised Controlled Feeding Study | Human Subjects | N = 36 | N = 12 | CD4+ CD+ T cells | 8 Weeks | McDonald Criteria | Baseline < 6.5 Intermittent CR = 1.75 (0.72) Daily CR = 1.67 (0.91) Control CR = 1.08 (1.14) | 37.4 | 37.4 | The findings show that an intermittent CR diet reduced T cell subsets and specific biologically relevant lipid markers, causing a significant reduction in the effector memory for MS markers. | [15] |
| Visweswaran, M., Hendrawan, K., Massey, J. C., Khoo, M. L., Ford, C. D., Zaunders, J. J., Withers, B., Sutton, I. J., Ma, D. D. F., & Moore, J. J. (2022). Sustained immunotolerance in multiple sclerosis after stem cell transplant. Annals of Clinical and Translational Neurology, 9(2), 206–220. | Randomized Clinical Trial | Human Subjects | N = 22 | N = 18 | CD4+ CD8+ CD57+ T cells | 36 Months | McDonald Criteria | Baseline = 7.0 After Treatment = 2.0 | 34 | 34.5 | The study findings revealed that the Autologous Hematopoietic Stem Cell Transplantation increased the CD4+ Tregs and CD39+ Treg percentages, lowering disease symptoms. | [13] |
| Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta neuropathologica communications. 2014 Dec;2:1–7. | Experimental Trial | EAE (DEREG Mice) | N = 11 | N = 5 | CD45+ CD4+ FoxP3− T cells | 6 Days | Not Stated | Not Indicated | Not Indicated | Not Indicated | The study findings showed that the absence of regulatory T cells decreased the velocity of effector T cells. The study concludes that regulatory T cells mediate recovery from EAE. | [56] |
| Leavenworth JW, Luo L, Hu X, Dixon ML, Pope BJ, Leavenworth JD, Raman C, Meador WR. Dysregulated follicular regulatory T cells and antibody responses exacerbate CNS autoimmunity. The Journal of Immunology. 2021 May 1;206(1_Supplement):51–11. | Experimental Trial | EAE (Mice) | N = 5 | N = 5 | CD103 (C) CD69 (D) FoxP3+ Tregs | 20 Days | Not Stated | Not Indicated | 7 Weeks | 7 Weeks | The study revealed that mice with FoxP3-specific deletion of Blimp1 developed severe EAE and did not recover compared with the mice in the control group. | [53] |
| McIntyre LL, Greilach SA, Othy S, Sears-Kraxberger I, Wi B, Ayala-Angulo J, Vu E, Pham Q, Silva J, Dang K, Rezk F. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiology of disease. 2020 Jul 1;140:104868. | Experimental Studies | EAE (Mice) | eGFP-mNSCs (N = 12) | PBS (N = 13) | CD4+ CD25+ FoxP3+ regulatory T cells | 21 Days | Not Stated | Not Indicated | 8 Weeks | 8 Weeks | The study showed that eight weeks of mice receiving hNSCs at the chronic stage experienced reduced neuroinflammation, remyelination, and an increase in CD4+, CD25+, FoxP3+ regulatory T cells. | [57] |
| Kashi VP, Ortega SB, Karandikar NJ. Neuroantigen-specific autoregulatory CD8+ T cells inhibit autoimmune demyelination through modulation of dendritic cell function. Plos one. 2014 Aug 21;9(8): e105763. | Experimental Studies | EAE (Mice) | N = 15 | N = 15 | CD8+ CD4+ T cells | 12 Days | Not Stated | Not Indicated | 7 Weeks | 7 Weeks | The study found that CD8+ T cells inhibit autoimmune demyelination in EAE, relieving the disease symptoms. | [60] |
| Ghosh, D., Curtis, A. D., Wilkinson, D. S., & Mannie, M. D. (2016). Depletion of CD4+ CD25+ regulatory T cells confers susceptibility to experimental autoimmune encephalomyelitis (EAE) in GM-CSF-deficient csf2−/− mice. Journal of Leukocyte Biology, 100(4), 747–760. https://doi.org/10.1189/jlb.3a0815-359r | Experimental Studies | EAE (Mice) | N = 18 | N = 19 | e CD4+ CD25+ FoxP3+ T cell | 41 Days | Not Stated | Not Indicated | Not Indicated | Not Indicated | The study findings show that Csf2-deficient mice resisted EAE due to the imbalance between T cells and effector T cells. | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B. Regulatory T Cells in Multiple Sclerosis Diagnostics—What Do We Know So Far? J. Pers. Med. 2024, 14, 29. https://doi.org/10.3390/jpm14010029
Arneth B. Regulatory T Cells in Multiple Sclerosis Diagnostics—What Do We Know So Far? Journal of Personalized Medicine. 2024; 14(1):29. https://doi.org/10.3390/jpm14010029
Chicago/Turabian StyleArneth, Borros. 2024. "Regulatory T Cells in Multiple Sclerosis Diagnostics—What Do We Know So Far?" Journal of Personalized Medicine 14, no. 1: 29. https://doi.org/10.3390/jpm14010029
APA StyleArneth, B. (2024). Regulatory T Cells in Multiple Sclerosis Diagnostics—What Do We Know So Far? Journal of Personalized Medicine, 14(1), 29. https://doi.org/10.3390/jpm14010029





